Abstract
Background
Repetitive transcranial magnetic stimulation (RTMS) targeting the left dorsolateral prefrontal cortex (DLPFC) is a treatment option for patients with medication-resistant major depressive disorder (MDD). However, antidepressant response is variable and there are currently no response predictors with sufficient accuracy for clinical use.
Objective
We report on results of an observational open-label study to determine whether the modulatory effect of 10 Hz motor cortex (MC) rTMS is predictive of the antidepressant effect of 10 Hz DLPFC rTMS.
Methods
Fifty-one medication-resistant MDD patients were enrolled for a 10-day treatment course of DLPFC rTMS and antidepressant response was assessed according to post-treatment reduction of the 17-item Hamilton Rating Scale for Depression score. Prior to treatment, we assessed the modulation of motor evoked potential (MEP) amplitude by MC rTMS. MEP’s were induced with single TMS pulses and measured using surface electromyography. MEP modulation was calculated as the change of mean MEP amplitude after MC rTMS.
Results
MEP modulation proved to be a robust predictor of reduction of clinician-rated depression severity following the course of DLPFC rTMS: larger MC rTMS-induced increase of corticospinal excitability anticipated a better antidepressant response. This was found both in univariate analyses (Spearman regression: rho=0.43, p<0.005) and a multivariable linear regression model (β=0.25, p<0.0001) controlling for baseline depression severity, age and resting motor threshold.
Conclusions
These findings suggest that MC rTMS-induced modulation of corticospinal excitability warrants further evaluation as a potential predictive biomarker of antidepressant response to left DLPFC 10 Hz rTMS.
Keywords: MDD, rTMS, dorsolateral prefrontal cortex, motor cortex, excitability, antidepressant response prediction
Introduction
Major depressive disorder (MDD) is a common disorder, frequently with a chronic and disabling course (1), and partial or non-response to first-line treatment options (2, 3). Transcranial magnetic stimulation (TMS), a technique based on electromagnetic induction, allows for focal non-invasive modulation of neural activity in discrete cortical regions (4). Repetitive TMS (rTMS) has therapeutic effects in MDD when applied at high frequencies (10 or 20Hz) to the left dorsolateral prefrontal cortex (DLPFC) (5–7), and is useful in patients with medication-resistant MDD (8). However, not all DLPFC rTMS candidates respond to treatment, with certain factors, such as age, medication resistance and episode duration (8–10), predicting poor antidepressant response, and others, such as psychomotor retardation and baseline sleep disturbance (9, 11), predicting enhanced response. Unfortunately, while these factors predict antidepressant response to rTMS at a group level, they are not sufficiently accurate to guide decisions regarding individual patients (e.g., patient selection).
Variability in antidepressant efficacy of rTMS also depends on treatment parameters, namely stimulation intensity (12) and stimulation site (13), raising the possibility of individualizing such parameters in order to optimize antidepressant response (12, 13). To this end, definition of rTMS-related biomarkers will be instrumental for accurate identification of patients in need of parameter adjustment (i.e., those who would otherwise not improve with DLPFC rTMS) and for correct definition of individual parameter adjustments (14). Intrinsic connectivity has been proposed as a biomarker for individualization of the stimulation target (15, 16), but strategies to optimize rTMS stimulation intensity are lacking. Currently, in an attempt to balance treatment efficacy and safety, intensity is adjusted for each patient as a percentage of the resting motor threshold (RMT), i.e the minimum intensity needed to reliably produce an electromyographic (EMG) or movement response in a finger, when the contralateral motor cortex (MC) is stimulated (17). RMT-adjustment of stimulation intensity for safety purposes is unquestioned (17). However, the relationship of RMT with final antidepressant response is equivocal (10, 18), possibly because rTMS intensity is associated with antidepressant response (19), and absolute intensity is defined according to RMT. Finally, other biomarkers proposed for rTMS intensity adjustment, namely coil-to-cortex distance, an indirect measure of cerebral atrophy, were of limited success (20).
It is thought that the therapeutic antidepressant effects of rTMS are mediated by modulation of prefrontal cortex excitability (5, 21). However, measurements of the relationship between rTMS-induced modulation of cortical excitability and clinical response to DLPFC rTMS have not been performed. Such studies could provide novel biomarkers for patient selection and individualization of treatment parameters and, in addition, contribute towards a better understanding of the mechanisms underlying rTMS efficacy. Here we examined whether modulation of motor cortex excitability by rTMS, measured prior to DLPFC rTMS treatment, is predictive of antidepressant treatment efficacy. Excitability modulation of the motor cortex, rather than the prefrontal cortex, was tested because it can be readily assessed by measures of corticospinal excitability, such as the amplitude of TMS-induced motor evoked potentials (MEP) (22). We hypothesized that facilitatory modulation of corticospinal excitability would be related to an enhancement of antidepressant response.
Material and Methods
Subjects
To address our hypothesis, an observational open-label study was conducted in medication-resistant outpatients, fulfilling DSM-IV criteria for the diagnosis of MDD, and who had failed at least three trials of adequate psychopharmacology treatment. Exclusion criteria were based on international safety guidelines for use of TMS (17). Participants were selected from 73 patients referred to the Berenson-Allen Center for Noninvasive Brain Stimulation for rTMS for treatment of MDD (Figure 1), 51 of who were eligible and consented to participate. In these participants, a stable antidepressant medication regimen was maintained 4 weeks prior to the trial and throughout rTMS treatment. Five participants did not complete the rTMS treatment protocol and one had missing data regarding primary and secondary outcomes. The study was carried out in accordance with the Declaration of Helsinki and approved by the Beth Israel Deaconess Medical Center’s Internal Review Board. Informed consent for experimentation with human subjects was obtained from all subjects.
Figure 1.
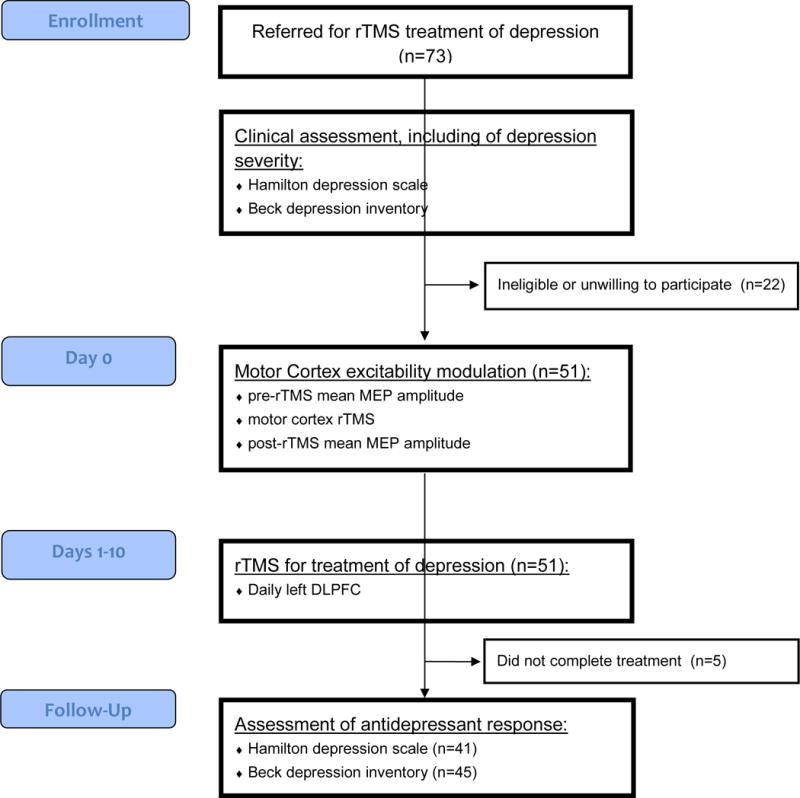
Flowchart and timeline of experimental procedures.
Eligible patients were assessed for depression severity and motor cortex excitability, prior to 10 daily sessions of DLPFC rTMS, performed over 2 weeks. After treatment, depression severity was assessed again, to measure clinical response.
Clinical ratings and response classification
Severity of depression was assessed at baseline and after 2 weeks of rTMS treatment, with the clinician-rated 17-item Hamilton Rating Scale for Depression (HAM-D-17 (23, 24)), administered by a board-certified psychiatrist, and the self-report 21-item Beck Depression Inventory-II (BDI-II (25, 26)). Clinical response to rTMS was calculated as the percentage of score reduction after the second week of treatment, relative to baseline, on the HAM-D-17 and BDI-II scores . Positive values reflect a decrease in HAM-D-17 or BDI-II scores after treatment, representing improvement in depression symptoms after rTMS, while negative values denote worsening of severity of symptoms. Exploratory analyses were conducted on the number of patients responding to treatment (responders), defined according to a reduction of symptom severity of at least 50% after 2 weeks of treatment, as measured by HAM-D-17 total scores.
TMS procedures
TMS was performed using a Magstim SuperRapid Stimulator (Magstim Company Ltd., UK) equipped with a commercially available 70-mm figure-of-eight coil. Sites for TMS were marked on a tightly fitting swimming cap placed on each patient’s head, to ensure accurate repositioning of the coil. For all procedures, the coil was held at approximately 45° to the midline and positioned tangentially to the skull with the handle pointing backward. Patients were seated in a comfortable chair with the elbow semi-flexed, and instructed to keep their hands as relaxed as possible. Resting motor threshold, established prior to all rTMS sessions, was defined using EMG techniques and according to international recommendations (27), as the lowest intensity of a single TMS pulse capable of eliciting at least 5 MEPs, with amplitude of at least 50 μV peak-to-peak, in a series of 10 consecutive single pulses delivered to the MC. Muscle activity was recorded with surface electrodes (Ag-AgCl, 10 mm diameter) overlying the right abductor pollicis brevis (APB) muscle, and surface EMG signals were amplified (×1000), filtered (20–1000 Hz) and sampled at 2000 Hz (PowerLab 4/25T, AD Instruments Ltd., Australia; Scope, version 4.0). The optimal scalp position over the MC to elicit maximal amplitude MEPs in the APB was identified (APB ‘hotspot’), and pulses were delivered with an inter-stimulus interval of at least 7s.
In an initial rTMS session (day 0), we assessed the modulation of MC excitability by rTMS (22), in accordance with methods previously applied by Maeda and colleagues to obtain mostly, but not exclusively, MEP facilitation in a sample of healthy individuals (28). For that purpose, MEPs were induced using single TMS pulses, delivered to the MC at an intensity of 120% of RMT, with a random stimulus interval of approximately 10 seconds (±1 second). Muscle relaxation was monitored through visual inspection of EMG signal, to ensure that single-pulses were delivered in the absence of active muscle contraction. MEP amplitude was measured peak-to-peak and averaged across 10 consecutive MEPs. Patients then received a single rTMS session over the APB ‘hotspot’ with the same parameters as those used for treatment: twenty 8-second long 10 Hz stimulation trains at 90% RMT intensity, with 52-second inter-train intervals (1600 stimuli). Approximately 30 s after completion of MC rTMS MEP amplitude was measured again, in the same manner as prior to rTMS. During both the MEP amplitude assessments and the delivery of MC rTMS, muscle relaxation was carefully monitored through visual inspection of hand and wrist muscle twitching, which was not found. An index of modulation of MC excitability was calculated as the percentage change of mean MEP amplitude, post-rTMS relative to pre-rTMS , with positive values (MEP amplitude increase) reflecting facilitation of cortical excitability by rTMS, and negative values (MEP amplitude decrease) representing suppression (table 1). Both patients and the investigators administering therapeutic rTMS were kept blind to these results. The therapeutic rTMS protocol consisted of 10 daily sessions (delivered in 5 consecutive sessions per week over the DLPFC, defined as a site 5 cm anterior to the APB ‘hotspot’, in the same parasagittal plane. In each treatment session, rTMS was delivered as described for MC rTMS.
Table 1.
Demographic, neurophysiologic and clinical characteristics of the study sample. Relationship of the main outcome variable with the remaining variables.
| Variables | n | Mean ± SD | Range | Relationship with %reduction HAM-D-17 | |
|---|---|---|---|---|---|
| % reduction HAM-D-17 | 41 | 22.7 ± 23.7 | 19.4 – 91.3 | ||
| % reduction BDI-II | 43 | 18.2 ± 29.7 | −48.4 – 82.1 | r=0.69a | p<0.001 |
| Gender (% male) | 51 | 43.1% | t=−0.3c | p=0.8 | |
| Handedness (% right) | 51 | 92.2% | t=1.3c | p=0.2 | |
| Age (years) | 51 | 46.5 ± 12.4 | 18–78 | r=−0.14a | p=0.4 |
| % change MEP amplitude | 51 | 8 ± 49 | −78–190 | rho=0.43b | p<0.005 |
| RMT (baseline) | 49 | 57 ± 12.3 | 27–86 | r=−0.15a | p=0.4 |
| HAM-D-17 (baseline) | 43 | 31.3 ± 7.5 | 16–44 | r=−0.39a | p<0.015 |
| BDI-II (baseline) | 46 | 29.9 ± 9.8 | 10–49 | r=0.02a | p=0.9 |
n – number of valid observations for each variable; SD – standard deviation.
Statistical methods
Analyses were performed using SAS software (version 9.3, SAS Institute, Cary, North Carolina). Data for continuous measurements is presented as the mean ± standard deviation (SD). Assessment of normal distribution of continuous measurements was performed according to analysis of kurtosis, skewness and comparison of mean and median. Only % change of MEP amplitude was not normally distributed. For univariate analyses of binary predictors of clinical response (gender and handedness), outcome measures were compared between groups using unpaired t-tests. For continuous predictor variables, univariate analyses of correlation with outcome measures were conducted using Pearson r correlation coefficients (age, baseline depression severity scores and RMT) or Spearman rho correlation coefficients (for % change of MEP amplitude). Hierarchical multivariable linear regression models were used for adjusted analyses of the relationship between outcome variables and potential response predictors. Initial models for each outcome were based on prior knowledge, with age and baseline depression severity included as potential predictors of antidepressant response, and neurophysiologic variables of interest (% change of MEP amplitude and RMT) were then sequentially added to the initial models. Gender was included in model building of all models, but was neither a significant predictor nor a confounder, and thus was dropped. Data transformations and polynomial models were used to test the better alternative to fit continuous predictors, model assumptions were tested by analyses of residuals, and influence diagnostics were conducted using Cook’s distance. Exploratory receiver operating characteristic (ROC) analyses were performed using logistic regression, to determine the accuracy of % change of MEP amplitude to discriminate between responders and non-responders (as defined above). The area under the curve (AUC) was computed as a quantitative measure of test performance. All statistical tests were two-tailed, with statistical significance defined at p < 0.05.
Results
In the original study sample, participants were 18 to 78 years old and 56.9% were women. The pre-treatment measurement of % change of MEP amplitude after MC rTMS was collected in all participants and was highly variable between individuals. Six patients were excluded from outcome analyses due to not completing the treatment protocol (n=5) or to missing data on both outcome measures (n=1). Of the 45 remaining patients, data were missing on the primary outcome in 4 and the secondary outcome in 1 (see Figure 1 for a full description of experimental timeline). The primary and secondary outcomes were strongly correlated (r=0.69, p<0.001) suggesting that, as expected, they were expressions of a similar construct. Mean % reduction HAM-D-17 score (22.7%) and mean % reduction BDI-II score (18.2%) were moderate relative to other DLPFC rTMS studies (6, 7), as was the number of responders (n=7, 17.1%), possibly due to the relatively low stimulation intensity used here (19) and to the high refractoriness of MDD in these patients, who had failed at least three good trials of different antidepressants). A full description of the data collected for this study is given in Table 1 and Supplementary table 1.
In univariate analyses, the primary outcome measure (% reduction HAM-D-17) was found to correlate with the secondary outcome (% reduction BDI-17; r=0.69, p<0.001) and with baseline HAM-D-17 (r=−0.39, p<0.015). However, correlations with age, baseline RMT or baseline BDI-II were not significant (−0.4<r<-0.1, p>0.3), and % reduction HAM-D-17 also did not differ according to gender or handedness (t-tests, p>0.1; Table 1). Importantly, our main predictor of interest (% change MEP amplitude) correlated significantly both with the primary (rho=0.43, p<0.005; Figure 2) and secondary outcomes (rho=0.51, p<0.005, Figure 2).
Figure 2.
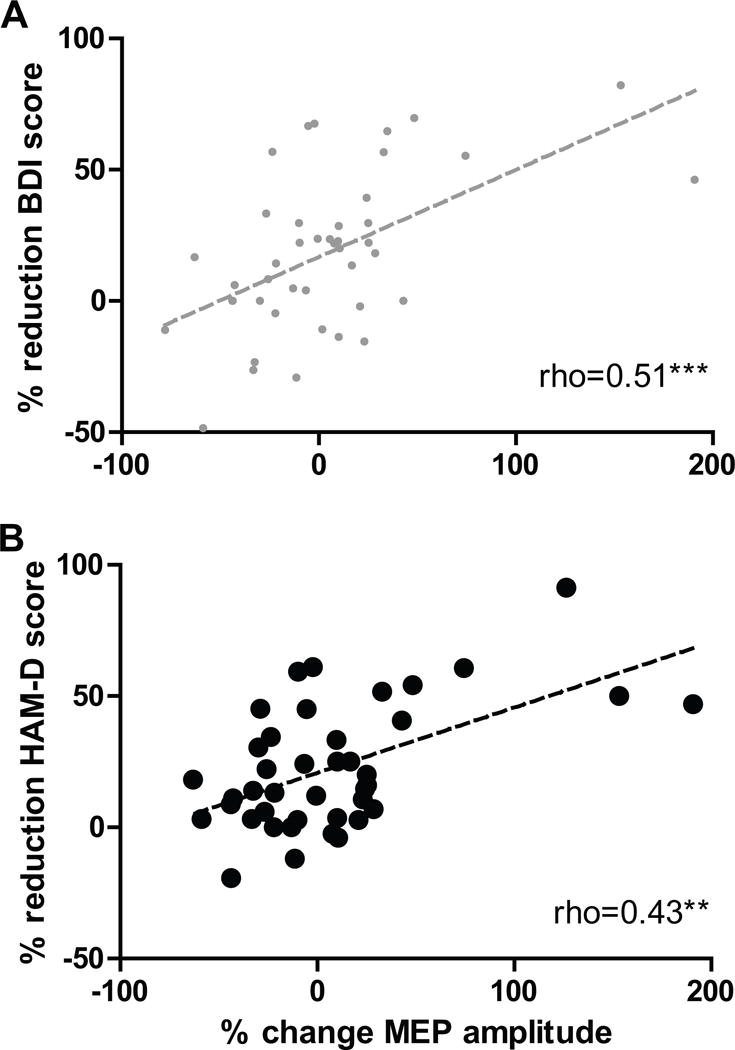
Relationship between modulation of corticospinal excitability by MC rTMS and antidepressant response to DLPFC rTMS.
Significant correlations were found between the percentage change of MEP amplitude after MC rTMS and the percentage reduction of depression severity after 10 days of DLPFC rTMS, measured both using the self-report Beck Depression Inventory-II (BDI; rho=0.51, p<0.005; panel A) and the clinician-rated Hamilton Rating Scale for Depression (HAM-D; rho=0.43, p<0.005; panel B). The values for these correlations were similar when calculated using parametric analyses (r=0.55, p<0.0005 in both cases).
Hierarchical multiple linear regression models were then used for adjusted analyses of the relationship between the primary outcome and potential response predictors (Table 2). As expected, in the initial model (model 1, adjusted R2=0.18) baseline depression severity (HAM-D-17) was a significant predictor of worse antidepressant response, and age had a significant quadratic relationship with outcome, suggesting enhanced antidepressant responses in patients at the center of the age distribution. In a sequential model including also MEP amplitude change, this variable was found to be a very significant predictor of enhanced antidepressant response (β=0.21±0.06, p<0.001), more than doubling the predictive potential of the model (model 2, adjusted R2=0.39). This association was robust to inclusion of baseline RMT in a third exploratory model, where RMT was also a significant predictor of outcome (β=−0.7±0.3, p<0.05) and the predictive potential of the model was further enhanced (model 3, adjusted R2=0.48; Table 2). In additional multivariable models for prediction of the secondary outcome (BDI-II change), MEP amplitude change, but not age, baseline depression severity (BDI-II) and baseline RMT, was still a significant predictor of response (models 4 and 5; Table 2).
Table 2.
Hierarchical multiple linear regression models to predict clinical response (% reduction HAM-D-17 or BDI-II) according to baseline demographic, neurophysiologic and clinical characteristics.
| Models predicting HAM-D-17 response | Models predicting BDI-II response | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Model 1a (R2=0.18) |
Model 2 (R2=0.39) |
Model 3 (R2=0.48) |
Model 4b (R2=0.28) |
Model 5 b (R2=0.25) |
|||||
| β (SE) | p | β (SE) | p | β (SE) | p | β (SE) | p | β (SE) | p | |
| Severityc | −1.6 (0.5) | 0.004 | −1.3 (0.5) | 0.007 | −1.3 (0.4) | 0.005 | −0.46 (0.4) | 0.26 | −0.47 (0.42) | 0.27 |
| Age | 3.8 (1.9) | 0.058 | 2.9 (1.7) | 0.096 | 2.7 (1.6) | 0.11 | 0.23 (0.33) | 0.48 | 0.23 (0.34) | 0.5 |
| (Age)2 | −0.04 (0.02) | 0.049 | −0.03 (0.02) | 0.086 | −0.03 (0.02) | 0.09 | ||||
| MEP change | 0.21 (0.06) | 0.0007 | 0.25 (0.06) | <0.0001 | 0.35 (0.08) | 0.0001 | 0.35 (0.09) | 0.0003 | ||
| RMT | −0.7 (0.3) | 0.026 | −0.009 (0.36) | 0.98 | ||||||
For model 1, improved fit was obtained when age was included as a quadratic term.
Contrary to model 1, no advantage was obtained from including age as a quadratic term.
Baseline HAM-D-17 for models 1–3 and Baseline BDI-II for models 4 and 5.
Data from several participants was excluded from multivariable modelling analysis for prediction of models 1 to 3. This was due to failure to complete rTMS treatment or absent depression severity assessments (HAM-D-17). Control analyses were thus conducted to compare demographic, neurophysiologic and clinical characteristics between these participants (n=10), and those who completed rTMS treatment and in whom both pre- and post-treatment HAM-D-17 assessments were available (n=41). No differences were found between the two groups regarding gender or handedness (Fisher’s exact test, p>0.2), nor regarding age, baseline depression severity (BDI-II), % reduction BDI-II, baseline RMT or % change MEP amplitude (t-tests, p>0.1; Supplementary table 1).
In this study, of the 41 patients completing DLPFC rTMS treatment and depression severity assessments (HAM-D-17) approximately 17% were responders to treatment, i.e., experienced a reduction of symptom severity of at least 50%. This relatively low number of responders limits the utility of ROC analyses to determine the accuracy of MEP amplitude change to predict those that will be responders to rTMS. Nevertheless, an exploratory analysis was conducted, and the ROC curve of MEP amplitude change to identify responders to DLPFC rTMS was calculated. Interestingly, the area under the ROC curve was 0.84, suggesting good to very good performance of MEP amplitude change in the prediction of response to DLPFC rTMS (i.e., 84% accuracy).
Discussion
Here we found that in individuals suffering from major depressive disorder, modulation of corticospinal excitability by rTMS delivered to the MC, measured prior to DLPFC rTMS treatment of depression, is correlated with antidepressant response to DLPFC rTMS (Table 1, Figure 2). This finding was robust to measurement of antidepressant response using two different depression severity scales (HAM-D-17 and BDI-II; Table 1, Figure 2) and to adjustment according to demographic and clinical factors, in multivariable analyses (Table 2). The mechanisms underlying interindividual variability in modulation of corticospinal excitability could be related to factors such as age (29), MDD severity (30–35) and coil-to-MC distance (20, 36) (that correlates with RMT (36)). However, as shown in model 3 and 4, age, severity and RMT do not confound the relationship between modulation of corticospinal excitability and antidepressant response. Thus, these findings support our primary hypothesis, i.e., that the degree of modulation of corticospinal excitability by rTMS delivered to the left MC is predictive of the antidepressant effects of rTMS delivered to the left DLPFC.
These results are consistent with findings of altered cortical function in MDD, initially described using functional brain imaging and consisting mainly of reduced activity in prefrontal areas, particularly in the left hemisphere (37, 38). TMS has since been used as a tool for in vivo measurements of cortical excitability in several neuropsychiatric disorders, including MDD (39), with most studies specifically assessing MC excitability given the ease of measurement and interpretation of MC output, in terms of corticospinal excitability (21). Several authors have reported altered MC excitability in MDD (39), namely excitability differences between patients and controls, and/or interhemispheric asymmetry of excitability in patients, but not in controls (30–32). However, these findings have not been consistent across all studies (40, 41), possibly reflecting heterogeneity in the pathophysiology of MDD (42, 43) or confounding medication effects. Nevertheless, even though the motor cortex is not typically regarded as a critical brain area in the pathophysiology of MDD, altered MC excitability in depressed patients may represent more widespread pathological and neuroplastic changes due to altered of glutamatergic or GABAergic neurotransmission (44, 45). The assay reported here allowed not only measurement of MC excitability but, critically, of corticospinal excitability modulation by rTMS. To our knowledge, such measures have not been systematically compared between depressed and control individuals in a single study. However, Maeda et al (28) used an identical method to the one used here in order to assess corticospinal excitability modulation by rTMS in healthy volunteers. Exploratory comparisons of the MEP facilitation obtained here in 51 depressed patients (8 ± 49%) and by Maeda et al in 14 healthy volunteers (37.9 ± 53.6%) reveal a borderline significant difference between the two datasets (p=0.05; unpaired two-sample t-test). While this difference cannot be interpreted in the absence of a direct comparison in a single study, it suggests that, in the context of MDD, the MC may be less sensitive to the neuromodulatory effects of 10Hz rTMS. Future research should explicitly address this hypothesis, and it is important in this context to consider the potential effect of antidepressant and other medications that may have influenced this finding.
There is also evidence that corticospinal excitability is modified after effective antidepressant treatment with DLPFC rTMS (33–35), electroconvulsive therapy (46, 47) and vagus nerve stimulation (48), suggesting that it may be a modifiable state marker for the depressive state, rather than a trait marker for susceptibility to depression. Furthermore, antidepressant treatments such as rTMS, as well as ketamine, an experimental rapid-acting drug (5, 21), are thought to act through the modulation of synaptic function (49). Evidence for a direct association between antidepressant efficacy of these treatments and effects on synaptic function and neuroplasticity would thus provide critical added support for the relevance of such mechanisms in the context of depression pathophysiology and antidepressant affects. Prior studies attempting to identify measures of excitability to serve as biomarkers for antidepressant treatment with rTMS tested methods to assess excitability proper, such as RMT (10), MEP potential amplitude, cortical silent period and intracortical inhibition (18). This research had limited success (50) and, in positive studies, excitability was a weak and/or inconsistent predictor of antidepressant response (10, 18). Other approaches for assessment of cerebral activity, such as PET imaging (51) and electroencephalography (52), have also been used as predictive biomarkers of antidepressant response to rTMS, and corticospinal excitability has been tested as a predictor of response to other treatments, namely fluoxetine (53), sleep deprivation and light therapy (54), with only moderate success.
While prior studies had limited success in identification of rTMS treatment biomarkers, the research presented here is, to our knowledge, the first study to use measures of corticospinal excitability modulation by rTMS, rather than excitability proper. Importantly, we found that modulation of corticospinal excitability by rTMS delivered to the MC was a robust predictor of antidepressant response to DLPFC rTMS. In the most similar approach described in the available literature, magnetoencephalography was used to show that, relative to pre-treatment, ketamine infusion increases excitability of the somatosensory cortex in response to tactile stimulation, specifically in patients with the most robust antidepressant responses (55). Use of such response predictors, reflecting individual modulation of motor or sensory reactivity in response to a proposed treatment course, in addition to contributing towards patient selection, could allow for diverse interventions to enhance treatment efficacy. One possibility would be to individualize rTMS treatment parameters, namely frequency, intensity (12, 20) and/or the stimulation paradigm proper (e.g., theta burst stimulation (56)), in order to identify the conditions that induce sufficient corticospinal excitability modulation. If, as proposed above, MDD patients are less sensitive to the neuromodulatory effects of MC rTMS than healthy subjects, another possibility would be to increase the likelihood of excitability modulation by rTMS, for example using concomitant interventions that may independently enhance cortical excitability, such as ketamine, caffeine or glucose (55, 57). Nevertheless, these proposals are speculative, and randomized trials will be required to compare antidepressant outcomes between current standard rTMS treatment and individualized or enhanced treatment options. Furthermore, since antidepressant drugs in current clinical use have been shown to modify MC excitability after a single dose (58, 59), it is tempting to hypothesize that similar approaches, i.e., of treatment-induced changes of cortical excitability, could be useful for prediction of antidepressant response and/or adjustment of parameters (e.g., dosage), for treatments other than DLPFC rTMS.
The results of this study should be interpreted in the context of the experimental design. In fact, a relatively low number of MEPs was recorded before and after the MC rTMS session to assess modulation of corticospinal excitability (22), which could have increased variability due to a greater impact of outliers on the mean pre and post-treatment excitability. Nevertheless, since use of a higher number of MEPs has been shown to increase reliability of this measure (60), we expect that future research using such methodology will have a greater power to confirm the findings described here. The rTMS treatment protocol used was also atypical relative to those currently approved for clinical use, with only 10 and relatively short sessions of rTMS delivered at a low stimulation intensity (6, 7) to a DLPFC target that was not optimal (13), which could explain the low clinical response that was observed in these patients. Importantly, the parameters for DLPFC rTMS were chosen according to the parameters for MC rTMS, to enhance comparability between the neuromodulatory effects of the latter and the antidepressant effects of the former. However, rTMS effects may differ between the MC and the DLPFC (20, 36), which could limit interpretation of our findings. In any case, the protocol for MC rTMS was chosen to follow those previously reported for assessment of corticospinal excitability (28), including delivery of MC rTMS at a low intensity for safety concerns, and explaining, in part, the choice of atypical parameters for DLPFC rTMS. Finally, while the results described here have been interpreted as a reflection of the relevance of cortical excitability for rTMS treatment of depression, the contribution of spinal cord and peripheral nerve excitability towards the effects of MC rTMS, and/or the amplitude of MEPs, should be considered. To minimize this possibility, during TMS procedures participants were instructed to keep their hands relaxed. Muscle relaxation was carefully monitored through visual inspection of hand and wrist muscle twitching, which was not found. Thus, while contributions from non-cortical excitability are unlikely, they cannot be fully excluded. Follow-up studies should consider methods to address this problem, such as the use of concurrent TMS and electroencephalography (TMS-EEG) for direct cortical measurements (14), or assessment of the H-reflex to disentangle contributions from spinal excitability (61).
Conclusions
In conclusion, the findings reported here demonstrate that measures of motor cortex excitability modulation by rTMS predict antidepressant response to prefrontal cortex rTMS. Depending on further refinement of these measures, we propose they could be used for patient selection and optimization of rTMS parameters (20), in order to obtain an individualized level of modulation, and thus contribute towards optimization of rTMS treatment efficacy (14) and safety (17). While it is possible that measures of modulation of cortical excitability performed in the prefrontal cortex (62) could perform even better as predictors of response, this would require the use of concurrent TMS-EEG, which poses additional technical and conceptual challenges (14). On the contrary, measures of corticospinal excitability, such as those used here, are well established, readily available in depressed patients under consideration for rTMS, and easier to interpret (21). Future research should confirm these findings in alternate rTMS centers and with other treatment parameters, and further explore details regarding how this approach can be used for patient selection and optimization of rTMS parameters.
Supplementary Material
Highlights.
10Hz rTMS of the dorsolateral prefrontal cortex (DLPFC) is used to treat depression.
Motor evoked potential amplitude can be modified after 10Hz motor cortex (MC) rTMS.
This index of excitability modulation predicts antidepressant response to DLPFC rTMS.
rTMS-induced modulation of excitability is a potential antidepressant biomarker.
Acknowledgments
The authors would like to thank Catarina Freitas for assistance in this project and comments on an initial version of the manuscript.
Funding
This work was supported in part by the Berenson-Allen Foundation, the Sidney R. Baer Jr. Foundation, the National Institutes of Health (R01HD069776, R21 NS082870, R01NS073601, R21 MH099196, R21 NS085491, R21 HD07616), and Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758). AJO-M was funded by Fundação para a Ciência e Tecnologia (Portugal) through a Junior Research and Career Development Award from the Harvard Medical School - Portugal Program to (HMSP-ICJ/0020/2011). The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the Fundação para a Ciência e Tecnologia, Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Institutes of Health, or the Sidney R. Baer Jr. Foundation.
Abbreviations
- APB
abductorpollicis brevis muscle
- AUC
area under the curve
- BDI-II
21-item Beck Depression Inventory-II
- DLPFC
dorsolateral prefrontal cortex
- EMG
electromyography
- HAM-D-17
17-item Hamilton Rating Scale for Depression
- MC
motor cortex
- MDD
major depressive disorder
- MEP
motor evoked potentials
- RMT
resting motor threshold
- ROC
receiver operating characteristic
- rTMS
repetitive TMS
- TMS
Transcranial Magnetic Stimulation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure:
Dr. A. Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, Axilum Robotics, Magstim Inc., and Neosync; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging. Dr. Oliveira-Maia and Dr. Press declare no potential conflict of interest.
Previously presented at the 16th World Congress of Psychiatry.
References
- 1.Ustun TB, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJ. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 2004;184:386–92. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- 2.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 3.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 4.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106–7. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 5.Pascual-Leone A, Rubio B, Pallardo F, Catala MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348(9022):233–7. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- 6.O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208–16. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 7.George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67(5):507–16. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 8.Fregni F, Marcolin MA, Myczkowski M, Amiaz R, Hasey G, Rumi DO, et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2006;9(6):641–54. doi: 10.1017/S1461145705006280. [DOI] [PubMed] [Google Scholar]
- 9.Brakemeier EL, Luborzewski A, Danker-Hopfe H, Kathmann N, Bajbouj M. Positive predictors for antidepressive response to prefrontal repetitive transcranial magnetic stimulation (rTMS) J Psychiatr Res. 2007;41(5):395–403. doi: 10.1016/j.jpsychires.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Lisanby SH, Husain MM, Rosenquist PB, Maixner D, Gutierrez R, Krystal A, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34(2):522–34. doi: 10.1038/npp.2008.118. [DOI] [PubMed] [Google Scholar]
- 11.Brakemeier EL, Wilbertz G, Rodax S, Danker-Hopfe H, Zinka B, Zwanzger P, et al. Patterns of response to repetitive transcranial magnetic stimulation (rTMS) in major depression: replication study in drug-free patients. J Affect Disord. 2008;108(1–2):59–70. doi: 10.1016/j.jad.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Padberg F, Zwanzger P, Keck ME, Kathmann N, Mikhaiel P, Ella R, et al. Repetitive transcranial magnetic stimulation (rTMS) in major depression: relation between efficacy and stimulation intensity. Neuropsychopharmacology. 2002;27(4):638–45. doi: 10.1016/S0893-133X(02)00338-X. [DOI] [PubMed] [Google Scholar]
- 13.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72(7):595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fidalgo TM, Morales-Quezada JL, Muzy GS, Chiavetta NM, Mendonca ME, Santana MV, et al. Biological Markers in Noninvasive Brain Stimulation Trials in Major Depressive Disorder: A Systematic Review. J ECT. 2013 doi: 10.1097/YCT.0b013e31828b34d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, Pascual-Leone A. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci U S A. 2014;111(41):E4367–75. doi: 10.1073/pnas.1405003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox MD, Liu H, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage. 2013;66:151–60. doi: 10.1016/j.neuroimage.2012.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMSCG Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzgerald PB, Brown TL, Marston NA, Daskalakis ZJ, de Castella A, Bradshaw JL, et al. Motor cortical excitability and clinical response to rTMS in depression. J Affect Disord. 2004;82(1):71–6. doi: 10.1016/j.jad.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am J Psychiatry. 2003;160(5):835–45. doi: 10.1176/appi.ajp.160.5.835. [DOI] [PubMed] [Google Scholar]
- 20.Nahas Z, Li X, Kozel FA, Mirzki D, Memon M, Miller K, et al. Safety and benefits of distance-adjusted prefrontal transcranial magnetic stimulation in depressed patients 55–75 years of age: a pilot study. Depress Anxiety. 2004;19(4):249–56. doi: 10.1002/da.20015. [DOI] [PubMed] [Google Scholar]
- 21.Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15(4):333–43. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117(Pt 4):847–58. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 26.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 27.Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91(2):79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 28.Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000;133(4):425–30. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
- 29.Muller-Dahlhaus JF, Orekhov Y, Liu Y, Ziemann U. Interindividual variability and age-dependency of motor cortical plasticity induced by paired associative stimulation. Exp Brain Res. 2008;187(3):467–75. doi: 10.1007/s00221-008-1319-7. [DOI] [PubMed] [Google Scholar]
- 30.Bajwa S, Bermpohl F, Rigonatti SP, Pascual-Leone A, Boggio PS, Fregni F. Impaired interhemispheric interactions in patients with major depression. J Nerv Ment Dis. 2008;196(9):671–7. doi: 10.1097/NMD.0b013e318183f86f. [DOI] [PubMed] [Google Scholar]
- 31.Lefaucheur JP, Lucas B, Andraud F, Hogrel JY, Bellivier F, Del Cul A, et al. Inter-hemispheric asymmetry of motor corticospinal excitability in major depression studied by transcranial magnetic stimulation. J Psychiatr Res. 2008;42(5):389–98. doi: 10.1016/j.jpsychires.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Maeda F, Keenan JP, Pascual-Leone A. Interhemispheric asymmetry of motor cortical excitability in major depression as measured by transcranial magnetic stimulation. Br J Psychiatry. 2000;177:169–73. doi: 10.1192/bjp.177.2.169. [DOI] [PubMed] [Google Scholar]
- 33.Bajbouj M, Brakemeier EL, Schubert F, Lang UE, Neu P, Schindowski C, et al. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex and cortical excitability in patients with major depressive disorder. Exp Neurol. 2005;196(2):332–8. doi: 10.1016/j.expneurol.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Chistyakov AV, Kaplan B, Rubichek O, Kreinin I, Koren D, Feinsod M, et al. Antidepressant effects of different schedules of repetitive transcranial magnetic stimulation vs. clomipramine in patients with major depression: relationship to changes in cortical excitability. Int J Neuropsychopharmacol. 2005;8(2):223–33. doi: 10.1017/S1461145704004912. [DOI] [PubMed] [Google Scholar]
- 35.Triggs WJ, McCoy KJ, Greer R, Rossi F, Bowers D, Kortenkamp S, et al. Effects of left frontal transcranial magnetic stimulation on depressed mood, cognition, and corticomotor threshold. Biol Psychiatry. 1999;45(11):1440–6. doi: 10.1016/s0006-3223(99)00031-1. [DOI] [PubMed] [Google Scholar]
- 36.McConnell KA, Nahas Z, Shastri A, Lorberbaum JP, Kozel FA, Bohning DE, et al. The transcranial magnetic stimulation motor threshold depends on the distance from coil to underlying cortex: a replication in healthy adults comparing two methods of assessing the distance to cortex. Biol Psychiatry. 2001;49(5):454–9. doi: 10.1016/s0006-3223(00)01039-8. [DOI] [PubMed] [Google Scholar]
- 37.Baxter LR, Jr, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46(3):243–50. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- 38.Kocmur M, Milcinski M, Budihna NV. Evaluation of brain perfusion with technetium-99m bicisate single-photon emission tomography in patients with depressive disorder before and after drug treatment. Eur J Nucl Med. 1998;25(10):1412–4. doi: 10.1007/s002590050316. [DOI] [PubMed] [Google Scholar]
- 39.Radhu N, de Jesus DR, Ravindran LN, Zanjani A, Fitzgerald PB, Daskalakis ZJ. A meta-analysis of cortical inhibition and excitability using transcranial magnetic stimulation in psychiatric disorders. Clin Neurophysiol. 2013;124(7):1309–20. doi: 10.1016/j.clinph.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Grunhaus L, Polak D, Amiaz R, Dannon PN. Motor-evoked potential amplitudes elicited by transcranial magnetic stimulation do not differentiate between patients and normal controls. Int J Neuropsychopharmacol. 2003;6(4):371–8. doi: 10.1017/S1461145703003705. [DOI] [PubMed] [Google Scholar]
- 41.Navarro R, Zarkowski P, Sporn A, Avery D. Hemispheric asymmetry in resting motor threshold in major depression. J ECT. 2009;25(1):39–43. doi: 10.1097/YCT.0b013e3181761cf5. [DOI] [PubMed] [Google Scholar]
- 42.Bella R, Ferri R, Cantone M, Pennisi M, Lanza G, Malaguarnera G, et al. Motor cortex excitability in vascular depression. Int J Psychophysiol. 2011;82(3):248–53. doi: 10.1016/j.ijpsycho.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Concerto C, Lanza G, Cantone M, Pennisi M, Giordano D, Spampinato C, et al. Different patterns of cortical excitability in major depression and vascular depression: a transcranial magnetic stimulation study. BMC Psychiatry. 2013;13:300. doi: 10.1186/1471-244X-13-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bajbouj M, Lisanby SH, Lang UE, Danker-Hopfe H, Heuser I, Neu P. Evidence for impaired cortical inhibition in patients with unipolar major depression. Biol Psychiatry. 2006;59(5):395–400. doi: 10.1016/j.biopsych.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 45.Croarkin PE, Nakonezny PA, Husain MM, Melton T, Buyukdura JS, Kennard BD, et al. Evidence for increased glutamatergic cortical facilitation in children and adolescents with major depressive disorder. JAMA Psychiatry. 2013;70(3):291–9. doi: 10.1001/2013.jamapsychiatry.24. [DOI] [PubMed] [Google Scholar]
- 46.Bajbouj M, Lang UE, Niehaus L, Hellen FE, Heuser I, Neu P. Effects of right unilateral electroconvulsive therapy on motor cortical excitability in depressive patients. J Psychiatr Res. 2006;40(4):322–7. doi: 10.1016/j.jpsychires.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Chistyakov AV, Kaplan B, Rubichek O, Kreinin I, Koren D, Hafner H, et al. Effect of electroconvulsive therapy on cortical excitability in patients with major depression: a transcranial magnetic stimulation study. Clin Neurophysiol. 2005;116(2):386–92. doi: 10.1016/j.clinph.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Bajbouj M, Gallinat J, Lang UE, Hellen F, Vesper J, Lisanby SH, et al. Motor cortex excitability after vagus nerve stimulation in major depression. J Clin Psychopharmacol. 2007;27(2):156–9. doi: 10.1097/JCP.0b013e31803308f3. [DOI] [PubMed] [Google Scholar]
- 49.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dolberg OT, Dannon PN, Schreiber S, Grunhaus L. Magnetic motor threshold and response to TMS in major depressive disorder. Acta Psychiatr Scand. 2002;106(3):220–3. doi: 10.1034/j.1600-0447.2002.01334.x. [DOI] [PubMed] [Google Scholar]
- 51.Speer AM, Benson BE, Kimbrell TK, Wassermann EM, Willis MW, Herscovitch P, et al. Opposite effects of high and low frequency rTMS on mood in depressed patients: relationship to baseline cerebral activity on PET. J Affect Disord. 2009;115(3):386–94. doi: 10.1016/j.jad.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olbrich S, Arns M. EEG biomarkers in major depressive disorder: discriminative power and prediction of treatment response. Int Rev Psychiatry. 2013;25(5):604–18. doi: 10.3109/09540261.2013.816269. [DOI] [PubMed] [Google Scholar]
- 53.Croarkin PE, Nakonezny PA, Husain MM, Port JD, Melton T, Kennard BD, et al. Evidence for pretreatment LICI deficits among depressed children and adolescents with nonresponse to fluoxetine. Brain Stimul. 2014;7(2):243–51. doi: 10.1016/j.brs.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Canali P, Sferrazza Papa G, Casali AG, Schiena G, Fecchio M, Pigorini A, et al. Changes of cortical excitability as markers of antidepressant response in bipolar depression: preliminary data obtained by combining transcranial magnetic stimulation (TMS) and electroencephalography (EEG) Bipolar Disord. 2014;16(8):809–19. doi: 10.1111/bdi.12249. [DOI] [PubMed] [Google Scholar]
- 55.Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, et al. Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psychiatry. 2012;72(7):555–61. doi: 10.1016/j.biopsych.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li CT, Chen MH, Juan CH, Huang HH, Chen LF, Hsieh JC, et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. Brain. 2014;137(Pt 7):2088–98. doi: 10.1093/brain/awu109. [DOI] [PubMed] [Google Scholar]
- 57.Specterman M, Bhuiya A, Kuppuswamy A, Strutton PH, Catley M, Davey NJ. The effect of an energy drink containing glucose and caffeine on human corticospinal excitability. Physiol Behav. 2005;83(5):723–8. doi: 10.1016/j.physbeh.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Minelli A, Bortolomasi M, Scassellati C, Salvoro B, Avesani M, Manganotti P. Effects of intravenous antidepressant drugs on the excitability of human motor cortex: a study with paired magnetic stimulation on depressed patients. Brain Stimul. 2010;3(1):15–21. doi: 10.1016/j.brs.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 59.Munchau A, Langosch JM, Gerschlager W, Rothwell JC, Orth M, Trimble MR. Mirtazapine increases cortical excitability in healthy controls and epilepsy patients with major depression. J Neurol Neurosurg Psychiatry. 2005;76(4):527–33. doi: 10.1136/jnnp.2004.037010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang WH, Fried PJ, Saxena S, Jannati A, Gomes-Osman J, Kim YH, et al. Optimal number of pulses as outcome measures of neuronavigated transcranial magnetic stimulation. Clin Neurophysiol. 2016;127(8):2892–7. doi: 10.1016/j.clinph.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valero-Cabre A, Pascual-Leone A. Impact of TMS on the primary motor cortex and associated spinal systems. IEEE Eng Med Biol Mag. 2005;24(1):29–35. doi: 10.1109/memb.2005.1384097. [DOI] [PubMed] [Google Scholar]
- 62.Casarotto S, Canali P, Rosanova M, Pigorini A, Fecchio M, Mariotti M, et al. Assessing the effects of electroconvulsive therapy on cortical excitability by means of transcranial magnetic stimulation and electroencephalography. Brain Topogr. 2013;26(2):326–37. doi: 10.1007/s10548-012-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


