Abstract
Stem cell-based therapies are steadily gaining traction for regenerative medicine approaches to treating disease and injury throughout the body. While a significant body of work has shown success in preclinical studies, results often fail to translate in clinical settings. One potential cause is the massive transplanted cell death that occurs post injection, preventing functional integration with host tissue. Therefore, current research is focusing on developing injectable hydrogel materials to protect cells during delivery and to stimulate endogenous regeneration through interactions of transplanted cells and host tissue. This review explores the design of targeted injectable hydrogel systems for improving the therapeutic potential of stem cells across a variety of tissue engineering applications with a focus on hydrogel materials that have progressed to the stage of preclinical testing.
Keywords: injectable hydrogels, stem cells, regenerative medicine, cell survival, functional recovery
1. Introduction
One of the largest challenges facing stem cell-derived therapies is the survival and engraftment of transplanted cells into host tissue. In general, only approximately 1–20% of transplanted cells survive, significantly limiting their therapeutic potential [1–5]. While many stem cell sources (adult, embryonic, induced pluripotent) are being investigated for regenerative applications, all share universal challenges that occur at each stage of transplantation. It is useful to divide an injectable hydrogel therapy into the following phases: pre-injection, injection, acute post-injection, and long-term survival and function, in order to identify the distinct challenges and potential solutions to maintaining cell survival (Table 1). While hydrogel design strategies can help mitigate the challenges of each phase of transplantation, no single material currently exists that addresses all of these issues simultaneously.
Table 1.
Challenges that reduce stem cell survival during transplantation and biomaterials strategies to address these challenges
| Transplant Phases | Challenges to Stem Cell Survival | Hydrogel Design Approaches | References |
|---|---|---|---|
| Pre-injection | Prolonged exposure to cell encapsulation reagents |
|
11,12,15 |
| Prolonged exposure to non- physiological encapsulation conditions |
|
11, 12, 15, 20, 23, 26 | |
| Injection | Cell damage during injection |
|
16, 18, 19, 20, 22, 23, 25, 26, 28–30, 31–35 |
| Acute post-injection | Cell dispersal from target area |
|
38–45 |
| Anoikis |
|
4, 36–38, 60, 61, 62–68 | |
| Hypoxia |
|
46, 47, 50–54 | |
| Poor nutrient transport |
|
56–59, 88, 89 | |
| Immune response |
|
69–76 | |
| Long-term survival and function | Limited cell migration |
|
58, 59, 66, 77,78, 86, 87, 88, 89 |
| Reduced/inappropriate cell secretome |
|
90–93 | |
| Poor cell differentiation |
|
78, 79, 86, 87, 88, 89, 90, 93, 99 |
In the first section of this review, we will describe each of the transplant phases individually with a focus on which hydrogel design approaches can be used to promote cell viability during that specific phase. The relative priority ranking of the various cell survival challenges will be different for each clinical indication and site of transplantation. No single hydrogel formulation will be optimal for all stem cell transplantation therapies. In order to select an appropriate injectable material for a specific application, one must first carefully assess and prioritize the specific challenges and requirements for that particular tissue. Thus, in the second section of this review, we will focus on applying the hydrogel design strategies discussed in the first section to develop therapies for specific tissue applications, with an emphasis on hydrogels that have been evaluated in preclinical models.
2. General Hydrogel Design Approaches to Address Cell Viability Challenges at Each Stage of Transplantation
2.1. Pre-injection Phase
While most hydrogel designs have focused on cell-material interactions that occur after injection, recently, more studies have begun to also consider how cells interact with the material prior to and during the injection process. Even the choice of the hydrogel crosslinking mechanism, chemical or physical, can influence cell survival in the syringe pre-injection. For chemical gels, crosslinking involves the formation of irreversible, covalent bonds between polymer chains using chemical crosslinking reagents. Physical hydrogels, on the other hand, form through temporary, reversible associations between chains, including hydrogen and ionic bonds [6–9]. Both crosslinking methods can be potentially detrimental to cell survival. For example, many chemical crosslinking reactions often have cross-reactivity with biomolecules presented on the cell surface or utilize reagents that have some degree of cytotoxicity prior to crosslinking. For physically crosslinked hydrogels, frequently an external trigger is used to induce gelation (e.g., changing solvent pH or ionic strength), which exposes cells to non-physiological conditions [10]. As long-term exposure to these crosslinking mechanisms can decrease cell viability, one way to overcome this concern is the use of dual barrel syringes to isolate cells from the potentially cytotoxic component until immediately before delivery [11–15]. Unfortunately, these crosslinking strategies can be difficult to reproducibly control in a clinical setting, as each injection site has its own local microenvironment that can impact crosslinking kinetics. Current focus has shifted to a new strategy in which cells are pre-encapsulated in injectable hydrogels that are already in the gel phase ex vivo. Such hydrogels contain reversible crosslinks, including the use of dynamic covalent bonds or supramolecular assembly, that allow them to shear thin upon application of shear force to enable injection of the gel through syringe needles and catheters [16–20].
2.2. Injection Phase
Studies suggest that cells exposed to syringe needle flow experience mechanical shear and extensional forces that can damage the cell membrane. Interestingly, this effect is exacerbated when cells are injected in low viscosity solutions such as saline or cell culture medium [21, 22]. Recent work has demonstrated that some injectable hydrogels can protect cells from membrane damage; however, this effect seems to be limited to weak gels only (< 100 Pa), although the exact rheological requirements are still unknown [20, 21, 23–28]. Data suggests that mechanical protection is likely due to hydrogel “plug flow”, where shear-thinning at the walls forms a lubricating layer that enables the rest of the gel and encapsulated cells to slip through the needle relatively undeformed [16, 22, 27, 29, 30]. An alternative strategy is the encapsulation of individual cells (or small clusters of cells) into microbeads that are able to protect cells from the extensional forces that can damage cell membranes [31–35].
2.3. Acute Post-Injection Phase
Achieving cell retention at the target site, and thus therapeutic efficacy, requires rapid gelation of the pre-polymer solution or rapid self-healing of an injectable gel. If the gelation is too slow, the cells can become dispersed. The gelation kinetics must be carefully tuned, though, because if gelation is too rapid, it can clog the needle [4, 10, 36–38]. A variety of strategies have been developed for different crosslinking mechanisms. For example, for supramolecular gels such as MAX peptide gels, the kinetics of assembly can be tuned by altering the association energy of the self-assembling components [39, 40]. For ionic crosslinking, an interesting strategy is the controlled release of the crosslinking reagent, Ca2+ into alginate [41]. Finally, external triggers, such as light-activated crosslinking of diacrylate and methacrylate-modified hydrogels, can also be used to initiate the crosslinking process and maintain cell retention in the target area [42–45].
In the acute post-injection phase, transplanted cells can be confronted with a host of survival challenges including hypoxia, lack of nutrients, and lack of a three-dimensional supporting matrix. Depending on the specific clinical application, the relative importance of these different challenges can vary dramatically. Thus, biomaterials designed for different potential therapies often focus on addressing different challenges. For example, transplanted cells that are delivered into ischemic tissue face the acute challenges of hypoxia and lack of nutrient delivery. To combat hypoxia, the material can be engineered to release oxygen directly [46]. Alternatively, the material can deliver factors that assist cells in surviving hypoxia, such as HIF-1α [47–50]. A longer-term strategy is to design the material to promote vascularization in situ; for example, through the delivery of VEGF to induce angiogenesis from neighboring blood vessels [51–54]. While this serves the dual purpose of providing both oxygen and nutrients to the target site, the process of angiogenisis typically requires days to weeks. An alternative strategy to enhance the rate of vascularization is to deliver “pre-vascularized” biomaterials to the target site [55], although this strategy is usually incompatible with injectable material delivery. In the absence of an integrated vasculature structure, nutrient transport can still be promoted through the use of macroporous materials that contain interconnected channels to permit rapid diffusion [56–58]. For example, one recently reported system used an injectable composite of two different materials with very different biodegradation rates. As the rapidly degrading component (i.e. the sacrificial porogen) breaks down, a series of large pores are left behind. These large pores not only facilitate the diffusion of nutrients, but they also were found to promote the migration of transplanted cells [59].
Another common challenge at many transplantation sites is the lack of a healthy extracellular matrix. This is especially problematic for adherent cell types that undergo anoikis without proper matrix signaling cues. To combat this challenge, recent work has investigated the use of decellularized matrix, either purified or as complex mixtures, for cell delivery [4, 38]. Injectable materials that utilize decellularized matrix offer plenty of cell-binding sites necessary to anchor transplanted cells and prevent apoptosis. Furthermore, decellularized matrix can be harvested from the donor tissue of interest providing appropriate, tissue-specific biochemical cues necessary for transplanted cell engraftment and function [4, 38, 60, 61]. Alternatively, synthetic scaffolds can be decorated with known matrix ligands to elicit specific interactions with cell-surface receptors [62–68].
The immune and inflammatory response after tissue injury results in a harsh environment that transplanted cells must overcome to survive. In order to achieve this, materials that were once thought to be “bioinert” can be used to modulate the immune system [69]. An exciting recent advance within biomaterials design is the use of elements to stimulate or suppress the immune system. These design elements include release of soluble cytokines [70, 71], delivery of immuno-engineered cells, and presentation of immunomodulatory peptide sequences [72, 73]. A thorough discussion of this topic is beyond the scope of this review, and the interested reader is pointed to several recent excellent reviews on immunomodulatory biomaterials [53, 69, 74–76].
2.4. Long-term Survival and Function Phase
Transplanted stem cells have different mechanisms through which they can have therapeutic effects. For some clinical applications, stem cells are thought to directly participate in the formation and function of new tissue. In other applications, the primary therapeutic function is thought to be indirect through the secretion of pro-regenerative factors, which act on the host tissue. Thus, depending on the underlying therapeutic mechanism, the biomaterial can be designed to enhance the cellular function. A variety of biomaterial properties are known to influence cell differentiation and maturation including mechanical properties such as stiffness and stress relaxation rate [77–80], presentation of biochemical ligands [81–83], delivery of soluble factors or morphogens [84, 85], and material degradation [86–89]. Each combination of stem cell type and tissue type will likely require different optimal biomaterial properties to influence differentiation, and the underlying mechanisms governing these interactions are only just beginning to be elucidated. Much of the work to date on biomaterials-guided differentiation has focused on studies of bone marrow-derived stem cells in an in vitro setting, and it remains to be seen if these insights hold true for other cell types and if they can be translated to in vivo applications. Even less work has been done to identify which biomaterials cues influence the stem cell secretome, although early work is promising [90–92]. For example, in vitro studies suggest that biomaterial mechanical properties can modulate the pro-angiogenic secretome of mesenchymal stem cells [92].
Developing mechanistic causal relationships between biomaterial parameters and stem cell function (either differentiation or secretion) is challenging for two main reasons. First, stem cells are simultaneously receiving multiple input signals from the matrix where the signal transduction pathways that propagate and amplify these signals have many points of cross-talk, resulting in non-linear relationships. For example, cells may have a different sensitivity to a range of material stiffness depending on the density of biochemical ligands that is presented [78]. Second, manipulating one biomaterial property often has the unintended consequence of also changing several other biomaterial properties. For example, a common technique to increase biomaterial stiffness is to increase the crosslinking density, but this is usually accompanied by a decrease in biodegradation rate and a decrease in the diffusion rate of paracrine secreted signals [86, 87, 93]. Thus, studies in the area of biomaterials-guided differentiation and secretion require careful design to tease apart the intersecting mechanistic relationships. This area of research is likely to continue to expand for the next several decades.
Current research aims to address these issues, but there is no one hydrogel formula that is able solve all of the challenges stem cells face during the transplantation process. A single material property has the ability to impact several different challenges. For example, different hydrogel mechanical properties may be appropriate for different phases of the transplantation process (Fig. 1). While a weak hydrogel may be optimal for shielding cells from forces exerted during injection, the mechanics may prove insufficient for long-term cell retention and function. Furthermore, these properties are highly dependent on specific, applications and thus potential materials must be tunable in order to be optimized for a given therapy. In the next section, we will highlight injectable hydrogel design strategies based on tissue specific needs and applications. In particular, we will place an emphasis on those materials evaluated in preclinical models.
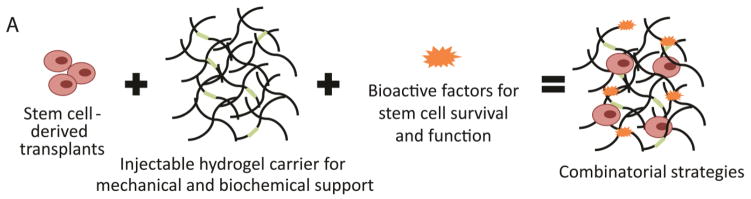
Figure 1. Design of injectable hydrogel delivery platforms for improved stem cell-derived therapeutics.
A) Combinatorial regenerative medicine strategies often include encapsulation of stem cell-derived transplants within injectable hydrogels designed to provide cell appropriate mechanical support and biochemical cues along with co-encapsulation of bioactive factors. B) The design of injectable hydrogels must consider four separate phases of hydrogel use. In the first and second, some injectable hydrogels can protect cells during the potentially harmful pre-injection and injection processes, which exposes cells to a variety of crosslinking mechanisms and mechanical forces. Third, some injectable hydrogels can improve acute cell survival and functionality by providing appropriate mechanical and biochemical matrix cues along with soluble bioactive factors. Fourth, carefully developed injectable materials can promote grafted cell function within host tissue as it degrades.
3. Specific Hydrogel Design Choices for Specific Tissue Applications
3.1. Cardiovascular Stem Cell Transplantation Therapies
Stem cell therapies have been studied extensively in cardiovascular applications such as myocardial infarction (MI) and peripheral arterial disease (PAD) [94]. Researchers have attempted to offset the irreversible cell death from ischemia that occurs in the myocardium during MI or endothelium in PAD through the introduction of stem cells into the injury site in hopes of replacing lost cells and/or encouraging native tissue remodeling through the secretion of regenerative growth factors [95–97].
The cardiac tissue environment includes several cell types including cardiomyocytes, pacemaking cells, fibroblasts, and endothelial cells, as well as, extracellular matrix (ECM) proteins such as collagen, fibronectin, hyaluronic acid, and proteoglycans [98]. Collagen, the most common component of cardiac ECM, forms fibrils that contribute to the mechanical properties of the heart with an approximate physiological stiffness of ~10–20 kPa [99]. While it is unclear if an optimal injectable material would have mechanical properties that match this physiological stiffness or would be weaker or stiffer, it is clear that cells sense and respond to matrix material properties. For example, functional output of embryonic and neonatal cardiomyocytes (CMs) or hiPSC-derived CMs in vitro depends heavily on substrate mechanical stiffness, with increased electrical output and contractile beating observed on 8–14 kPa substrates [100, 101]. Thus, any material used to improve stem cell-derived therapies for cardiovascular tissue must be designed with these mechanical properties taken into consideration.
Alginate has been used as an injectable, naturally occurring biomaterial to deliver stem cells for cardiac tissue regeneration [102, 103]. Alginate is a polysaccharide from seaweed that crosslinks when exposed to calcium ions, making it an ideal injectable material as gel formation will not occur until it comes into contact with physiological calcium. This would prevent clogging in the long catheters used in cardiac injection methods. Since alginate is a non-fouling and non-adhesive biomaterial, functionalization with cell-adhesive domains must take place to encourage cell attachment and matrix remodeling. Alginate, modified with the cell-binding ligand RGD found in collagen and fibronectin, has also improved human mesenchymal stem cell (hMSC) retention from 9% in saline controls to 60% in a direct injection model [104]. Further studies have shown that the concentration of alginate, and thus mechanical stiffness, also plays a significant role in cell retention. MSCs transplanted in a 2% alginate solution (~2 kPa) were found to be retained and survive 4x higher than that of cells transplanted with saline or even 1% alginate (~700 Pa) [105].
Whereas alginate crosslinks in the presence of Ca2+, other materials can form hydrogels at physiological temperatures making them potential injectable hydrogel cell carriers. The study by Roche et al. also examined the use of a thermosensitive, injectable chitosan/β-glycerophosphate (β-GP) gel as the hMSC vehicle and found cell retention also significantly improved to 50%. Chitosan has also been used as a cell carrier for brown adipose derived stem cells (ASCs) in myocardial repair, with a reported 70% increase in cell retention as well as improved angiogenesis and preserved heart function [104]. Unfortunately, chitosan/β-GP and other thermosensitive hydrogels may prove difficult for use in non-direct injection methods that make use of catheters. While catheter-delivery of transplanted cells is less invasive than direct injection, it requires the cell/gel mixture to travel a long distance through the body, which can potentially result in early gelation and failure to inject into the damaged tissue [106]. Gelfoam, an FDA-approved, gelatin-based gel, has also been shown to successfully transplant MSCs as a heat-sensitive injectable material [107]. As an alternative to naturally occurring biomaterials, Xia et al. designed a synthetic, injectable, thermosensitive copolymer composed of poly (N-isopropylacrylamide (PNIPAAM)/acrylic acid/2-hydroxylethyl methacrylate-poly(ε-caprolactone) and functionalized with collagen I to deliver hMSCs to an infarcted heart. Cell retention within the heart was 4x higher with the injectable hydrogel compared to cells alone and this corresponded with increased heart function, increased angiogenesis, and decreased fibrous scarring [108]. As with other injectable, thermosensitive composites, the copolymer suffers from the potential to gel in catheters, but may prove a more useful tool for direct myocardial injection therapies. Another interesting synthetic injectable material is self-assembling nanofibers, which have been used in mini-pig models to improve bone marrow-mononuclear cell retention 10-fold in treating MI and improving both diastolic and systolic functional outcomes [43].
In treating PAD, transplanted MSCs have been used to produce pro-angiogenic factors needed for regeneration and are more commonly delivered systemically [109]. However, embryonic derived stem cell (ESC)- and induced pluripotent derived stem cell (iPSC)-derived endothelial cells (EC) have been used to improve endothelialization and vascular regeneration of the occluded arteries in ischemic tissue through intramusclar injection [110–112]. Unfortunately, cell survival after intramuscular transplantation is poor due to the immunoreactive, ischemic, and necrotic host environment. The Heilshorn group showed the use of a weak, shear-thinning, protein-based hydrogel with cell-adhesive domains improved iPSC-EC viability during the injection process by protecting the cells from mechanical forces within the needle [20]. Furthermore, incorporation of vascular endothelial growth factor (VEGF) into the hydrogel cell carrier improved muscle regeneration while minimizing inflammation and necrosis. Finally, collagen, which gels at body temperature, has been used to deliver BMSCs intramuscularly in a PAD model. Improved angiogenesis and hind limb perfusion was observed with an increase in local blood vessel density [113]. Xu et al. designed a synthetic, injectable hydrogel with a PNIPAAM backbone that exhibited strong mechanical properties (~17 kPa) when raised to body temperature. The incorporation of the pro-survival factor basic fibroblast growth factor (bFGF) with the hydrogel improved MSC survival after intramuscular injection, as well as increased blood vessel density, limb perfusion, and muscle diameter [114].
3.2. Cartilage Stem Cell Transplantation Therapies
Cartilage degeneration occurs through the break down of the connective tissue that covers bones at joints, particular in joints at the knees, elbows, and spine. This can result from diseases such as osteoarthritis, mechanical wear, crystal formation from gout, diabetes, and rheumatoid arthritis [115]. Current stem cell therapies used to treat cartilage degeneration include the use of MSCs and ASCs differentiated into chondrocytes, the main cellular component of cartilage, in order to replace lost cells [116]. Unfortunately, many of these cells die within this avascular environment [117].
While chondrocytes make up the main cellular component of cartilage tissue, the main structural component is composed of ECM proteins including collagens I and II and a significant fraction of proteoglycans. Cartilage tissue must have significant mechanical properties to withstand the high forces that occur in joints as they minimize the friction between connected bones [118]. Therefore, any injectable material intended for long-term presence in the joint must also be able to withstand these mechanical forces. In addition, the ideal material would provide pro-survival cues, which might include native-like protein and proteoglycan content, in order to encourage transplanted cell survival and integration, as well as promote endogenous tissue remodeling.
Photopolymerizing hydrogels have shown promise for delivery of stem cells in cartilage regeneration work [119]. Studies have shown advances in the use of chitosan-based injectable hydrogels for improving ASC and human synovial MSC survival in articular cartilage regeneration. This group has developed a methacrylated-chitosan-based material (MeCG) that allows for injection followed by photopolymerization in situ under visible blue light. To specify this material for cartilage applications, the group modified the hydrogel by conjugating transforming growth factor-βTGF-β) and incorporating collagen II and the proteoglycan, chondroitin sulfate (CS). This material improved chondrogenic differentiation as well as improved cartilage ECM deposition in a rat chondral defect model [120]. This material may be an improvement upon other pure mammalian-based materials such as collagen or CS alone, which would be rapidly degraded by the body [121]. Similar visible light-crosslinking hydrogels have been investigated, in which methacrylated-gelatin hydrogels used to inject MSCs showed strong mechanical properties (~30 kPa) at physiological conditions and strong integration with native cartilage compared to cells delivered in faster degrading agarose [43]. Finally, MSCs have been delivered via a cartilage specific, hydrogel carrier system composed of a UV-crosslinking, synthetic polymer base (poly(ethylene oxide) diacrylate) incorporating hyaluronic acid and TGF-β. This system has demonstrated successful in vitro differentiation of MSCs into chondrocytes and generation of cartilage-like tissue when injected subcutaneously and transdermally UV-crosslinked [44, 45]. The design of this system using the proteoglycan hyaluronic acid improved the viscosity of the solution, preventing dispersion of the injected MSCs and improving cartilage formation [44].
Thermosensitive hydrogels have also been utilized for increasing stem cell efficacy in cartilage repair [45]. Thermoreversable chitosan/β-GP/hydroxyethyl cellulose hydrogels were shown to support human and mouse MSC survival and proliferation, while further incorporation of TGF- β3 improved chondrogenic differentiation [122]. Similarly, chitosan-poly(vinyl alcohol) copolymer hydrogels used to deliver MSCs showed significant regeneration of rabbit articular cartilage defects, particularly when TGF-β was introduced through MSC adenoviral overexpression [45].
These studies highlight current methods in improving the regenerative potential of stem cell-based therapies for cartilage repair with encouraging preclinical results. Many more studies have made strides in developing promising, novel injectable hydrogel systems for cartilage applications; however, these materials have not yet progressed to preclinical experiments and have only been shown effective in in vitro models [123–126].
3.3. Nervous System Stem Cell Transplantation Therapies
Diseases and injuries to the nervous system impact a significant portion of the population with devastating implications. While much of the underlying etymology behind neurological diseases such as Parkinson’s and Alzheimer’s is unknown, each results in irrevocable loss of specific neuron populations. In addition, injury and ischemia to the spinal cord (SCI) and brain results in significant neural cell death, leading to substantially diminished movement and sensation as well as impaired mental and cognitive function [84, 127–129]. Stem cell-derived therapies are currently being investigated in numerous neurological-based clinical trials looking to either replace lost neuron populations or provide supporting glial cell types, such as oligodendrocytes and astrocytes [127, 130]. Unfortunately, despite promising pre-clinical data, no stem cell-based therapies have been able to move to the market or clinic, often due to failure to show improvement in humans and limited cell characterization [131]. The neural microenvironment post injury can be incredibly cytotoxic due to ischemia, presence of inhibitory myelin debris, and release of excitotoxic molecules [130]. Furthermore, neural cells tend to be very sensitive to handling, thus the injection process itself may be decreasing cell survival.
Therefore, in order to improve transplanted cell survival, engraftment, and integration with host tissue, researchers have designed injectable hydrogel systems that are specifically tailored to improve neural and glial cell phenotypic function within injured nervous tissue. The neural tissue environment has several unique characteristics that need to be taken into account when developing therapeutic strategies. Unlike musculoskeletal or connective tissues, neural tissues are mechanically very compliant, with stiffness ranging from 100–1000 Pa [79, 132, 133]. This property of the in vivo matrix appears to translate to preferred in vitro substrates. For example, stem cells cultured on softer substrates tend to differentiate down a neural lineage compared to cells on stiffer substrates, and primary neurons respond to more compliant materials by producing longer neurite extensions [63, 65, 79, 134]. Therefore, using hydrogel carriers that are significantly stiffer than native neural tissue would likely limit integration of transplanted cells due to both failure of transplanted cells to differentiate into appropriate cell types in the stiff matrix as well as failure to promote host cell penetration and remodeling. ECM proteins expressed in neural tissue are primarily laminins, collagens, and fibronectin [135]. Laminin contains two cell binding sites, IKVAV and YIGSR, that neurons have an affinity for, with IKVAV promoting significant neurite outgrowth and YISGR promoting neural cell attachment and survival [63, 136, 137]. Neurons are also dependent on several soluble signaling factors for survival and regeneration including neurotrophin-3 (NT3), glial cell line-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), and platelet-derived growth factor-A (PDGF-A) [138, 139]. Many groups have therefore attempted to incorporate these factors into injectable hydrogels to improve functional recovery after disease or injury to the nervous system.
In part due to their mechanically compliant properties, naturally derived biomaterials have been historically favored in the development of injectable hydrogel carriers for neural applications. For example, fibrin hydrogels have been used to deliver ESC-derived neural progenitors for treating SCI [138, 139]. Fibrin hydrogel properties can be easily tuned to mimic native environmental properties simply by altering the concentration of fibrinogen. Fibrin delivery also improved neural progenitor cell (NPC) survival and influenced differentiation into neural phenotypes when modified with a growth factor delivery system [138]. Sustained delivery of GDNF, NT3, PDGF-A as well as other growth factors from injectable fibrin scaffolds with mouse NPCs impacted astroglial scar formation and macrophage response and improved neuronal differentiation [139]. Matrigel, another naturally derived material that is rich in laminin and collagen, has also been investigated for cell delivery, as it can be injected due to its thermal gelation property. In vitro, Matrigel is used extensively to support stem cell survival, proliferation, and differentiation, especially into neural lineages. In an ischemic stroke model, delivery of ESC-derived NPCs with Matrigel significantly improved cell survival and outcomes in sensimotor and cognitive function [140]. Furthermore, Matrigel (growth factor reduced) improved ESC-derived NPC survival compared to artificial cerebrospinal fluid and increased dopaminergic neuron differentiation for treating Parkinson’s disease [141]. The authors suggested that use of Matrigel suppressed the normal immune response to grafted cells, thus increasing the number of dopaminergic neurons, rather than the material itself inducing differentiation. Unfortunately, Matrigel is derived from mouse sarcoma and cannot be used in clinical applications.
Hyaluronan or hyaluronic acid (HA) is commonly found in the nervous system and therefore is a promising material for delivering cells to neural tissues [142]. The Shoichet group has designed and extensively studied hyaluronic acid-methylcellulose (HAMC) hydrogels for cell delivery and have found encouraging results for SCI and stroke therapies [142–144]. This family of hydrogels can be tuned to match native neural mechanical properties and has been shown to reduce scarring and inflammation in SCI [145]. In addition, these hydrogels modified with recombinant PDGF-A led to increased survival of transplanted adult NPCs and improved differentiation into oligodendrocytes after SCI. This combination therapy led to increased graft survival, improved host tissue sparing, and decreased SCI pathology, which correlated with increased behavioral recovery [146]. HAMC similarly led to increased oligodendrocyte differentiation of transplanted iPSC-derived oligodendrocyte progenitor cells, but most importantly, it attenuated teratoma formation compared to cells delivered in saline alone [144]. Degradable HA hydrogels modified with the cell-binding domain RGD have also been used to deliver iPSC-derived NPCs for treating stroke. While the hydrogels did not improve cell survival post cerebral injection compared to cells alone, differentiation of iPSC-NPCs into doublecortin positive neuroblasts was significantly increased with HA delivery [147, 148]. Another HA variant, modified with heparin sulfate and collagen, has similarly been tested as an ESC-derived NPC delivery vehicle for ischemic stroke therapy. The use of the support matrix increased transplanted cell survival and decreased microglia response. Unfortunately, while cell survival increased two-fold, it only increased from 300 to 600 cells out of the original 100,000 cells transplanted [149]. Lastly, HA carriers modified with poly-L-lysine (PLL) for enhanced cell attachment were shown to improve transplanted BMSC survival and differentiation in a thoracic SCI model. Compared to cells transplanted alone, cells with HA-PLL gels led to improved hind limb locomotion, a result rarely seen with MSC therapies alone in SCI [150].
Other biomaterials that are naturally derived or bio-inspired have shown promise in enhancing the therapeutic potential of stem cells for treatment of neural injury and disease. For example, the self-assembling peptide K2(QL)6K2 (QL6) has previously been shown to reduce the associated pathology observed after SCI with decreased inflammation, glial scarring, and cell death [151]. Therefore, Iwasaki et al. probed the synergistic effect of QL6 transplanted in succession with adult NPCs in a cervical compression SCI. QL6 did not statistically improve NPC survival in vivo (0.25% for cells only versus 0.62% for cells and QL6), yet the addition of the self assembling peptide led to decreased microglia activation and gliosis and increased motoneuron and neuron sparring resulting in improved forelimb function [2]. It is of note that the NPCs were not embedded within the QL6 solution, instead the treatments were injected separately, and thus it is uncertain whether the peptide would improve cell survival to greater effect if they were co-delivered. Composite scaffolds made of poly(lactic acid) nanofibers embedded in an injectable xyloglucan hydrogel improved the survival and reinnervation of dopamine progenitor cells in Parkinson’s debilitated mice, with significant improvement observed when the scaffolds were optimized with GDNF and BDNF co-delivery [152].
3.4. Osteoinductive Stem Cell Transplantation Therapies
Due to our increasingly aging population, bone related diseases and injuries are on the rise with an increasing need for bone grafting technologies. An extensive amount of research is being undertaken to replace lost bone cells and encourage native tissue regeneration using stem cell-derived technologies [153, 154]. There are currently dozens of clinical trials probing the use of stem cells, primarily MSCs, for bone regeneration applications in a variety of indications including cancer, osteonecrosis, pseudo/osteoarthritis, fractures, periodontal disease, and spinal fusions [154, 155]. The goal of stem cell-derived therapies is to initiate new tissue remodeling and growth with cohesive integration of grafts and host tissue allowing for proper movement and function. Unfortunately, lack of nutrients from blood supply and poor mechanical support can lead to graft failure to integrate and potential morbidity [154]. In addition, while cell-seeded hydrogels have been investigated broadly for bone tissue engineering, a non-invasive, injectable cell carrier system is needed in order to deliver cells to difficult-to-reach and non-uniform injury sites.
In response, researchers are developing a variety of supporting scaffolding systems to facilitate engraftment of transplanted cells with host tissue and differentiation into osteogenic phenotypes. Key matrix properties of bone that must be considered include their protein and mineral composition and their structural and mechanical strength properties. Bone is characterized as cortical and trabecular bone, with cortical bone being dense, compact tissue and trabecular bone being spongey and porous. On the ultrastructural level, bone is composed of compacted collagen fibrils that are mineralized with hydroxyapatite (Ca5(PO4)3) microparticles. Cortical and trabecular bone stiffness can range between 100–2000 MPa, with substrate stiffness in the 100 kPa range supporting enhanced osteogenic differentiation of MSCs in vitro [79, 156].
Many groups have investigated the use of PNIPAAM, a thermosensitive polymer, for injectable cell delivery in bone applications. PNIPAAM is a synthetic polymer that can be tuned to match the mechanical stiffness of bone once injected at body temperature [157–159]. Watson et al. demonstrated that decorating PNIPAAM with pendant phosphate groups improved biodegradation and biointegration of the hydrogel with host tissue, as well as support enhanced mineralization, MSC differentiation, and bone formation in a rat cranial defect model [159]. Further work has shown promising results with MSC delivery using PNIPAAM hydrogels either grafted with gelatin or incorporating gelatin microspheres [157, 158]. Gelatin, which is denatured collagen, is an excellent source of natively relevant bioactive adhesion sites for both transplanted and host cells. The use of gelatin microspheres within injectable PNIPAAM and encapsulated MSCs led to increased direct bone-to-hydrogel contact, cell infiltration, and osteoid formation [158]. In addition, direct grafting of gelatin to PNIPAAM increased the rate of new bone formation with extensive graft integration into the host tissue [157–159].
Alginate has also been investigated as a stem cell carrier for bone regeneration applications. Functionalizing alginate with the RGD cell-binding domain for co-delivery of MSCs with bone morphogenetic protein-2 (BMP-2) led to increased mineralization in vitro and increased bone formation in vivo in a critically sized femoral defect model [160]. Alginate hydrogels have also been shown to support hMSC migration and osteodifferentiation when chemically modified with osteoinductive growth peptides [161] [161]. Unfortunately, this work was only performed in vitro, and efficacy in vivo has yet to be evaluated. Indeed, there are dozens of promising new injectable hydrogel stem cell carrier technologies being investigated for bone regeneration, including Pluronic F127, chitosan/collagen/β-GP, calcium phosphate cement, and several synthetic polymers, yet they have only been tested in vitro or in non-bone defect, subcutaneous in vivo models [162–165].
3.5. Other Targets for Injectable Stem Cell Therapeutics
Recent advances in stem cell biology have opened new doors in developing therapies for less high prolife diseases and organ systems than those discussed above. Accordingly, with the increased attention given to stem cell-derived treatments for other applications, interesting new methods to improve their efficacy have arisen using injectable, combinatorial hydrogel strategies. For example, in treating retinal degenerative diseases, stem cell therapies routinely fail to survive and integrate. Recent research has shown, however, that encapsulation of retinal progenitor/stem cells (RPCs) in hyaluronan-methyl cellulose (HAMC) hydrogels supports robust survival and proliferation. Most importantly, when transplanting cells in vivo, the use of the HAMC hydrogel improved RPC distribution through the impacted area compared to saline, as well as improved grafted retinal rod survival and functional visual integration [142, 166]. Engineering new muscle through regenerative medicine strategies has also shown promise through enhanced delivery and survival of MSCs, muscle stem cells, and skeletal muscle satellite cells using thermosensitive, injectable hydrogels such as collagen/chitosan/βGP [167], composite synthetic polymers PNIAPPAM/acrylic acid/2-hydroxyethyl methacrylate-oligomers [168], small intestinal submucosa [169], and fibrin [33]. Wound healing has also been a targeted research area for potential stem cell-derived therapies. Delivery of hASCs for full thickness skin wounds using injectable gelatin microspheres was shown to significantly improve the wound healing rate, stem cell retention, and growth factor secretion levels compared to delivery of cells alone [170]. Similar functional results in wound healing have been observed when BMSCs were delivered using cell-protective alginate beads within injectable hydrophobic poly(ether urethane) hydrogels compared to implanting pre-formed scaffolds [171] or transplanting cells within gelatin-poly(ethylene glycol) hydrogels [172]. Lastly, chitosan-based polymers have been shown to improve iPSC-derived hepatocyte (iPSC-Heps) survival and integration for liver tissue engineering as well as ASCs for acute kidney failure. Carboxymethyl-hexanoyl chitosan hydrogels were shown to successfully engraft iPSC-Heps through direct intrahepatic delivery and to reduce necrotic tissue area and to improve liver function [173]. Thermosensitive chitosan chloride hydrogels were capable of supporting enhanced ASC survival and proliferation in an acute kidney injury model, as well as reducing host renal cell apoptosis and improving microvessel density and renal function [174]. While research in these areas is limited to only a handful of studies in each case, promising in vitro and in vivo data suggest combining stem cell-derived therapies with injectable hydrogels can significantly improve their therapeutic potential.
4. Conclusion
Throughout these different areas of regenerative medicine application, a common theme has emerged indicating that stem cells hold great potential for pronounced therapeutic benefits. Unfortunately, harsh conditions after injury and disease, as well as the delivery process itself, can significantly hinder the functional impact of transplanted cells. Therefore, delivering cells in carefully designed, cell-protective and cell-supporting injectable hydrogels may significantly enhance therapeutic efficacy for several different regenerative medicine applications. Looking forward, these injectable materials are expected to improve the rate of clinical translation for stem cell-derived therapies by increasing grafted cell survival and functionality.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Laura M. Marquardt declares that she has no conflict of interest.
Sarah C. Heilshorn reports she has two patents that have been issued to her (20150290329 [SHIELD] and 20100183720 [MITCH]).
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Sortwell CE, Pitzer MR, Collier TJ. Time course of apoptotic cell death within mesencephalic cell suspension grafts: implications for improving grafted dopamine neuron survival. Exp Neurol. 2000;165(2):268–77. doi: 10.1006/exnr.2000.7476. [DOI] [PubMed] [Google Scholar]
- 2.Iwasaki M, et al. Synergistic effects of self-assembling peptide and neural stem/progenitor cells to promote tissue repair and forelimb functional recovery in cervical spinal cord injury. Biomaterials. 2014;35(9):2617–29. doi: 10.1016/j.biomaterials.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Malliaras K, Kreke M, Marban E. The stuttering progress of cell therapy for heart disease. Clin Pharmacol Ther. 2011;90(4):532–41. doi: 10.1038/clpt.2011.175. [DOI] [PubMed] [Google Scholar]
- 4.Singelyn JM, Christman KL. Injectable materials for the treatment of myocardial infarction and heart failure: the promise of decellularized matrices. J Cardiovasc Transl Res. 2010;3(5):478–86. doi: 10.1007/s12265-010-9202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Templin C, Luscher TF, Landmesser U. Cell-based cardiovascular repair and regeneration in acute myocardial infarction and chronic ischemic cardiomyopathy-current status and future developments. Int J Dev Biol. 2011;55(4–5):407–17. doi: 10.1387/ijdb.103219ct. [DOI] [PubMed] [Google Scholar]
- 6.Ebara M, et al. Smart Biomaterials. Springer Japan; Japan: 2014. Smart Hydrogels. [Google Scholar]
- 7.Hennink WE, van Nostrum CF. Novel crosslinking methods to design hydrogels. Adv Drug Deliv Rev. 2002;54(1):13–36. doi: 10.1016/s0169-409x(01)00240-x. [DOI] [PubMed] [Google Scholar]
- 8.Jen AC, Wake MC, Mikos AG. Review: Hydrogels for cell immobilization. Biotechnol Bioeng. 1996;50(4):357–64. doi: 10.1002/(SICI)1097-0290(19960520)50:4<357::AID-BIT2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 9.Omidian H, Park K. Hydrogels. In: Siepmann J, Siegel AR, Rathbone JM, editors. Fundamentals and Applications of Controlled Release Drug Delivery. Springer US; Boston, MA: 2012. pp. 75–105. [Google Scholar]
- 10.Boesel LF, RRL . Biodegradable systems in tissue engineering and regenerative medicine. Boca Raton, FL: CRC Press; 2004. Injectable biodegradable systems. [Google Scholar]
- 11.Christman KL, et al. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10(3–4):403–9. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 12.Christman KL, et al. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44(3):654–60. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 13.Giano MC, et al. Injectable bioadhesive hydrogels with innate antibacterial properties. Nat Commun. 2014;5:4095. doi: 10.1038/ncomms5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoare TR, Kohane DS. Hydrogels in drug delivery: Progress and challenges. Polymer. 2008;49(8):1993–2007. [Google Scholar]
- 15.Purcell BP, et al. Injectable and bioresponsive hydrogels for on-demand matrix metalloproteinase inhibition. Nat Mater. 2014;13(6):653–61. doi: 10.1038/nmat3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glassman MJ, Chan J, Olsen BD. Reinforcement of Shear Thinning Protein Hydrogels by Responsive Block Copolymer Self-Assembly. Adv Funct Mater. 2013;23(9):1182–1193. doi: 10.1002/adfm.201202034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin Y, et al. Recent advances in dynamic covalent chemistry. Chem Soc Rev. 2013;42(16):6634–54. doi: 10.1039/c3cs60044k. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Ni X, Leong KW. Injectable drug-delivery systems based on supramolecular hydrogels formed by poly(ethylene oxide)s and alpha-cyclodextrin. J Biomed Mater Res A. 2003;65(2):196–202. doi: 10.1002/jbm.a.10444. [DOI] [PubMed] [Google Scholar]
- 19.Lu HD, et al. Injectable shear-thinning hydrogels engineered with a self-assembling Dock-and-Lock mechanism. Biomaterials. 2012;33(7):2145–53. doi: 10.1016/j.biomaterials.2011.11.076. [DOI] [PubMed] [Google Scholar]
- 20••.Mulyasasmita W, et al. Avidity-controlled hydrogels for injectable co-delivery of induced pluripotent stem cell-derived endothelial cells and growth factors. J Control Release. 2014;191:71–81. doi: 10.1016/j.jconrel.2014.05.015. This study showed the design of protein-engineered, physical hydrogels that gently encapsulate and protect iPSC-derived endothelial cells during the injection process and improve pathology in a hindlimb ischemia model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aguado BA, et al. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A. 2012;18(7–8):806–15. doi: 10.1089/ten.tea.2011.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan C, et al. Injectable solid hydrogel: mechanism of shear-thinning and immediate recovery of injectable beta-hairpin peptide hydrogels. Soft Matter. 2010;6(20):5143–5156. doi: 10.1039/C0SM00642D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Cai L, Dewi RE, Heilshorn SC. Injectable Hydrogels with In Situ Double Network Formation Enhance Retention of Transplanted Stem Cells. Adv Funct Mater. 2015;25(9):1344–1351. doi: 10.1002/adfm.201403631. This study demonstrated a novel family of injectable hydrogels comprised of an engineered protein and a thermoresponsive synthetic component protect cells during the injection process and imrpove long-term cell retention in a subcutaneous injection model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai L, Heilshorn SC. Designing ECM-mimetic materials using protein engineering. Acta Biomater. 2014;10(4):1751–60. doi: 10.1016/j.actbio.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Appel EA, et al. Self-assembled hydrogels utilizing polymer-nanoparticle interactions. Nat Commun. 2015;6:6295. doi: 10.1038/ncomms7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta D, Tator CH, Shoichet MS. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials. 2006;27(11):2370–9. doi: 10.1016/j.biomaterials.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Yan C, Pochan DJ. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem Soc Rev. 2010;39(9):3528–40. doi: 10.1039/b919449p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan J, et al. Biocompatibility evaluation of chitosan-based injectable hydrogels for the culturing mice mesenchymal stem cells in vitro. J Biomater Appl. 2010;24(7):625–37. doi: 10.1177/0885328208100536. [DOI] [PubMed] [Google Scholar]
- 29•.Gaffey AC, et al. Injectable shear-thinning hydrogels used to deliver endothelial progenitor cells, enhance cell engraftment, and improve ischemic myocardium. J Thorac Cardiovasc Surg. 2015;150(5):1268–76. doi: 10.1016/j.jtcvs.2015.07.035. This study demonstrated a shear-thinning hyaluronic acid hydrogel improved transplated endothelial progenitor cell survival in an ischemic MI model, with increased vasculogensis and ventricular function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guvendiren M, Lu HD, Burdick JA. Shear-thinning hydrogels for biomedical applications. Soft Matter. 2012;8(2):260–272. [Google Scholar]
- 31.Leslie SK, et al. Controlled release of rat adipose-derived stem cells from alginate microbeads. Biomaterials. 2013;34(33):8172–84. doi: 10.1016/j.biomaterials.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 32.Leslie SK, et al. Development of a cell delivery system using alginate microbeads for tissue regeneration. Journal of Materials Chemistry B. 2016 doi: 10.1039/c6tb00035e. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, et al. Human umbilical cord stem cell encapsulation in novel macroporous and injectable fibrin for muscle tissue engineering. Acta Biomater. 2013;9(1):4688–97. doi: 10.1016/j.actbio.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Rao RR, Stegemann JP. Delivery of mesenchymal stem cells in chitosan/collagen microbeads for orthopedic tissue repair. Cells Tissues Organs. 2013;197(5):333–43. doi: 10.1159/000348359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson JL, et al. Alginate encapsulation parameters influence the differentiation of microencapsulated embryonic stem cell aggregates. Biotechnol Bioeng. 2014;111(3):618–31. doi: 10.1002/bit.25121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson TD, Braden RL, Christman KL. Injectable ECM scaffolds for cardiac repair. Methods Mol Biol. 2014;1181:109–20. doi: 10.1007/978-1-4939-1047-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson TD, Christman KL. Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert Opin Drug Deliv. 2013;10(1):59–72. doi: 10.1517/17425247.2013.739156. [DOI] [PubMed] [Google Scholar]
- 38.Ungerleider JL, Christman KL. Concise review: injectable biomaterials for the treatment of myocardial infarction and peripheral artery disease: translational challenges and progress. Stem Cells Transl Med. 2014;3(9):1090–9. doi: 10.5966/sctm.2014-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dooling LJ, et al. Programming Molecular Association and Viscoelastic Behavior in Protein Networks. Adv Mater. 2016 doi: 10.1002/adma.201506216. [DOI] [PubMed] [Google Scholar]
- 40.Seow WY, Hauser CAE. Short to ultrashort peptide hydrogels for biomedical uses. Materials Today. 2014;17(8):381–388. [Google Scholar]
- 41.Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromol Biosci. 2006;6(8):623–33. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 42.Lin H, et al. Cartilage tissue engineering application of injectable gelatin hydrogel with in situ visible-light-activated gelation capability in both air and aqueous solution. Tissue Eng Part A. 2014;20(17–18):2402–11. doi: 10.1089/ten.tea.2013.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin YD, et al. Intramyocardial peptide nanofiber injection improves postinfarction ventricular remodeling and efficacy of bone marrow cell therapy in pigs. Circulation. 2010;122(11 Suppl):S132–41. doi: 10.1161/CIRCULATIONAHA.110.939512. [DOI] [PubMed] [Google Scholar]
- 44.Sharma B, et al. In vivo chondrogenesis of mesenchymal stem cells in a photopolymerized hydrogel. Plast Reconstr Surg. 2007;119(1):112–20. doi: 10.1097/01.prs.0000236896.22479.52. [DOI] [PubMed] [Google Scholar]
- 45.Williams CG, et al. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;9(4):679–88. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]
- 46.Camci-Unal G, et al. Oxygen Releasing Biomaterials for Tissue Engineering. Polym Int. 2013;62(6):843–848. doi: 10.1002/pi.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hadjipanayi E, et al. Injectable system for spatio-temporally controlled delivery of hypoxia-induced angiogenic signalling. J Control Release. 2012;161(3):852–60. doi: 10.1016/j.jconrel.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 48.Ladet SG, et al. Multi-membrane chitosan hydrogels as chondrocytic cell bioreactors. Biomaterials. 2011;32(23):5354–64. doi: 10.1016/j.biomaterials.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 49.Park KM, Gerecht S. Hypoxia-inducible hydrogels. Nat Commun. 2014;5:4075. doi: 10.1038/ncomms5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szot CS, et al. 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials. 2011;32(31):7905–12. doi: 10.1016/j.biomaterials.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.des Rieux A, et al. Vascular endothelial growth factor-loaded injectable hydrogel enhances plasticity in the injured spinal cord. J Biomed Mater Res A. 2014;102(7):2345–55. doi: 10.1002/jbm.a.34915. [DOI] [PubMed] [Google Scholar]
- 52.Emerich DF, et al. Injectable VEGF hydrogels produce near complete neurological and anatomical protection following cerebral ischemia in rats. Cell Transplant. 2010;19(9):1063–71. doi: 10.3727/096368910X498278. [DOI] [PubMed] [Google Scholar]
- 53.Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5(3):590–8. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 54.Wu J, et al. Infarct stabilization and cardiac repair with a VEGF-conjugated, injectable hydrogel. Biomaterials. 2011;32(2):579–86. doi: 10.1016/j.biomaterials.2010.08.098. [DOI] [PubMed] [Google Scholar]
- 55.Moya M, Brey E. Vascularization in Engineered Tissues. In: Fisher JP, et al., editors. Tissue Engineering: Principles and Practices. CRC; 2012. [Google Scholar]
- 56.Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4(7):518–24. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 57.Hwang CM, et al. Fabrication of three-dimensional porous cell-laden hydrogel for tissue engineering. Biofabrication. 2010;2(3):035003. doi: 10.1088/1758-5082/2/3/035003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scott EA, et al. Modular scaffolds assembled around living cells using poly(ethylene glycol) microspheres with macroporation via a non-cytotoxic porogen. Acta Biomater. 2010;6(1):29–38. doi: 10.1016/j.actbio.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Huebsch N, et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat Mater. 2015;14(12):1269–77. doi: 10.1038/nmat4407. This study described the formation of void-forming hydrogels by the incorporation of sacrificial porogens that allow for increased nutrient diffusion, which resulted in imrpoved transplanted MSC migration and differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang S, et al. Adipose Tissue and Extracellular Matrix Development by Injectable Decellularized Adipose Matrix Loaded with Basic Fibroblast Growth Factor. Plast Reconstr Surg. 2016;137(4):1171–80. doi: 10.1097/PRS.0000000000002019. [DOI] [PubMed] [Google Scholar]
- 61.Hudson TW, et al. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004;10(11–12):1641–51. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 62.Hern DL, Hubbell JA. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39(2):266–76. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 63.Marquardt L, Willits RK. Student award winner in the undergraduate’s degree category for the society for biomaterials 35th annual meeting, Orlando, Florida, April 13–16, 2011. Journal of Biomedical Materials Research Part A. 2011;98A(1):1–6. doi: 10.1002/jbm.a.33044. [DOI] [PubMed] [Google Scholar]
- 64.Schense JC, Hubbell JA. Three-dimensional migration of neurites is mediated by adhesion site density and affinity. J Biol Chem. 2000;275(10):6813–8. doi: 10.1074/jbc.275.10.6813. [DOI] [PubMed] [Google Scholar]
- 65.Scott R, Marquardt L, Willits RK. Characterization of poly(ethylene glycol) gels with added collagen for neural tissue engineering. J Biomed Mater Res A. 2010;93(3):817–23. doi: 10.1002/jbm.a.32775. [DOI] [PubMed] [Google Scholar]
- 66.Yu X, Dillon GP, Bellamkonda RB. A laminin and nerve growth factor- laden three-dimensional scaffold for enhanced neurite extension. Tissue Eng. 1999;5(4):291–304. doi: 10.1089/ten.1999.5.291. [DOI] [PubMed] [Google Scholar]
- 67.Zhu J, et al. Design and synthesis of biomimetic hydrogel scaffolds with controlled organization of cyclic RGD peptides. Bioconjug Chem. 2009;20(2):333–9. doi: 10.1021/bc800441v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zustiak SP, Durbal R, Leach JB. Influence of cell-adhesive peptide ligands on poly(ethylene glycol) hydrogel physical, mechanical and transport properties. Acta Biomater. 2010;6(9):3404–14. doi: 10.1016/j.actbio.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hotaling NA, et al. Biomaterial Strategies for Immunomodulation. Annu Rev Biomed Eng. 2015;17:317–49. doi: 10.1146/annurev-bioeng-071813-104814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bos GW, et al. In situ crosslinked biodegradable hydrogels loaded with IL-2 are effective tools for local IL-2 therapy. Eur J Pharm Sci. 2004;21(4):561–7. doi: 10.1016/j.ejps.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 71.Soranno DE, et al. Immunotherapy with injectable hydrogels to treat obstructive nephropathy. J Biomed Mater Res A. 2014;102(7):2173–80. doi: 10.1002/jbm.a.34902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mora-Solano C, Collier JH. Engaging adaptive immunity with biomaterials. J Mater Chem B Mater Biol Med. 2014;2(17):2409–2421. doi: 10.1039/C3TB21549K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rudra JS, et al. Modulating adaptive immune responses to peptide self-assemblies. ACS Nano. 2012;6(2):1557–64. doi: 10.1021/nn204530r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh A, Peppas NA. Hydrogels and scaffolds for immunomodulation. Adv Mater. 2014;26(38):6530–41. doi: 10.1002/adma.201402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vishwakarma A, et al. Engineering Immunomodulatory Biomaterials To Tune the Inflammatory Response. Trends Biotechnol. 2016 doi: 10.1016/j.tibtech.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 76.Wen Y, Collier JH. Supramolecular peptide vaccines: tuning adaptive immunity. Curr Opin Immunol. 2015;35:73–9. doi: 10.1016/j.coi.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chaudhuri O, et al. Substrate stress relaxation regulates cell spreading. Nat Commun. 2015;6:6364. doi: 10.1038/ncomms7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78•.Chaudhuri O, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2016;15(3):326–34. doi: 10.1038/nmat4489. This study reported that MSC differentiation, spreading, and proliferation is dependent on a hydrogel’s relaxation rate, independent of the material’s inital stiffness. Furthermore, ligand clustering plays a substantial role in the effect of stress relaxation on cell behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Engler AJ, et al. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 80.Guilak F, et al. Control of Stem Cell Fate by Physical Interactions with the Extracellular Matrix. Cell Stem Cell. 2009;5(1):17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cosson S, Lutolf MP. Microfluidic patterning of protein gradients on biomimetic hydrogel substrates. Methods Cell Biol. 2014;121:91–102. doi: 10.1016/B978-0-12-800281-0.00007-5. [DOI] [PubMed] [Google Scholar]
- 82.Caiazzo M, et al. Defined three-dimensional microenvironments boost induction of pluripotency. Nat Mater. 2016;15(3):344–52. doi: 10.1038/nmat4536. [DOI] [PubMed] [Google Scholar]
- 83.Tysseling-Mattiace VM, et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci. 2008;28(14):3814–23. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choe AS, et al. Extensive neurological recovery from a complete spinal cord injury: a case report and hypothesis on the role of cortical plasticity. Frontiers in Human Neuroscience. 2013;7:290. doi: 10.3389/fnhum.2013.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wylie RG, et al. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nat Mater. 2011;10(10):799–806. doi: 10.1038/nmat3101. [DOI] [PubMed] [Google Scholar]
- 86.Chung C, et al. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials. 2009;30(26):4287–96. doi: 10.1016/j.biomaterials.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chung C, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009;15(2):243–54. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burdick JA, Murphy WL. Moving from static to dynamic complexity in hydrogel design. Nat Commun. 2012;3:1269. doi: 10.1038/ncomms2271. [DOI] [PubMed] [Google Scholar]
- 89.Kloxin AM, et al. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324(5923):59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bakota EL, et al. Injectable multidomain peptide nanofiber hydrogel as a delivery agent for stem cell secretome. Biomacromolecules. 2011;12(5):1651–7. doi: 10.1021/bm200035r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y, et al. Peptide nanofibers preconditioned with stem cell secretome are renoprotective. J Am Soc Nephrol. 2011;22(4):704–17. doi: 10.1681/ASN.2010040403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abdeen AA, et al. Matrix composition and mechanics direct proangiogenic signaling from mesenchymal stem cells. Tissue Eng Part A. 2014;20(19–20):2737–45. doi: 10.1089/ten.tea.2013.0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dhote V, et al. On the role of hydrogel structure and degradation in controlling the transport of cell-secreted matrix molecules for engineered cartilage. J Mech Behav Biomed Mater. 2013;19:61–74. doi: 10.1016/j.jmbbm.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mozaffarian D, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 95.Chong JJ, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510(7504):273–7. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85(4):1373–416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 97.Nasseri BA, et al. Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: the Cardio133 trial. Eur Heart J. 2014;35(19):1263–74. doi: 10.1093/eurheartj/ehu007. [DOI] [PubMed] [Google Scholar]
- 98.Lockhart M, et al. Extracellular matrix and heart development. Birth Defects Res A Clin Mol Teratol. 2011;91(6):535–50. doi: 10.1002/bdra.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Engler AJ, et al. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121(Pt 22):3794–802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bhana B, et al. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol Bioeng. 2010;105(6):1148–60. doi: 10.1002/bit.22647. [DOI] [PubMed] [Google Scholar]
- 101.Ribeiro AJ, et al. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci U S A. 2015;112(41):12705–10. doi: 10.1073/pnas.1508073112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Levit RD, et al. Cellular encapsulation enhances cardiac repair. J Am Heart Assoc. 2013;2(5):e000367. doi: 10.1161/JAHA.113.000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ruvinov E, Cohen S. Alginate biomaterial for the treatment of myocardial infarction: Progress, translational strategies, and clinical outlook: From ocean algae to patient bedside. Adv Drug Deliv Rev. 2016;96:54–76. doi: 10.1016/j.addr.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 104.Roche ET, et al. Comparison of biomaterial delivery vehicles for improving acute retention of stem cells in the infarcted heart. Biomaterials. 2014;35(25):6850–8. doi: 10.1016/j.biomaterials.2014.04.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105•.Panda NC, et al. Improved conduction and increased cell retention in healed MI using mesenchymal stem cells suspended in alginate hydrogel. J Interv Card Electrophysiol. 2014;41(2):117–27. doi: 10.1007/s10840-014-9940-9. This study describes the use of alginate hydrogels for MSC delivery in an MI model and their effects on cellular and cardiac functions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Assaad E, Maire M, Lerouge S. Injectable thermosensitive chitosan hydrogels with controlled gelation kinetics and enhanced mechanical resistance. Carbohydr Polym. 2015;130:87–96. doi: 10.1016/j.carbpol.2015.04.063. [DOI] [PubMed] [Google Scholar]
- 107.Ladage D, et al. Delivery of gelfoam-enabled cells and vectors into the pericardial space using a percutaneous approach in a porcine model. Gene Ther. 2011;18(10):979–85. doi: 10.1038/gt.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xia Y, et al. Enhanced infarct myocardium repair mediated by thermosensitive copolymer hydrogel-based stem cell transplantation. Exp Biol Med (Maywood) 2015;240(5):593–600. doi: 10.1177/1535370214560957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kinnaird T, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109(12):1543–9. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 110.Hou L, et al. Stem Cell-Based Therapies to Promote Angiogenesis in Ischemic Cardiovascular Disease. Am J Physiol Heart Circ Physiol. 2015 doi: 10.1152/ajpheart.00726.2015. ajpheart 00726 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang NF, et al. Embryonic stem cell-derived endothelial cells for treatment of hindlimb ischemia. J Vis Exp. 2009;(23) doi: 10.3791/1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang NF, et al. Embryonic stem cell-derived endothelial cells engraft into the ischemic hindlimb and restore perfusion. Arterioscler Thromb Vasc Biol. 2010;30(5):984–91. doi: 10.1161/ATVBAHA.110.202796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang J, et al. A cellular delivery system fabricated with autologous BMSCs and collagen scaffold enhances angiogenesis and perfusion in ischemic hind limb. J Biomed Mater Res A. 2012;100(6):1438–47. doi: 10.1002/jbm.a.34081. [DOI] [PubMed] [Google Scholar]
- 114.Xu Y, et al. A prosurvival and proangiogenic stem cell delivery system to promote ischemic limb regeneration. Acta Biomater. 2015 doi: 10.1016/j.actbio.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. [PubMed] [Google Scholar]
- 116.Park IK, Cho CS. Stem Cell-assisted Approaches for Cartilage Tissue Engineering. Int J Stem Cells. 2010;3(2):96–102. doi: 10.15283/ijsc.2010.3.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Prestwich GD. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J Control Release. 2011;155(2):193–9. doi: 10.1016/j.jconrel.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang L, Hu J, Athanasiou KA. The role of tissue engineering in articular cartilage repair and regeneration. Crit Rev Biomed Eng. 2009;37(1–2):1–57. doi: 10.1615/critrevbiomedeng.v37.i1-2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Frith JE, et al. An injectable hydrogel incorporating mesenchymal precursor cells and pentosan polysulphate for intervertebral disc regeneration. Biomaterials. 2013;34(37):9430–40. doi: 10.1016/j.biomaterials.2013.08.072. [DOI] [PubMed] [Google Scholar]
- 120•.Choi B, et al. Covalently conjugated transforming growth factor-beta1 in modular chitosan hydrogels for the effective treatment of articular cartilage defects. Biomater Sci. 2015;3(5):742–52. doi: 10.1039/c4bm00431k. This study developed a visible light crosslinkable hydrogel to delvier both stem cells and biochemical cues for chondrol defect repair. [DOI] [PubMed] [Google Scholar]
- 121.Choi B, et al. Cartilaginous Extracellular Matrix-Modified Chitosan Hydrogels for Cartilage Tissue Engineering. ACS Applied Materials & Interfaces. 2014;6(22):20110–20121. doi: 10.1021/am505723k. [DOI] [PubMed] [Google Scholar]
- 122•.Qi BW, et al. Chitosan/poly(vinyl alcohol) hydrogel combined with Ad-hTGF-beta1 transfected mesenchymal stem cells to repair rabbit articular cartilage defects. Exp Biol Med (Maywood) 2013;238(1):23–30. doi: 10.1258/ebm.2012.012223. This study demonstrated a combinatorial therapy of injectable hydrogel and growth factor-modified stem cells can improve articular cartilage repair with extensive histological analysis. [DOI] [PubMed] [Google Scholar]
- 123.Collin EC, et al. An injectable vehicle for nucleus pulposus cell-based therapy. Biomaterials. 2011;32(11):2862–70. doi: 10.1016/j.biomaterials.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 124.Endres M, et al. Microencapsulation and chondrogenic differentiation of human mesenchymal progenitor cells from subchondral bone marrow in Ca-alginate for cell injection. Acta Biomater. 2010;6(2):436–44. doi: 10.1016/j.actbio.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 125.Park H, et al. Effect of dual growth factor delivery on chondrogenic differentiation of rabbit marrow mesenchymal stem cells encapsulated in injectable hydrogel composites. J Biomed Mater Res A. 2009;88(4):889–97. doi: 10.1002/jbm.a.31948. [DOI] [PubMed] [Google Scholar]
- 126.Toh WS, et al. Modulation of mesenchymal stem cell chondrogenesis in a tunable hyaluronic acid hydrogel microenvironment. Biomaterials. 2012;33(15):3835–45. doi: 10.1016/j.biomaterials.2012.01.065. [DOI] [PubMed] [Google Scholar]
- 127.Tetzlaff W, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28(8):1611–82. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. British Journal of Anaesthesia. 2007;99(1):4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 129.World Health Organization. Neurological disorders: public health challenges. Geneva: World Health Organization; 2006. p. xi.p. 218. [Google Scholar]
- 130.Ruff CA, Wilcox JT, Fehlings MG. Cell-based transplantation strategies to promote plasticity following spinal cord injury. Exp Neurol. 2012;235(1):78–90. doi: 10.1016/j.expneurol.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 131.Baker M. Stem-cell pioneer bows out. Nature. 2011;479(7374):459. doi: 10.1038/479459a. [DOI] [PubMed] [Google Scholar]
- 132.Elkin BS, et al. Mechanical heterogeneity of the rat hippocampus measured by atomic force microscope indentation. J Neurotrauma. 2007;24(5):812–22. doi: 10.1089/neu.2006.0169. [DOI] [PubMed] [Google Scholar]
- 133.Saha K, et al. Substrate Modulus Directs Neural Stem Cell Behavior. Biophysical Journal. 2008;95(9):4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lampe KJ, Antaris AL, Heilshorn SC. Design of three-dimensional engineered protein hydrogels for tailored control of neurite growth. Acta Biomater. 2013;9(3):5590–9. doi: 10.1016/j.actbio.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barros CS, Franco SJ, Muller U. Extracellular matrix: functions in the nervous system. Cold Spring Harb Perspect Biol. 2011;3(1):a005108. doi: 10.1101/cshperspect.a005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ranieri JP, et al. Neuronal cell attachment to fluorinated ethylene propylene films with covalently immobilized laminin oligopeptides YIGSR and IKVAV. II. J Biomed Mater Res. 1995;29(6):779–85. doi: 10.1002/jbm.820290614. [DOI] [PubMed] [Google Scholar]
- 137.Tashiro K, et al. A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J Biol Chem. 1989;264(27):16174–82. [PubMed] [Google Scholar]
- 138.Johnson PJ, et al. Tissue-engineered fibrin scaffolds containing neural progenitors enhance functional recovery in a subacute model of SCI. Soft Matter. 2010;6(20):5127–5137. doi: 10.1039/c0sm00173b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Johnson PJ, et al. Controlled release of neurotrophin-3 and platelet-derived growth factor from fibrin scaffolds containing neural progenitor cells enhances survival and differentiation into neurons in a subacute model of SCI. Cell Transplant. 2010;19(1):89–101. doi: 10.3727/096368909X477273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jin K, et al. Transplantation of human neural precursor cells in Matrigel scaffolding improves outcome from focal cerebral ischemia after delayed postischemic treatment in rats. J Cereb Blood Flow Metab. 2010;30(3):534–44. doi: 10.1038/jcbfm.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Uemura M, et al. Matrigel supports survival and neuronal differentiation of grafted embryonic stem cell-derived neural precursor cells. J Neurosci Res. 2010;88(3):542–51. doi: 10.1002/jnr.22223. [DOI] [PubMed] [Google Scholar]
- 142.Ballios BG, et al. A Hyaluronan-Based Injectable Hydrogel Improves the Survival and Integration of Stem Cell Progeny following Transplantation. Stem Cell Reports. 2015;4(6):1031–45. doi: 10.1016/j.stemcr.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Caicco MJ, et al. Characterization of hyaluronan-methylcellulose hydrogels for cell delivery to the injured spinal cord. J Biomed Mater Res A. 2013;101(5):1472–7. doi: 10.1002/jbm.a.34454. [DOI] [PubMed] [Google Scholar]
- 144••.Fuhrmann T, et al. Injectable hydrogel promotes early survival of induced pluripotent stem cell-derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials. 2016;83:23–36. doi: 10.1016/j.biomaterials.2015.12.032. This study demonstrated a designed hyaluronic acid-methylcellulose hydrogel that improved iPSC-derived oligodendrocyte progentior cell survival and differentiation after spinal cord injury. Notable for potential clinical application, hydrogel delivery attenuated teratoma formation compared to cells in saline. [DOI] [PubMed] [Google Scholar]
- 145.Austin JW, et al. The effects of intrathecal injection of a hyaluronan-based hydrogel on inflammation, scarring and neurobehavioural outcomes in a rat model of severe spinal cord injury associated with arachnoiditis. Biomaterials. 2012;33(18):4555–64. doi: 10.1016/j.biomaterials.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 146.Mothe AJ, et al. Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials. 2013;34(15):3775–83. doi: 10.1016/j.biomaterials.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 147.Lam J, et al. Delivery of iPS-NPCs to the Stroke Cavity within a Hyaluronic Acid Matrix Promotes the Differentiation of Transplanted Cells. Adv Funct Mater. 2014;24(44):7053–7062. doi: 10.1002/adfm.201401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moshayedi P, Carmichael ST. Hyaluronan, neural stem cells and tissue reconstruction after acute ischemic stroke. Biomatter. 2013;3(1) doi: 10.4161/biom.23863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhong J, et al. Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehabil Neural Repair. 2010;24(7):636–44. doi: 10.1177/1545968310361958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Raynald, et al. The hetero-transplantation of human bone marrow stromal cells carried by hydrogel unexpectedly demonstrates a significant role in the functional recovery in the injured spinal cord of rats. Brain Res. 2015 doi: 10.1016/j.brainres.2015.10.038. [DOI] [PubMed] [Google Scholar]
- 151.Liu Y, et al. A self-assembling peptide reduces glial scarring, attenuates post-traumatic inflammation and promotes neurological recovery following spinal cord injury. Acta Biomater. 2013;9(9):8075–88. doi: 10.1016/j.actbio.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 152.Wang TY, et al. Functionalized composite scaffolds improve the engraftment of transplanted dopaminergic progenitors in a mouse model of Parkinson’s disease. Biomaterials. 2016;74:89–98. doi: 10.1016/j.biomaterials.2015.09.039. [DOI] [PubMed] [Google Scholar]
- 153.Gomez-Barrena E, et al. Bone regeneration: stem cell therapies and clinical studies in orthopaedics and traumatology. J Cell Mol Med. 2011;15(6):1266–86. doi: 10.1111/j.1582-4934.2011.01265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Marolt D, et al. Engineering bone tissue from human embryonic stem cells. Proc Natl Acad Sci U S A. 2012;109(22):8705–9. doi: 10.1073/pnas.1201830109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kaigler D, et al. Stem cell therapy for craniofacial bone regeneration: a randomized, controlled feasibility trial. Cell Transplant. 2013;22(5):767–77. doi: 10.3727/096368912X652968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rho JY, Ashman RB, Turner CH. Young’s modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. Journal of Biomechanics. 1993;26(2):111–119. doi: 10.1016/0021-9290(93)90042-d. [DOI] [PubMed] [Google Scholar]
- 157.Ren Z, et al. Effective Bone Regeneration Using Thermosensitive Poly(N-Isopropylacrylamide) Grafted Gelatin as Injectable Carrier for Bone Mesenchymal Stem Cells. ACS Appl Mater Interfaces. 2015;7(34):19006–15. doi: 10.1021/acsami.5b02821. [DOI] [PubMed] [Google Scholar]
- 158•.Vo TN, et al. Injectable dual-gelling cell-laden composite hydrogels for bone tissue engineering. Biomaterials. 2016;83:1–11. doi: 10.1016/j.biomaterials.2015.12.026. This study demonstrated that MSC dual encapsulation with gelatin microspheres and a thermosensitive polymer protects cells during delivery and improves bone regeneration in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Watson BM, et al. Biodegradable, phosphate-containing, dual-gelling macromers for cellular delivery in bone tissue engineering. Biomaterials. 2015;67:286–96. doi: 10.1016/j.biomaterials.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dosier CR, et al. Effect of cell origin and timing of delivery for stem cell-based bone tissue engineering using biologically functionalized hydrogels. Tissue Eng Part A. 2015;21(1–2):156–65. doi: 10.1089/ten.tea.2014.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Maia FR, et al. Hydrogel depots for local co-delivery of osteoinductive peptides and mesenchymal stem cells. J Control Release. 2014;189:158–68. doi: 10.1016/j.jconrel.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 162.Kwon JS, et al. In vivo osteogenic differentiation of human turbinate mesenchymal stem cells in an injectable in situ-forming hydrogel. Biomaterials. 2014;35(20):5337–46. doi: 10.1016/j.biomaterials.2014.03.045. [DOI] [PubMed] [Google Scholar]
- 163.Sun B, et al. The osteogenic differentiation of dog bone marrow mesenchymal stem cells in a thermo-sensitive injectable chitosan/collagen/beta-glycerophosphate hydrogel: in vitro and in vivo. J Mater Sci Mater Med. 2011;22(9):2111–8. doi: 10.1007/s10856-011-4386-4. [DOI] [PubMed] [Google Scholar]
- 164.Zhao L, et al. Osteogenic media and rhBMP-2-induced differentiation of umbilical cord mesenchymal stem cells encapsulated in alginate microbeads and integrated in an injectable calcium phosphate-chitosan fibrous scaffold. Tissue Eng Part A. 2011;17(7–8):969–79. doi: 10.1089/ten.tea.2010.0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhao L, Weir MD, Xu HH. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials. 2010;31(25):6502–10. doi: 10.1016/j.biomaterials.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ballios BG, et al. A hydrogel-based stem cell delivery system to treat retinal degenerative diseases. Biomaterials. 2010;31(9):2555–64. doi: 10.1016/j.biomaterials.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 167.Ding K, et al. Injectable thermosensitive chitosan/beta-glycerophosphate/collagen hydrogel maintains the plasticity of skeletal muscle satellite cells and supports their in vivo viability. Cell Biol Int. 2013;37(9):977–87. doi: 10.1002/cbin.10123. [DOI] [PubMed] [Google Scholar]
- 168.Xu Y, et al. Regulating myogenic differentiation of mesenchymal stem cells using thermosensitive hydrogels. Acta Biomater. 2015;26:23–33. doi: 10.1016/j.actbio.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 169.Kim K, Kim MS. An injectable hydrogel derived from small intestine submucosa as a stem cell carrier. J Biomed Mater Res B Appl Biomater. 2015 doi: 10.1002/jbm.b.33504. [DOI] [PubMed] [Google Scholar]
- 170.Zeng Y, et al. Preformed gelatin microcryogels as injectable cell carriers for enhanced skin wound healing. Acta Biomater. 2015;25:291–303. doi: 10.1016/j.actbio.2015.07.042. [DOI] [PubMed] [Google Scholar]
- 171•.Guo R, et al. A transient cell-shielding method for viable MSC delivery within hydrophobic scaffolds polymerized in situ. Biomaterials. 2015;54:21–33. doi: 10.1016/j.biomaterials.2015.03.010. This study demonstrated a combinatorial approach using synthetic and natural materials to provide optimal conditions for transplanting BMSCs for wound healing applications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xu K, et al. Thiol-ene Michael-type formation of gelatin/poly(ethylene glycol) biomatrices for three-dimensional mesenchymal stromal/stem cell administration to cutaneous wounds. Acta Biomater. 2013;9(11):8802–14. doi: 10.1016/j.actbio.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173••.Chiang CH, et al. Enhanced antioxidant capacity of dental pulp-derived iPSC-differentiated hepatocytes and liver regeneration by injectable HGF-releasing hydrogel in fulminant hepatic failure. Cell Transplant. 2015;24(3):541–59. doi: 10.3727/096368915X686986. This study showed the significant role a growth factor modified hydrogel carrier can play in improving transplanted iPsc-hepatocyte viability and increasing functional liver output. [DOI] [PubMed] [Google Scholar]
- 174.Gao J, et al. The use of chitosan based hydrogel for enhancing the therapeutic benefits of adipose-derived MSCs for acute kidney injury. Biomaterials. 2012;33(14):3673–81. doi: 10.1016/j.biomaterials.2012.01.061. [DOI] [PubMed] [Google Scholar]