Abstract
Pneumonia is caused by both viral and bacterial pathogens and is responsible for a significant health burden in the Unites States. The innate immune system is the human body’s first line of defense against these pathogens. The recognition of invading pathogens via pattern recognition receptors leads to proinflammatory cytokine and chemokine production, followed by recruitment and activation of effector immune cells. The nonspecific inflammatory nature of the innate immune response can result in immunopathology that is detrimental to the host. In this review, we focus on one class of pattern recognition receptors, the nucleotide-binding oligomerization domain (NOD)-like receptors, specifically NOD1 and NOD2, and their role in host defense against viral and bacterial pathogens of the lung, including influenza, respiratory syncytial virus, Streptococcus pneumoniae, Chlamydophila pneumoniae, and Staphylococcus aureus. It is hoped that improved understanding of NOD1 and NOD2 activity in pneumonia will facilitate the development of novel therapies and promote improved patient outcomes.
Keywords: NOD like receptors, innate immunity, pulmonary infections, influenza, Streptococcus pneumoniae
Pneumonia is responsible for considerable morbidity and mortality in the United States (1). The annual incidence of community-acquired pneumonia (CAP) requiring hospitalization in the United States is 24.8 cases per 10,000 adults (1). For patients requiring hospital admission, a rigorous diagnostic evaluation will fail to identify a causative pathogen in a majority of cases. In the recent Etiology of Pneumonia in the Community (EPIC) study, a prospective, population-based study of hospitalized adults with CAP, only 38% of patients had a detectable pathogen identified, of which 27% were viral and 14% were bacterial in origin, respectively. Human rhinovirus, influenza virus, and human metapneumovirus were among the most common viral pathogens detected, whereas Streptococcus pneumoniae, Mycoplasma pneumoniae, and Staphylococcus aureus were among the most common bacterial pathogens (1).
Even when a causative organism is identified and the appropriate therapy is initiated, pneumonia can be fatal. According to the 2014 national vital statistics report, pneumonia continues to be the leading cause of death from an infectious disease in the United States, with a death rate of 17.3 per 100,000 persons (2). Likewise, the economic burden of pneumonia is substantial, with more than $17 billion spent each year (3). The morbidity and mortality is related in part to the virulence of the infecting organism, but another important contributing factor is the immunopathology caused by the nonspecific inflammatory nature of the innate immune system’s response to these pathogens (4). This phenomenon was demonstrated effectively by Cheng and colleagues with a transgenic mouse model in which lung epithelial cells underwent selective activation of nuclear factor-κB (NF-κB), a common downstream transcriptional activator that drives the proinflammatory response of the innate immune system. This activation resulted in high-protein pulmonary edema, neutrophilic lung inflammation, hypoxemia, and death, all in the absence of an infectious insult (5). Furthermore, inhibition of NF-κB in lung epithelial cells prevented gram-negative bacterial LPS-induced lung inflammation and injury (5). These findings support the premise that the host’s inflammatory response contributes to the injurious nature of pneumonia, irrespective of the pathogenicity of the invading organism.
To improve the pneumonia-related mortality rate and its associated economic burden, it is imperative that we better understand this potentially deleterious inflammatory response of the innate immune system and how in a subset of pneumonia patients it may contribute to excessive inflammation and worse outcomes. Moreover, it will be critical that future studies aim to elucidate whether targeted interruption of innate immunity signaling pathways or individual cytokines can protect the host from this immunopathology.
Pulmonary Innate Immunity
The lungs represent the largest epithelial surface area to come into contact with environmental foreign substances and microbes. The innate immune response is the nonspecific first line of defense against these potential pathogens. Although they are important to pathogen control, the nonspecific nature of the innate immune effectors can result in significant immunopathology.
The innate immune system is composed of the physical and chemical barriers that impede the entry of invading organisms, as well as the immune cells and their effector cytokines and chemokines. Respiratory epithelial cells provide the primary mechanical barrier between the atmosphere and the pulmonary parenchyma. Secreted mucins form a mucoid layer that lies on top of the epithelium and acts to disrupt microbial binding to the epithelial cell surface and to promote mucociliary clearance. In addition to providing a structural barrier to invading pathogens, epithelial cells secrete microbicidal substances, including lactoferrin and lysozyme, which destabilize the bacterial cell wall. These promote bacterial cell degradation and secretion of immunoglobulin A, which is important in blocking bacterial entry and in the neutralization of toxins and viruses (6–8).
A microbe that is able to escape the physical and chemical defense mechanisms of the branching conducting airways will ultimately reach the end-respiratory unit, the alveoli, where type II epithelial cells produce pulmonary surfactant. Surfactant proteins SP-A and SP-D have been recognized as important mediators of innate immunity, enhancing alveolar macrophage opsonization as well as modulating the inflammatory response to viral infection (9).
The Role of Pattern Recognition Receptors in the Pulmonary Innate Immune Response
The conducting airway cells and the alveolar type II epithelial cells not only provide a physical barrier and produce protective secretory chemicals, but also express germline-encoded pattern recognition receptors (PRRs). These PRRs detect conserved microbial components called pathogen-associated molecular patterns as well as host-derived danger-associated molecular patterns after tissue injury (10, 11). Binding of pathogen-associated molecular patterns or danger-associated molecular patterns to PRRs promotes a downstream inflammatory response involving cytokine/chemokine production as well as the up-regulation of adhesion molecules required for the influx of inflammatory cells such as neutrophils (11).
The three classes of PRRs are the membrane-bound Toll-like receptors (TLRs), the intracellular retinoic acid-inducible gene (RIG)-1-like receptors (RLRs), and the nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) (12). The TLRs are the most extensively studied PRRs. They reside on the cell surface and within endosomes and are responsible for recognizing microbial and self ligands including LPS, peptidoglycan (PGN), flagellin, RNA, and the unmethylated cytosine and CpG (unmethylated cytosine and guanine separated by one phosphate) DNA of viruses and bacteria (13–16). Unlike the TLRs, the RLRs and the NLRs are cytoplasmic PRRs. RLRs are well recognized for their important role in viral RNA detection and the subsequent activation of the innate and adaptive immune responses (11). In recent years, emerging data have allowed clearer elucidation of the role of the cytoplasmic NLRs, including their function in pulmonary infections.
NOD-Like Receptors
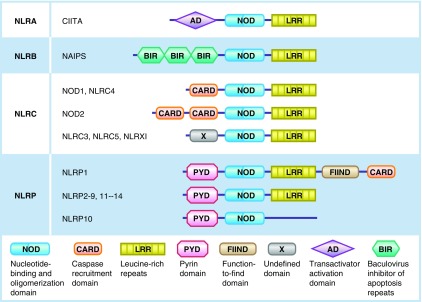
There are >20 receptors in the NLR family, all of which share three structural domains: a carboxy terminal leucine-rich repeat (LRR), which is responsible for the detection of conserved microbial motifs; the central NOD domain, which promotes self-oligomerization; and the N-terminal effector domain (17, 18). The NLRs are further classified into four groups on the basis of their N-terminal effector domain: the transactivator activation domain containing NLRAs, the baculovirus-inhibitor-of apoptosis repeats (BIR) containing NLRBs or neuronal apoptosis inhibitor proteins (NAIPS), the caspase-recruitment domain (CARD) containing NODs or NLRCs, and the pyrin domain containing NLRPs (NACHT, LRR, and PYD domain-containing proteins, also called NALPs) (19) (Figure 1).
Figure 1.
Graphic depiction of the nucleotide-binding oligomerization domain (NOD)–like receptor (NLR) family. All the NLRs compose a central NOD domain with a carboxy terminal leucine-rich repeat (LRR), with the exception of NLRP10, which lacks an LRR. The NLR family is further classified into four subfamilies on the basis of their N-terminal effector domains: the transactivator activation domain (AD) containing NLRAs, the baculovirus-inhibitor-of apoptosis repeats (BIR) containing NLRBs, the caspase-recruitment domain (CARD) containing NLRCs, and the pyrin-domain (PYD) containing NLRPs. CIITA, class II transactivator; FIIND, function-to-find domain; NAIPS, neuronal apoptosis inhibitor proteins; X, undefined domain.
In the steady state, the carboxy terminal LRR binds to the NOD domain to prevent self-oligomerization and thus autoactivation (20, 21). Once a PAMP is recognized by the C-terminal LRR, a conformational change occurs that allows self-oligomerization of the NOD domain (22). This results in the exposure of the effector domain, which promotes the recruitment and activation of the effector molecules.
Bacterial Activation of the NOD1/NOD2 Directed Innate Immune Response
There are two main CARD-containing NODs, NOD1 and NOD2. Although NOD1 and NOD2 share similar structure, they differ in their activating ligands and in the role they play in pulmonary immunity. NOD1 is expressed ubiquitously, with recognized expression in the human lung epithelium (23). It is activated by PGN-derived peptides containing γ-d-glutamyl-meso-diaminopimelic acid (iE-DAP), which is found in many gram-negative bacteria as well as some gram-positive bacteria (24, 25). NOD2 expression has been confirmed in phagocytic cells including, macrophages, monocytes, and dendritic cells, as well as in the lung epithelium and in Paneth cells of the small intestine (26–28). NOD2 is activated by muramyl-dipeptide MurNAc-l-Ala-d-isoGln (MDP), a conserved PGN found in almost all bacteria (25, 27).
Both intracellular and extracellular pathogens have been recognized as activating the cytoplasmic NLRs. The exact mechanism by which the extracellular pathogen NOD ligands obtain access to the intracellular compartment to activate these NLRs is not completely clear. Host cell phagocytosis of the pathogenic bacteria may be one mechanism that permits iE-DAP and MDP to be sensed by cytosolic NOD1 and NOD2, respectively (29). But there is also new evidence by Ismair and colleagues that suggests that the NOD2-activating ligand MDP can enter epithelial cells via an active transport mechanism, the peptide transporter hPepT1, which is specific for MDP and does not have the capacity to transport the NOD1-activating ligand iE-DAP (30). Outer membrane vesicles, which are shed naturally from gram-negative bacteria, have also been recognized recently for their ability to enter nonphagocytic epithelial cells and activate the NOD1 signaling pathway (31, 32). More research is necessary to expound on alternative mechanisms of NOD ligand transport into the intracellular space.
After ligand binding, the hemophilic CARD–CARD interaction of NOD1 and NOD2 results in the recruitment of receptor interacting protein 2 (RIP2) (also called RIP-like interacting CLARP [caspase-like apoptosis-regulatory protein] kinase), a serine-threonine kinase (22, 33, 34). The interaction of RIP2 with the effector domain of NOD results in the ubiquitination of RIP2, with the resulting activation of downstream NF-κB, mitogen-activated protein kinase, extracellular signal-regulated kinase, and c-Jun N-terminal kinase (27, 35–39) (Figure 2). It was established by Kobayashi and colleagues that the presence of RIP2 is essential for the NOD-specific bacterial activation of NF-κB (36). NOD1- and NOD2-mediated activation of NF-κB was completely eliminated in mouse embryonic fibroblasts deficient in RIP2. The subsequent ectopic expression of RIP2 in these fibroblasts resulted in the restoration of NOD-mediated NF-κB signaling (36). It was similarly shown by Chin and colleagues that mice deficient in RIP2 do not activate NF-κB in response to NOD1 or TLR4 signaling (33). NF-κB signaling is responsible for the up-regulation of proinflammatory molecules and neutrophilic lung inflammation (40, 41). When this signaling pathway is inhibited in the airway epithelium, there is a reduced inflammatory response and an increased susceptibility to infection to certain pulmonary pathogens including Chlamydophila pneumoniae, Escherichia coli, and Acinetobacter baumannii (42–45).
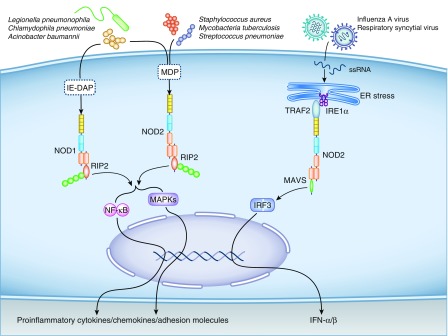
Figure 2.
Activation of Nod-like receptors, NOD1 and NOD2, by bacterial and viral pathogens. Intracellular NOD1 and NOD2 detect bacterial peptidoglycan-derived peptides containing γ-d-glutamyl-meso-diaminopimelic acid (iE-DAP) and the conserved peptidoglycan, muramyl-dipeptide MurNAc-l-Ala-d-isoGln (MDP), respectively. After activation, NOD1 and NOD2 recruit receptor interacting protein 2 (RIP2), a serine-threonine kinase that promotes the activation of downstream nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPKs), resulting in the production of proinflammatory cytokines, chemokines, and adhesion molecules. Although NOD2 can also be activated after infection with single-stranded RNA (ssRNA) viruses, the exact mechanism remains poorly elucidated. One potential mechanism includes viral-induced endoplasmic reticulum (ER) stress promoting activation of the inositol-requiring enzyme 1α (IRE1α) transmembrane receptor. IRE1α recruits the tumor necrosis factor receptor–associated factor 2 (TRAF2), which associates with the TRAF2 binding motif on NOD2, causing its activation. Activated NOD2 acts downstream on IFN regulatory factor 3 (IRF3), promoting its translocation to the nucleus, where it promotes transcription of the type 1 IFNs (IFN-α/β). MAVS, mitochondrial antiviral-signaling protein.
NOD1 activation via NOD1-specific stimulatory synthetic compounds results in the production of chemokines and the recruitment of neutrophils (46). It is well recognized that NOD1 knockout mice have increased vulnerability to several pathogens, including C. pneumoniae, Shigella flexneri, enteroinvasive E. coli, Campylobacter jejuni, and Listeria monocytogenes (38, 47, 48).
Similarly, NOD2 activation by MDP results in the induction of multiple downstream cytokines (tumor necrosis factor [TNF]-α, IL-1α, and IL-1β), chemokines (IL-6 and granulocyte-macrophage colony-stimulating factor), and adhesive molecules (ICAM1, CD44, and TNFAIP6), all of which are critical for the recruitment and activation of inflammatory cells (49–51). NOD2 is involved in the response to many bacterial pathogens, including Mycobacterium tuberculosis, Salmonella enterica serovar Typhimurium, L. monocytogenes, S. pneumoniae, and S. aureus (47, 48, 52–54).
Viral Activation of NOD2-Mediated Innate Immunity
Recently, there has been evidence to suggest that NOD2, unlike NOD1, is also involved in the innate immune response to RNA viruses (55). The mechanism by which RNA viruses activate NOD2 remains elusive. Unlike bacteria, viruses do not contain the NOD2-activating ligand MDP, but data by Keestra-Gounder and colleagues suggest that endoplasmic reticulum (ER) stress, which can be seen after viral infection, may be responsible for the activation of this NOD2-mediated pathway (56). ER stress causes activation of the inositol-requiring enzyme 1α transmembrane receptor, which recruits the TNF receptor-associated factor 2 (TRAF2) (56, 57). Because NOD2 contains a major TRAF2 binding motif, it is plausible that an association between TRAF2 and NOD2 could stimulate downstream mediators to promote viral clearance and recovery. It was demonstrated by Keestra-Gounder that mice deficient in NOD1 and NOD2 have a diminished proinflammatory response when challenged with the ER stress inducer thapsigargin, as evidenced by decreased IL-6, macrophage inflammatory protein (MIP-1β), and keratinocyte chemoattractant (56). It remains unclear whether this dampened proinflammatory response corresponds to worse outcomes after viral infection. Likewise, additional research is required to distinguish whether ER stress is uniquely responsible for the activation of NOD2 after viral infection or whether there are additional inducers of this pathway.
It was observed by Sabbah and colleagues that viral single-stranded RNA (ssRNA) exposure results in NOD2-mediated activation of IFN regulatory factor 3 (IRF3), prompting its translocation to the nucleus, where it is responsible for the transcription of IFN-β (55). In contrast to bacterial activation of NOD2, the innate antiviral activity of NOD2 does not require RIP2 to mediate its downstream mediators. NOD2 instead requires interaction with mitochondrial antiviral-signaling protein to promote this antiviral response (55). Sabbah and colleagues went on to speculate that NOD2 can interact with either RIP2 or mitochondrial antiviral-signaling protein, depending on the stimulus, to activate NF-κB or IRF3, respectively (Figure 2). NOD2-deficient bone marrow–derived macrophages exposed to ssRNA viruses had decreased IFN-β production. In addition, NOD2-deficient cells exposed to respiratory syncytial virus (RSV) and vesicular stomatitis virus produced higher viral titers than did NOD2-expressing cells, suggesting viral clearance is mediated in part by the activation of NOD2 (55).
PRRs in Adaptive Immunity
Adaptive immunity provides a highly specific response to a precise pathogen and creates immunologic memory of that pathogen. Unlike innate immunity, the targeted response of T cells and B cells limits the potential for immunopathology. Dendritic cells serve as the bridge between the innate and the adaptive immune responses. Dendritic cells, with their dendrites extended between the pulmonary epithelial cells, survey the environment for pathogens (58). Once a pathogen is encountered, dendritic cells phagocytose it and carry the associated pathogenic antigens on their major histocompatibility complex molecules to the peripheral lymphoid organs to stimulate T lymphocytes (59). There is increasing evidence to suggest that the PRRs play a role in promoting this T-helper cell response.
The organized migration of dendritic cells to their draining lymph nodes is mediated by the TLR-induced up-regulation of the lymphoid chemokine receptors, with the concomitant down-regulation of inflammatory chemokine receptors (60). It was demonstrated by Schnare and colleagues that TLRs also play a role in the differentiation of naive T cells toward a Th1 response via TLR-induced cytokines. Mice deficient in MyD88, a downstream adapter protein of all TLRs, fail to activate the Th1 response when stimulated with TLR ligands (61). The CARD-containing NODs are now being recognized as mediators in the adaptive immune response as well, although the spectrum of cellular processes involved in promoting this response is not well understood. NOD1 activation contributes to a predominately Th2 response, as demonstrated by Fritz and colleagues, who showed that the application of the NOD1-specific agonist FK156 resulted in increased expression of monocyte chemoattractant protein-1 (MCP-1), a chemokine important in the promotion of the Th2 response. This response was not present in NOD1-deficient mice (62). When NOD1 is activated together with the TLRs, they work in synergy, causing an amplified NF-κB response that promotes the production of important cytokines, including IL-12, IL-1β, and IL-6, essential for the Th1 and Th17 immune response (62).
NOD2 also contributes to the activation of the Th2 response. It was shown by Kobayashi and colleagues that NOD2-deficient mice demonstrate significantly decreased levels of antigen-specific immunoglobulin production after immunization with the NOD2-specific ligand, MDP (36). Van Beelen and colleagues provided evidence that NOD2 also regulates a Th17 response via the up-regulation of the IL-17–inducing cytokines, IL-23 and IL-1. Moreover, dendritic cells from patients with Crohn’s disease, who have NOD2 deficiency, have a compromised ability to express IL-17 after MDP exposure (63). Future studies aimed at identifying the mechanisms by which NOD1 or NOD2 directly promote the activation of the adaptive immune response are needed.
Role of NOD in Specific Viral Pulmonary Infections
The innate immune response to viral infections has been attributed historically to the activation of the TLRs and RIG-like helicase receptors. Recently, the NLRs, namely, NLRP3 and NOD2, have been established as playing a role in the host’s defense against viral pulmonary infections (4, 64). One of the first groups to demonstrate NOD2 activation after viral ssRNA exposure was Sabbah and colleagues (55). NOD2 is now recognized as an important component of the innate signaling pathway, responsible for the synthesis of type 1 IFNs (IFN-α and IFN-β), which are vital in the production of proinflammatory cytokines and the restriction of viral replication (55). The most compelling data supporting the role of NOD2 in viral respiratory infections have been in the setting of influenza and RSV infections (Table 1).
Table 1.
The Host Response to Pulmonary Pathogens in Mice Deficient in NOD Signaling
| Pathogen | Model | Pathogen Clearance | Cytokine/Chemokine Expression | Neutrophil Influx | Reference | ||
|---|---|---|---|---|---|---|---|
| Influenza virus | NOD2 KO | ↓ | (Days 2, 7, 12) | ↓ | IFN-β (Day 2) | 55, 66 | |
| ↑ | KC (Day 2) | ||||||
| IP-10 (Days 2, 7) | |||||||
| MIP-1α (Days 2, 7) | |||||||
| MCP-1 (Days 2, 7) | |||||||
| IFN-γ (Day 7 | |||||||
| Respiratory syncytial virus | NOD2 KO | ↓ | (Day 3) | ↓ | IFN-β (Day 2) | Increased at 48 h | 55 |
| ↑ | TNF (Days 1, 6) | ||||||
| IL-10 (Days 1, 6) | |||||||
| RANTES (Days 1, 6) | |||||||
| Streptococcus pneumoniae | NOD2 KO | ↔ | (6, 25, 48 h) | ↑ | IL-6 (Days 1, 2) | No change at 6, 24, or 48 h | 72, 73 |
| MIP-2 (Days 1, 2) | |||||||
| ↔ | TNF-α (6, 24, 48 h) | ||||||
| IL-1β (6, 24, 48 h) | |||||||
| KC (6, 24, 48 h) | |||||||
| CCL2 (6, 24, 48 h) | |||||||
| Staphylococcus aureus | NOD2 KO | ↑ | (Day 2: BAL) | ↓ | IL-1β (Day 2) | Decreased at 48 h | 53 |
| ↔ | (Days 1, 7: BAL) | IL-6 (Day 2) | |||||
| ↔ | (Days 1, 2, 7: lung homogenates) | IL-10 (Day 2) | |||||
| IFN-у (Day 2) | |||||||
| MIP-2 (Day 2) | |||||||
| TNF (Day 2) | |||||||
| KC (Day 2) | |||||||
| ↔ | IL-1β (Day 7) | ||||||
| IL-6 (Day 7) | |||||||
| IL-10 (Day 7) | |||||||
| IFN-у (Day 7) | |||||||
| MIP-2 (Day 7) | |||||||
| TNF (Day 7) | |||||||
| KC (Day 7) | |||||||
| Mycobacterium tuberculosis | NOD2 KO | ↓ | (Week 24) | ↓ | TNF-α (24 h, Day 28) | 52, 85 | |
| IFN-γ (Day 28) | |||||||
| ↔ | IL-12 p40 (Day 28) | ||||||
| IL-10 (Day 28) | |||||||
| IL-6 (24 h) | |||||||
| Chlamydophila pneumoniae | NOD2 KO | ↓ | (Day 5) | 42 | |||
| RIP2 KO | ↓ | (Days 5, 14) | ↓ | IL-6 (Day 3) | Decreased at Day 3 Increased at Days 5, 14 | ||
| IL12 p40 (Day 3) | |||||||
| IFNγ (Day 3) | |||||||
| IL-12 p40 (Day 3) | |||||||
| ↑ | IFNγ (Day 5) | ||||||
| KC (Day 5) | |||||||
| MIP-2 (Day 5) | |||||||
| IL-6 (Days 5, 14) | |||||||
| IL-12 p40 (Days 5, 14) | |||||||
| Legionella pneumophila | NOD1 KO | ↓ | (Day 3) | ↔ | KC (9 h) | Decreased at 4, 9, 24 h Increased at Day 3 | 78, 79 |
| G-CSF (9 h) | |||||||
| IL-1β (9 h) | |||||||
| IL-12 (9 h) | |||||||
| IL-13 (9 h) | |||||||
| Eotaxin (9 h) | |||||||
| TNF-α (9 h) | |||||||
| IL-1α (9 h) | |||||||
| IL-18 (9 h) | |||||||
| IL-6 (9 h) | |||||||
| MCP-1 (9 h) | |||||||
| MIP-2 (9 h) | |||||||
| ↓ | IL-1β (4 h) | ||||||
| KC (4 h) | |||||||
| ↑ | IL-6 (24 h) | ||||||
| NOD2 KO | ↔ | (4 h, Days 1, 3, 30) | ↑ | IL-6 (4, 24 h) | Decreased at 9 h; increased at 24 h | ||
| MCP-1 (4, 24 h) | |||||||
| IL-1β (24 h) | |||||||
| RIP2 KO | ↓ | KC (9 h) | Decreased at 9 h | ||||
| IL-6 (9 h) | |||||||
| G-CSF (9 h) | |||||||
Definition of abbreviations: BAL, bronchoalveolar lavage; CCL2, chemokine (C-C motif) ligand 2; G-CSF, granulocyte colony-stimulating factor; IP-10, interferon γ-inducible protein 10; KC, keratinocyte chemoattractant; KO, knockout; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; NOD, nucleotide-binding oligomerization domain; RANTES, regulated upon activation, normal; RIP, receptor interacting protein.
Arrows indicate levels relative to wild-type mice: (↓) decreased, (↑) increased, (↔) similar levels between wild-type and KO mice; empty boxes indicate no available data.
Influenza Virus
Influenza A virus (IAV) is a negative-sense ssRNA virus that is highly contagious and is responsible for >200,000 hospitalizations per year in the United States (65). It was demonstrated by Sabbah and colleagues that NOD2-deficient cells stimulated with IAV had a diminished IFN-β response. This was also confirmed in vivo, with decreased IFN-β production in the bronchoalveolar lavage (BAL) fluid of IAV-infected NOD2-deficient mice (55). These findings were supported by Lupfer and colleagues, who showed that NOD2-deficient mice were more susceptible to IAV infection, with these mice having higher viral titers and lower IFN-β levels at all time points (66). In addition, the study by Lupfer and colleagues revealed that the loss of NOD2 signaling resulted in a decreased number of dendritic cells in the lungs and in increased dendritic cell death after IAV infection. This reduction in the dendritic cell population resulted in diminished dendritic cell priming of CD8+ T cells, resulting in a suboptimal IAV-specific CD8+ T cell response (66).
Further supporting the role of NOD2 signaling in response to IAV infection, Sabbah and colleagues showed an increase in NOD2 expression after infection of human lung epithelial A549 cells with synthetic ssRNA (55). In contrast, Wu and colleagues did not show an induction of NOD2 after infection of human type II alveolar epithelial cells at 24 hours after IAV infection (67). One possible reason for these inconsistent findings is treatment-related differences (i.e., synthetic ssRNA versus live IAV). Another possibility is that increases in NOD2 expression follow an early time course after infection. Indeed, Sabbah and colleagues identified peak NOD2 expression at 6 hours after ssRNA treatment, with decreased expression by 24 hours (55). This may have been the reason for the null results found by Wu and colleagues (67). Collectively, the data suggest that NOD2 contributes to viral clearance and improved survival after IAV infection by promoting an early IFN-β inflammatory response and by establishing optimal adaptive immunity through dendritic cell priming of CD8+ T cells.
RSV
RSV is a negative-sense ssRNA virus that can cause pneumonia in children and immunosuppressed adults (68). It was documented by Sabbah and colleagues that RSV infection induces NOD2 expression in human bronchial epithelial cells. The up-regulation of NOD2 expression occurred early after infection with RSV. RIG-1, the PRR historically accredited for its role in promoting the antiviral innate immune response, was detectable at later time points than was NOD2. These findings suggest that NOD2 is critical in the early antiviral response, whereas RIG-1 plays a role in the later response (55). Knockdown of NOD2 via small interfering RNA (siRNA) results in dampened early IFN-β production and higher RSV titers than in NOD2-expressing cells after infection, suggesting that viral clearance is mediated in part by the activation of NOD2 (55). These findings were confirmed in vivo, where the BAL fluid of NOD2-deficient mice infected with RSV had increased cytokine and chemokine levels and increased histologic lung inflammation on Days 3 and 5 after infection (55). NOD2-deficient mice also demonstrated increased morbidity and mortality after RSV infection (55).
The type I IFN response to RSV was identified by Vissers and colleagues as resulting in the up-regulation of NOD2 expression (69). This amplified expression was associated with increased signaling through the NOD2 pathway when the host was exposed subsequently to a secondary bacterial infection. This NOD2-specific synergy results in an increase in proinflammatory cytokine production and potentially contributes to the increased severity and lethality of postviral pneumonia superinfection (69). The findings of Vissers and colleagues (69) likely apply to other viral infections, as supported by the conclusions of Kim and colleagues, who demonstrated that type I IFNs induce the expression of both NOD1 and NOD2 (70). Mice treated previously with poly(I:C), a synthetic analog of double-stranded RNA, have increased susceptibility to secondary bacterial infection with E. coli and intranasal Pseudomonas aeruginosa (70). The increased mortality induced by previous poly(I:C) exposure was associated with increased levels of TNF-α. Mice deficient in both NOD1 and NOD2 did not experience this same increased mortality and had lower levels of TNF-α (70).
NOD2 signaling in RSV infection, as in IAV infection, results in improved viral clearance via the up-regulation of IFN-β production and limits the inflammatory parenchymal damage and its associated morbidity and mortality. Furthermore, the type I IFN response of NOD2 potentiates increased NOD2 expression, which may result in increased immunopathology in the event of a superinfection.
Role of NOD in Specific Bacterial Pulmonary Infections
There is now sound evidence to support the importance of NLRs, specifically NOD1 and NOD2, in the body’s immune response to bacterial pulmonary infection. Several studies have attempted to clarify the NOD-specific contributions to distinct bacterial pulmonary pathogens, some of which are reviewed here (Table 1).
S. Pneumoniae
S. pneumoniae is the leading bacterial cause of CAP (1). The amplified expression of NOD2 after pneumococcal infection was demonstrated by Opitz and colleagues (23). The activation of NOD2 by S. pneumoniae mediates downstream NF-κB activity and contributes to the generation of important cytokines and chemokines, including IL-8 and granulocyte-macrophage colony-stimulating factor (23, 71). Details of this extracellular pathogen’s activation of the intracellular PRR NOD2 was elucidated by Davis and colleagues, who demonstrated that lysozyme, a PGN-degrading enzyme expressed within pulmonary phagocytes, digests S. pneumoniae, producing pneumococcal PGN fragments that can be sensed by NOD2. These fragments gain access into the host cell cytosol via the S. pneumoniae virulence factor, the pore-forming pneumolysin (72). NOD2-mediated expression of MCP-1 and IL-6 are important in the recruitment of monocytes/macrophages to the site of inflammation as well as in the differentiation of naive CD4+ cells to Th17 cells, which contribute to the clearance of pneumococcal infection (72).
The in vivo role of NOD2 during pneumococcal infection was first characterized by Hommes and colleagues. After intranasal infection with 107 colony-forming units (CFUs) of S. pneumoniae (D39 strain), IL-6 and MIP-2 levels were elevated in the lung homogenates of NOD2-deficient mice at 24 and 48 hours after infection compared with those of wild-type (WT) mice. This increase in proinflammatory cytokines and chemokines did not correlate with increased neutrophil recruitment or improved bacterial clearance, as evidenced by similar bacterial loads in the blood, lung homogenates, and spleen between the NOD2-deficient mice and the WT mice at 6, 24, and 48 hours after infection. Likewise, there was no appreciable difference in histologic lung inflammation between the two groups, leading the authors to conclude that there was no clear role of NOD2 in the in vivo host defense against pneumococcal pneumonia (73). It is plausible that the lack of appreciable difference between the two groups in this study was caused by redundant roles of the PRRs. TLR2 plays a well-established role in the clearance of pneumococcal infection. Davis and colleagues showed that mice lacking both TLR2 and NOD2 receptors were unable to clear S. pneumoniae 21 days after inoculation, whereas mice lacking only one of these receptors were able to clear the colonization without difficulty (72). These findings suggest that NOD2 contributes to the proinflammatory cytokine response after infection with S. pneumoniae, but that its role is redundant. The TLR2 receptor is able to compensate in the absence of NOD2, leaving the host without obvious vulnerability to this pathogen.
C. Pneumoniae
C. pneumoniae is a gram-negative obligate intracellular pathogen that is estimated to cause ∼10% of CAP cases each year (74). C. pneumoniae can reside intracellularly for prolonged periods because of its unique life cycle. The bacterium enters the host cell as an elementary body via phagocytosis, but, unlike most phagocytosed organisms, it does not undergo fusion with lysosomes, with subsequent destruction. Instead, the elementary body transforms into a reticulate body and begins replication. C. pneumoniae is recognized by the innate immune system, triggering several PRRs, including TLR2, TLR4, NOD1, and NOD2 (42, 47). In an in vivo murine model, Shimada and colleagues demonstrated that mice deficient in the downstream adaptor protein of NOD1 and NOD2, RIP2, had impaired early chemokine expression and decreased polymorphonuclear neutrophil (PMN) recruitment on Day 3 after infection with C. pneumoniae. This delayed PMN response correlated with a higher bacterial burden on Days 5 and 14 after infection (42). In response to the higher bacterial burden in these mice, which lacked an intact NOD signaling pathway, there was a delayed and exaggerated production of cytokines and chemokines, with a subsequent increase in PMN recruitment that correlated with more severe lung inflammation, which persisted for up to 35 days after infection and was associated with increased lethality compared with WT mice (42). Shimada and colleagues went on to demonstrate that the upstream receptors of RIP2, NOD1, and NOD2 play a role in the host recognition of C. pneumoniae, because mice deficient in NOD1 or NOD2 exhibited delayed bacterial clearance after C. pneumoniae infection (42).
Legionella Pneumophila
Legionella pneumophila is a gram-negative facultative intracellular organism estimated to account for ∼4% of CAP cases (75). Legionellosis can result in severe pneumonia; of those cases of reported legionellosis from 2011 through 2013, ∼40% had severe symptoms requiring intensive care unit admission, and 9% resulted in death (76). L. pneumophila replicates intracellularly and uses the Dot/Icm Type IVB secretion system to deliver bacterial proteins into the host cell cytoplasm, where they are recognized subsequently by NLRs and contribute to cytokine/chemokine production and the subsequent neutrophil recruitment response (77). Frutuoso and colleagues established that the dual deficiency of both NOD1 and NOD2 in a mouse model resulted in reduced neutrophil recruitment to the lung at 9 hours after infection with L. pneumophila. In concordance with this finding, mice deficient in RIP2, the downstream kinase of both NOD1 and NOD2, demonstrated an increase in pulmonary bacterial load on Days 2 and 4 after infection, although this increase was relatively small and of unclear clinical significance (78). However, the deficiency of NOD1 or NOD2 alone did not hamper the animal’s ability to clear the infection, as evidenced by similar pulmonary bacterial loads.
Berrington and colleagues documented similarly that mice deficient in either NOD1 or NOD2 had no significant differences from WT mice in L. pneumophila bacterial clearance at 4 and 24 hours after infection (79). It was not until 72 hours after infection that the authors identified an increase in bacterial CFUs in NOD1-deficient mice, although this difference was small and of unclear clinical significance (79).
Berrington and colleagues showed an impaired neutrophilic response in NOD1-deficient mice at 4 and 24 hours after infection (79). However, unlike Frutuoso and colleagues (78), they found that the blunted recruitment of neutrophils in NOD1-deficient mice was associated with significant improvement in histologic inflammation at 24 hours after infection, although this improvement was transient because it was no longer significant at 72 hours after infection. Interestingly, the NOD2-deficient mice had an increased neutrophilic response at 24 hours after infection, which did not correlate with a change in histologic inflammation (79).
The lack of completely concordant results between the above-mentioned studies may be related in part to the fact that multiple PRRs, not only NOD, contribute to the innate host response to L. pneumophila. The TLR proteins, namely, TLR2, TLR5, and TLR9, have also been identified as contributors of host defense against L. pneumophila (80). Furthermore, the flagellin protein of L. pneumophila is sensed by the NLR protein NAIP5, which goes on to promote the physical interaction of NAIP5 with NLRC4 and subsequent inflammasome activation with cleavage of caspase-1 and production of IL-1β and IL-18 (81).
RIP2 and MyD88, the downstream adapter proteins of the CARD-containing NODs and TLRs, respectively, both participate in the activation of NF-κB and play a cooperative role in cytokine/chemokine production and cellular recruitment after infection with L. pneumophila. To better understand the relative contributions and potential interactions of each of these PRRs in the host’s response to infection with L. pneumophila, Archer and colleagues evaluated the immune response after intranasal infection with WT L. pneumophila, as well as a flagellin-deficient strain of L. pneumophila to circumvent activation of the NAIP5-NLRC4 pathway (82). These two strains of L. pneumophila were administered to WT mice, as well as to mice deficient in the downstream adaptor protein to the TLRs, MyD88; mice deficient in the downstream adaptor protein to both NOD1 and NOD2, RIP2; and mice deficient in both MyD88 and RIP2 (82). Their group established that mice that lacked signaling through NOD1 and NOD2 as a result of RIP2 deficiency did not experience hindered bacterial clearance or host susceptibility. When RIP2-deficient mice underwent infection with the flagellin-deficient strain of L. pneumophila, thus bypassing the innate immune activity of NAIP5-NLRC4 as well, they continued to retain their ability to clear bacteria from the lung via MyD88 signaling alone. This finding suggests that TLR signaling via MyD88 plays a prominent role in pulmonary bacterial clearance of L. pneumophila. However, in the setting of MyD88 deficiency, NOD1 and NOD2 signaling through RIP2 plays an important role in host survival (82). Together these findings indicate that the principle PRR response to L. pneumophila is the TLR response, yet the individualized roles of the other PRRs, including NOD1 and NOD2, contribute to the successful immune response to L. pneumophila.
S. Aureus
S. aureus, including methicillin-resistant S. aureus, is one of the leading causes of pneumonia in health care–exposed patients. S. aureus is an extracellular gram-positive organism that is recognized by NOD2 as well as the TLRs. Kapetanovic and colleagues showed that in TLR2-deficient mice exposed to S. aureus, adequate cytokine production relied on intact phagocytosis, so that intracellular sensing of whole microorganisms could activate the NOD pathway (83). Interestingly NOD1, although it does not interact with gram-positive PGN directly, also appeared to play a role in the activation of S. aureus–mediated NF-κB activity via the modulation of NOD2 activity (83). Further in vivo studies by Kapetanovic and colleagues demonstrated that NOD2-deficient mice infected with 109 CFUs of intranasal S. aureus had a decreased inflammatory response, as evident by fewer inflammatory cells (neutrophils and leukocytes) in the BAL fluid at 48 hours after infection, as well as by decreased levels of inflammatory cytokines (IL-1β, IL-6, IL-10, and IFN-у) and chemokines (MIP-2 and keratinocyte chemoattractant) at the same time point (53). The associated bacterial load in the BAL fluid at this time point was decreased compared with that in WT mice, but this improvement in bacterial clearance was not observed in the lung homogenate samples, which also failed to show a change in cytokine/chemokine concentrations between the two groups of mice. NOD2-deficient mice developed less severe histologic pulmonary lesions, but despite this, they had survival curves similar to those of WT mice (53). One limitation of this study is that C57BL/6 mice are relatively resistant to S. aureus, rapidly clearing the pathogen; thus, this experiment required a high infectious dose (109 CFUs), which may limit its clinical relevance.
M. Tuberculosis
M. tuberculosis is an intracellular pathogen against which NOD-mediated innate immunity plays a significant role in host defense. M. tuberculosis is responsible for one of the most prevalent infections in the world. It has been well recognized that multiple TLRs serve a purpose in the recognition of M. tuberculosis. Recent research by Ferwerda and colleagues demonstrates a NOD-specific function that is nonredundant to TLR activation but can act in synergy with the TLRs to promote a strong inflammatory response (52). Brooks and colleagues showed that the knockdown of NOD2 expression via siRNA in human macrophages resulted in an increased intracellular bacterial burden (84). Likewise, Divangahi and colleagues showed that NOD2-deficient alveolar macrophages and NOD2-deficient mice exhibit reduced cytokine production in the setting of M. tuberculosis (85). In vivo NOD2 deficiency was not only associated with an impaired innate immune response as evidenced by the reduction in cytokines, but also led to altered adaptive immunity, which was associated with increased pulmonary bacterial burden at 6 months after infection, as well as increased lethality of chronic mycobacterial infection compared with WT mice (85).
A. Baumannii
A. baumannii is a gram-negative pathogen known to cause nosocomial pneumonia, which is often drug resistant and associated with high mortality. A. baumannii is an extracellular pathogen that can invade and survive intracellularly in lung epithelial cells and macrophages. As with many other gram-negative bacteria, TLR signaling has been identified after A. baumannii infection, but only recently was it recognized that in vitro infection of the human lung epithelial cell line A549 with A. baumannii resulted in up-regulation of RIP2 expression and activation of NF-κB (44). Furthermore, knockdown of NOD1 and NOD2 by siRNA in lung epithelial cells resulted in increased intracellular bacterial loads. This finding was cell type specific, because similar knockdown experiments in THP1 cells and in bone marrow–derived macrophages from NOD2 knockout mice did not show a change in intracellular survival or bacterial levels (44).
Conclusions
There is now significant research demonstrating the important roles of NOD1 and NOD2 in the recognition of bacterial and viral pathogens. This recognition drives the host’s innate response via the activation of the NF-κB and IRF3 pathways, respectively, and also potentially aids in shaping the adaptive host defense. From the currently available research on bacterial pneumonia, it is clear that in some bacterial infections, specifically C. pneumoniae, M. tuberculosis, and A. baumannii, the complete deficiency of NOD1 or NOD2 results in fewer inflammatory cell infiltrates and a higher bacterial burden in the lungs, suggesting that the presence of NOD signaling is crucial to the host defense against these pulmonary pathogens. Conversely, in S. pneumoniae and L. pneumophila infection, the proinflammatory response of NOD appears to be redundant to that of the TLRs, and NOD deficiency has little influence on the host’s ability to fight the infection (Table 1). What drives the interaction between the TLRs and the NLRs remains poorly understood, and future studies will be crucial to enhance our comprehension of the regulatory networks that control the dominant PRR response after infection.
The available data suggest that in viral pneumonia, complete deficiency of NOD2 results in impaired early viral clearance and increased lethality. Some of these poor outcomes may be related to the dwindled population of antigen-presenting dendritic cells and the diminished ability to prime the adaptive immune response. Interestingly, increased NOD2 expression after viral infection may provide a potential explanation for the increased immunopathology and lethality of bacterial superinfections that follow viral infection. Thus, although it is evident that the complete absence of NOD signaling can be detrimental to the host, these findings would suggest that too much NOD signaling is also detrimental. Exactly how NOD signaling and the innate immune response are regulated and what upstream modulators control the positive and negative regulation of this signaling pathway are still to be determined. Many questions remain to be answered, including whether it is possible to modulate the activity of NOD and the other PRRs to minimize immunopathology while allowing adequate pathogen control. Recent work has established the ability of nanoparticle delivery of NOD ligands to promote immunostimulatory properties including the up-regulation of proinflammatory cytokines (86). It remains to be seen whether a similar approach can be used to down-regulate this signaling pathway, which could potentially improve patient outcomes by decreasing the likelihood of innate immunity-mediated immunopathology after pulmonary infection.
Acknowledgments
Acknowledgements
The authors thank Jacqueline Schaffer, Master of Associated Medical Sciences, for assistance in figure illustration.
Footnotes
Originally Published in Press as DOI: 10.1165/rcmb.2016-0375TR on February 3, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, Reed C, Grijalva CG, Anderson EJ, Courtney DM, et al. CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heron M. Deaths: leading causes for 2014. Natl Vital Stat Rep. 2016;65:1–96. [PubMed] [Google Scholar]
- 3.File TM, Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122:130–141. doi: 10.3810/pgm.2010.03.2130. [DOI] [PubMed] [Google Scholar]
- 4.Coates BM, Staricha KL, Wiese KM, Ridge KM. Influenza A virus infection, innate immunity, and childhood. JAMA Pediatr. 2015;169:956–963. doi: 10.1001/jamapediatrics.2015.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng DS, Han W, Chen SM, Sherrill TP, Chont M, Park GY, Sheller JR, Polosukhin VV, Christman JW, Yull FE, et al. Airway epithelium controls lung inflammation and injury through the NF-κB pathway. J Immunol. 2007;178:6504–6513. doi: 10.4049/jimmunol.178.10.6504. [DOI] [PubMed] [Google Scholar]
- 6.Underdown BJ, Schiff JM. Immunoglobulin A: strategic defense initiative at the mucosal surface. Annu Rev Immunol. 1986;4:389–417. doi: 10.1146/annurev.iy.04.040186.002133. [DOI] [PubMed] [Google Scholar]
- 7.Jacquot J, Puchelle E, Zahm JM, Beck G, Plotkowski MC. Effect of human airway lysozyme on the in vitro growth of type I Streptococcus pneumoniae. Eur J Respir Dis. 1987;71:295–305. [PubMed] [Google Scholar]
- 8.Ellison RT, III, Giehl TJ. Killing of gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest. 1991;88:1080–1091. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartshorn KL. Role of surfactant protein A and D (SP-A and SP-D) in human antiviral host defense. Front Biosci (Schol Ed) 2010;2:527–546. doi: 10.2741/s83. [DOI] [PubMed] [Google Scholar]
- 10.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 12.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 13.Takeda K, Akira S. Toll-like receptors. Curr Protoc Immunol. 2015;109:14.12.1–14.12.10. doi: 10.1002/0471142735.im1412s109. [DOI] [PubMed] [Google Scholar]
- 14.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 16.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 17.Girardin SE, Jéhanno M, Mengin-Lecreulx D, Sansonetti PJ, Alzari PM, Philpott DJ. Identification of the critical residues involved in peptidoglycan detection by Nod1. J Biol Chem. 2005;280:38648–38656. doi: 10.1074/jbc.M509537200. [DOI] [PubMed] [Google Scholar]
- 18.Tanabe T, Chamaillard M, Ogura Y, Zhu L, Qiu S, Masumoto J, Ghosh P, Moran A, Predergast MM, Tromp G, et al. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J. 2004;23:1587–1597. doi: 10.1038/sj.emboj.7600175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inohara C, Chamaillard, McDonald C, Nuñez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 20.Duncan JA, Bergstralh DT, Wang Y, Willingham SB, Ye Z, Zimmermann AG, Ting JP. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc Natl Acad Sci USA. 2007;104:8041–8046. doi: 10.1073/pnas.0611496104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 22.Inohara N, Koseki T, del Peso L, Hu Y, Yee C, Chen S, Carrio R, Merino J, Liu D, Ni J, et al. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-κB. J Biol Chem. 1999;274:14560–14567. doi: 10.1074/jbc.274.21.14560. [DOI] [PubMed] [Google Scholar]
- 23.Opitz B, Püschel A, Schmeck B, Hocke AC, Rosseau S, Hammerschmidt S, Schumann RR, Suttorp N, Hippenstiel S. Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J Biol Chem. 2004;279:36426–36432. doi: 10.1074/jbc.M403861200. [DOI] [PubMed] [Google Scholar]
- 24.Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, Ogura Y, Kawasaki A, Fukase K, Kusumoto S, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 25.Girardin SE, Boneca IG, Carneiro LA, Antignac A, Jéhanno M, Viala J, Tedin K, Taha MK, Labigne A, Zähringer U, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 26.Correa RG, Milutinovic S, Reed JC. Roles of NOD1 (NLRC1) and NOD2 (NLRC2) in innate immunity and inflammatory diseases. Biosci Rep. 2012;32:597–608. doi: 10.1042/BSR20120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inohara N, Ogura Y, Nuñez G. Nods: a family of cytosolic proteins that regulate the host response to pathogens. Curr Opin Microbiol. 2002;5:76–80. doi: 10.1016/s1369-5274(02)00289-8. [DOI] [PubMed] [Google Scholar]
- 28.Ogura Y, Lala S, Xin W, Smith E, Dowds TA, Chen FF, Zimmermann E, Tretiakova M, Cho JH, Hart J, et al. Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut. 2003;52:1591–1597. doi: 10.1136/gut.52.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herskovits AA, Auerbuch V, Portnoy DA. Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system. PLoS Pathog. 2007;3:e51. doi: 10.1371/journal.ppat.0030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ismair MG, Vavricka SR, Kullak-Ublick GA, Fried M, Mengin-Lecreulx D, Girardin SE. hPepT1 selectively transports muramyl dipeptide but not Nod1-activating muramyl peptides. Can J Physiol Pharmacol. 2006;84:1313–1319. doi: 10.1139/y06-076. [DOI] [PubMed] [Google Scholar]
- 31.Kaparakis M, Turnbull L, Carneiro L, Firth S, Coleman HA, Parkington HC, Le Bourhis L, Karrar A, Viala J, Mak J, et al. Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell Microbiol. 2010;12:372–385. doi: 10.1111/j.1462-5822.2009.01404.x. [DOI] [PubMed] [Google Scholar]
- 32.Irving AT, Mimuro H, Kufer TA, Lo C, Wheeler R, Turner LJ, Thomas BJ, Malosse C, Gantier MP, Casillas LN, et al. The immune receptor NOD1 and kinase RIP2 interact with bacterial peptidoglycan on early endosomes to promote autophagy and inflammatory signaling. Cell Host Microbe. 2014;15:623–635. doi: 10.1016/j.chom.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Chin AI, Dempsey PW, Bruhn K, Miller JF, Xu Y, Cheng G. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature. 2002;416:190–194. doi: 10.1038/416190a. [DOI] [PubMed] [Google Scholar]
- 34.Park JH, Kim YG, McDonald C, Kanneganti TD, Hasegawa M, Body-Malapel M, Inohara N, Núñez G. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178:2380–2386. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- 35.Pauleau AL, Murray PJ. Role of nod2 in the response of macrophages to toll-like receptor agonists. Mol Cell Biol. 2003;23:7531–7539. doi: 10.1128/MCB.23.21.7531-7539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi K, Inohara N, Hernandez LD, Galán JE, Núñez G, Janeway CA, Medzhitov R, Flavell RA. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 37.Hasegawa M, Fujimoto Y, Lucas PC, Nakano H, Fukase K, Núñez G, Inohara N. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-κB activation. EMBO J. 2008;27:373–383. doi: 10.1038/sj.emboj.7601962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Girardin SE, Tournebize R, Mavris M, Page AL, Li X, Stark GR, Bertin J, DiStefano PS, Yaniv M, Sansonetti PJ, et al. CARD4/Nod1 mediates NF-κB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2001;2:736–742. doi: 10.1093/embo-reports/kve155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayle S, Boyle JP, Sekine E, Zurek B, Kufer TA, Monie TP. Engagement of nucleotide-binding oligomerization domain-containing protein 1 (NOD1) by receptor-interacting protein 2 (RIP2) is insufficient for signal transduction. J Biol Chem. 2014;289:22900–22914. doi: 10.1074/jbc.M114.557900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadikot RT, Han W, Everhart MB, Zoia O, Peebles RS, Jansen ED, Yull FE, Christman JW, Blackwell TS. Selective IκB kinase expression in airway epithelium generates neutrophilic lung inflammation. J Immunol. 2003;170:1091–1098. doi: 10.4049/jimmunol.170.2.1091. [DOI] [PubMed] [Google Scholar]
- 41.Sadikot RT, Zeng H, Joo M, Everhart MB, Sherrill TP, Li B, Cheng DS, Yull FE, Christman JW, Blackwell TS. Targeted immunomodulation of the NF-κB pathway in airway epithelium impacts host defense against Pseudomonas aeruginosa. J Immunol. 2006;176:4923–4930. doi: 10.4049/jimmunol.176.8.4923. [DOI] [PubMed] [Google Scholar]
- 42.Shimada K, Chen S, Dempsey PW, Sorrentino R, Alsabeh R, Slepenkin AV, Peterson E, Doherty TM, Underhill D, Crother TR, et al. The NOD/RIP2 pathway is essential for host defenses against Chlamydophila pneumoniae lung infection. PLoS Pathog. 2009;5:e1000379. doi: 10.1371/journal.ppat.1000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poynter ME, Irvin CG, Janssen-Heininger YM. A prominent role for airway epithelial NF-κB activation in lipopolysaccharide-induced airway inflammation. J Immunol. 2003;170:6257–6265. doi: 10.4049/jimmunol.170.12.6257. [DOI] [PubMed] [Google Scholar]
- 44.Bist P, Dikshit N, Koh TH, Mortellaro A, Tan TT, Sukumaran B. The Nod1, Nod2, and Rip2 axis contributes to host immune defense against intracellular Acinetobacter baumannii infection. Infect Immun. 2014;82:1112–1122. doi: 10.1128/IAI.01459-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balamayooran T, Batra S, Balamayooran G, Cai S, Kobayashi KS, Flavell RA, Jeyaseelan S. Receptor-interacting protein 2 controls pulmonary host defense to Escherichia coli infection via the regulation of interleukin-17A. Infect Immun. 2011;79:4588–4599. doi: 10.1128/IAI.05641-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Masumoto J, Yang K, Varambally S, Hasegawa M, Tomlins SA, Qiu S, Fujimoto Y, Kawasaki A, Foster SJ, Horie Y, et al. Nod1 acts as an intracellular receptor to stimulate chemokine production and neutrophil recruitment in vivo. J Exp Med. 2006;203:203–213. doi: 10.1084/jem.20051229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Opitz B, Förster S, Hocke AC, Maass M, Schmeck B, Hippenstiel S, Suttorp N, Krüll M. Nod1-mediated endothelial cell activation by Chlamydophila pneumoniae. Circ Res. 2005;96:319–326. doi: 10.1161/01.RES.0000155721.83594.2c. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 49.Fritz JH, Girardin SE, Fitting C, Werts C, Mengin-Lecreulx D, Caroff M, Cavaillon JM, Philpott DJ, Adib-Conquy M. Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists. Eur J Immunol. 2005;35:2459–2470. doi: 10.1002/eji.200526286. [DOI] [PubMed] [Google Scholar]
- 50.Nau GJ, Richmond JF, Schlesinger A, Jennings EG, Lander ES, Young RA. Human macrophage activation programs induced by bacterial pathogens. Proc Natl Acad Sci USA. 2002;99:1503–1508. doi: 10.1073/pnas.022649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubino SJ, Selvanantham T, Girardin SE, Philpott DJ. Nod-like receptors in the control of intestinal inflammation. Curr Opin Immunol. 2012;24:398–404. doi: 10.1016/j.coi.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 52.Ferwerda G, Girardin SE, Kullberg BJ, Le Bourhis L, de Jong DJ, Langenberg DM, van Crevel R, Adema GJ, Ottenhoff TH, Van der Meer JW, et al. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 2005;1:279–285. doi: 10.1371/journal.ppat.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kapetanovic R, Jouvion G, Fitting C, Parlato M, Blanchet C, Huerre M, Cavaillon JM, Adib-Conquy M. Contribution of NOD2 to lung inflammation during Staphylococcus aureus-induced pneumonia. Microbes Infect. 2010;12:759–767. doi: 10.1016/j.micinf.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 54.Hisamatsu T, Suzuki M, Reinecker HC, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993–1000. doi: 10.1053/gast.2003.50153. [DOI] [PubMed] [Google Scholar]
- 55.Sabbah A, Chang TH, Harnack R, Frohlich V, Tominaga K, Dube PH, Xiang Y, Bose S. Activation of innate immune antiviral responses by Nod2. Nat Immunol. 2009;10:1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keestra-Gounder AM, Byndloss MX, Seyffert N, Young BM, Chávez-Arroyo A, Tsai AY, Cevallos SA, Winter MG, Pham OH, Tiffany CR, et al. NOD1 and NOD2 signalling links ER stress with inflammation. Nature. 2016;532:394–397. doi: 10.1038/nature17631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roberson EC, Tully JE, Guala AS, Reiss JN, Godburn KE, Pociask DA, Alcorn JF, Riches DW, Dienz O, Janssen-Heininger YM, et al. Influenza induces endoplasmic reticulum stress, caspase-12-dependent apoptosis, and c-Jun N-terminal kinase-mediated transforming growth factor-β release in lung epithelial cells. Am J Respir Cell Mol Biol. 2012;46:573–581. doi: 10.1165/rcmb.2010-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sertl K, Takemura T, Tschachler E, Ferrans VJ, Kaliner MA, Shevach EM. Dendritic cells with antigen-presenting capability reside in airway epithelium, lung parenchyma, and visceral pleura. J Exp Med. 1986;163:436–451. doi: 10.1084/jem.163.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holt PG, Haining S, Nelson DJ, Sedgwick JD. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J Immunol. 1994;153:256–261. [PubMed] [Google Scholar]
- 60.Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, Qin S, Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 61.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 62.Fritz JH, Le Bourhis L, Sellge G, Magalhaes JG, Fsihi H, Kufer TA, Collins C, Viala J, Ferrero RL, Girardin SE, et al. Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity. Immunity. 2007;26:445–459. doi: 10.1016/j.immuni.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 63.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, Kapsenberg ML, de Jong EC. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 64.dos Santos G, Rogel MR, Baker MA, Troken JR, Urich D, Morales-Nebreda L, Sennello JA, Kutuzov MA, Sitikov A, Davis JM, et al. Vimentin regulates activation of the NLRP3 inflammasome. Nat Commun. 2015;6:6574. doi: 10.1038/ncomms7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reed C, Chaves SS, Daily Kirley P, Emerson R, Aragon D, Hancock EB, Butler L, Baumbach J, Hollick G, Bennett NM, et al. Estimating influenza disease burden from population-based surveillance data in the United States. PLoS One. 2015;10:e0118369. doi: 10.1371/journal.pone.0118369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lupfer C, Thomas PG, Kanneganti TD. Nucleotide oligomerization and binding domain 2-dependent dendritic cell activation is necessary for innate immunity and optimal CD8+ T cell responses to influenza A virus infection. J Virol. 2014;88:8946–8955. doi: 10.1128/JVI.01110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu W, Zhang W, Duggan ES, Booth JL, Zou MH, Metcalf JP. RIG-I and TLR3 are both required for maximum interferon induction by influenza virus in human lung alveolar epithelial cells. Virology. 2015;482:181–188. doi: 10.1016/j.virol.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farrag MA, Almajhdi FN. Human respiratory syncytial virus: role of innate immunity in clearance and disease progression. Viral Immunol. 2016;29:11–26. doi: 10.1089/vim.2015.0098. [DOI] [PubMed] [Google Scholar]
- 69.Vissers M, Remijn T, Oosting M, de Jong DJ, Diavatopoulos DA, Hermans PW, Ferwerda G. Respiratory syncytial virus infection augments NOD2 signaling in an IFN-β-dependent manner in human primary cells. Eur J Immunol. 2012;42:2727–2735. doi: 10.1002/eji.201242396. [DOI] [PubMed] [Google Scholar]
- 70.Kim YG, Park JH, Reimer T, Baker DP, Kawai T, Kumar H, Akira S, Wobus C, Núñez G. Viral infection augments Nod1/2 signaling to potentiate lethality associated with secondary bacterial infections. Cell Host Microbe. 2011;9:496–507. doi: 10.1016/j.chom.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmeck B, Zahlten J, Moog K, van Laak V, Huber S, Hocke AC, Opitz B, Hoffmann E, Kracht M, Zerrahn J, et al. Streptococcus pneumoniae-induced p38 MAPK-dependent phosphorylation of RelA at the interleukin-8 promotor. J Biol Chem. 2004;279:53241–53247. doi: 10.1074/jbc.M313702200. [DOI] [PubMed] [Google Scholar]
- 72.Davis KM, Nakamura S, Weiser JN. Nod2 sensing of lysozyme-digested peptidoglycan promotes macrophage recruitment and clearance of S. pneumoniae colonization in mice. J Clin Invest. 2011;121:3666–3676. doi: 10.1172/JCI57761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hommes TJ, van Lieshout MH, van ’t Veer C, Florquin S, Bootsma HJ, Hermans PW, de Vos AF, van der Poll T. Role of nucleotide-binding oligomerization domain-containing (NOD) 2 in host defense during Pneumococcal pneumonia. PLoS One. 2015;10:e0145138. doi: 10.1371/journal.pone.0145138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burillo A, Bouza E. Chlamydophila pneumoniae. Infect Dis Clin North Am. 2010;24:61–71. doi: 10.1016/j.idc.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 75.Carratalà J, Garcia-Vidal C. An update on Legionella. Curr Opin Infect Dis. 2010;23:152–157. doi: 10.1097/QCO.0b013e328336835b. [DOI] [PubMed] [Google Scholar]
- 76.Dooling KL, Toews KA, Hicks LA, Garrison LE, Bachaus B, Zansky S, Carpenter LR, Schaffner B, Parker E, Petit S, et al. Active bacterial core surveillance for Legionellosis - United States, 2011-2013. MMWR Morb Mortal Wkly Rep. 2015;64:1190–1193. doi: 10.15585/mmwr.mm6442a2. [DOI] [PubMed] [Google Scholar]
- 77.Zhu W, Banga S, Tan Y, Zheng C, Stephenson R, Gately J, Luo ZQ. Comprehensive identification of protein substrates of the Dot/Icm type IV transporter of Legionella pneumophila. PLoS One. 2011;6:e17638. doi: 10.1371/journal.pone.0017638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Frutuoso MS, Hori JI, Pereira MS, Junior DS, Sônego F, Kobayashi KS, Flavell RA, Cunha FQ, Zamboni DS. The pattern recognition receptors Nod1 and Nod2 account for neutrophil recruitment to the lungs of mice infected with Legionella pneumophila. Microbes Infect. 2010;12:819–827. doi: 10.1016/j.micinf.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 79.Berrington WR, Iyer R, Wells RD, Smith KD, Skerrett SJ, Hawn TR. NOD1 and NOD2 regulation of pulmonary innate immunity to Legionella pneumophila. Eur J Immunol. 2010;40:3519–3527. doi: 10.1002/eji.201040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Akamine M, Higa F, Arakaki N, Kawakami K, Takeda K, Akira S, Saito A. Differential roles of Toll-like receptors 2 and 4 in in vitro responses of macrophages to Legionella pneumophila. Infect Immun. 2005;73:352–361. doi: 10.1128/IAI.73.1.352-361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, Shao F. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 82.Archer KA, Ader F, Kobayashi KS, Flavell RA, Roy CR. Cooperation between multiple microbial pattern recognition systems is important for host protection against the intracellular pathogen Legionella pneumophila. Infect Immun. 2010;78:2477–2487. doi: 10.1128/IAI.00243-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kapetanovic R, Nahori MA, Balloy V, Fitting C, Philpott DJ, Cavaillon JM, Adib-Conquy M. Contribution of phagocytosis and intracellular sensing for cytokine production by Staphylococcus aureus-activated macrophages. Infect Immun. 2007;75:830–837. doi: 10.1128/IAI.01199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brooks MN, Rajaram MV, Azad AK, Amer AO, Valdivia-Arenas MA, Park JH, Núñez G, Schlesinger LS. NOD2 controls the nature of the inflammatory response and subsequent fate of Mycobacterium tuberculosis and M. bovis BCG in human macrophages. Cell Microbiol. 2011;13:402–418. doi: 10.1111/j.1462-5822.2010.01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Divangahi M, Mostowy S, Coulombe F, Kozak R, Guillot L, Veyrier F, Kobayashi KS, Flavell RA, Gros P, Behr MA. NOD2-deficient mice have impaired resistance to Mycobacterium tuberculosis infection through defective innate and adaptive immunity. J Immunol. 2008;181:7157–7165. doi: 10.4049/jimmunol.181.10.7157. [DOI] [PubMed] [Google Scholar]
- 86.Pavot V, Rochereau N, Primard C, Genin C, Perouzel E, Lioux T, Paul S, Verrier B. Encapsulation of Nod1 and Nod2 receptor ligands into poly(lactic acid) nanoparticles potentiates their immune properties. J Control Release. 2013;167:60–67. doi: 10.1016/j.jconrel.2013.01.015. [DOI] [PubMed] [Google Scholar]