Abstract
Repair of the lung epithelium after injury is a critical component for resolution; however, the processes necessary to drive epithelial resolution are not clearly defined. Published data demonstrate that Foxp3+ regulatory T cells (Tregs) enhance alveolar epithelial proliferation after injury, and Tregs in vitro directly promote type II alveolar epithelial cell (AT2) proliferation, in part by a contact-independent mechanism. Therefore, we sought to determine the contribution of Treg-specific expression of a growth factor that is known to be important in lung repair, keratinocyte growth factor (kgf). The data demonstrate that Tregs express kgf and that Treg-specific expression of kgf regulates alveolar epithelial proliferation during the resolution phase of acute lung injury and in a model of regenerative alveologenesis in vivo. In vitro experiments demonstrate that AT2 cells cocultured with Tregs lacking kgf have decreased rates of proliferation compared with AT2 cells cocultured with wild-type Tregs. Moreover, Tregs isolated from lung tissue and grown in culture express higher levels of two growth factors that are important for lung repair (kgf and amphiregulin) compared with Tregs isolated from splenic tissue. Lastly, Tregs isolated from human lung tissue can be stimulated ex vivo to induce kgf expression. This study reveals mechanisms by which Tregs direct tissue-reparative effects during resolution after acute lung injury, further supporting the emerging role of Tregs in tissue repair.
Keywords: regulatory T cells, Foxp3, acute lung injury, keratinocyte growth factor, alveolar epithelial repair
Clinical Relevance
Epithelial repair is an essential component for optimal resolution from acute lung injury. Foxp3+ regulatory T cells enhance alveolar epithelial proliferation through their expression of keratinocyte growth factor. This study supports an expanding role for Foxp3+ Treg cells in lung tissue repair.
In acute or chronic injury, the lung epithelium is often injured and its normal functions are impeded. Failure to regenerate the lung epithelium contributes to poor resolution and inadequate reparative processes in lung diseases such as acute respiratory distress syndrome (ARDS) and pneumonia, and leads to pulmonary scarring and fibrosis (1). Resolution from injury is not simply relief from injurious stimuli, but rather an actively regulated program involving removal of apoptotic neutrophils, remodeling of matrix, repair of alveolar epithelium, and reabsorption of alveolar edema (2–5).
The epithelial cells involved in repair include local progenitor cells that migrate to the site of injury, where they proliferate and differentiate to repair the epithelial layer. Lung epithelial cells renew slowly in the absence of injury, but are stimulated to self-renew during injury (6, 7). In the alveolar space, type II alveolar epithelial (AT2) cells proliferate both at steady state and in response to injury, whereas type I alveolar epithelial (AT1) cells are generally regarded as nonproliferating (8, 9). Pulse-labeling and lineage-tracing experiments have shown that AT2 cells give rise to AT1 cells during development and in response to injury (8–10).
It has been proposed that the immune system plays an important role in enhancing barrier function and promoting resolution (1, 11, 12). Tregs are a distinct population of CD4+ lymphocytes that are identified by their expression of the transcription factor Forkhead homeobox protein-3 (Foxp3), and suppress or downregulate immune responses (13, 14). Others have shown a central role for Tregs in the resolution of experimental acute lung injury (ALI) (15, 16).
More recently, several groups have shown that Tregs play a role in tissue repair through expression of the growth factor amphiregulin (Areg) (17, 18). In a previous study, we demonstrated that Tregs enhance alveolar epithelial proliferation after injury in vivo and directly promote AT2 proliferation in vitro, in part by a contact-independent mechanism, suggesting that a soluble factor may be important in this process (15). However, the mechanisms by which Tregs facilitate resolution are far from clear.
In the study presented here, we sought to determine whether Treg expression of a growth factor, keratinocyte growth factor (kgf), is a potential mechanism by which Tregs promote lung repair. kgf (also called fibroblast growth factor 7 [Fgf-7]) can be expressed by several cell types (19, 20). One lymphocyte subset, γδ+ T cells, has also been shown to express kgf in the skin and intestine (21, 22). kgf plays an important role in lung repair and mediates protective effects on epithelial cells, as previously described (19, 23–25). Interestingly, human subjects administered recombinant human KGF and then exposed to inhaled lipopolysaccharide (LPS) were found to have an increased phagocytic uptake of bacteria by alveolar macrophages and increased bronchoalveolar lavage (BAL) concentrations of surfactant protein D, matrix metallopeptidase 9 (MMP-9), granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-1Rα (23). These studies led to a clinical trial for recombinant KGF in ARDS (26). Given the increase of Foxp3+ Tregs in the lung after injury and our previous report of a contact-independent effect on AT2 proliferation, we hypothesized that one mechanism by which Tregs promote epithelial repair is through direct expression of kgf.
The data herein demonstrate that Tregs express kgf, and Treg-specific expression of kgf plays a role in enhancing alveolar epithelial proliferation both in vivo and in vitro. Additionally, Tregs isolated from human lungs also expressed KGF and AREG after stimulation. This study enhances our mechanistic understanding of Treg-directed effects on the reparative process during lung resolution and document an emerging role for Foxp3+ Tregs in tissue repair.
Materials and Methods
Mice
C57BL/6J and 129S1/SvlmJ mice (8–12 wk of age) were obtained from The Jackson Laboratory (Bar Harbor, ME) and colonies were maintained at the University of North Carolina. SP-CGFP mice were a gift from Dr. John K. Heath (University of Birmingham) (27). Foxp3DTR and Foxp3EGFP mice were generated by Dr. Alexander Y. Rudensky and Dr. Talal Chatila, respectively (obtained from The Jackson Laboratory) (28, 29). Foxp3EGFP mice express green fluorescent protein (GFP) downstream of the endogenous Foxp3 stop codon, resulting in fluorescence of all Tregs (28). The Foxp3DTR mice express the human diphtheria toxin receptor (DTR) along with GFP, and genes for both DTR and GPF have been inserted into the 3′ untranslated region of the Foxp3 locus (29). Foxp3DTR mice allow for specific elimination of Foxp3+ Tregs in vivo through intraperitoneal administration of diphtheria toxin (DT) (29, 30). This results in selective depletion of Tregs without significant systemic toxicity (30). Fgf7−/− mice (B6;129-Fgf7tm1Efu/J, designated kgf null hereafter) were obtained courtesy of Dr. Wendy Havran and Dr. Elaine Fuchs (20).
Preparation of Mice
Male mice were anesthetized with tribromoethanol before tracheal intubation as previously described (15, 16). The mice were administered LPS or DT, or underwent left-lung pneumonectomy (PNX) as previously described (18). Details regarding the methods used in this study and other procedures are provided in the online supplement.
RNA Isolation and Analysis of Relative Gene Expression
RNA was isolated from snap-frozen cells stored at −80°C using the Zymo Direct-zol RNA MiniPrep with TRI-Reagent (Zymo Research, Irvine, CA) according to the manufacturer’s protocol with the modifications described in the online supplement. TaqMan Universal PCR Master Mix, no AmpErase UNG from ThermoFisher Scientific (Waltham, MA) was used for RT-PCR analysis.
Immunoblot Analysis
Whole-lung samples were homogenized and cells were lysed with radioimmunoprecipitation assay buffer (RIPA buffer; Santa Cruz Biotechnology Inc., Dallas, TX) as previously described (15).
Isolation of WT and kgf Null CD4+CD25+ Tregs, and Adoptive Transfer
Spleens from male and female C57BL/6J or kgf null mice were removed and prepared for single-cell suspensions, and Tregs were isolated as detailed in the online supplement. Adoptive transfer (AdTr) of Tregs (1 × 106) was performed 1 h after LPS treatment or PNX by retro-orbital injection into Treg-depleted Foxp3DTR mice as previously described (30).
AT2 and Treg Coculture Experiments
Single-cell suspensions were labeled for surface markers, and sorting was performed as detailed in the online supplement.
Lymphocyte Culture
Splenic or lung CD4+CD25+ cells were isolated by magnetic-bead separation (Stemcell Technologies, Vancouver, Canada) and cultured as detailed in the online supplement.
Isolation of Lymphocytes from Human Lungs and Immunophenotyping
Human lung tissue was procured through the Cystic Fibrosis Center Tissue Procurement and Cell Culture Core of the Marsico Lung Institute under protocols approved by The University of North Carolina Office of Research Ethics Biomedical Institutional Review Board. Human lymphocytes were isolated from distal human lung tissue from donor lungs that were not accepted for transplantation by enzymatic digestions to obtain single-cell suspensions for flow-cytometry analysis. The methods used are described in detail in the online supplement.
Statistics and Sample Size Calculations
Pairwise comparisons were made by using Student’s t-test or the Mann–Whitney rank sum test. When more than two conditions were compared, P values determined by Kruskal–Wallis ANOVA with post hoc Dunn’s test were used to identify specific differences between groups. Data are expressed as the mean ± SEM. Statistical analysis was performed using GraphPad Prism 5 software (La Jolla, CA). Statistical difference was accepted at P < 0.05.
Results
kgf Increases during Resolution of LPS-Induced ALI, and CD4+Foxp3+ Cells Express kgf
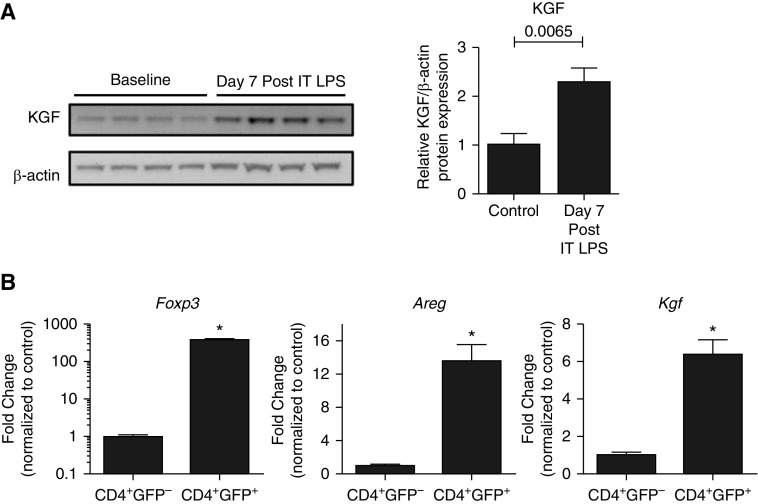
After intratracheal administration of LPS to wild-type (WT) mice, kgf expression increased more than 2-fold by 7 days, as determined by immunoblot analysis of whole-lung lysates, compared with uninjured WT controls (Figure 1A). To determine whether Tregs directly express kgf, single-cell suspensions from enzymatically digested lung tissue from Foxp3EGFP mice were utilized for cell sorting to obtain purified populations of Tregs for transcriptional analysis. CD4+GFP− (CD4+ lymphocyte control) and CD4+GFP+ (Foxp3+ Tregs) cells were sorted 7 days after LPS. RNA was isolated from the two populations for real-time RT-PCR analysis to determine Foxp3, Areg, and Kgf transcription levels normalized to 18s ribosomal RNA. As expected, the CD4+GFP+ sorted cells were greatly enriched in Foxp3 expression. Importantly, these Tregs produced manyfold more Areg and Kgf transcripts compared with CD4+GFP− lymphocyte controls (Figure 1B). These observations indicate that LPS induces kgf expression and Foxp3+ cells express Areg and Kgf.
Figure 1.
Keratinocyte growth factor (kgf) expression increases in the lung during acute lung injury (ALI) resolution. (A) Wild-type (WT; C57BL/6J) mice were challenged with LPS (3 mg/kg) intratracheally (IT), and whole-lung lysates were measured for kgf and β-actin expression by immunoblot analysis 7 days after injury (n = 4 per group, blots representative of two separate experiments). P value determined by Mann–Whitney. (B) CD4+GFP− (CD4+ lymphocyte control) and CD4+GFP+ (Foxp3+ Tregs) cells were sorted from the lungs of Foxp3EGFP mice (cells pooled from > 10 mice) 7 days after LPS administration. Real-time PCR quantification of RNA obtained from both cell populations was used to quantitate forkhead box p3 (Foxp3), Amphiregulin (Areg), and Kgf transcription levels, which were then normalized to 18s ribosomal RNA (n = 3 replicates; data shown are representative of three independent experiments). *P < 0.001 by Student’s t-test. Tregs, regulatory T cells.
Epithelial Proliferation after LPS-Induced ALI Is Impaired in kgf Null Mice
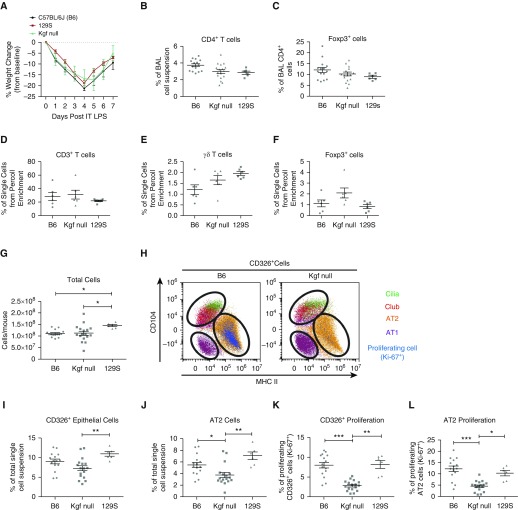
To examine the role of kgf in lung repair, we compared injury and epithelial kinetics during resolution from LPS-induced injury in kgf null mice and two WT strains, C57BL/6J (B6) and 129S1/SvlmJ (129S). These studies were based on previous reports that kgf can affect alveolar innate immune functions and AT2 epithelial proliferation (23–25). The kgf null mice were constructed on a mixed C57BL/6 and 129S background (20). Both WT strains were used to determine potential differences in specific cell populations compared with kgf null mice, as different strains may have variation in epitopes for the antibodies used for immunophenotyping with flow cytometry. The LPS-induced lung injury resolved similarly in all three strains, as measured by recovery of weight loss after injury (Figure 2A). There were no differences among the three strains with regard to the percentages of CD4+ or CD4+Foxp3+ cells in the BAL compartment (Figures 2B and 2C), and no differences in CD3+, γδ+, or Foxp3+ T cell percentages among the three strains after Percoll enrichment for lymphocyte populations in whole-lung single-cell suspensions (Figures 2D–2F). Overall, these observations indicate that all three strains resolved from LPS-induced inflammation similarly, with no difference in the observed percentages in Foxp3+ cells.
Figure 2.
Alveolar epithelial proliferation is markedly impaired in kgf null mice during ALI resolution. C57BL/6J (B6), 129S, and kgf null mice (n = 6 – 17 per group) were challenged with LPS (3 mg/kg) intratracheally and harvested at Day 7 after LPS treatment. (A) Body weight relative to baseline plotted after injury. (B and C) Percentages of alveolar CD4+ and CD4+Foxp3+ cells 7 days after treatment with LPS. (D and E) Single-cell lung suspensions subjected to Percoll gradients for lymphocyte enrichment were then used with flow cytometry to determine the percentages of (D) CD3+ T, (E) γδ+ T, and (F) Foxp3+ cells in each strain. Data are representative of two separate experiments. No significant difference was determined by Kruskal–Wallis ANOVA in the three populations. (G–L) Single-cell lung suspensions were obtained from harvested lungs at Day 7 after LPS treatment for identification of epithelial populations: (G) total lung cell count; (H) representative dot plot labeling the subgating of CD326+ cells and identifying cilia, club, type II alveolar epithelial cells (AT2), and type I alveolar epithelial cells (AT1) cells; and the percentage of lung digest that consisted of (I) CD326+ epithelial cells or (J) AT2 cells. The percentage of cells that were proliferating was identified by Ki-67 expression for (K) epithelial cells (CD326+Ki-67+) and (L) AT2 cells using flow cytometry. Gating and dot-plot results are representative of at least three independent experiments. P values were determined by Kruskal–Wallis ANOVA followed by post hoc Dunn’s test to determine specific differences between groups. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. BAL, bronchoalveolar lavage; MHC, major histocompatibility complex.
To examine potential changes in specific lung epithelial cell populations, we built upon published methods of examining alveolar epithelial populations using multicolor flow cytometry of lung single-cell digests during resolution of lung injury (see Figure E1A in the online supplement for the gating scheme) (15, 31–35). In the single-cell lung suspensions, there was an increase in the total number of cells obtained from the 129S background strain compared with the other two strains (Figure 2G). The percentages of hematopoietic (CD45+), endothelial (CD31+), and CD31−CD45− populations did not differ among the three strains at 7 days after injury (Figure E1B).
The CD31−CD45− population includes epithelial cells, which can be identified using the pan-epithelial marker CD326+ (Figure E1A), as well as all mesenchymal and neural cells (15). Specific epithelial populations (AT1, AT2, club, and cilia cells) can be further delineated in CD326+ cells by using antibodies to the cell-surface proteins CD104, CD24, MHCII, and T1α (Figure 2H; further description in the online supplement and Figure E1A) (15, 35). The lungs of kgf null mice given LPS contained fewer total epithelial cells, as measured by CD326 expression, compared with the 129S strain, but not the C57BL/6 strain (Figure 2I). Importantly, the percentage of AT2 cells was significantly lower in the kgf null strain compared with the two WT strains (Figure 2J). The percentage of AT1 cells was similar among the three strains (Figure E1B). The 129S mice had lower percentages of club and cilia cells compared with the other two strains by this antibody-based identification method (Figure E1B).
To determine the contribution of kgf to epithelial proliferation after injury, the percentages of epithelial cell populations staining for Ki-67, a marker of proliferation, were quantified (Figures E1A and E1B) (36). We previously detected an increase in epithelial cell (CD326+) proliferation (Ki-67+), which peaked 7 days after LPS injury, compared with proliferation in uninjured lungs (15). At 7 days after LPS injury, the kgf null mice had lower percentages of proliferating CD326+ cells and AT2 cells compared with the two WT strains (Figures 2K and 2L). These results indicate that kgf plays a critical role in modulating alveolar epithelial proliferation after injury.
Transfer of kgf Null Tregs into Treg-Depleted Mice Fails to Augment Epithelial Proliferation after LPS-Induced ALI
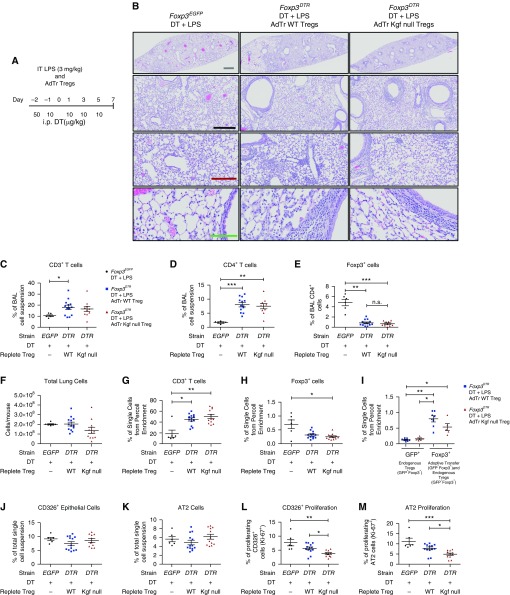
To determine the role of kgf expressed by Tregs in mediating epithelial proliferation, we isolated CD4+CD25+ cells from uninjured spleens of WT or kgf null mice and performed intravenous AdTr into Treg-depleted mice 1 h after exposure to LPS (Figure 3A) (15, 30). Previous studies showed that AdTr of WT Tregs (CD4+CD25+ lymphocytes) into Rag-1−/− mice restored the percentage of proliferating CD326+ Ki-67+ cells back to WT levels, whereas AdTr of CD4+CD25− lymphocytes did not (15).
Figure 3.
Adoptive transfer (AdTr) of WT Tregs, but not kgf null Tregs, augments epithelial proliferation after ALI. (A) Foxp3EGFP (EGFP) or Foxp3DTR (DTR) mice (n = 6–11 per group) were challenged with LPS (Day 0) intratracheally and 50 μg/kg diphtheria toxin (DT) administered intraperitoneally (i.p.) at Day −2, and then 10 μg/kg administered on Days −1, 1, 3, and 5. One hour after LPS challenge, the Foxp3DTR mice received 1 × 106 CD4+CD25+ cells from either C57BL/6J (WT) or kgf null mice, and lungs were harvested on Day 7 after LPS administration. (B) Hematoxylin and eosin (H&E) stain of representative lung sections on Day 7 after LPS, intraperitoneal DT, and AdTr of the designated lymphocyte subsets. Gray scale bar, 1 mM; black scale bar, 500 μM; maroon scale bar, 250 μM; green scale bar, 100 μM. (C–E) Identification of BAL immune cells in Foxp3EGFP, Foxp3DTR AdTr WT Treg, and Foxp3DTR AdTr kgf null Treg mice after treatment with LPS and intraperitoneal DT 7 days after LPS. (C) BAL percentage of CD3+ T cells, (D) CD4+ T cells, and (E) CD4+Foxp3+ cells. (F) Total lung cell count from single-cell suspensions. (G–I) Lung single-cell suspensions were subjected to Percoll lymphocyte enrichment and then used with flow cytometry to determine the percentages of (G) CD3+, (H) Foxp3+, and (I) endogenous Treg (GFP+) versus AdTr Treg and endogenous (Foxp3+) cells. (J–M) Single-cell lung suspensions were obtained from harvested lungs at Day 7 after LPS for identification of epithelial populations. (J) Total CD326+ and (K) AT2 cell percentages along with the percentages of (L) proliferating CD326+ cells (CD326+Ki-67+) and (M) AT2 cells as determined by flow cytometry. P values were calculated by Kruskal–Wallis ANOVA followed by post hoc Dunn’s test to determine specific differences between groups. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. n.s., not significant.
To assess whether kgf expression by Tregs is important for alveolar epithelial proliferation, we studied two AdTr groups. Foxp3DTR mice depleted of endogenous Tregs received either WT Tregs (AdTr WT Treg) or kgf null Tregs (AdTr kgf null Treg) 1 h after LPS treatment. Foxp3EGFP mice given intraperitoneal DT and LPS served as controls.
Histopathology of lung tissue at 7 days after LPS-induced injury demonstrated elevated immune cells in a peribronchial distribution in both the WT and kgf null Treg AdTr groups compared with the Foxp3EGFP control group that was not depleted of Tregs (Figure 3B). In the BAL compartment, there was an increase in the percentage of CD3+ cells in the AdTr WT Treg group, and CD4+ cells also had an increase in percentage in both the AdTr WT Treg and AdTr kgf null Treg groups (Figures 3C and 3D). Foxp3+ cells were decreased in both the AdTr groups compared with the control Foxp3EGFP mice; however, no significant difference between the two AdTr groups was detected in the BAL (Figure 3E).
No difference was seen in the total number of cells obtained from the enzymatic single-cell suspensions 7 days after LPS and AdTr among the three conditions (Figure 3F). The percentages of CD3+ lymphocytes were elevated in both AdTr groups compared with the Foxp3EGFP control mice as measured in Percoll-enriched lymphocyte populations in whole-lung, single-cell suspensions (Figure 3G). No difference was detected in the percentage of Foxp3+ lymphocytes in either the AdTr WT Treg or AdTr kgf null Treg groups in these Percoll-enriched lymphocyte preparations; however, the fact that the Foxp3EGFP mice had a greater percentage compared with the AdTr groups demonstrated the incomplete repletion of Tregs by AdTr (Figure 3H). The AdTr Tregs were present 7 days after administration, as detected by measuring the percentage difference between endogenous GFP+ Tregs and total Foxp3+ Tregs, which includes both endogenous GFP+ and exogenous Foxp3+ cells (Figure 3I). There was no difference in the percentage of γδ+ T cells among the three groups in the Percoll preparations (data not shown). Treg expression of CD103, which was previously demonstrated to retain Tregs on epithelial surfaces during LPS exposure (15), did not differ among the groups (data not shown). All three groups recovered weight after injury and had no difference in mortality after LPS injury (data not shown).
Flow cytometry of lung digests showed no difference in the percentage of CD326+ or AT2 cells between conditions (Figures 3J and 3K), and no difference in other epithelial population percentages (AT1, club, or cilia) was detected at 7 days after injury (data not shown).
Importantly, AdTr of WT Tregs into Treg-depleted Foxp3DTR mice restored the percentage of proliferating CD326+ and AT2 cells back to control levels (Figures 3L and 3M), with no difference in overall CD326+ and AT2 cells (Figures 3J and 3K). These results reinforce the notion that Foxp3+ Tregs have a role in lung epithelial tissue repair, and indicate that Treg expression of kgf, at least in part, promotes proliferation of AT2 cells in this model.
kgf Is Critical to Enhance Epithelial Proliferation after Left-Lung PNX
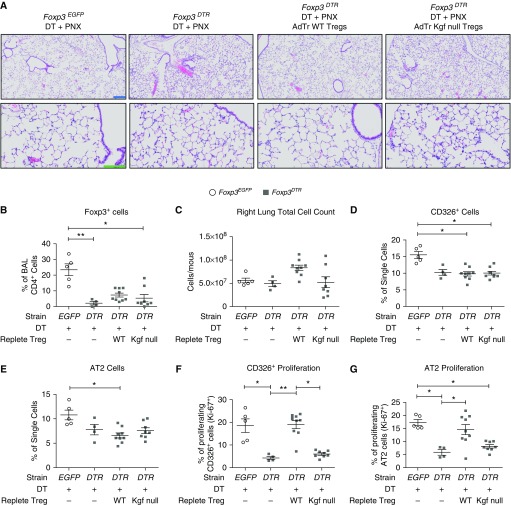
To determine whether Treg expression of kgf can promote epithelial proliferation independently of its ability to dampen alveolar inflammation, we studied lung growth after single-lung PNX. Previous studies showed that after left-lung PNX in mice, contralateral lung growth occurred without significant inflammation (15, 37, 38). Increased levels of epithelial proliferation occurred by 7 days after PNX, and Foxp3+ Tregs were increased in the right lung (15). To further examine the contribution of Treg-expressed kgf to epithelial proliferation in a noninflammatory model of lung growth, we administered DT to Foxp3EGFP and Foxp3DTR mice using the same depletion scheme employed for LPS (Figure 3A) and subjected them to left-lung PNX. The pneumonectomized and DT-treated Foxp3DTR mice were repleted with either WT Tregs or kgf null Tregs 1 h after PNX. There were no differences in markers of inflammation, including BAL total protein and BAL total cell count (data not shown). No histological differences between conditions were detected (Figure 4A). These observations indicate lower levels of barrier disruption and immune cell influx compared with the LPS model. The Treg-depleted PNX Foxp3DTR mice had lower percentages of Foxp3+ cells in the BAL compared with the Foxp3EGFP control group (Figure 4B). Repletion with either WT or kgf null Tregs resulted in similar percentages of BAL Foxp3+ cells (Figure 4B). Repletion was far from complete.
Figure 4.
kgf is critical to enhance epithelial proliferation after left-lung pneumonectomy (PNX). Adoptive transfer (AdTr) of C57BL/6J (WT) Tregs augments epithelial proliferation after left-lung PNX compared with AdTr of kgf null Tregs. Foxp3EGFP (EGFP) or Foxp3DTR (DTR) mice underwent left-lung PNX (Day 0) after intraperitoneal administration of DT (50 μg/kg on Day −2 and then 10 μg/kg on Days −1, 1, 3, and 5). One hour after PNX, the Foxp3DTR mice received either 1 × 106 CD4+CD25+ WT or kgf null cells (or no cells), and the right lung was harvested on Day 7 after PNX. (A) H&E stain of representative lung sections after PNX, intraperitoneal DT, and AdTr of the designated lymphocyte subsets to reveal morphologic changes 7 days after the procedure. Blue scale bar, 200 μM; green scale bar, 100 μM. (B) BAL CD4+Foxp3+ cells were identified by flow cytometry for all four conditions after PNX, DT administration, and AdTr of either WT or kgf null Tregs at 7 days after PNX. (C–G) Single-cell lung suspensions were obtained from harvested lungs on Day 7 after LPS treatment for identification of epithelial populations. (C) Total right-lung cell count, (D) percentages of CD326+ and (E) AT2 single cells, and percentages of (F) proliferating CD326+ cells (CD326+Ki-67+) and (G) AT2 cells were determined by flow cytometry 7 days after PNX (n = 4–9 per group). P values were calculated using Kruskal–Wallis ANOVA followed by post hoc Dunn’s test to determine specific differences between groups. *P ≤ 0.05, **P ≤ 0.01.
No difference in the number of total cells in the right lung was seen, as measured in single-cell lung suspensions (Figure 4C). However, there was a significantly smaller percentage of CD326+ cells in the Foxp3DTR AdTr groups depleted of endogenous Tregs compared with the Foxp3EGFP control (Figure 4D). There was also a decrease in AT2 cells in the AdTr WT Treg group compared with the Foxp3EGFP control (Figure 4E). This decrease in total CD326+ cells in Foxp3DTR depleted mice is due to the elimination of endogenous Tregs (15, 29).
Strikingly, at 7 days after PNX, the mice lacking Tregs (PNX Foxp3DTR and no AdTr) or mice repleted with kgf null Tregs had significantly lower percentages of CD326+ proliferation (CD326+ Ki-67+) and AT2 proliferation when compared with either mice without Treg depletion (Foxp3EGFP control group) or depleted mice repleted with WT Tregs 7 days after PNX (Figures 4F and 4G). Even the partial repletion of WT Tregs fully restored the proliferative capability of CD326+ and AT2 cells. These observations indicate a critical role for Treg expression of kgf in enhancing epithelial proliferation in a noninflammatory model of alveolar epithelial growth that is similar to its role in repair after an inflammatory injury.
Treg Expression of kgf Enhances Proliferation of AT2 Cells In Vitro
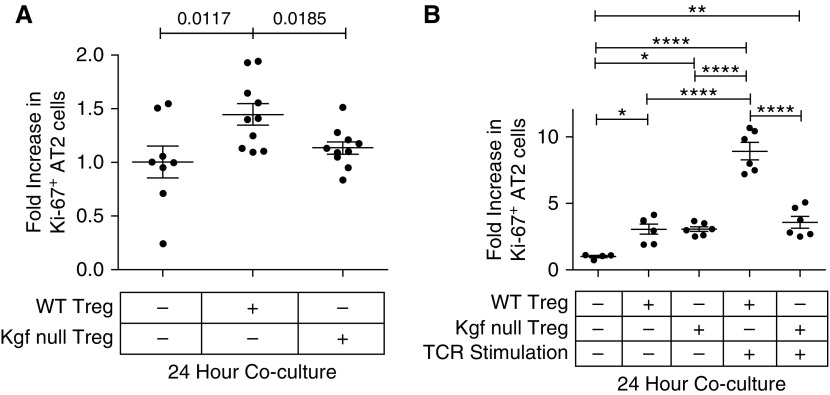
Our previous work demonstrated a direct pro-proliferative effect of Tregs on primary AT2 cells when grown together in vitro (15). To determine whether Treg-expressed kgf could modify epithelial proliferation, we sorted primary AT2 cells from SP-CGFP mice, and cocultured with either WT or kgf null Tregs (CD4+CD25+) at a 1:5 ratio of lymphocytes to AT2 cells similar to previously described (15). AT2 cells cocultured for 24 h with unstimulated WT Tregs had higher rates of proliferation compared with those cultured with kgf null Tregs or AT2 cells grown in media alone (Figure 5A).
Figure 5.
C57BL/6J (WT) Tregs, but not kgf null Tregs, augment epithelial proliferation of AT2 cells in vitro. Primary AT2 cells were isolated by sorting GFP+ cells from SP-CGFP mice and cocultured with either WT or kgf null CD4+CD25+ lymphocytes at a lymphocyte/AT2 ratio of 1:5. The fold increase (compared with media alone) in proliferation (CD326+Ki-67+) was determined after 24 h of coculture of freshly sorted AT2 cells with either purified WT or kgf null CD4+CD25+ lymphocytes isolated from splenocytes. (A) Cocultures of unstimulated lymphocytes and AT2 cells immediately after isolation of both cell types (n ≥ 8, from three independent experiments combined for each condition). P values determined by Mann–Whitney. (B) TCR stimulated (anti-CD3/CD28 beads and IL-2 [500 IU/ml]) or unstimulated WT or kgf null CD4+CD25+ splenic lymphocytes were grown in vitro for 72 h and then cocultured for 24 h with AT2 cells sorted the day of coculture. Data are representative of one of two independent experiments, n ≥ 4 for each condition. P values were determined by Kruskal–Wallis ANOVA with post hoc Dunn’s test used to determine specific differences between groups. *P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.0001. TCR, T-cell receptor.
WT or kgf null Tregs were isolated, grown, and expanded by T cell receptor (TCR) stimulation (anti-CD3/CD28 and recombinant IL-2 500 IU/ml) in vitro for 3 days and then cocultured with freshly sorted AT2 cells for 24 h. AT2 cells cocultured with TCR-stimulated WT Tregs proliferated more than AT2 cells cultured with TCR-stimulated kgf null Tregs (Figure 5B). AT2 cells cocultured with either TCR-unstimulated WT or kgf null Tregs after 3 days of in vitro growth had similar, albeit smaller, increases in rates of AT2 proliferation (Figure 5B). These data demonstrate that Treg-derived kgf expression directly promotes primary AT2 cell proliferation in vitro.
Treg Expression of kgf Varies Depending on the Tissue Source of Treg Isolation
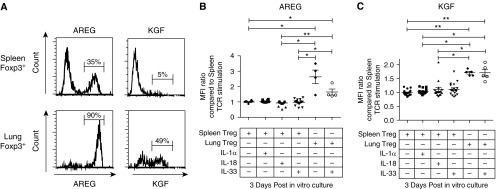
Previous studies have demonstrated that specific cytokines, IL-18 and IL-33, increase Treg expression of the growth factor Areg (17, 18). To assess the potential influence of these cytokines on Treg-derived kgf expression, Tregs were isolated and pooled from the spleens or lungs of mice and then incubated in vitro with the cytokine IL-1α, IL-18, or IL-33 along with TCR stimulation using anti-CD3 and anti-CD28 antibody-coated beads in the presence of IL-2. Quantification of both Areg and kgf was performed using flow cytometry. A larger percentage of TCR-stimulated Tregs isolated from the lung tissue expressed Areg and kgf compared with Tregs isolated from splenic tissue (Figure 6A) after 3 days of in vitro culture.
Figure 6.
The Treg expression levels of the growth factors AREG and KGF after 3 days of in vitro expansion are dependent on the tissue from which the lymphocytes were isolated. CD4+CD25+ lymphocytes were isolated from either lung or splenic tissue and grown in TCR-stimulating conditions (anti-CD3/CD28 beads) and IL-2 (500 IU/ml) in the presence or absence of IL-1α, IL-18, or IL-33 (cytokine concentration: 100 ng/ml). (A) Representative histogram of intracellular staining for AREG or KGF expression in Foxp3+ cells. CD4+CD25+ lymphocytes were first isolated from either lung or splenic tissue and then expanded in vitro by TCR stimulation for 3 days. (B and C) Median fluorescent intensity (MFI) of AREG (B) or KGF (C) in Foxp3+ cells after 3 days of in vitro growth with TCR stimulation, IL-2 (500 IU/ml), and in some conditions the cytokine IL-1α, IL-18, or IL-33 at a concentration of 100 ng/ml. The data combine the results of four independent experiments. P values were determined by Kruskal–Wallis ANOVA with post hoc Dunn’s test used to determine specific differences between groups. *P ≤ 0.05, **P ≤ 0.01.
Culture of Tregs with the cytokine IL-1α, IL-18, or IL-33 along with TCR stimulation did not lead to an increase in Areg or kgf expression when compared with TCR stimulation alone (Figures 6B and 6C). In addition, these cytokines did not lead to an increase in Areg or kgf expression in the absence of TCR stimulation after 3 days of in vitro culture (data not shown). Interestingly, Tregs from lung tissue had a marked increase in expression of both growth factors when compared with Tregs isolated from the splenic tissue from the same mice, as quantified by the median fluorescent intensity using flow cytometry (Figures 6B and 6C). The alarmin IL-33 did not augment expression of either growth factor by either lung or splenic Tregs (Figures 6B and 6C). These results suggest that there is a differential expression pattern for Treg-expressed Areg and kgf depending on the tissue of isolation.
Tregs Isolated from Human Distal Lung Samples Express Areg and kgf with Ex Vivo Stimulation
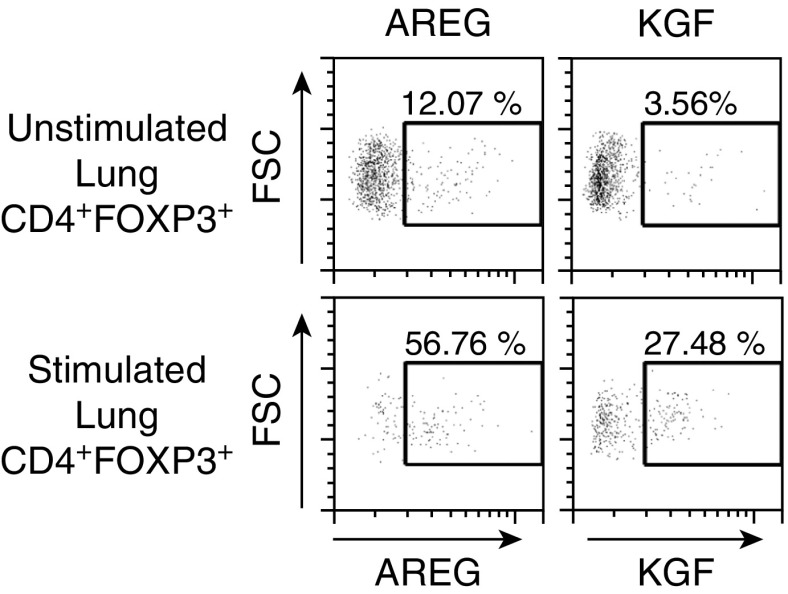
Previous studies demonstrated that Tregs are present in the BAL of humans with ALI (19). To determine whether our findings extend to human Tregs, we sought evidence of AREG or KGF growth factor expression in Tregs present in distal human lung tissue. Human lung tissue was obtained from donor lungs that were deemed not acceptable for transplant, and Tregs were isolated by enzymatic digestion and lymphocyte enrichment. Subsets of these lymphocyte enrichments underwent cell activation for 3 h with phorbol 12-myristate 13-acetate, ionomycin, and brefeldin A before evaluation of AREG or KGF expression by intracellular flow cytometry. Under stimulated conditions, the percentage of CD4+FOXP3+ cells expressing either AREG or KGF increased compared with unstimulated controls (Figure 7). These findings indicate that human Tregs have the ability to express these growth factors under stimulated conditions ex vivo.
Figure 7.
Tregs (CD4+FOXP3+) isolated from human lung tissue and stimulated ex vivo express AREG and KGF. Human lymphocytes were isolated from distal human lung tissue from donor lungs that were deemed not acceptable for transplant by enzymatic digestions and Percoll enrichment for lymphocytes. Lymphocytes were unstimulated or stimulated for 3 h with phorbol 12-myristate 13-acetate and ionomycin in the presence of brefeldin A. CD4+FOXP3+ cells expressing AREG or KGF were identified by flow cytometry. Data are representative of one of three independent experiments for KGF and two independent experiments for AREG. FSC, forward scatter.
Discussion
Earlier studies demonstrated that Foxp3+ Treg cells enhanced alveolar epithelial proliferation in two mouse models of lung regeneration (15). The models were LPS-induced injury, which has been well characterized for eliciting alveolar epithelial damage (15, 39, 40), and left-lung PNX, in which epithelial proliferation and alveolar regeneration occur in the absence of almost any inflammation (15). Furthermore, our previous work demonstrated a direct effect of Tregs in enhancing AT2 cell proliferation in vitro in a contact-independent manner (15). These findings led us to examine soluble factors. Several growth factors are important for lung epithelial repair, with kgf being one of the better-characterized factors (17, 25). Now, in this report we pursue the mechanisms by which Foxp3+ Tregs act, importantly demonstrating that Foxp3+ Tregs express kgf and that Treg-derived kgf enhanced the rate of epithelial proliferation in both models. Additionally, in vitro cocultures of AT2 cell with Tregs lacking expression of kgf failed to augment AT2 proliferation. These findings broaden our understanding of Treg-promoted epithelial repair and reveal the underlying mechanism.
The mechanisms underlying epithelial regeneration after injury involve cellular interactions with extracellular matrix, paracrine and autocrine signaling factors, immune cells, and progenitor cell populations (41–43). The paradigm for alveolar epithelial repair has been that AT1 cells are damaged and AT2 cells undergo hyperplasia, cell spreading, and proliferation at sites of injury to reestablish an intact functional epithelial barrier for gas exchange (10, 44, 45). Recent reports highlight that not only the etiology of the injury but also the severity of the injury may influence the reparative dynamics in the lungs, along with influencing the contribution of certain progenitor populations in epithelial regeneration (35, 42, 43, 46).
Fgfs are a family of signaling molecules that are involved in embryonic development, wound healing, cellular proliferation, and differentiation (47). There are 22 known Fgfs and 4 Fgf receptors (47). kgf (Fgf-7) signals through the Fgfr2b receptor (47). Administration of recombinant kgf in numerous mouse models of lung injury has been found to be protective (for review see Ref. 25). Interestingly, human subjects administered recombinant human KGF and then exposed to inhaled LPS were shown to have increased phagocytic uptake of bacteria by alveolar macrophages and increased BAL concentrations of surfactant protein D, MMP-9, GM-CSF, and IL-1Rα (23). The numerous protective effects demonstrated in animal and human models led to a clinical trial for recombinant KGF in ARDS, the results of which are pending publication (26).
Remarkably, while this work was in progress, Arpaia and colleagues (17) demonstrated that Tregs express another growth factor, Areg, which was found to be important for viral clearance in an influenza model of lung injury. Tregs are not the first lymphocyte population that has been demonstrated to express kgf: previous studies found that intraepithelial γδ T cells can induce epithelial proliferation by expression of kgf (21). To our knowledge, this is the first report to demonstrate kgf expression by Foxp3+ lymphocytes, and to show a role for Treg-specific expression of kgf in promoting repair after ALI.
The AdTr experiments we performed here in both LPS and PNX models demonstrate that a deficiency of Treg-derived kgf expression affects AT2 proliferation. For these studies, we elected to use Tregs obtained from spleens, which are predominantly natural Tregs (nTregs; also known as thymically derived Tregs), because our previous work demonstrated a clear role for them in resolution. Using nTregs also allows the lung microenvironment to generate the tissue-specific effects that influence Treg function during injury. Using nTregs from the spleen provides a purer population of Tregs than would be obtained from the lungs or generated by in vitro induction. The percent purity of Foxp3+ cells in the splenic CD4+CD25+ population is 85–90%, compared with 55–65% in the lung (16). Importantly, recent published studies by several groups have demonstrated a tissue-specific influence on the transcriptional prolife of Foxp3+ Tregs (17, 18). However, the contribution of nTregs and induced Tregs may vary in both the immune-suppression and tissue-repair functions of Foxp3+ cells.
In the initial study of Foxp3DTR mice in which Tregs were selectively depleted, the lack of Tregs led to expanded immune-cell subsets, including CD4+ and CD8+ lymphocytes (29). Notably, in our studies, histology sections of both AdTr groups showed an increase in immune cells around airways and blood vessels compared with the Foxp3EGFP controls. This increase in immune cells was further quantitated by flow-cytometric measurements, which documented an increased percentage of CD3+ T cells in lung single-cell suspensions for the AdTr groups compared with Foxp3EGFP controls. The AdTr of exogenous Tregs did not fully replete the depletion of endogenous Treg in Foxp3DTR mice (Figure 3I) and thus did not prevent the expansion of immune subsets in our experiments. These AdTr results suggest that far fewer Foxp3+ Tregs are required for the production of sufficient kgf to restore epithelial cell proliferation than are needed to induce immunosuppressive effects.
Fgfr2b signaling has been demonstrated to be important for lung repair in an influenza model of lung injury (34). Both kgf and Fgf10 signal through the Fgfr2b receptor (47). Quantius and colleagues (34) also evaluated AT2 proliferation in kgf null mice 7 days after influenza infection (an early time point in such injury) and detected no difference between kgf null and WT mice. These data support the emerging concept that different stimuli induce different host responses, and thus different mechanisms may underlie the reparative processes. Interestingly, Fgf10 has been demonstrated to play a larger role in signaling for cell migration, whereas kgf promotes a more cell-proliferative response through Fgfr2B (47).
A contribution of Treg-expressed kgf to epithelial repair through modulation of another cell type cannot be completely excluded, but the in vitro coculture experiments support a direct interaction and effect of Treg-expressed kgf on AT2 proliferation. A yet-to-be examined possibility that would be in line with our prior contact-independent findings is that Treg-derived microparticles or exosomes that are able to pass through transwell pores may be enriched for growth factors such as kgf or contain RNA, which could enhance proliferation and repair (15). Tregs have been reported to produce more exosomes than other lymphocyte subsets, and packaging of KGF RNA by human mesenchymal stem cell–derived microvesicles has been demonstrated to reduce inflammation in LPS-treated mice (48, 49).
Arpaia and colleagues (17) recently concluded that lung Tregs play a distinct role in tissue repair separate from suppression of inflammation, and further demonstrated that expression of Areg by Tregs is enhanced by the cytokines IL-18 and IL-33, but not TCR stimulation. Our findings differ in that TCR stimulation did enhance Areg and kgf expression in Foxp3+ cells, and the addition of IL-18 or IL-33 did not further augment expression (Figures 5B, 6B, and 6C). There are several possible explanations for these findings. First, the majority of our in vitro experiments were performed using Tregs isolated from spleens, due to the increased number of Foxp3+ cells from this source. However, when lung resident Tregs were isolated, the level of expression of Areg and kgf was higher than that found for splenic Tregs as measured by flow cytometry (Figures 6B and 6C). Areg or kgf expression did not increase further after addition of IL-33 in the presence of TCR stimulation. There are also differences in methodologies between the two studies. For example, we did not add recombinant TGF-β1 or IL-7 to our in vitro cocultures, we used different IL-2 concentrations, we did not stimulate lymphocytes with phorbol 12-myristate 13-acetate and ionomycin before administering brefeldin A, and we did not use the metalloproteinase inhibitor marimastat. These differences likely explain the discrepancies between our results and those of Arpaia and colleagues (17).
Others have demonstrated in studies of experimental muscle injury in mice that a distinct population of Tregs accumulate and function to suppress inflammation, and these muscle-localized Tregs express Areg, which is pro-reparative toward muscle satellite cells (18). The Tregs in the injured muscle tissue were found to be clonally expanded and had a transcriptome distinct from that of other Treg populations (18). Our in vitro studies show that kgf and Areg expression differs in Tregs isolated from uninjured lungs compared with spleens. Given these findings, the reparative response of Tregs appears to be strikingly organ or tissue specific (17, 18).
One potential limitation of this study is that we did not backcross the kgf null mice to the C57BL/6J strain, which is the background strain for Foxp3DTR mice. However, the lack of difference between the two founder strains (C57BL/6J and 129S) in response to LPS-induced injury and subsequent resolution suggests that little strain variation exists between these lines with respect to lung resolution. This notion is supported by a previous study in which kgf null mice were backcrossed several generations to C57BL/6J, and a similar decrease in AT2 cell proliferation was detected without a significant difference in inflammation, as determined by cytokine expression or immune cell percentages in BAL fluid at baseline or after bacterial-induced lung injury (19).
In summary, lung resolution is an active process, and understanding the roles of different cell types and their interactions in the reparative process is critical for intelligent development of therapeutic interventions. Lung Tregs express kgf, and Treg-specific expression of kgf plays a role in promoting alveolar epithelial proliferation both during the resolution phase in an experimental model of lung injury and during regenerative alveologenesis after PNX. In vitro experiments demonstrate that AT2 cells cocultured with kgf null Tregs proliferate less than AT2 cells cocultured with WT Tregs. Moreover, Tregs isolated from the lung express higher levels of two growth factors, kgf and Areg, compared with Tregs isolated from splenic tissue. Lastly, Tregs isolated from human lung tissue can be stimulated ex vivo to induce both AREG and KGF expression. These findings enhance our understanding of Treg-directed effects on the lung epithelial reparative processes during resolution of ALI, and further support the emerging role of Tregs in tissue repair (17, 50).
Acknowledgments
Acknowledgments
We thank the UNC Flow Cytometry Core Facility and Dr. Evan Trudeau for assistance with cell sorting. The UNC Flow Cytometry Core Facility is supported in part by Cancer Center Core Support Grant P30 CA016086 to the UNC Lineberger Comprehensive Cancer Center. Studies with human lung tissue were supported by the Marsico Lung Institute Cystic Fibrosis Tissue Procurement and Cell Culture Core, which is supported by the NIH (P30 DK065988) and the Cystic Fibrosis Foundation (BOUCHE15R0). The SP-CGFP mice were a gift from Dr. John Heath and Dr. Jo Rae Wright, and obtained from Dr. Enid Neptune. We thank Dr. Alexander Rudensky and Dr. Talal Chatila for use of the Foxp3DTR and Foxp3EGFP strains, respectively. We thank Dr. Wendy Havran and Dr. Elaine Fuchs for the kgf null mice (B6;129-Fgf7tm1Efu/J). We thank Dr. Beverly Koller for the 129S strain and helpful discussions. We thank Kim Burns in the Marsico Lung Institute Histology Core for lung histology sections.
Footnotes
This work was supported by grants from the Parker B. Francis Foundation (J.R.M.) and the National Institutes of Health (5K12HL119998 to C.M.D. and 1K08HL129075-01A1 to J.R.M.).
Author Contributions: C.F.D., C.M.D., and J.R.M. conceived and designed experiments, wrote the manuscript, and provided creative input. C.F.D., M.K.T., and J.R.M. performed experiments and analysis.
This article has an online data supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2017-0019OC on March 15, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Mizgerd JP. Respiratory infection and the impact of pulmonary immunity on lung health and disease. Am J Respir Crit Care Med. 2012;186:824–829. doi: 10.1164/rccm.201206-1063PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kieran NE, Maderna P, Godson C. Lipoxins: potential anti-inflammatory, proresolution, and antifibrotic mediators in renal disease. Kidney Int. 2004;65:1145–1154. doi: 10.1111/j.1523-1755.2004.00487.x. [DOI] [PubMed] [Google Scholar]
- 4.Savill J. Apoptosis in resolution of inflammation. J Leukoc Biol. 1997;61:375–380. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- 5.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 6.Giangreco A, Arwert EN, Rosewell IR, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci USA. 2009;106:9286–9291. doi: 10.1073/pnas.0900668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 8.Uhal BD. Cell cycle kinetics in the alveolar epithelium. Am J Physiol. 1997;272:L1031–L1045. doi: 10.1152/ajplung.1997.272.6.L1031. [DOI] [PubMed] [Google Scholar]
- 9.Kauffman SL. Alterations in cell proliferation in mouse lung following urethane exposure. 3. Effects of chronic exposure on type 2 alveolar epithelial cell. Am J Pathol. 1972;68:317–326. [PMC free article] [PubMed] [Google Scholar]
- 10.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BLM. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto K, Ferrari JD, Cao Y, Ramirez MI, Jones MR, Quinton LJ, Mizgerd JP. Type I alveolar epithelial cells mount innate immune responses during pneumococcal pneumonia. J Immunol. 2012;189:2450–2459. doi: 10.4049/jimmunol.1200634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol. 2013;75:593–615. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- 13.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 14.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Mock JR, Garibaldi BT, Aggarwal NR, Jenkins J, Limjunyawong N, Singer BD, Chau E, Rabold R, Files DC, Sidhaye V, et al. Foxp3+ regulatory T cells promote lung epithelial proliferation. Mucosal Immunol. 2014;7:1440–1451. doi: 10.1038/mi.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, Rudensky AY. A distinct function of regulatory t cells in tissue protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner JC, Wu H, Noel JG, Ramser BJ, Pitstick L, Saito A, Nikolaidis NM, McCormack FX. Keratinocyte growth factor supports pulmonary innate immune defense through maintenance of alveolar antimicrobial protein levels and macrophage function. Am J Physiol Lung Cell Mol Physiol. 2016;310:L868–L879. doi: 10.1152/ajplung.00363.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo L, Degenstein L, Fuchs E. Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev. 1996;10:165–175. doi: 10.1101/gad.10.2.165. [DOI] [PubMed] [Google Scholar]
- 21.Boismenu R, Havran WL. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994;266:1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- 22.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, Havran WL. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 23.Shyamsundar M, McAuley DF, Ingram RJ, Gibson DS, O’Kane D, McKeown ST, Edwards A, Taggart C, Elborn JS, Calfee CS, et al. Keratinocyte growth factor promotes epithelial survival and resolution in a human model of lung injury. Am J Respir Crit Care Med. 2014;189:1520–1529. doi: 10.1164/rccm.201310-1892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atabai K, Ishigaki M, Geiser T, Ueki I, Matthay MA, Ware LB. Keratinocyte growth factor can enhance alveolar epithelial repair by nonmitogenic mechanisms. Am J Physiol Lung Cell Mol Physiol. 2002;283:L163–L169. doi: 10.1152/ajplung.00396.2001. [DOI] [PubMed] [Google Scholar]
- 25.Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol. 2002;282:L924–L940. doi: 10.1152/ajplung.00439.2001. [DOI] [PubMed] [Google Scholar]
- 26.Cross LJ, O’Kane CM, McDowell C, Elborn JJ, Matthay MA, McAuley DF. Keratinocyte growth factor in acute lung injury to reduce pulmonary dysfunction—a randomised placebo-controlled trial (KARE): study protocol. Trials. 2013;14:51. doi: 10.1186/1745-6215-14-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo B, Hansen S, Evans K, Heath JK, Wright JR. Alveolar epithelial type II cells induce T cell tolerance to specific antigen. J Immunol. 2008;180:881–888. doi: 10.4049/jimmunol.180.2.881. [DOI] [PubMed] [Google Scholar]
- 28.Haribhai D, Lin W, Relland LM, Truong N, Williams CB, Chatila TA. Regulatory T cells dynamically control the primary immune response to foreign antigen. J Immunol. 2007;178:2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- 29.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 30.Singer BD, Mock JR, Aggarwal NR, Garibaldi BT, Sidhaye VK, Florez MA, Chau E, Gibbs KW, Mandke P, Tripathi A, et al. Regulatory T cell DNA methyltransferase inhibition accelerates resolution of lung inflammation. Am J Respir Cell Mol Biol. 2015;52:641–652. doi: 10.1165/rcmb.2014-0327OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 32.Lee JH, Kim J, Gludish D, Roach RR, Saunders AH, Barrios J, Woo AJ, Chen H, Conner DA, Fujiwara Y, et al. Surfactant protein-C chromatin-bound green fluorescence protein reporter mice reveal heterogeneity of surfactant protein c-expressing lung cells. Am J Respir Cell Mol Biol. 2013;48:288–298. doi: 10.1165/rcmb.2011-0403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujino N, Kubo H, Ota C, Suzuki T, Suzuki S, Yamada M, Takahashi T, He M, Suzuki T, Kondo T, et al. A novel method for isolating individual cellular components from the adult human distal lung. Am J Respir Cell Mol Biol. 2012;46:422–430. doi: 10.1165/rcmb.2011-0172OC. [DOI] [PubMed] [Google Scholar]
- 34.Quantius J, Schmoldt C, Vazquez-Armendariz AI, Becker C, El Agha E, Wilhelm J, Morty RE, Vadász I, Mayer K, Gattenloehner S, et al. Influenza virus infects epithelial stem/progenitor cells of the distal lung: Impact on fgfr2b-driven epithelial repair. PLoS Pathog. 2016;12:e1005544. doi: 10.1371/journal.ppat.1005544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, Tan K, Tan V, Liu FC, Looney MR, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517:621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuylen S, Blaukopf C, Politi AZ, Müller-Reichert T, Neumann B, Poser I, Ellenberg J, Hyman AA, Gerlich DW. Ki-67 acts as a biological surfactant to disperse mitotic chromosomes. Nature. 2016;535:308–312. doi: 10.1038/nature18610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, Crystal RG, Simons M, Sato TN, Worgall S, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsia CC. Signals and mechanisms of compensatory lung growth. J Appl Physiol (1985) 2004;97:1992–1998. doi: 10.1152/japplphysiol.00530.2004. [DOI] [PubMed] [Google Scholar]
- 39.Rojas M, Woods CR, Mora AL, Xu J, Brigham KL. Endotoxin-induced lung injury in mice: structural, functional, and biochemical responses. Am J Physiol Lung Cell Mol Physiol. 2005;288:L333–L341. doi: 10.1152/ajplung.00334.2004. [DOI] [PubMed] [Google Scholar]
- 40.Su X, Looney MR, Gupta N, Matthay MA. Receptor for advanced glycation end-products (RAGE) is an indicator of direct lung injury in models of experimental lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1–L5. doi: 10.1152/ajplung.90546.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rock J, Königshoff M. Endogenous lung regeneration: potential and limitations. Am J Respir Crit Care Med. 2012;186:1213–1219. doi: 10.1164/rccm.201207-1151PP. [DOI] [PubMed] [Google Scholar]
- 42.Zemans RL, Henson PM, Henson JE, Janssen WJ. Conceptual approaches to lung injury and repair. Ann Am Thorac Soc. 2015;12:S9–S15. doi: 10.1513/AnnalsATS.201408-402MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donne ML, Lechner AJ, Rock JR. Evidence for lung epithelial stem cell niches. BMC Dev Biol. 2015;15:32. doi: 10.1186/s12861-015-0082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975;22:142–150. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- 45.Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res. 2001;2:33–46. doi: 10.1186/rr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanegai CM, Xi Y, Donne ML, Gotts JE, Driver IH, Amidzic G, Lechner AJ, Jones KD, Vaughan AE, Chapman HA, et al. Persistent pathology in influenza-infected mouse lungs. Am J Respir Cell Mol Biol. 2016;55:613–615. doi: 10.1165/rcmb.2015-0387LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Francavilla C, Rigbolt KT, Emdal KB, Carraro G, Vernet E, Bekker-Jensen DB, Streicher W, Wikström M, Sundström M, Bellusci S, et al. Functional proteomics defines the molecular switch underlying FGF receptor trafficking and cellular outputs. Mol Cell. 2013;51:707–722. doi: 10.1016/j.molcel.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, Qu JM, Matthay MA, Lee JW. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32:116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, Seabra MC, Wilson MS. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014;41:89–103. doi: 10.1016/j.immuni.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burzyn D, Benoist C, Mathis D. Regulatory T cells in nonlymphoid tissues. Nat Immunol. 2013;14:1007–1013. doi: 10.1038/ni.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]