Abstract
Allergic asthma is a chronic Th2 inflammation in the lungs that constricts the airways and presents as coughing and wheezing. Asthma mostly affects boys in childhood and women in adulthood, suggesting that shifts in sex hormones alter the course of the disease. Alveolar macrophages have emerged as major mediators of allergic lung inflammation in animal models as well as humans. Whether sex differences exist in macrophage polarization and the molecular mechanism(s) which drive differential responses are not well understood. We found that IL-4- stimulated bone marrow-derived and alveolar macrophages from female mice exhibited greater expression of M2 genes in vitro and after allergen challenge in vivo. Alveolar macrophages from female mice exhibited greater expression of the IL-4 receptor (IL-4R)-α and estrogen receptor (ER)-α compared to macrophages from male mice following allergen challenge. An ERα-specific agonist enhanced IL-4-induced M2 gene expression in macrophages from both sexes but more so in macrophages from female mice. Further, IL-4-stimulated macrophages from female mice exhibited more transcriptionally active histone modifications at M2 gene promoters than did macrophages from male mice. We found that supplementation of estrogen into ovariectomized female mice enhanced M2-polarization in vivo upon challenge with allergen and that macrophage-specific deletion of ERα impaired this M2-polarization. The effects of estrogen are long-lasting; bone marrow derived macrophages from ovariectomized mice implanted with estrogen exhibited enhanced IL-4-induced M2-gene expression compared to macrophages from placebo-implanted littermates. Taken together, our findings suggest that estrogen enhances IL-4-induced M2-gene expression and thereby contributes to sex differences observed in asthma.
Keywords: Monocytes/Macrophages, Cytokine Receptors, Lung, Allergy, Rodent, IL-4, Estrogen Receptor, Sex Differences, Asthma, Epigenetics
INTRODUCTION
Asthma is a chronic inflammatory constricting of the airways that causes wheezing, coughing and shortness of breath. Over 300 million people world-wide are reported to suffer from asthma (1). Asthma is a heterogeneous disorder consisting of allergic, corticosteroid-sensitive, Th2-driven and corticosteroid-resistant Th17-driven neutrophilic endotypes. The incidence and severity of asthma exhibit sex differences, affecting mostly boys in childhood and women in adulthood (2–4). Several lines of clinical and experimental evidence suggest that sex hormones, specifically estrogen, play a role in asthma development and progression (5–7). Fluctuations in circulating estrogen reflect changes in severity and incidence of asthma. Roughly 50% of women hospitalized for asthma-related symptoms are reported to be premenstrual and 33–52% of asthmatic women report premenstrual worsening of symptoms (8–12). Spikes in menstrual hormones correlate with impaired lung function, indicated by a decline in forced expiratory volume in one second (FEV1), largely during the late follicular phase where circulating estrogen levels peak (13, 14). In mouse models of allergic lung inflammation, female mice have been shown to exhibit worsened eosinophilic inflammation, increased B and T cell infiltration, increased Th2 cytokines in the lung and more serum IgE compared to male mice following allergen challenge (15, 16).
Alveolar macrophages have emerged as major cellular mediators of allergic lung inflammation and tissue remodeling. The bronchoalveolar lavage fluid (BALF) and airway walls from asthmatic patients has more M2-polarized macrophages and this correlates with impaired lung function (17, 18). In mice, the intratracheal instillation of M2-polarized alveolar macrophages prior to OVA-challenge results in enhanced eosinophilia in the bronchoalveolar lavage (BAL) and effector T cells in the interstitium (19). Likewise, the intratracheal instillation of alveolar macrophages from OVA-sensitized mice resulted in enhanced eosinophilic and T cell infiltration into the lungs following OVA challenge compared to mice receiving macrophages from naive animals (20). The depletion of alveolar macrophages prior to allergen challenge results in a dramatic reduction of the classical features of allergic inflammation including eosinophil influx into the BAL and Th2 cytokine production as well as tissue remodeling and fibrosis (21). Since the incidence and severity of asthma exhibit sex and sex hormone-mediated differences in humans and mice and M2-polarized alveolar macrophages confer disease severity, we reasoned that there may be underlying sex differences in M2-polarization in the allergic lung. Thus, we hypothesized that enhanced M2 polarization of alveolar macrophages contributes to the sex differences in allergic lung inflammation described in asthma. We also sought to explain the molecular basis for sex differences in M2-polarization in vivo and in vitro.
MATERIALS AND METHODS
Reagents
Ovalbumin and Avertin used for mouse experiments were obtained from Sigma-Aldrich (St Louis, MO). LIVE/DEAD Zombie UV and LIVE/DEAD yellow stains and antibodies specific for mouse CD11c-PECy7 (clone N418), CD45-APC (clone 30F11), CD11b-APCCy7 (clone M1/70), Ly6C-PerCPCy5.5 (clone HK1.4), Ly6G-APCCy7 (clone 1A8), CD16/CD32, and anti-mouse-PercPCy5.5 were obtained from Biolegend (San Diego, CA). SIGLEC F-BV421 (clone E50-2440) and Arg1 (clone 19) antibodies, Fix/Perm and Perm buffers were obtained from BD Biosciences (San Jose, CA) and anti-rabbit-PE antibodies were obtained from Biolegend and R&D systems (Minneapolis, MN). A polyclonal antibody against mouse YM1 was obtained from Stemcell technologies (Vancouver, BC) and mouse iNOS and FIZZ1 from Abcam (Cambridge, MA). Antibodies against mouse ERα (MC-20) and β-actin as well as recombinant ERα were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA). Pierce BCA protein assay reagents, SuperScript III First Strand Synthesis System, and 2x SYBR green PCR master mix were obtained from Thermo Scientific (Rockford, IL). Criterion TGX 10% and 4–20% polyacrylamide gels, anti-rabbit-horse radish peroxidase (HRP), anti-mouse-HRP, and polyvinylidene difluoride (PVDF) membrane were obtained from Bio-Rad (Hercules, CA). Primers for qPCR were obtained from IDT (Coralville, IA). Amersham ECL reagent was obtained from GE Healthcare (Piscataway, NJ). RNeasy kit was obtained from Qiagen (Valencia, CA). Imject alum adjuvant was obtained from Thermo Fisher (Grand Island, NY).
Mice
C57BL/6 and LysM-CRE mice were obtained from Jackson Laboratories. ERαflox/flox mice were generously provided by Jan-Åke Gustafsson (Karolinska Institutet, Stockholm, Sweden) (22). All study protocols using mice were in compliance with and approved by the Johns Hopkins University Animal Care and Use Committee. For ovalbumin-induced allergic lung inflammation studies, the protocol of Wang et al 2000 was followed (23). Briefly, male and female adult 8-week-old mice were intraperitoneally (IP) injected with 100 µg OVA in Imject Alum (Thermo Scientific; Grand Island, NY) on days 1 and 6. On days 12 and 14, the mice were nebulized with 1% OVA in PBS for 40 minutes. On day 16, the mice were anesthetized with 2.5% Avertin and BAL was collected by lavage for further analysis. For qPCR analysis, alveolar macrophages were isolated by two hour adhesion in culture and eosinophils and lymphocytes were removed by washing with cold PBS. Ovariectomized C57BL/6 mice were purchased from Charles River Laboratories. The bilateral ovariectomy surgeries were carried out at 3 weeks of age. Estrogen and placebo pellets (Innovative Research of America, Sarasota, FL) were implanted on day 7 of the OVA protocol and contain 0.5 mg E2 or placebo. This was done after sensitization to avoid interfering with T cell priming. Serum estrogen was measured by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core. The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver NICHD/NIH (NCTRI) Grant P50-HD28934.
Cell culture
Bone marrow cells from 5–6 week old male and female C57BL/6 mice were lavaged from femurs and tibias and collected into αMEM obtained from Lonza (Basel, Switzerland) supplemented with 10% FBS, 4 mM L-glutamate and 100 units/ml Penicillin and 100 mg/ml Streptomycin. After an overnight culture at 37 °C, 5% CO2, the non-adherent cells were collected and treated with Ammonium-Chloride-Potassium (ACK) buffer for RBC lysis. The cells were then cultured in αMEM medium containing 40 ng/ml recombinant mouse M-CSF for 10 days prior to experimentation. M-CSF was replaced in the medium every 2 days. For IL-4-responsiveness experiments, the cells were treated with IL-4 at the indicated concentrations for 48 h prior to collection. For ERα agonist studies, cells were placed in serum- and phenol red-free αMEM 24 hours prior to IL-4 stimulation. Pre-treatment with PPT occurred 16 h prior to IL-4 stimulation (1 ng/ml for 48 h).
Flow cytometry
LIVE/DEAD staining was carried out on BAL suspensions for 30 min at room temperature in the dark. The cells were stained for CD11c, CD45, and SIGLEC F for 30 min on ice in the dark. The cells were then fixed and permeabilized overnight at 4 °C. Intracellular staining for YM1 and iNOS was carried out with anti-CD16/CD32 mouse block for 40 min at RT followed by anti-mouse and anti-rabbit secondary antibody staining for 20 min at RT. Stained cells were then analyzed with an LSR II (BD Biosciences), courtesy of the Ross Flow Cytometry Core Facility. Alternatively samples were analyzed using a CytoFLEX (Beckman-Coulter; Brea, CA), courtesy of the Anesthesiology and Critical Care Medicine (ACCM) Flow Cytometry Core. Analysis was carried out on FlowJo single cell analysis software (Ashland, OR) or CytExpert software (Beckman-Coulter). Alveolar macrophages were defined as LIVE/DEAD−CD45+CD11c+SIGLEC F+ CD11b−Ly6C−Ly6G− cells and eosinophils were defined as LIVE/DEAD−CD45+CD11c−SIGLEC F+ cells in the BAL.
Histology
Lungs were inflated under constant pressure with 10% Formalin as previously described (24). Inflated lungs were fixed for 24 h and then resuspended in 70% ethanol. Lungs were paraffin embedded, sectioned and stained with Periodic acid–Schiff (PAS) and hematoxylin and eosin (H&E) by the Molecular and Comparative Pathobiology Histology Laboratory at the Johns Hopkins University School of Medicine. PAS staining analysis was carried out blindly. First, the samples were number coded. Then 5 alveoli from each sample were randomly chosen for analysis. PAS+ cells were counted and normalized to the pixel circumference of each selected alveolus. The data were then represented as PAS+ cells / 1000 pixels.
Western Blot
Cells were lysed in lysis buffer containing 1% NP-40, 50 mM HEPES (pH 8.0), 5 mM EDTA, 10 mM sodium pyrophosphate, 50 mM NaF, 0.25 % sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 mM benzamidine, 1 µg/ml pepstatin, 10 µg/ml leupeptin, 20mM iodoacetimide, and 100 µg/ml soy bean trypsin inhibitor. The lysates were centrifuged at 14,000 rpm for 15 min at 4 °C. RIPA buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 1% Triton-X-100, 1% NP40, and 0.1% SDS) was used for the detection of sex hormone receptors. The protein concentration of the supernatants were then measured using the Pierce BCA protein assay reagents and 25 µg of protein was loaded in each well of either 10% or 4–20% Criterion TGX polyacrylamide gels (Biorad, Hercules, CA). The gels were transferred onto a 0.2 mm PVDF membrane that was then probed with antibodies against YM1, ARG1, FIZZ1, and β-actin. Secondary antibodies conjugated to HRP were used to detect target proteins, which was visualized with ECL reagent and autoradiograph film. Lightly exposed x-ray films were used for densitometric analysis. Films were scanned and band intensities were measured using ImageJ software (NIH). Target proteins were normalized to β-actin internal control. To assess fold changes in signal, the target protein/β-actin values were then normalized to the control group within each experiment.
Chromatin Immunoprecipitation
ChIP analysis was carried out on 2 × 15cm BMM dishes per group using the ChIP-IT kit obtained from Active Motif (Carlsberg, CA) per manufacturer’s protocol. Primer sequences for qPCR analysis of immunoprecipitated chromatin is previously published (25).
Quantitative PCR
RNA was collected in RLT or Trizol buffer and processed using RNeasy mini kit (Qiagen, Valencia, CA). Quantitative PCR was performed using the indicated primers and the 7500 fast real time PCR instrument from Applied Biosystems (Grand Island, NY). Primer sequences for M2 genes have been previously published (26).
ELISA
Blood was collected from allergen challenged male and female mice by cardiac puncture. Serum was obtained by centrifugation. Total IgE was measured using the OptEIA kit from BD Biosciences (San Jose, CA).
Statistical Analyses
All graphs and statistical analyses were generated using Prism graphpad software (La Jolla, CA). Unless otherwise indicated, each experiment was carried out at least 3 independent times. Mouse experiments contain four mice per group and were repeated at least twice. Statistical significance was measured using a parametric Student t test and a p value less than 0.05 was considered significant.
RESULTS
Alveolar macrophages from female mice exhibit enhanced M2 responses in allergic lung inflammation
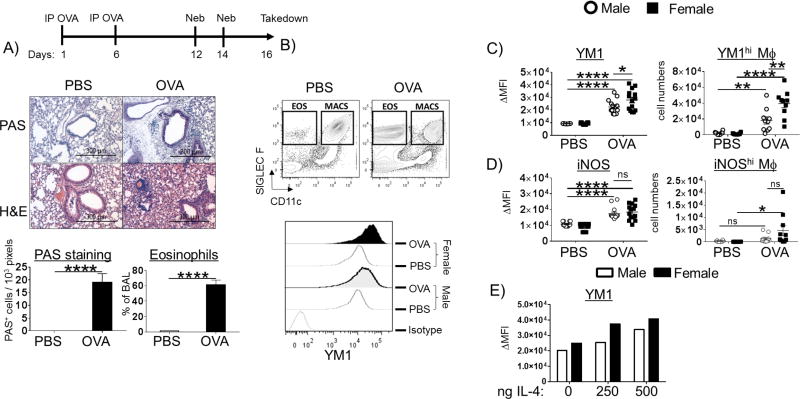
Asthma afflicts women more than men. Mouse models reflect this sex difference. Female animals have more eosinophils and M2-macrophages in their lungs and BALF, higher serum IgE, greater Th2 cytokine production and T cell numbers, and impaired lung function compared to males following allergen challenge (15, 16, 19, 27). Earlier reports of sex differences in M2-responses in allergic mice were based on immunohistochemistry staining for YM1 as an M2 marker and IRF5 as an M1 marker in lung sections, which is not a quantitative technique (27). Therefore, while it is clear that female mice contain more YM1-expressing cells, it is not known whether YM1 expression is higher in M2-polarized macrophages from female mice compared to those from male mice. In order to assess quantitative and qualitative sex differences in M2-polarization during allergic lung inflammation, we used FACS analysis on whole lung and BAL from allergic and healthy mice. We conducted these studies in C57BL/6 mice, knowing that we would employ macrophage-specific ERαflox/flox animals on the B6 background as a part of these studies. Male and female C57BL/6 mice were sensitized to OVA twice 5 days apart and then aerosol-challenged with OVA twice two days apart (28). We chose to use this acute OVA model to measure sex differences in macrophage polarization as alveolar macrophages are critical initiators of allergic lung inflammation (21). Despite reports that suggest C57BL/6 mice are resistant to Th2-inflammation, mice sensitized and challenged by this protocol show enhanced inflammation by H&E staining, increased mucus production by PAS staining, Th2 cytokines, serum IgE, and eosinophil influx into the airways (Figure 1 A, Figure S1). To measure sex differences after OVA challenge, alveolar macrophages were collected by lung lavage at 48 h and the M2 marker, YM1, was measured by FACS. Alveolar macrophages were identified in the BAL based on their coexpression of CD11c, SIGLEC F, and CD45 as well as the lack of CD11b, Ly6C, and Ly6G (Figure 1 B). YM1 is a canonical M2 marker in mice that has been shown to promote Th2 responses and eosinophil inflammation (29). OVA challenge induced robust M2-responses in the OVA-sensitized mice compared to PBS controls (Figure 1 C). Alveolar macrophages from female mice expressed significantly higher YM1 following OVA challenge compared to male macrophages as indicated by a shift in mean fluorescence intensity (MFI) (Figure 1 B and C). Further, the BAL from female mice contained significantly more YM1hi macrophages compared to male mice while the number of iNOShi macrophages did not exhibit a sex difference (Figure 1 D). No induction of YM1 was observed in interstitial macrophages (data not shown). This sex difference was also observed when male and female mice were intratracheally instilled with IL-4 in a dose-dependent manner (Figure 1 E, Figure S2).
Figure 1. Sex-specific alveolar macrophage polarization in vivo following ovalbumin (OVA) challenge.
(A) Schematic of the OVA model: C57BL/6 mice were sensitized on days 1 and 6 and challenged with OVA on days 12 and 14. Two days post final challenge (on day 16), the mice were euthanized and lung inflammation was analyzed by hitochemistry and FACS. The left lobe from each mouse was inflated with 10% formalin under constant pressure and paraffin embedded and sectioned. Mucus production (Periodic Schiff-Acid stain), hemotoxylin/eosin (H&E) stain, and relative abundance of eosinophils (LIVE/DEAD−CD11c−SIGLEC F+ cells) in the BALF were quantified. (B) Gating strategy for FACS: LIVE/DEAD−CD11c+SIGLEC F+ alveolar macrophages were used for analysis of YM1 and iNOS expression (upper panel). The fluorescence intensity of YM1 in LIVE/DEAD−CD11c+Siglec F+ alveolar macrophages is shown in a representative FACS histogram (lower panel). The delta mean fluorescent intensity (MFI target – MFI isotype) of (C) YM1 (left panel) and (D) iNOS (left panel) in alveolar macrophages. The relative abundance of intracellular (C) YM1hi (right panel) and (D) iNOShi (right panel) macrophages are graphed. (E) Male and female C57BL/6 mice were intratracheally instilled with increasing doses of IL-4. Two days after the instillation, the bronchoalveolar lavage was collected and YM1 expression ΔMFI in macrophages was measured using intracellular FACS, (n = 1 mouse /group). N = 3; *p < 0.05; ** p <0.01, *** p <0.001, **** p < 0.0001
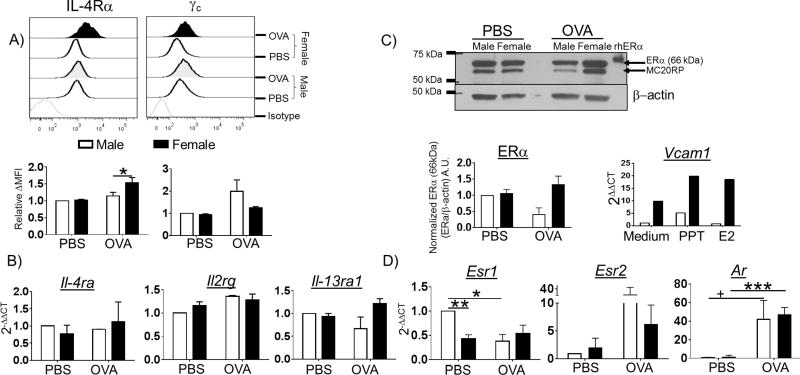
We wanted to understand the molecular basis of this sex difference in male and female alveolar macrophages. Since IL-4 and IL-13 can polarize macrophages to an M2 phenotype, we hypothesized that differences in expression of IL-4 receptors on the surface of alveolar macrophages might explain the observed enhanced M2 polarization in female mice following OVA challenge. Therefore, we quantified the amount of IL-4Rα, γc and IL-13 receptors by FACS and qPCR in control and allergen-sensitized and -challenged male and female mice. Alveolar macrophages from female mice exhibited a statistically significant increase in surface IL-4Rα expression following OVA challenge while male alveolar macrophages did not (Figure 2 A). Although this is a small shift in MFI for IL-4Rα, our previous studies and those of others have shown that the ratio of IL-4R chains has profound impacts on IL-4 signaling and downstream M2 gene expression (26, 30). OVA challenge did not significantly alter γc expression on either male or female alveolar macrophages (Figure 2 A). Because there is no reliable antibody to detect mouse IL-13Rα1 by FACS analysis, we measured IL-13Rα1 and IL-13Rα2 transcripts by qPCR in RNA extracted from macrophages lavaged from male and female mouse lungs following OVA challenge. We found more mRNA for the IL-13Rα1 chain in female macrophages than in male macrophages following OVA challenge although this difference was not statistically significant (Figure 2 B). Although sex differences in IL-13Rα2 expression have been reported in splenic macrophages (31), IL-13Rα2 mRNA was not detectable in alveolar macrophages from either group (data not shown). Expression of mRNA for IL-4Rα and γc mirrored the surface protein expression found on alveolar macrophages by FACS (Figure 2 B).
Figure 2. IL-4 Receptor expression on alveolar macrophages is increased in female mice following allergen challenge.
(A) C57BL/6 mice were sensitized and challenged with OVA as in Figure 1. Two days after the final challenge, the mice were euthanized and the BAL cells were collected, alveolar macrophages were selected by a LIVE/DEAD−CD11c+SIGLEC F+ gate and stained for IL-4Rα and γc by FACS. A representative histogram of IL-4Rα and γc expression (upper panel) and the relative delta MFI (MFI target – MFI isotype) for each receptor relative to the male PBS group (middle panel) are shown. Alveolar macrophages were enriched by a two hour culture in αMEM, in which all non-adherent cells (lymphocytes, eosinophils and other cell types) were removed by extensive washing. Total RNA was collected and receptor expression was measured by qPCR. (B) Relative expression of IL-4Rα, γc and IL-13Rα1 transcripts (normalized to male PBS) are shown (lower panel). (C) Expression of ERα was measured by western blot (upper panel). ERα protein was normalized to β-actin, A.U. = arbitrary units, N = 4 independent experiments. Alveolar macrophages were collected from male and female C57BL/6 mice by bronchoalveolar lavage. The cells were cultured in hormone-free, PPT-containing, or E2-containing αMEM for 24 h. Total RNA was collected and qPCR was used to measure the expression of Vcam1, n = 1 mouse /group. (D) The expression of Esr1 (ERα), Esr2 (ERβ), and Ar (AR, androgen receptor) mRNA was measured by qPCR using the ΔΔCT method, normalized to the amount in the male PBS sample. N = 3 independent experiments with n = 4 mice / group. *p < 0.05; ** p <0.01, *** p <0.001, + p = 0.083.
The engagement of estrogen receptors has been associated with wound healing activity in macrophages and M2-gene expression in vitro (32). In order to determine whether differential expression of sex hormone receptors could account for the sex differences in M2-responses following OVA challenge, we measured the expression of ERα, ERβ, and androgen receptor (AR) by western blot and qPCR. We used an adherence protocol to enrich alveolar macrophages from other cellular infiltrates found in the BAL of allergic animals from ∼10% to ∼80% of total cellular content (Figure S3). For detection of ERα by western blot, we used the polyclonal ERα antibody (MC-20) raised against the C-terminus of mouse ERα that detects the full-length 66 kDa isoform of ERα as well as at the previously described 61 kDa MC-20 reactive protein (MC20RP; Figure 2 C, upper panel)(33). There were no differences in ERα expression in alveolar macrophages in naïve male and female animals (Figure 2 C, left panel). However, the expression of ERα in alveolar macrophages from male mice was reduced following challenge with OVA (Figure 2 C, right panel). ERα consists of several isoforms in humans and mice that arise from splice variants and the use of alternative transcription start sites (reviewed in (34)). We therefore used a qPCR primer set that recognizes the hinge and dimerization regions present in all ERα isoforms (66, 46, and 36 kDa in mice) for this analysis. Intriguingly, female alveolar macrophages from control C57BL/6 mice expressed less mRNA for ERα (Esr1) than did male macrophages (Figure 2 D). There was a trend to increased ERα mRNA expression in alveolar macrophages from female mice following OVA challenge (Figure 2 D). Again, male alveolar macrophages exhibited a statistically significant reduction in expression of mRNA for ERα expression following OVA challenge (Figure 2 D).
Alveolar macrophages from naïve male and female mice express similar levels of ERα. However, differences in the amount of ERα in alveolar macrophages may not necessarily reflect responsiveness to ERα ligands. Therefore, we tested the responsiveness of alveolar macrophages from male and female mice to estrogen and the ERα agonist 4,4',4"-(4-Propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol (PPT). BAL macrophages from male and female mice were stimulated with either estrogen or PPT for 24 h. The expression of a known estrogen-responsive gene in macrophages, VCAM1 (35), was measured by qPCR. Alveolar macrophages from female mice exhibited enhanced basal expression of VCAM1, likely reflecting differences in circulating estrogen (Figure 2 C, right panel). Stimulation with PPT increased VCAM1 expression in both sexes, although female alveolar macrophages exhibited much higher expression. While male alveolar macrophages did not exhibit overt induction of VCAM1 in response to estrogen, alveolar macrophages from female mice did.
ERβ has been shown to enhance M1 gene expression and suppress M2 gene expression (32). Expression of mRNA for ERβ (Esr2) increased with OVA challenge in both sexes, particularly in macrophages from male animals, although this was not statistically significant (Figure 2 D). Interestingly, AR expression was dramatically increased in alveolar macrophages from both males and females following OVA challenge. Taken together these data indicate that female mice exhibit enhanced M2-gene expression in vivo during allergic lung inflammation. This may be at least partially accounted for by the increased surface expression of IL-4Rα and ERα following OVA challenge in alveolar macrophages from female mice.
Macrophages from female mice exhibit enhanced M2-gene expression in response to IL-4 in vitro
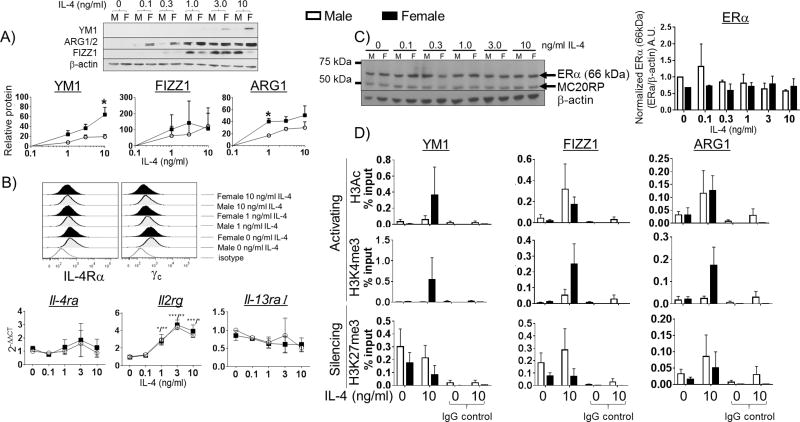
After observing that there was a sex difference in the M2 polarization of alveolar macrophages in the mouse model of allergic lung inflammation, we used a reductionist approach to determine if the sex differences were an intrinsic property of macrophages derived from male or female animals rather than the environment within the allergic lung. To address this, we employed an in vitro model using bone marrow-derived macrophages (BMMs) from male and female C57BL/6 mice. These cells were differentiated in culture for ten days (in the absence of exogenous hormones) prior to stimulation with IL-4. We measured M2-gene expression by western blot following stimulation with increasing concentrations of IL-4 for 48 h. BMMs from female mice exhibited significantly higher expression of the canonical mouse M2 genes, YM1 and ARG1, in a concentration-dependent manner in response to IL-4 compared to BMMS from male mice (Figure 3 A). Interestingly, the induction of FIZZ1, another hallmark mouse M2 gene, in response to IL-4 did not seem to exhibit female dominance. FIZZ1 has been demonstrated to limit Th2 responses to worm infection in vivo (36, 37), although its precise role in allergic lung inflammation is not fully understood. We also looked for sex differences in M2 polarization in response to IL-13. While a similar female-dominant M2-gene expression is observed with IL-13 stimulation, the magnitude of gene induction was much lower than it is with IL-4 (data not shown) as we observed previously (26). These data suggest that macrophages exhibit sex hormone-independent differences in the degree of M2 polarization in response to IL-4. To test whether changes in IL-4R expression accompanied the increased M2-gene expression in these in vitro experiments, we measured the expression of IL-4Rα, γc, and IL-13Rα1 by FACS and qPCR. As expected, IL-4 treatment resulted in the progressive down regulation of surface IL-4Rα and γc in BMMs due to receptor internalization, with no apparent sex differences (Figure 3 B, upper panel) (38, 39). Interestingly, the amount of mRNA for γc, but not IL-4Rα or IL-13Rα1, increased in a concentration-dependent manner with IL-4 stimulation in both male and female macrophages (Figure 3 B, lower panel).We also used western blot to measure expression of ERα in the BMM to determine sex differences in the effect of IL-4 exposure on estrogen receptor expression. Treatment with IL-4 did not change ERα expression in BMMs from male or female mice (Figure 3 C).
Figure 3. Sex differences in IL-4 responsiveness in vitro.
(A) BMMs from male and female C57BL/6 mice were differentiated in vitro for 10 days with M-CSF, then stimulated with increasing concentrations of IL-4 for 48h. Protein lysates were collected and measured for the expression of the canonical mouse M2 genes YM1, FIZZ1, and ARG1 by western blot. All target protein bands were normalized to β-actin, AU = arbitrary units. (B) BMMs from male and female mice were grown on low-adhesion plates, then detached using Cell Stripper and assayed for surface IL-4 receptor expression by FACS and mRNA for the IL-4R by qPCR. A representative histogram showing IL-4Rα and γc expression on BMMs is shown above (upper panel). Il4ra, Il2rg, and Il13ra1 expression following stimulation with increasing concentrations of IL-4 was measured by qPCR (lower panel). N = 3 independent experiments. *p < 0.05; ** p <0.01, *** p <0.001. (C) ERα expression was measured by western blot at the indicated concentrations of IL-4. ERα band was normalized to β-actin, AU = arbitrary unit. (D) BMMs from male and female mice were stimulated with 10 ng/ml IL-4 for 48 h. The cells were fixed in 1 % formaldehyde and processed for ChIP-qPCR analysis. Immunoprecipitations were carried out using antibodies against pan-acetyl H3, H3K4me3, H3K27me3 and IgG control. The abundance of Chi3l3 (YM1), Retnla (FIZZ1), and Arg1 promoters in each IP was measured by qPCR. The % input was determined using the ΔΔCT method and is reported above for each histone modification. N = 3 independent experiments.
Epigenetic regulation of M2-gene expression exhibits sex differences
Previous studies have demonstrated that the induction of M2 genes by IL-4 involves epigenetic control of M2 gene expression, involving histone H3 acetylation and trimethylation of H3 lysine 4 as well as the reciprocal reduction of trimethylated H3 K27 (25, 40, 41). Acetylation of H3 and trimethylation of H3 lysine 4 are characteristic of transcriptionally active chromatin (reviewed in (42)). Since we had determined that M2 gene expression was different between female and male macrophages following IL-4 stimulation we hypothesized that there were epigenetic differences in the promoters of the M2 genes in macrophages from males and females. BMMs from male and female mice were stimulated with IL-4 for 48 h and chromatin was harvested and analyzed for histone modifications at the YM1, FIZZ1, and ARG1 promoters by chromatin immunoprecipitation (ChIP)-qPCR. Female BMMs exhibited dramatic increases in H3Ac and H3K4me3 at the YM1 promoter relative to male BMMs (Figure 3 D, upper and middle panels, black bars). Likewise, females exhibited reduced repressive H3K27me3 at the YM1 promoter (Figure 3 D, bottom panel, black bar). Acetylation of H3 at the FIZZ1 and ARG1 promoters did not exhibit sex differences, although the Arg1 promoter in female macrophages did exhibit enhanced trimethlation of H3K4 and a reduction of trimethylation of H3K27 compared to macrophages from male mice (Figure 3 D, middle and lower panels). Together these data suggest that M2 genes promoters are epigenetically regulated in a sex-specific manner when macrophages are stimulated with IL-4.
Engaging ERα enhances sex differences in M2-gene expression in vitro
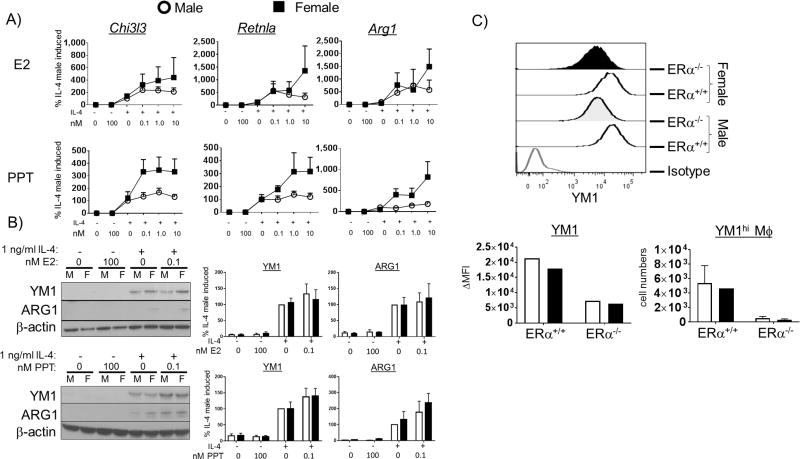
We have shown that M2-polarization, in vitro with IL-4 and in vivo in a mouse model of allergic lung inflammation, exhibits sex differences. ERα signaling has previously been shown to enhance M2-gene expression and wound healing responses in macrophages (32). Next, we determined the effect of engagement of ERα with an ERα-specific agonist on M2-gene expression in IL-4-polarized BMMs from male and female mice. To this end, we exposed male and female BMMs to increasing concentrations of estrogen or the selective ERα agonist PPT for 16 h prior to a 48 h stimulation with IL-4. We then measured M2-gene expression by qPCR and western blot. Estrogen modestly increased M2-gene expression in both male and female BMMs (Figure 4 A and B, upper panels). PPT-treated BMMs from female mice (Figure 4 A and B, lower panels, black squares) exhibited a concentration-dependent increase in the amount of IL-4-induced mRNA for all three M2-genes compared to PPT treatment alone. Male BMMs, however, did not exhibit PPT enhanced M2-gene mRNA following IL-4 treatment (Figure 4 A, lower panels, open circles). Although, IL-4-stimulated BMMs from both male and female mice exhibited increased YM1 and ARG1 protein following pre-treatment with PPT (Figure 4 B, lower panels). The different responses to estrogen and PPT may be due to either regulatory signaling through other inhibitory estrogen receptors, such as ERβ and/or G protein-coupled estrogen receptor (GPER)-30, or to differences in half maximum effective concentration (EC50) between PPT and E2 on ERα activity (32, 43–45). The ERβ agonist, diarylpropionitrile (DPN), had no effect on IL-4-induced M2 gene expression (data not shown). These data suggest that engaging ERα enhanced the capacity of female macrophages to polarize to an M2 phenotype while having little effect on male macrophages.
Figure 4. ERα ligands enhance M2 gene expression.
(A) BMMs from male and female C57BL/6 mice were cultured for 16h in increasing concentrations of either E2 or PPT prior to stimulation with 1 ng/ml IL-4. Two days after the addition of IL-4, mRNA was collected and M2 gene expression was measured by qPCR. Expression of Chi3l3 (YM1), Retnla (FIZZ1) and Arg1 is shown. (B) Western blot was used to measure protein expression of YM1 and ARG1 as well. N = 4 independent experiments. (C) LysMCRE-ERαflox/flox mice underwent OVA sensitization and challenge as in Figure 1 with ERαflox/flox mice as littermate controls. Two days after the last challenge, the mice were sacrificed and YM1 expression was measured on alveolar macrophages from the BAL by intracellular FACS (n = 1–2 mice/group).
Our in vitro ER-agonist data suggest that ERα is the estrogen-recognizing receptor that enhances M2-gene expression. We hypothesized that mice lacking functional ERα in their macrophages would exhibit impaired M2-polarization in vivo when challenged with OVA. To test this, we employed a LysM-CRE ERαflox/flox mouse to compare M2 polarization in an ERα-sufficient and-deficient model. We challenged LysM-CRE ERαflox/flox as well as CRE− ERαflox/flox littermate control mice with OVA and measured YM1 expression in alveolar macrophages. ERα deficiency resulted in a dramatic reduction of YM1 expression in both male and female mice (Figure 4 C). The abundance of YM1hi alveolar macrophages was also dramatically reduced in ERα-deficient mice following challenge with OVA (Figure 4 C). Taken together, these data suggest that ERα is instrumental in promoting M2 polarization in vivo. Thus, an estrogen-rich environment may enhance M2 polarization through engagement of ERα in women and can thereby contribute to sex differences in asthma in humans.
Estrogen enhances M2-polarization in vivo
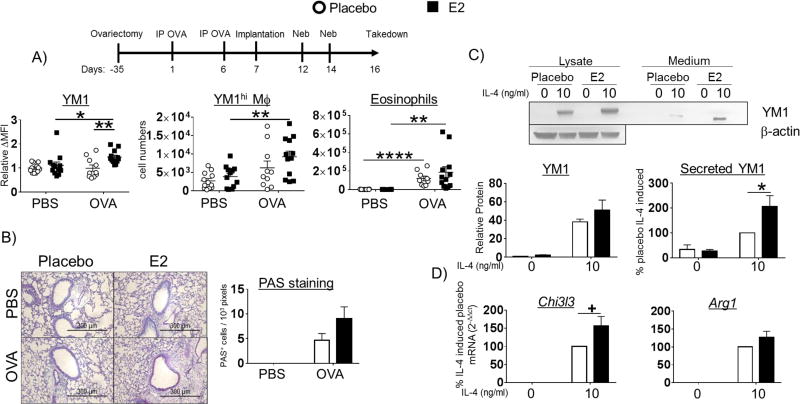
We have demonstrated female-dominant M2-polarization in vivo and in vitro as well as the enhancing effects of estrogen and PPT on M2 macrophage polarization in macrophages from female mice in vitro. Next, we asked whether estrogen would enhance M2-polarization in vivo following allergen challenge. To address this, we implanted female mice that had undergone bilateral ovariectomy surgery (OVx mice) with either placebo- or estrogen-secreting subcutaneous pellets (see scheme of protocol, Figure 5 A). Bilateral ovariectomy surgery was performed at 3 weeks of age, prior to reproductive maturation and estrogen and progesterone production to avoid the epigenetic effects of estrogen on M2 promoters that we observed in vitro (Figure 3 C). Furthermore, to avoid confounding effects of estrogen on T cell priming, we implanted pellets after the mice were sensitized to OVA on day 7. After two challenges with aerosolized OVA, the mice were euthanized and assessed for allergic lung inflammation by histology as well as macrophage polarization by FACS. First, to validate successful pellet implantation, we collected serum from each mouse and measured estrogen concentrations. Placebo-implanted mice exhibited a mean serum estrogen concentration of 8.236 ± 1.322 pg/ml (n = 24 mice) and E2-implanted mice exhibited a mean serum estrogen concentration of 990.167 ± 87.813 pg/ml (n = 24 mice). This corresponded to a mean uterine weight of 54.077± 3.199 mg (n = 24 mice) in the E2-implanted mice whereas the placebo group lacked defined uterine structure on day 16.
Figure 5. Estrogen enhances M2-polarization in ovariectomized mice.
(A) C57BL/6 mice underwent ovariectomy surgery at 3 weeks of age. The standard OVA protocol was initiated at 8 weeks of age. One day after the second IP delivery of OVA, the mice were implanted with pellets that secrete either placebo or E2 and were then challenged with aerosol OVA. Expression of YM1 ΔMFI (MFI target - MFI isotype) in LIVE/DEAD−CD11c+Siglec F+CD45+ alveolar macrophages normalized to placebo-implanted PBS group and absolute numbers of YM1hi alveolar macrophages and CD11c−Siglec F+CD45+ eosinophils are reported above (upper panels). N = 3 independent experiments with 4 mice / group; *p < 0.05; ** p <0.01 (B) PAS staining was carried out as in Figure 1 A. Representative PAS stained alveoli are shown above (left panel). (C) BMM from pellet E2- and placebo-implanted OVx mice were stimulated with IL-4 for 48h. Protein lysates and culture medium were collected and YM1 expression and secretion was measured by western blot. YM1 in cell lysates was normalized to β-actin, AU = arbitrary unit. (D) Expression of mRNA for Chi3l3, Retnla, and Arg1 in BMMs from placebo- or E2-implanted mice stimulated with IL-4 were measured by qPCR. The data is reported as % placebo IL-4-induced; all samples are normalized to the placebo group stimulated with IL-4 and then multiplied by 100. A representative blot (upper panel) and quantified band intensities are graphed (lower panels). N = 3 independent experiments; *p < 0.05, +p = 0.0705.
The alveolar macrophages from female OVx mice implanted with E2-secreting pellets exhibited increased YM1 expression compared to placebo pellet-implanted mice (Figure 5 A, left panel). Estrogen-implanted mice also had more YM1hi alveolar macrophages following allergen challenge compared to placebo-implanted mice (Figure 5 A, middle panel). Estrogen implantation resulted in increased eosinophil infiltration into the alveolar space compared to mice lacking estrogen, after allergen challenge (Figure 5 A, right panel). Mucus production quantified by PAS staining was elevated in the estrogen-implanted allergic mice compared to the placebo group (Figure 5 B). To test whether estrogen had long-term effects on macrophage polarization, we measured YM1 in IL-4-stimulated BMMs harvested from OVx mice implanted with either placebo- or E2-secreting pellets. These BMMs were differentiated in the absence of exogenous hormones for ten days prior to stimulation. Cellular expression and secretion of YM1 into the culture medium were significantly increased in BMMs from E2 pellet-implanted mice compared to cells from placebo-implanted mice (Figure 5 C, upper and middle panel). IL-4-stimulated BMMs from E2 pellet-implanted mice also exhibited increased expression of Chi3l3 and Arg1 mRNA compared to the placebo-implanted group (Figure 5 D). These data suggest that estrogen has long-lasting effects on M2 gene promoter activity even after removal of the cells from the animal for 10 days and when exogenous hormone is absent. These data may also explain the sex differences that we observed in histone modifications at M2-gene promoters in IL-4-stimulated BMMs from male and female mice.
DISCUSSION
Asthma is a major public health issue that burdens more women than men. Asthma exhibits sex differences with respect to prevalence and severity, affecting mostly boys in childhood and women in adulthood. These sex differences implicate sex hormones like estrogen in enhancing allergic inflammation in the lung. Others have shown that M2-activated alveolar macrophages are critical in recruiting inflammatory cells like eosinophils and in secreting factors that promote tissue remodeling and fibrosis in animal models of asthma (19, 21, 46). Likewise, the sputum and airway walls of asthmatic patients is rich with M2-polarized alveolar macrophages and their expression of M2 markers like CD206 and CCL17 positively correlates with asthma severity (47, 48). Inhaled glucocorticoid treatment is a mainstay therapy used to dampen inflammatory gene expression by leukocytes, including macrophages, in asthmatic patients (49). However, women are more likely to develop severe, steroid-resistant asthma than men and this may be due in part to interactions between estrogen and glucocorticoid receptors (50, 51). It is therefore paramount to develop a detailed understanding of how sex hormones like estrogen skew macrophage polarization and function and how hormones contribute to sex differences in alveolar macrophage polarization and function in asthma.
We employed an acute mouse model of allergen challenge to assess sex differences in macrophage polarization, as these are key early events that determine the outcome of allergic lung inflammation in the chronic setting (21). There are differences between acute and chronic models of allergic lung inflammation in mice. Notably, repeated challenge with allergen leads to a dampening of Th2 cytokine production and cellular infiltration into the lungs of mice (52, 53). We were especially interested in the early cellular and molecular events that shape the inflammatory response in the allergic lungs. However, it is clear that M2 macrophages are enriched and persist chronically in the lungs of people with asthma (18, 47, 54–58).
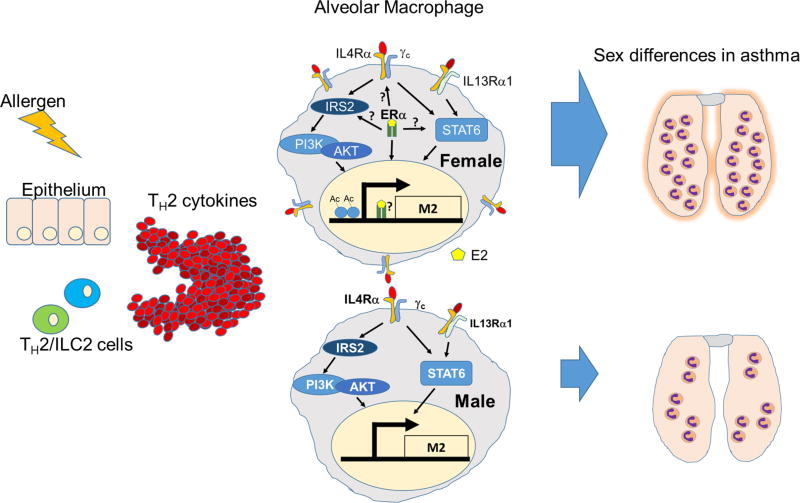
In this report, we undertook that endeavor using in vitro and in vivo models of M2-polarization. Here we are the first to show differences in macrophage polarization, at the cellular and molecular level, depending on the sex of the animal from which the cells were obtained. We report that alveolar and bone marrow-derived macrophages from female mice expressed higher amounts of the classical M2-markers, YM1 and ARG1, following stimulation with IL-4 than do macrophages from male mice (summarized in Figure 6). We further demonstrate that engagement of ERα by estrogen or the ERα agonist, PPT, enhanced M2-gene expression to a greater degree in macrophages from female mice compared to macrophages from male mice. Finally, we report that this sex difference is relevant in vivo in a mouse model of allergic lung inflammation, where female mice exhibit enhanced YM1 expression in alveolar macrophages following allergen challenge, compared to male mice. The expression of the M1 marker iNOS was not different between male and female macrophages following allergen challenge, suggesting that M2- polarization maybe uniquely regulated in a sex-specific manner. Further, ovariectomy reduced M2-polarization in vivo and the reintroduction of estrogen into OVx mice restored M2 polarization of BAL macrophages. ERα is likely the major estrogen-recognizing receptor to drive M2-polarization as we show that mice with ERα deficiency in their macrophages exhibited dramatic reduction in YM1 expression in vivo following challenge with OVA. We show that these sex differences coincide with increased expression of IL-4Rα and ERα on female macrophages following challenge with aerosolized OVA. The differential pattern of IL-4R and ER expression in males and females suggest that these two networks may function synergistically to promote M2 polarization. Finally, we are the first to demonstrate that macrophages from female mice exhibit more histone marks of transcriptionally active chromatin at M2 gene promoters compared to males. This likely reflects chromatin that is poised to respond to IL-4/−13 signals in macrophages from female mice. It is also possible that ERs modulate the promoters of M2 genes, as they are known to interact with histone acetyl transferases, and thus regulate macrophage polarization through epigenetic mechanisms. Our work also underscores the importance and necessity of the new NIH directive to understand relevant biological variables such as sex in biomedical research. It is clear that sex and sex hormones have biological consequences on immune cells (59). Separating studies by sex, and understanding and reporting which sex was used for experiments is critical for interpreting data.
Figure 6. A schematic of the current model.
Alveolar macrophages become activated in the Th2 cytokine environment elicited by allergen challenge. These macrophages are estrogen-primed in women to produce an enhanced amount of inflammatory factors that recruit downstream cellular mediators and promote fibrosis and tissue remodeling.
The increase of IL-4Rα expression in alveolar macrophages from allergic female mice and the reduction of ERα expression in alveolar macrophages from allergic male mice may result in an overall female-dominant expression of M2 genes in vivo following challenge with OVA. More work needs to be done to dissect sex differences in IL-4 signaling in macrophages and how estrogen can affect these signaling events. Estrogen signaling is complex, involving genomic and non-genomic pathways. Therefore such studies would have to be comprehensive enough to accommodate these features and would have to acknowledge that acute exposure to estrogen may have different consequences on M2 signaling than chronic exposure to the hormone. We cannot detect any effects of acute or chronic estrogen exposure on IL-4-induced STAT6 phosphorylation in macrophages (data not shown). However, estrogen may also impact the IRS2/Akt pathway and likely also regulates the assembly of histone modifying factors at M2 promoters. These questions will be addressed in further studies.
These findings have major implications for diseases in which skewing of macrophages serve a pathological role and for which sex is a factor. For example, tumor associated macrophages (TAMs) acquire an M2-like phenotype and remodel the extracellular matrix in order to promote tumor metastasis. TAM presence is enhanced in ER+ breast cancers and leads to increased epithelial-mesenchymal transition (60). It is not clear whether estrogen antagonism of TAMs would be of therapeutic value but it may diminish the tumor-promoting properties in these cells. Consistently, women are at greater risk of small cell and adenocarcinoma lung cancers than men (61, 62). Furthermore, these cancers are promoted by factors like hormone replacement therapy and are characterized by M2-polarized macrophages (63, 64).
Understanding the impact of estrogen on sex-biased diseases is paramount for guiding the development of effective therapies. One major challenge to estrogen-based therapeutics is maximizing cell specificity and minimizing unintended impact on reproductive function. Women are burdened with the highest incidence of allergy (65, 66) and autoimmune diseases (67–69). Generally speaking, women respond to infection and vaccination with increased antibody production compared to men and this reflects a predisposition to a Th2 bias in women compared to a Th1 bias in men (70–72). However, estrogen receptors are expressed in virtually all leukocytes and can thus impact all aspects of immunobiology. Therefore, understanding the molecular mechanisms that cause estrogen sensitivity and alter cellular function in macrophages in women is an important goal. For example, estrogen is known to downregulate the expression of the autoimmune regulator (AIRE), thereby compromising central tolerance and promoting autoimmune disease in women (73). The impact of estrogen on innate and adaptive immunity is context- and cell-specific. Estrogen enhances Th1 and Th2 cytokine production by T cells and stimulates antibody production by B cells, in vitro (74–77). However, estrogen pellet implantation in EAE mice suppresses Th1 cytokine production by CD4 T cells in an ERα-dependent manner (74). Estrogen enhances IL-6 and IL-10 production in splenic DCs and LPS/CpG-induced IL-12 production in bone marrow-derived DCs (78, 79). In BMMs, estrogen seems to promote M2 gene expression while suppressing M1 gene expression (32, 80–82). In our model, estrogen primed macrophages for enhanced M2-gene induction following stimulation with IL-4 and this effect is long-lived. Macrophages from female mice are more sensitive to this priming than their male counterparts. Consistent with the idea that estrogen skews macrophages to an M2 phenotype, estrogen treatment downregulates TNFα, CCR2, and CCL3 in the spinal cord to alleviate Th1-/Th17-mediated pathology associated with multiple sclerosis in a mouse experimental autoimmune encephalomyelitis (EAE) model (83). This propensity to an enhanced M2-phenotype in female mice in our model of allergic lung inflammation needs to be addressed in asthmatic women. Our work points to alternative therapeutic avenues that could be harnessed to effectively antagonize this estrogen-enhanced macrophage phenotype by pharmacological intervention. Women are less responsive to corticosteroid treatment than men and may therefore benefit from therapy that targets the ER or pathways downstream of the ER (84).
The work discussed here has shed light onto the effects of estrogen on the cellular and molecular function of alveolar and bone marrow-derived macrophages. However, there are many avenues left to explore in this regard. It remains unclear exactly how estrogen modulates macrophage responsiveness to Th2 cytokines. Estrogen receptors can alter gene expression by directly engaging estrogen response elements, heterodimerizing with other transcriptional regulators, and signaling through protein kinases. Thus, the molecular biology behind the sex differences in macrophage polarization reported here is likely complex and multifaceted. Estrogen receptors may enhance macrophage polarization by recruiting histone acetylases and demethylases to M2 gene promoters. It is critical to identify the histone modifying- and transcription factor-binding partners associated with the estrogen receptors in initiating the transcription of M2-genes in macrophages and understanding how estrogen affects this interaction. This will enable the targeting of a specific protein-protein interaction using small molecule inhibitors to suppress M2-gene expression in alveolar macrophages in the lungs of asthmatic women. Understanding the complex interaction between IL-4 and estrogen signaling in macrophages will help develop therapies for various allergic and autoimmune diseases that disproportionately burden women. Further research is needed to develop an in-depth molecular understanding of estrogen signaling in macrophages and its role in contributing to sex differences in many different diseases.
Supplementary Material
Acknowledgments
We thank Mireya Becerra-Diaz and Sarah M. McCormick for assisting with sample collection, processing, and intellectual contributions to this project. We extend our gratitude to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for serum estrogen measurements. We also thank Patricia Wilcox at the Molecular and Comparative Pathobiology Histology Laboratory at the Johns Hopkins University School of Medicine for her help with paraffin embedding, slicing, and staining tissue for histological analysis.
Funding support: R01 HL124477
References
- 1.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Govaere E, Van Gysel D, Verhamme KM, Doli E, De Baets F. The association of allergic symptoms with sensitization to inhalant allergens in childhood. Pediatr. Allergy Immunol. 2009;20:448–457. doi: 10.1111/j.1399-3038.2008.00805.x. [DOI] [PubMed] [Google Scholar]
- 3.Jang Y, Shin A. Sex-Based Differences in Asthma among Preschool and School-Aged Children in Korea. PLoS One. 2015;10:e0140057. doi: 10.1371/journal.pone.0140057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen S, Probst-Hensch N, Keidel D, Dratva J, Bettschart R, Pons M, Burdet L, Bridevaux PO, Schikowski T, Schindler C, Rochat T, Zemp E. Gender differences in adult-onset asthma: results from the Swiss SAPALDIA cohort study. Eur. Respir. J. 2015;46:1011–1020. doi: 10.1183/13993003.02278-2014. [DOI] [PubMed] [Google Scholar]
- 5.Matteis M, Polverino F, Spaziano G, Roviezzo F, Santoriello C, Sullo N, Bucci MR, Rossi F, Polverino M, Owen CA, D’Agostino B. Effects of sex hormones on bronchial reactivity during the menstrual cycle. BMC pulmonary medicine. 2014;14:108. doi: 10.1186/1471-2466-14-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macsali F, Svanes C, Sothern RB, Benediktsdottir B, Bjorge L, Dratva J, Franklin KA, Holm M, Janson C, Johannessen A, Lindberg E, Omenaas ER, Schlunssen V, Zemp E, Real FG. Menstrual cycle and respiratory symptoms in a general Nordic-Baltic population. Am. J. Respir. Crit. Care Med. 2013;187:366–373. doi: 10.1164/rccm.201206-1112OC. [DOI] [PubMed] [Google Scholar]
- 7.Tam A, Wadsworth S, Dorscheid D, Man SF, Sin DD. Estradiol increases mucus synthesis in bronchial epithelial cells. PLoS One. 2014;9:e100633. doi: 10.1371/journal.pone.0100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandler MH, Schuldheisz S, Phillips BA, Muse KN. Premenstrual asthma: the effect of estrogen on symptoms, pulmonary function, and beta 2-receptors. Pharmacotherapy. 1997;17:224–234. [PubMed] [Google Scholar]
- 9.Eliasson O, Scherzer HH, DeGraff AC., Jr Morbidity in asthma in relation to the menstrual cycle. J. Allergy Clin. Immunol. 1986;77:87–94. doi: 10.1016/0091-6749(86)90328-3. [DOI] [PubMed] [Google Scholar]
- 10.Gibbs CJ, Coutts II, Lock R, Finnegan OC, White RJ. Premenstrual exacerbation of asthma. Thorax. 1984;39:833–836. doi: 10.1136/thx.39.11.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wulfsohn NL, Politzer WM. BRONCHIAL ASTHMA DURING MENSES AND PREGNANCY. S. Afr. Med. J. 1964;38:173. [PubMed] [Google Scholar]
- 12.Skobeloff EM, Spivey WH, Silverman R, Eskin BA, Harchelroad F, Alessi TV. The effect of the menstrual cycle on asthma presentations in the emergency department. Arch. Intern. Med. 1996;156:1837–1840. [PubMed] [Google Scholar]
- 13.Lam SM, Huang SC. Premenstrual asthma: report of a case with hormonal studies. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 1998;31:197–199. [PubMed] [Google Scholar]
- 14.Pauli BD, Reid RL, Munt PW, Wigle RD, Forkert L. Influence of the menstrual cycle on airway function in asthmatic and normal subjects. Am. Rev. Respir. Dis. 1989;140:358–362. doi: 10.1164/ajrccm/140.2.358. [DOI] [PubMed] [Google Scholar]
- 15.Blacquiere MJ, Hylkema MN, Postma DS, Geerlings M, Timens W, Melgert BN. Airway inflammation and remodeling in two mouse models of asthma: comparison of males and females. Int. Arch. Allergy Immunol. 2010;153:173–181. doi: 10.1159/000312635. [DOI] [PubMed] [Google Scholar]
- 16.Melgert BN, Postma DS, Kuipers I, Geerlings M, Luinge MA, van der Strate BW, Kerstjens HA, Timens W, Hylkema MN. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin. Exp. Allergy. 2005;35:1496–1503. doi: 10.1111/j.1365-2222.2005.02362.x. [DOI] [PubMed] [Google Scholar]
- 17.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, Cullen M, Grandsaigne M, Dombret MC, Aubier M, Pretolani M, Elias JA. A chitinase-like protein in the lung and circulation of patients with severe asthma. N. Engl. J. Med. 2007;357:2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 18.Melgert BN, ten Hacken NH, Rutgers B, Timens W, Postma DS, Hylkema MN. More alternative activation of macrophages in lungs of asthmatic patients. J. Allergy Clin. Immunol. 2011;127:831–833. doi: 10.1016/j.jaci.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 19.Melgert BN, Oriss TB, Qi Z, Dixon-McCarthy B, Geerlings M, Hylkema MN, Ray A. Macrophages: regulators of sex differences in asthma? Am. J. Respir. Cell Mol. Biol. 2010;42:595–603. doi: 10.1165/rcmb.2009-0016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bang BR, Chun E, Shim EJ, Lee HS, Lee SY, Cho SH, Min KU, Kim YY, Park HW. Alveolar macrophages modulate allergic inflammation in a murine model of asthma. Exp. Mol. Med. 2011;43:275–280. doi: 10.3858/emm.2011.43.5.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YG, Jeong JJ, Nyenhuis S, Berdyshev E, Chung S, Ranjan R, Karpurapu M, Deng J, Qian F, Kelly EA, Jarjour NN, Ackerman SJ, Natarajan V, Christman JW, Park GY. Recruited alveolar macrophages, in response to airway epithelial-derived monocyte chemoattractant protein 1/CCl2, regulate airway inflammation and remodeling in allergic asthma. Am. J. Respir. Cell Mol. Biol. 2015;52:772–784. doi: 10.1165/rcmb.2014-0255OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonson P, Humire P, Gustafsson JA. Estrogen Receptor-alpha Knockout Mice. Methods Mol. Biol. 2016;1366:425–430. doi: 10.1007/978-1-4939-3127-9_33. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Homer RJ, Hong L, Cohn L, Lee CG, Jung S, Elias JA. IL-11 selectively inhibits aeroallergen-induced pulmonary eosinophilia and Th2 cytokine production. J. Immunol. 2000;165:2222–2231. doi: 10.4049/jimmunol.165.4.2222. [DOI] [PubMed] [Google Scholar]
- 24.Limjunyawong N, Mock J, Mitzner W. Instillation and Fixation Methods Useful in Mouse Lung Cancer Research. Journal of visualized experiments : JoVE. 2015:e52964. doi: 10.3791/52964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishii M, Wen H, Corsa CA, Liu T, Coelho AL, Allen RM, Carson WFt, Cavassani KA, Li X, Lukacs NW, Hogaboam CM, Dou Y, Kunkel SL. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood. 2009;114:3244–3254. doi: 10.1182/blood-2009-04-217620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heller NM, Qi X, Junttila IS, Shirey KA, Vogel SN, Paul WE, Keegan AD. Type I IL-4Rs selectively activate IRS-2 to induce target gene expression in macrophages. Science signaling. 2008;1:ra17. doi: 10.1126/scisignal.1164795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Draijer C, Robbe P, Boorsma CE, Hylkema MN, Melgert BN. Characterization of macrophage phenotypes in three murine models of house-dust-mite-induced asthma. Mediators Inflamm. 2013;2013:632049. doi: 10.1155/2013/632049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dasgupta P, Qi X, Smith EP, Keegan AD. Absence of the common gamma chain (gamma(c)), a critical component of the Type I IL-4 receptor, increases the severity of allergic lung inflammation. PLoS One. 2013;8:e71344. doi: 10.1371/journal.pone.0071344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai Y, Kumar RK, Zhou J, Foster PS, Webb DC. Ym1/2 promotes Th2 cytokine expression by inhibiting 12/15(S)-lipoxygenase: identification of a novel pathway for regulating allergic inflammation. J. Immunol. 2009;182:5393–5399. doi: 10.4049/jimmunol.0803874. [DOI] [PubMed] [Google Scholar]
- 30.Junttila IS, Mizukami K, Dickensheets H, Meier-Schellersheim M, Yamane H, Donnelly RP, Paul WE. Tuning sensitivity to IL-4 and IL-13: differential expression of IL-4Ralpha, IL-13Ralpha1, and gammac regulates relative cytokine sensitivity. J. Exp. Med. 2008;205:2595–2608. doi: 10.1084/jem.20080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith-Bouvier DL, Divekar AA, Sasidhar M, Du S, Tiwari-Woodruff SK, King JK, Arnold AP, Singh RR, Voskuhl RR. A role for sex chromosome complement in the female bias in autoimmune disease. J. Exp. Med. 2008;205:1099–1108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell L, Emmerson E, Williams H, Saville CR, Krust A, Chambon P, Mace KA, Hardman MJ. Estrogen receptor-alpha promotes alternative macrophage activation during cutaneous repair. J. Invest. Dermatol. 2014;134:2447–2457. doi: 10.1038/jid.2014.175. [DOI] [PubMed] [Google Scholar]
- 33.Bollig-Fischer A, Thakur A, Sun Y, Wu J, Liao DJ. The Predominant Proteins that React to the MC-20 Estrogen Receptor Alpha Antibody Differ in Molecular Weight between the Mammary Gland and Uterus in the Mouse and Rat. International journal of biomedical science : IJBS. 2012;8:51–63. [PMC free article] [PubMed] [Google Scholar]
- 34.Keselman A, Heller N. Estrogen Signaling Modulates Allergic Inflammation and Contributes to Sex Differences in Asthma. Frontiers in immunology. 2015;6:568. doi: 10.3389/fimmu.2015.00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shchelkunova TA, Morozov IA, Rubtsov PM, Samokhodskaya LM, Andrianova IV, Rudimov EG, Sobenin IA, Orekhov AN, Smirnov AN. Effect of sex hormones on levels of mRNAs coding for proteins involved in lipid metabolism in macrophages. Biochemistry (Mosc) 2013;78:1342–1353. doi: 10.1134/S0006297913120043. [DOI] [PubMed] [Google Scholar]
- 36.Pesce JT, Ramalingam TR, Wilson MS, Mentink-Kane MM, Thompson RW, Cheever AW, Urban JF, Jr, Wynn TA. Retnla (relmalpha/fizz1) suppresses helminth-induced Th2-type immunity. PLoS Pathog. 2009;5:e1000393. doi: 10.1371/journal.ppat.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nair MG, Du Y, Perrigoue JG, Zaph C, Taylor JJ, Goldschmidt M, Swain GP, Yancopoulos GD, Valenzuela DM, Murphy A, Karow M, Stevens S, Pearce EJ, Artis D. Alternatively activated macrophage-derived RELM-{alpha} is a negative regulator of type 2 inflammation in the lung. J. Exp. Med. 2009;206:937–952. doi: 10.1084/jem.20082048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurgonaite K, Gandhi H, Kurth T, Pautot S, Schwille P, Weidemann T, Bokel C. Essential role of endocytosis for interleukin-4-receptor-mediated JAK/STAT signalling. J. Cell Sci. 2015;128:3781–3795. doi: 10.1242/jcs.170969. [DOI] [PubMed] [Google Scholar]
- 39.Gandhi H, Worch R, Kurgonaite K, Hintersteiner M, Schwille P, Bokel C, Weidemann T. Dynamics and interaction of interleukin-4 receptor subunits in living cells. Biophys. J. 2014;107:2515–2527. doi: 10.1016/j.bpj.2014.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang X, Wang X, Liu D, Yu L, Xue B, Shi H. Epigenetic regulation of macrophage polarization by DNA methyltransferase 3b. Mol. Endocrinol. 2014;28:565–574. doi: 10.1210/me.2013-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, Miyake T, Matsushita K, Okazaki T, Saitoh T, Honma K, Matsuyama T, Yui K, Tsujimura T, Standley DM, Nakanishi K, Nakai K, Akira S. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11:936–944. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- 42.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 43.He L, Hu XT, Lai YJ, Long Y, Liu L, Zhu BL, Chen GJ. Regulation and the Mechanism of Estrogen on Cav1.2 Gene in Rat-Cultured Cortical Astrocytes. J. Mol. Neurosci. 2016;60:205–213. doi: 10.1007/s12031-016-0803-y. [DOI] [PubMed] [Google Scholar]
- 44.Chu WL, Shiizaki K, Kawanishi M, Kondo M, Yagi T. Validation of a new yeast-based reporter assay consisting of human estrogen receptors alpha/beta and coactivator SRC-1: application for detection of estrogenic activity in environmental samples. Environ Toxicol. 2009;24:513–521. doi: 10.1002/tox.20473. [DOI] [PubMed] [Google Scholar]
- 45.Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERalpha and ERbeta, in estrogen target tissues in vivo through the use of an ERalpha-selective ligand. Endocrinology. 2002;143:4172–4177. doi: 10.1210/en.2002-220403. [DOI] [PubMed] [Google Scholar]
- 46.Voehringer D, van Rooijen N, Locksley RM. Eosinophils develop in distinct stages and are recruited to peripheral sites by alternatively activated macrophages. J. Leukoc. Biol. 2007;81:1434–1444. doi: 10.1189/jlb.1106686. [DOI] [PubMed] [Google Scholar]
- 47.Staples KJ, Hinks TS, Ward JA, Gunn V, Smith C, Djukanovic R. Phenotypic characterization of lung macrophages in asthmatic patients: overexpression of CCL17. J. Allergy Clin. Immunol. 2012;130:1404–1412. doi: 10.1016/j.jaci.2012.07.023. e1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Girodet PO, Nguyen D, Mancini JD, Hundal M, Zhou X, Israel E, Cernadas M. Alternative Macrophage Activation is Increased in Asthma. Am. J. Respir. Cell Mol. Biol. 2016 doi: 10.1165/rcmb.2015-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.John M, Lim S, Seybold J, Jose P, Robichaud A, O’Connor B, Barnes PJ, Chung KF. Inhaled corticosteroids increase interleukin-10 but reduce macrophage inflammatory protein-1alpha, granulocyte-macrophage colony-stimulating factor, and interferon-gamma release from alveolar macrophages in asthma. Am. J. Respir. Crit. Care Med. 1998;157:256–262. doi: 10.1164/ajrccm.157.1.9703079. [DOI] [PubMed] [Google Scholar]
- 50.Fagan JK, Scheff PA, Hryhorczuk D, Ramakrishnan V, Ross M, Persky V. Prevalence of asthma and other allergic diseases in an adolescent population: association with gender and race. Ann. Allergy. Asthma. Immunol. 2001;86:177–184. doi: 10.1016/S1081-1206(10)62688-9. [DOI] [PubMed] [Google Scholar]
- 51.Rohleder N, Schommer NC, Hellhammer DH, Engel R, Kirschbaum C. Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosom. Med. 2001;63:966–972. doi: 10.1097/00006842-200111000-00016. [DOI] [PubMed] [Google Scholar]
- 52.Wagh AD, Sharma M, Mahapatra J, Chatterjee A, Jain M, Addepalli V. Investigation into the Role of PI3K and JAK3 Kinase Inhibitors in Murine Models of Asthma. Frontiers in pharmacology. 2017;8:82. doi: 10.3389/fphar.2017.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Hove CL, Maes T, Cataldo DD, Gueders MM, Palmans E, Joos GF, Tournoy KG. Comparison of acute inflammatory and chronic structural asthma-like responses between C57BL/6 and BALB/c mice. Int. Arch. Allergy Immunol. 2009;149:195–207. doi: 10.1159/000199715. [DOI] [PubMed] [Google Scholar]
- 54.Girodet PO, Nguyen D, Mancini JD, Hundal M, Zhou X, Israel E, Cernadas M. Alternative Macrophage Activation Is Increased in Asthma. Am. J. Respir. Cell Mol. Biol. 2016;55:467–475. doi: 10.1165/rcmb.2015-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.St-Laurent J, Turmel V, Boulet LP, Bissonnette E. Alveolar macrophage subpopulations in bronchoalveolar lavage and induced sputum of asthmatic and control subjects. J. Asthma. 2009;46:1–8. doi: 10.1080/02770900802444211. [DOI] [PubMed] [Google Scholar]
- 56.Lensmar C, Katchar K, Eklund A, Grunewald J, Wahlstrom J. Phenotypic analysis of alveolar macrophages and lymphocytes following allergen inhalation by atopic subjects with mild asthma. Respir. Med. 2006;100:918–925. doi: 10.1016/j.rmed.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 57.Kim EY, Battaile JT, Patel AC, You Y, Agapov E, Grayson MH, Benoit LA, Byers DE, Alevy Y, Tucker J, Swanson S, Tidwell R, Tyner JW, Morton JD, Castro M, Polineni D, Patterson GA, Schwendener RA, Allard JD, Peltz G, Holtzman MJ. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat. Med. 2008;14:633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viksman MY, Bochner BS, Peebles RS, Schleimer RP, Liu MC. Expression of activation markers on alveolar macrophages in allergic asthmatics after endobronchial or whole-lung allergen challenge. Clin. Immunol. 2002;104:77–85. doi: 10.1006/clim.2002.5233. [DOI] [PubMed] [Google Scholar]
- 59.Klein SL. Immune cells have sex and so should journal articles. Endocrinology. 2012;153:2544–2550. doi: 10.1210/en.2011-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gwak JM, Jang MH, Kim DI, Seo AN, Park SY. Prognostic value of tumor-associated macrophages according to histologic locations and hormone receptor status in breast cancer. PLoS One. 2015;10:e0125728. doi: 10.1371/journal.pone.0125728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kiyohara C, Ohno Y. Sex differences in lung cancer susceptibility: a review. Gender medicine. 2010;7:381–401. doi: 10.1016/j.genm.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Wynder EL, Muscat JE. The changing epidemiology of smoking and lung cancer histology. Environ. Health Perspect. 1995;103(Suppl 8):143–148. doi: 10.1289/ehp.95103s8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poczobutt JM, De S, Yadav VK, Nguyen TT, Li H, Sippel TR, Weiser-Evans MC, Nemenoff RA. Expression Profiling of Macrophages Reveals Multiple Populations with Distinct Biological Roles in an Immunocompetent Orthotopic Model of Lung Cancer. J. Immunol. 2016;196:2847–2859. doi: 10.4049/jimmunol.1502364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taioli E, Wynder EL. Re: Endocrine factors and adenocarcinoma of the lung in women. J. Natl. Cancer Inst. 1994;86:869–870. doi: 10.1093/jnci/86.11.869. [DOI] [PubMed] [Google Scholar]
- 65.Baibergenova A, Thabane L, Akhtar-Danesh N, Levine M, Gafni A, Leeb K. Sex differences in hospital admissions from emergency departments in asthmatic adults: a population-based study. Ann. Allergy. Asthma. Immunol. 2006;96:666–672. doi: 10.1016/S1081-1206(10)61063-0. [DOI] [PubMed] [Google Scholar]
- 66.Emmett SE, Angus FJ, Fry JS, Lee PN. Perceived prevalence of peanut allergy in Great Britain and its association with other atopic conditions and with peanut allergy in other household members. Allergy. 1999;54:380–385. doi: 10.1034/j.1398-9995.1999.00768.x. [DOI] [PubMed] [Google Scholar]
- 67.Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin. Immunol. Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 68.Dooley MA, Hogan SL. Environmental epidemiology and risk factors for autoimmune disease. Curr. Opin. Rheumatol. 2003;15:99–103. doi: 10.1097/00002281-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 69.Gleicher N, Barad DH. Gender as risk factor for autoimmune diseases. J. Autoimmun. 2007;28:1–6. doi: 10.1016/j.jaut.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 70.Giron-Gonzalez JA, Moral FJ, Elvira J, Garcia-Gil D, Guerrero F, Gavilan I, Escobar L. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur. J. Endocrinol. 2000;143:31–36. doi: 10.1530/eje.0.1430031. [DOI] [PubMed] [Google Scholar]
- 71.Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiebaut R, Tibshirani RJ, Davis MM. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. U. S. A. 2014;111:869–874. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lorenzo ME, Hodgson A, Robinson DP, Kaplan JB, Pekosz A, Klein SL. Antibody responses and cross protection against lethal influenza A viruses differ between the sexes in C57BL/6 mice. Vaccine. 2011;29:9246–9255. doi: 10.1016/j.vaccine.2011.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dragin N, Bismuth J, Cizeron-Clairac G, Biferi MG, Berthault C, Serraf A, Nottin R, Klatzmann D, Cumano A, Barkats M, Le Panse R, Berrih-Aknin S. Estrogen-mediated downregulation of AIRE influences sexual dimorphism in autoimmune diseases. J. Clin. Invest. 2016;126:1525–1537. doi: 10.1172/JCI81894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lelu K, Laffont S, Delpy L, Paulet PE, Perinat T, Tschanz SA, Pelletier L, Engelhardt B, Guery JC. Estrogen receptor alpha signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J. Immunol. 2011;187:2386–2393. doi: 10.4049/jimmunol.1101578. [DOI] [PubMed] [Google Scholar]
- 75.Gilmore W, Weiner LP, Correale J. Effect of estradiol on cytokine secretion by proteolipid protein-specific T cell clones isolated from multiple sclerosis patients and normal control subjects. J. Immunol. 1997;158:446–451. [PubMed] [Google Scholar]
- 76.Karpuzoglu-Sahin E, Zhi-Jun Y, Lengi A, Sriranganathan N, Ansar Ahmed S. Effects of long-term estrogen treatment on IFN-gamma, IL-2 and IL-4 gene expression and protein synthesis in spleen and thymus of normal C57BL/6 mice. Cytokine. 2001;14:208–217. doi: 10.1006/cyto.2001.0876. [DOI] [PubMed] [Google Scholar]
- 77.Lambert KC, Curran EM, Judy BM, Milligan GN, Lubahn DB, Estes DM. Estrogen receptor alpha (ERalpha) deficiency in macrophages results in increased stimulation of CD4+ T cells while 17beta-estradiol acts through ERalpha to increase IL-4 and GATA-3 expression in CD4+ T cells independent of antigen presentation. J. Immunol. 2005;175:5716–5723. doi: 10.4049/jimmunol.175.9.5716. [DOI] [PubMed] [Google Scholar]
- 78.Yang L, Hu Y, Hou Y. Effects of 17beta-estradiol on the maturation, nuclear factor kappa B p65 and functions of murine spleen CD11c-positive dendritic cells. Mol. Immunol. 2006;43:357–366. doi: 10.1016/j.molimm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 79.Siracusa MC, Overstreet MG, Housseau F, Scott AL, Klein SL. 17beta-estradiol alters the activity of conventional and IFN-producing killer dendritic cells. J. Immunol. 2008;180:1423–1431. doi: 10.4049/jimmunol.180.3.1423. [DOI] [PubMed] [Google Scholar]
- 80.Deshpande R, Khalili H, Pergolizzi RG, Michael SD, Chang MD. Estradiol down-regulates LPS-induced cytokine production and NFkB activation in murine macrophages. Am. J. Reprod. Immunol. 1997;38:46–54. doi: 10.1111/j.1600-0897.1997.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 81.Zhang X, Wang L, Zhang H, Guo D, Qiao Z, Qiao J. Estrogen inhibits lipopolysaccharide-induced tumor necrosis factor-alpha release from murine macrophages. Methods Find. Exp. Clin. Pharmacol. 2001;23:169–173. doi: 10.1358/mf.2001.23.4.634640. [DOI] [PubMed] [Google Scholar]
- 82.Tomaszewska A, Guevara I, Wilczok T, Dembinska-Kiec A. 17beta-estradiol- and lipopolysaccharide-induced changes in nitric oxide, tumor necrosis factor-alpha and vascular endothelial growth factor release from RAW 264.7 macrophages. Gynecol. Obstet. Invest. 2003;56:152–159. doi: 10.1159/000073775. [DOI] [PubMed] [Google Scholar]
- 83.Ito A, Bebo BF, Jr, Matejuk A, Zamora A, Silverman M, Fyfe-Johnson A, Offner H. Estrogen treatment down-regulates TNF-alpha production and reduces the severity of experimental autoimmune encephalomyelitis in cytokine knockout mice. J. Immunol. 2001;167:542–552. doi: 10.4049/jimmunol.167.1.542. [DOI] [PubMed] [Google Scholar]
- 84.Sundberg R, Toren K, Franklin KA, Gislason T, Omenaas E, Svanes C, Janson C. Asthma in men and women: treatment adherence, anxiety, and quality of sleep. Respir. Med. 2010;104:337–344. doi: 10.1016/j.rmed.2009.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.