Summary
Nearly every cell in the human body contains a set of programmable gene-silencing proteins named Argonaute. Argonaute proteins mediate gene-regulation by small RNAs and thereby contribute to cellular homeostasis during diverse physiological process, such as stem cell maintenance, fertilization, and heart development. Over the last decade remarkable progress has been made towards understanding Argonaute proteins, small RNAs, and their roles in eukaryotic biology. Here, we review current understanding of Argonaute proteins from a structural prospective and discuss unanswered questions surrounding this fascinating class of enzymes.
Introduction
Since their discovery over 25 years ago, small RNAs (21–30 nt) have emerged as major regulators of gene expression in nearly all aspects of animal biology. The wide-spread utility of small RNAs in biology arises from the remarkable molecular assemblies in which small RNAs function: the RNA-Induced Silencing Complexes (RISC) [1]. RISC combines the natural capacity of nucleic acids to store and convey complex sequence information with the ability of proteins to fold into highly functional and dynamic molecular machines. The result is a family of fully programmable and highly efficient molecular complexes capable of identifying and silencing essentially any gene.
The functional core of RISC is composed of a small RNA loaded into a member of the Argonaute protein family [2–5]. Argonaute uses the sequence information encoded in the small RNA as a guide to identify complementary RNAs targeted for silencing. RISC is versatile in silencing diverse genes because Argonaute can be loaded with a small guide RNA of essentially any sequence. RISC is also remarkably fast and fastidious in finding its targets, with the ability to identify targets at rates approaching the limit of diffusion while avoiding the off-targets that are highly abundant in the cellular milieu [6]. Silencing mechanisms used by RISC are diverse, as a variety of distinct silencing pathways have evolved around the central ability of Argonaute proteins to rapidly and efficiently identify target RNAs.
The past ten years have seen major advances in structural and mechanistic understanding of the Argonaute proteins. Great strides have been made towards understanding how different forms of Argonaute are programmed through the assembly of RISC, how Argonaute reshapes its RNA guide to enable target searches, how targets recognized by RISC are silenced, and how RISC itself is regulated. In this review, we reflect upon these advances from a structural perspective and look forward to the major open questions and challenges ahead.
Classes of small RNAs and their biogenesis
miRNAs
microRNAs (miRNAs), which are typically 21–23 nt in length, regulate gene expression in eukaryotes and thereby participate in diverse physiological processes, including epithelial regeneration [7], cardiac development and function [8], ovulation [9], and neuronal plasticity [10]. In 1993, the first miRNA, lin-4, was discovered in Caenorhabditis elegans [11, 12]. Subsequently, over 2000 putative miRNAs have been identified in humans. The most evolutionarily conserved of these can be grouped into 87 miRNA families, which have predicted target sites in over half the protein-coding genes in the human transcriptome [13–15]. It is therefore unsurprising that dysregulation of miRNA silencing has been implicated in several pathologies, including diabetes, cardiovascular disease, and various forms of cancer [16].
miRNAs are transcribed endogenously by RNA polymerase II as long primary miRNA transcripts (pri-miRNAs) that contain a stem-loop structure that houses the mature miRNA [17–19]. The Microprocessor, composed of Drosha, an RNase III enzyme, and DGCR8 (DiGeorge syndrome chromosomal region 8, Pasha in flies), a double stranded RNA-binding protein that facilitates pri-miRNA recognition, removes the precursor miRNA (pre-miRNA) hairpin from this stem loop [20–25]. The hairpin is subsequently exported from the nucleus by Exportin-5 [26–28]. This pre-miRNA duplex is then processed by the cytoplasmic RNAse III enzyme Dicer, producing an RNA duplex about 22 nt long with 5′ phosphates and two nucleotide 3′ overhangs on both ends [29–31]. This duplex is loaded into Argonaute [29, 32–34] and one strand is preferentially retained, functioning thereafter as the guide strand for targeting.
siRNAs
Small interfering RNAs (siRNAs) are typically 21nt in length and generated by the cleavage of long double stranded RNAs by Dicer [35]. siRNAs silence extensively complementary target RNAs by endonucleolytic cleavage via a process called RNA interference (RNAi). RNAi was first identified in C. elegans [36], though it has since been demonstrated to be essential for viral defense and protection of the genome from transposable elements in many eukaryotic organisms [37–42]. In mammals, Ago2 is the only Argonaute capable of catalyzing siRNA-directed cleavage of target RNAs [3, 4]. This activity appears to be necessary during mammalian development, as Ago2 deficient mice are embryonic lethal [3]. Moreover, knock-in with a slicer-deficient Ago2 results in death shortly after birth. This observation revealed that miR-451, involved in erythropoiesis, requires endonucleolytic cleavage by Ago2 for maturation [43, 44]. In addition, siRNA mediated cleavage has also proven to be a powerful tool for exogenous gene manipulation [45].
piRNAs
Piwi-interacting RNAs (piRNAs) are the largest and most diverse class of small RNAs in metazoans [46]. Ranging in size from 24–31 nt in length, piRNAs are generated independently of the enzyme Dicer, and associate with members of a germline-specific subclade of the Argonaute family named Piwi [47–51]. piRNAs are initially transcribed as long precursors from defined genetic regions termed piRNA clusters [52, 53]. Precursors are fragmented, loaded into Piwi proteins and then trimmed from the 3′ end to their mature length [54–58]. Additionally, secondary piRNAs are generated from the 5′ end of cleaved piRNA targets, leading to amplification and sharpening of the cellular piRNA pool in a process termed the ping-pong pathway [52, 59]. Piwi proteins and piRNAs play an essential role in maintaining the integrity of the germline genome by repressing transposons via transcriptional or posttranscriptional mechanisms. A role for piRNAs controlling protein-coding genes early in development is emerging as well [60, 61].
The architecture of Argonaute
Small RNA classes possess distinct base-pairing requirements for target recognition as well as unique mechanisms of silencing [62]. These differences are imparted by the structure and dynamics of the Argonaute proteins associated with each small RNA class. Despite their diversity of function, however, all Argonaute proteins share a common global architecture composed of two lobes formed by four globular and two linker domains that together form a central RNA-binding cleft [63–67] (Figure 1, A and B). The N and PAZ (Piwi-Argonaute-Zwille) domains comprise the N-terminal lobe, while the C-terminal lobe contains the MID (middle) and PIWI (P-body-induced wimpy testes) domains. The two lobes are bridged by the L1 and L2 linker domains, which combined form the central cleft that cradles the guide and target nucleic acid molecules [68]. The slicer active site, which is formed by an RNase H-like fold located in the PIWI domain, resides in the center of the central cleft [66] (Figure 1B).
Figure 1. Architecture of Argonaute.

A. Linear schematic of the Argonaute primary sequence. Numbers correspond to residue numbers in human Ago2. Regions that contact the 5′ and 3′ ends of the small guide RNA are indicated. B. Surface representation of human Ago2 bound to an siRNA guide (red). C. Close up view of the binding site for the guide RNA 5′ end. D. Close up view of the seed region prior to target binding shows the majority of the seed splayed out in preparation for pairing to target RNAs. E. Surface representation of Ago2 reveals that only guide nucleotides g2–g4 are fully available for initiating interactions with target RNAs. F. Close up view of the binding site for the guide RNA 3′ end in the PAZ domain.
The guide RNA is held in an extended conformation through the central cleft and is contacted by all domains of the protein [69] (Figure 1B). In keeping with the notion that Argonaute can bind guide RNAs of any sequence, almost all interactions with the protein are mediated through the RNA sugar-phosphate backbone. The 5′-phosphate, which is critical for licensing small RNA entry into the RNA silencing pathway, is buried within a hydrophilic pocket at the interface of the MID and PIWI domains (Figure 1C). The 5′-nt of the guide binds to the MID domain in a manner that renders it unavailable for pairing to complementary target RNAs [70, 71]. This finding is in accordance with observations that the 5′-nt does not contribute to target recognition [72]. However, functional analysis demonstrated that human Argonaute proteins have a preference for 5′ uridines or adenosines [73–75]. This preference appears to be exerted by a nucleotide selectivity loop in the Argonaute MID domain that probes the Watson-Crick face of the 5′-nt [73, 76]. The selectivity loop serves as the only identified sequence-specific contact that Argonaute makes with its guide RNA.
In Argonautes that elicit siRNA or miRNA-mediated silencing, guide (g) nucleotides g2–g7 or g2–g8 form the seed region, which are the most evolutionarily conserved feature of miRNAs in animals and are the most critical for target recognition [77–82]. The base-pairing requirements for Piwi proteins remain less characterized, but current understandings of this pathway as well as future challenges are addressed below. For miRNAs and siRNA guides, g2–g7 are arranged in a helical conformation, largely enforced through contacts with the phosphate backbone, prior to target pairing [64, 65, 69, 83] (Figure 1D). Of these nucleotides, only g2–g4 are openly exposed to the bulk solvent prior to target binding (Figure 1E). The remainder of the guide RNA threads through the central cleft. Nucleotides g14–18 pass through a narrow channel formed between the N and PAZ domains [69]. In contrast to the seed region, helical stacking between these nucleotides is completely disrupted, with several bases facing the interior of the complex. Therefore, the 3′ half of the guide is effectively sequestered within the protein and thereby initially unavailable for pairing with complementary targets. After nucleotide g18, the guide RNA exits the N-PAZ channel and turns to approach a shallow, solvent-exposed binding pocket in the PAZ domain [69]. Interactions with the 3′-end are largely made with the sugar ring and are therefore independent of nucleotide identity [84] (Figure 1F).
Mechanisms for RISC assembly
Accurate and precise selection of the guide-RNA is essential, as once bound, the guide is sequestered stably in Argonaute and can have a lifetime on the order of days to weeks [30, 85, 86]. Only highly specialized processes, outlined below, are known to promote guide RNA release and turnover. It is therefore critical that proper guide strand selection occurs to ensure accurate gene regulation. Indeed, mutations that alter miRNA-biogenesis are associated with diverse forms of human tumors and cancers [87–96].
It is thought that small RNA strand selection occurs in a step-wise fashion, initiated by loading of a small RNA duplex into Argonaute (a complex termed pre-RISC), followed by wedging, in which the N-domain of the protein frays the duplex near the guide 3′ end, and finally ejection of the passenger strand, resulting in the formation of mature RISC [97]. Biochemically, this process is best characterized in flies. Drosophila possess distinct siRNA and miRNA silencing pathways elicited by homologous protein effectors and mechanisms that, although analogous, are functionally discrete [98–102]. These effectors include specialized Argonaute proteins into which miRNAs or siRNAs are preferentially sorted (dmAgo1 and dmAgo2, respectively). Precursor duplexes that contain strong central pairing at guide positions 9–11, as is typical of siRNAs, are sorted into dmAgo2, while central mismatches, a hallmark of pre-miRNA duplexes, are preferred by dmAgo1, thereby conferring specificity [103].
Mammals, conversely, do not possess a robust system for sorting small RNAs [4, 80]. There are four mammalian Argonaute homologs, Agos1-4, that associate with largely redundant pools of small RNAs [3, 4]. All four human Argonaute proteins can incorporate both siRNAs and miRNAs, although there tends to be a preference for duplexes that contain central mismatches, much like the miRNA pathway in flies [104], which is in accordance with the notion that siRNA silencing is less actively utilized by mammals [105].
Although substantial work in flies has revealed fundamental insights into loading of precursor duplexes into Argonaute and maintenance of guide strand selectivity, the structural basis for these processes, as well as a detailed model for the analogous pathway in mammals, still remain elusive. The sizeable technical challenge associated with structural analysis of protein effectors responsible for strand selection has largely contributed to unresolved gaps in understanding of the pathway. However, current models for the various steps of RISC assembly in flies are outlined below, alongside a comparison with what is known about the mammalian system and outstanding questions.
Loading of the precursor duplex and sensing of asymmetry
Small RNA duplexes generated by Dicer necessarily contain two RNA strands. The selection of one of these strands to guide downstream silencing processes is therefore a fundamental step in the miRNA and siRNA pathways. Guide strand selection is not random, but instead influenced by 5′ nucleotide identity and the relative thermodynamic stability of the two ends of the small RNA duplex [74, 75]. All available data suggest that the final determining step in guide selection involves the orientation in which the duplex is loaded into Argonaute, with the guide 5′ end engaged with the MID domain and the 3′ end with the PAZ domain.
In flies, loading of siRNAs into dmAgo2 is dependent upon the RISC-loading complex (RLC), which contains Dicer-2 (Dcr-2) and the dsRNA binding protein R2D2 [106]. Consequently, RNAi is impaired in dcr-2 and r2d2 null flies [100, 107]. The RLC is also involved in small RNA sorting as ablation of R2D2 leads to misloading of endogenous siRNAs (endo-siRNAs) into dmAgo1 [108–110].
Seminal work using a fully reconstituted system showed that the fly RLC is essential for loading of dmAgo2 with siRNAs [111]. Details for the molecular mechanism by which the RLC facilitate loading, however, remain unclear. It has been suggested that the RLC acts as a sensor for siRNA duplex asymmetry [106]. In this model, R2D2 was suggested to associate with the more thermodynamically stable end of the precursor duplex, orienting Dcr-2 at the opposite end and thereby establishing polarity. However, it has also been shown that depletion of R2D2 does not affect strand selection for both endogenous and exogenous siRNAs in flies, indicating that R2D2 is dispensable for sensing asymmetry in vivo [112]. The miRNA-associated Dicer, Dcr-1, and its counterpart Loquacious (Loqs), while suggested to work analogously to Dcr-2 and R2D2 in loading of dmAgo1, are also dispensable for miRNA incorporation [113]. Similarly, the mammalian homologs of Dcr-1 and Loqs, Dicer and TRBP, are not required for asymmetric loading of Argonaute [114, 115]. Therefore, the exact roles the dsRBD proteins play in loading as well as the molecular mechanism by which the RLC participates in sensing precursor polarity remain unclear.
Recent work has suggested that hAgo2 is itself the sensor for asymmetry [116, 117]. In this model, guide selection is driven predominantly by the Ago2 MID domain, and involves both selectivity of the 5′ nucleobase as well as thermodynamic stability and interactions with the 5′ phosphate [116]. The model suggests that distortions to helical stacking resulting from sequestration of the 5′-phosphate in the MID domain are facilitated if the nucleotide is unpaired, and therefore the less thermodynamically stable 5′ end is preferentially bound by Argonaute. Taken together, it is possible that strand selection relies on interplay between facilitation by the RLC followed by sensing of asymmetry and ultimately guide strand selection by Argonaute.
Wedging
Following duplex association, the N-domain of Argonaute destabilizes guide-passenger duplex pairing at the 3′ end of the guide strand [118]. Alanine scanning mutagenesis suggest that the N-domain is essential for passenger release, but is not necessary for initial association of the duplex with Argonaute [118]. Indeed, structures of prokaryotic Argonaute complexes indicate that the N-domain is highly motile and able to shift substantially relative to the rest of the protein in response to the size of the RNA duplex bound within the central cleft [119]. Most notably, even in the most open prokaryotic Argonaute structures, the N-domain is positioned to prevent base pairing beyond guide position 16. Modeling of a fully-complementary duplex onto human Ago2 structures reveals a similar clash, indicating that this structural element may be conserved. Taken together, it is likely that the N-domain moves to promote unwinding of the precursor duplex past g16, a checkpoint that appears to be necessary for subsequent passenger removal.
Passenger strand removal
Passenger strand ejection is promoted by two mechanisms: slicer-dependent cleavage or the by-pass mechanism, which is facilitated by mismatches in the duplex [32]. Perfectly complementary siRNA precursors that are loaded into endonucleolytically active Argonautes will typically employ slicing-dependent ejection, in which the passenger strand is cleaved, destabilizing the duplex. An endoribonuclease complex named C3PO has been suggested to facilitate removal and degradation of the passenger strand [120, 121].
Some Argonaute proteins, however, are not competent for slicing activity. Notable slicing-incompetent Argonautes include human Ago1, Ago3 and Ago4 [3, 4]. Because slicing-incompetent Argonautes cannot cleave their passenger strand these proteins must remove passenger strands via the bypass mechanism. The bypass mechanism is also used when central mismatches, which inhibit endonucleolytic cleavage and are characteristic of miRNA precursor duplexes, are present. The bypass mechanism is facilitated by mismatches in both the seed and supplemental regions of the guide [32, 104]. Notably, complementarity in these regions typically facilitate stable association with target RNAs [122]. Therefore, mismatches in this region may destabilize the ternary complex and promote passenger strand release. The PAZ domain has also been shown to be necessary for unwinding of the precursor duplex, indicating association of the guide 3′ end with Argonaute is likely involved in passenger ejection [123, 124]. The combined observations suggest that passenger strand removal involves an initial fraying of the guide-passenger duplex via wedging by the N-domain, followed by binding of the 3′ end of the guide strand in the PAZ domain, which imparts torsional strain on the paired duplex and results in melting and, ultimately, dissociation.
The role of chaperones in Drosophila RISC assembly
Although Argonaute does not use ATP to bind and cleave target RNAs, ATP hydrolysis has long been associated with establishing RISC activity in cell lysates [125]. For loading, ATP is likely necessary for the function of Hsc70/Hsp90, which has been shown to enable duplex loading [126, 127]. Currently, it is thought that chaperones stabilize a high-energy, RNA-free Argonaute, which, upon loading of a duplex, unwinding and ejection of the passenger strand, becomes stable in its low-energy, guide sequestered conformation [97, 111]. This model is supported by the finding that guide-free Argonaute is exceedingly difficult to isolate and is likely largely unstable [69, 128]. Moreover, unlike duplex loading, both wedging and passenger strand removal do not require ATP [104, 127]. These steps are thus likely facilitated by the intrinsic energy difference between a high-energy, RNA free Argonaute and a mature, guide-bound RISC.
Single molecule studies using purified recombinant components of Drosophila RISC assembly factors demonstrated that in the absence of chaperones, the RLC-siRNA complex only associated with dmAgo2 transiently [111]. Chaperone machinery extends the dwell-time of the RLC on Ago2, likely facilitating transfer of the precursor siRNA duplex, in a manner that requires recognition of the 5′ phosphate on the guide strand by dmAgo2. These studies support a model wherein chaperones stabilize RNA-free Argonaute, facilitating a conformation in which the protein is receptive to stable binding with the RLC. 5′ phosphate recognition then elicits loading of the duplex, after which the complex rolls down the energy landscape through wedging and passenger strand ejection toward its stable and mature form [97, 111].
Insights into assembly of mammalian RISC
As aforementioned, mammalian systems, unlike flies, do not possess stringently divergent pathways for siRNA and miRNA silencing. In addition, the RLC is disposable for loading and only minimally influences strand selection [114, 117, 129]. Efficient loading in mammals does require chaperone activity, however [111]. It appears, therefore, that mammals may utilize a mechanism that more closely corresponds to the miRNA pathway in Drosophila, which is in keeping with the idea that mammals rarely use siRNA-mediated silencing.
The role of chaperones in piRNA biogenesis
Hsp90 and its co-chaperones also play an important role in piRNA biogenesis. Disruption of the Hsp machinery reduces piRNA levels in cells, and can lead to aberrant production of shortened (~16 nt) piRNAs, presumably through defects in the ping-pong pathway [130–133]. In vitro experiments show that inhibition of Hsp90 in silkworm cells impairs piRNA loading as well as 5′ nucleotide selectivity [134]. Thus, all available evidence suggests a model in which Hsp90 and cochaperones interact directly with Piwi and facilitate accurate loading of piRNA precursors [132, 134].
Mysteries surrounding RISC assembly
Major unanswered questions involve understanding the structural basis for chaperone-mediated RISC assembly. It has been suggested that chaperones maintain Argonaute in a semi-folded state prior to guide-loading, in manner analogous to the chaperones acting on nuclear receptors prior to ligand binding [111]. The structural organization of this state is unknown. Indeed, exactly how Argonaute can recognize small RNA features involved in guide strand selection (such as 5′ nucleotide identity, the 5′ phosphate, and thermodynamic asymmetry) while existing in a partially folded state is not clear. A small subset of Drosophila chaperones is sufficient to simulate loading of fly Ago2 [135], but the domains or regions of Argonaute that are contacted by chaperones and which of these interactions are most important for loading is not known. It is also not clear whether or not chaperones act on Piwi proteins, which are loaded with single stranded precursors as opposed to RNA duplexes, in the same or different fashion as Argonautes. Moreover, the means by which chaperone proteins, which themselves are quite globular and bulky [136], engage the RNA-free Argonaute without substantially blocking access to the central cleft, and the extent to which the RNA-free Argonaute is unfolded, remains a mystery.
Argonaute targeting mechanisms
The predominant functional challenge that Argonaute faces is to efficiently and accurately identify complementary targets from the multitude of non-target RNAs within the cell. This feat is essentially the molecular equivalent of a search for a needle in a haystack as most non-target RNAs vastly outnumber any single miRNA target. Moreover, essentially all mRNAs contain within them, due to their length, an abundance of potential off-target binding sites. Mechanistic studies have suggested that Argonaute accomplishes this task by modulating the functional properties of its guide RNA such that it behaves similarly to an RNA-binding protein as opposed to a naked RNA [6]. Indeed, computational, structural, and biochemical studies have shown that various regions in the guide RNA contribute differently to target association, allowing for division of the guide into several functional domains [69, 122, 137].
Argonaute’s use of the seed region allows for efficient target recognition
All available evidence indicates that the seed region (g2–g7 or g2–g8) of small RNA guides is the primary determinant in target finding. It has been suggested that this is largely due to pre-ordering of the seed region, which lowers the entropic cost associated with hybridization [138–140]. In accordance with this idea, the crystal structure of T. thermophilus Argonaute revealed that the guide seed region is displayed in a pre-organized helical conformation [68]. Subsequent structures have found that pre-ordering of at least the 5′ end (g2–g5) of the seed is feature of all prokaryotic and eukaryotic Argonaute proteins studied thus far [64, 65, 67, 83, 141–143]. Moreover, single molecule studies have demonstrated that Argonaute binds substantially faster and remains more stably associated to seed-paired target RNAs than an equivalent naked RNA duplex [6, 144]. Use of a pre-ordered “seed”-like region for searching has been employed by several other RNA-guided proteins, indicating that this is an effective mechanism for nucleotide search processes [145, 146].
Functional studies have also shown that nucleotides within the seed region participate differentially in target searching, indicating that Argonaute actually uses a more complex mechanism in its search than can be explained by simple pre-payment of entropic losses associated with pairing [6]. Specifically, Argonaute’s ability to bind target is far more sensitive to mismatches at guide positions g2–g4 than g5–g6 [6, 144]. Structurally, this finding can be explained by the observation that prior to target binding, only nucleotides g2–g4 are exposed and available for target pairing, with nucleotides beyond g5 occluded by an α-helix (helix-7 in human Ago2) [64, 65] (Figure 1E, 2A). Moreover, structures of eukaryotic Argonaute proteins show that helical stacking in the seed is interrupted by insertion of a hydrophobic residue (I365 in human Ago2, located on helix-7) between the nucleobases of g6 and g7 [64, 65, 83]. Upon stable association with a seed paired target RNA, helix-7 shifts ~4A, releasing the structural constraints responsible for the kink between g6 and g7 [69] (Figure 2C). Helix-7 also docks in the minor groove of the seed-paired duplex, further stabilizing the opened, seed-paired conformation. This docking may serve as an additional assessment for proper pairing, as mismatches or G:U wobbles would distort the minor groove and preclude stable binding of helix-7, leading to a shift back toward the more closed, guide-only conformation and displacement of the mispaired target.
Figure 2. Model for Argonaute targeting.

A. Argonaute searches for target sites, in part via lateral diffusion, along single stranded regions of potential target RNAs. B. Semi-stable interactions are made between target and nucleotides g2–g4 of small RNA guide. C. Stable interactions are made when target (t) nucleotides match the full guide RNA seed (g2–g8). Adenosine nucleotides in the t1 position further stabilize the ternary complex through interactions with a surface pocket in Ago2. Full seed pairing requires movement of a-helix-7, which shifts to dock into the minor grove of the guide-taget duplex (lower panel). D. The shift in helix-7 also opens the central cleft, exposing guide nucleotides in the supplemental region (g13–g16) for interactions with target RNAs.
Target searches are enabled by non-specific interactions with potential target RNAs
It has also been suggested that Argonaute diffuses laterally along potential target RNAs, utilizing nt g2–g4 of the guide RNA to search for potential complementary sites [147] (Figure 2A, B). Binding and cross-linking studies suggest that human RISC associates transiently with single-stranded regions of RNA, and that this association accelerates identification of nearby target sites [148]. Indeed, single-molecule measurements demonstrated that human Ago2 pauses on sites with as little as three consecutive base pairs to the 5′ end of the seed region (g2–g4). Moreover, Ago2 was found to shuttle, via lateral diffusion, between neighboring target sites, effectively increasing its total dwell time on a target RNA [144]. Using one-dimensional diffusion while searching could facilitate efficiency significantly and is also employed by transcription factors and DNA repair and recombination machineries to great effect [145, 149–155]. Argonaute’s ability to laterally diffuse and sample a potential target RNA also helps explain why closely spaced miRNA binding sites silence target mRNAs in a cooperative fashion [137, 156].
Supplemental pairing for recognition of miRNA targets
Approximately 5% of annotated seed-matched human miRNA-target sites also possess complementarity to nucleotides in the miRNA 3′ end [15, 77]. This complementarity is typically located in the “supplemental region” (g13–16) and is thought to enhance seed pairing, especially when complementarity to the seed is imperfect or otherwise weak [77, 137] (Figure 2D). Indeed, biochemical studies have demonstrated that the supplemental region can impart a 2-fold increase in affinity as compared to a target that is restricted to seed-pairing only [122].
Structural studies of human Ago2 have provided insight into the mechanism of supplemental pairing. Upon pairing to the seed and movement of helix-7, Argonaute undergoes a conformational shift that results in a movement of the PAZ domain relative to the MID, PIWI, and N domains [69]. This positional shift is propagated along a hinge that is composed of helix-7 and a portion of the L2 domain, and results in a widening of the N-PAZ channel. This opening is coincident with a rearrangement of the 3′ half of the guide RNA such that g12–g16, which are largely occluded prior to target binding, adopt a helical conformation reminiscent of the seed region (Figure 2D). This observation suggests that targets that contain complementarity to the supplemental region exit the central cleft after pairing to the seed and then repair at this secondary site. This mechanism avoids the entropic costs and topological constraints associated with wrapping the guide and target strands around each other within the central cleft. In accordance with this idea, even in the seed-paired structure the central cleft of Ago2 narrows after g8 and thereby precludes pairing of nucleotides g9–g11 to target RNAs [69]. Moreover, Argonaute shows a decreased affinity for fully complementary targets compared to those containing seed and supplemental pairing [69, 122]. These observations explain why complementarity to g9–g12 is not an evolutionarily conserved feature of animal miRNA target sites [72, 78, 157].
The t1 nucleotide facilitates stable association with targets
Studies of miRNA target sequences in vertebrates have revealed that an adenosine (A) opposite guide nucleotide 1 (position 1 of the target, or t1) is a conserved feature in many seedmatched sites. However, from structural studies it is known that the 5′ nucleotide of the guide is unavailable for pairing. Computational studies have also indicated that t1A nucleotides appear to function independently of the identity of the miRNA g1 nucleotide. Recent crystal structures of human Ago2 bound to various targets revealed a t1A binding pocket formed by the interface of the L2 and MID domains [69, 158] (Figure 2C). This pocket is large enough to accommodate any nucleobase, but direct binding assays have shown that a t1A can contribute to roughly twofold increases in affinity over t1G, C, or U. This preference appears to be facilitated by a network of water-mediated hydrogen-bonds that recognize the N6 amine on adenosine [158]. Single-molecule studies show that t1A does not participate in the initial search, with little influence on rates of target association. However, t1A effectively increases dwell-time, and thereby may function as a molecular anchor that prevents Ago2 from diffusing off of target sites once seed-paired.
miRNA targeting
The cumulative data point to a model in which Argonaute searches for miRNA targets by dividing the seed sequence into two subdomains: an initial, “sub-seed” that Argonaute uses for fast, preliminary searches as it diffuses along a target RNA and which, when paired to a complementary target, promotes conformational changes that license pairing to the remaining seed nucleotides. Proper duplex formation is then stabilized by docking of helix-7 in the minor groove, ensuring fidelity and tight association with the target RNA. Upon stable seed-pairing, additional interactions through the supplemental region or t1A recognition can further increase the dwell time of Argonaute on the target site. This step-wise approach effectively flattens the search energy landscape, allowing Argonaute to identify target RNAs at a rate approaching the limit of diffusion [6, 144].
siRNA targeting
siRNA targeting likely involves a stepwise process similar to searches conducted by Argonaute for miRNA target sites. However, siRNAs are typically perfectly complementary to their targets [45]. Indeed, efficient siRNA-mediated cleavage requires extensive pairing in the seed, central, and supplemental regions [122, 159]. Therefore, after adopting the seed-paired conformation associated with miRNA targeting, RISC is predicted to undergo a series of additional conformational changes when engaging an siRNA target. These conformational changes almost certainly involve widening of the central cleft to allow guide-target pairing at positions g9–g12, as well as release of the siRNA 3′ end from the PAZ domain to allow guide and target to wrap around each other.
piRNA targeting
A recent structure of the silkworm Piwi (Siwi) has revealed that the protein shares an analogous overall structure to that of Argonaute [67]. Like Argonaute, the Piwi protein is bilobed, containing a central RNA binding channel that houses the piRNA guide (Figure 4A). The piRNA 3′ end is similarly bound to the PAZ domain and the 5′ phosphate is recognized in a binding pocket located between the MID and PIWI domains. However, unlike the eukaryotic Argonautes, Siwi utilizes a magnesium that coordinates the phosphates of guide nucleotides 1 and 3. Careful inspection reveals additional structural differences between Siwi and Ago2, which speak to the possibility of distinct functionalities between the Argonaute and Piwi families. Notably, while both proteins display the 5′ end of seed region a pseudo A-form conformation, the piRNA seed is heavily distorted from A-form at the 3′ end of the seed (Figure 4B). Indeed, in contrast to the miRNA bound to Ago2, nucleotides 6 and 7 of the piRNA are completely disordered, indicating that Siwi does not pre-organize the full seed region prior to target binding. These observations raise the intriguing possibility that piRNAs may have base pairing requirements for target recognition that are distinct from those of miRNAs. Indeed, bioinformatic analysis of piRNA cleavage products indicates that Miwi utilizes primary (nt 2–11) and secondary (nt. 12–21) seed regions for target cleavage [160]. Moreover, the central cleft of Siwi appears in a much more open conformation than in Ago2, indicating that piRNAs in Piwi proteins may form more stable extended guide:target duplex structures than miRNAs or siRNAs do in Ago proteins. This notion is consistent with the observation that Siwi employs an RNA helicase, Vasa, as a release factor for cleaved piRNA targets [161]. Thus, although many of the basic targeting principles learned through the study of Argonaute are likely shared with Piwi, current evidence indicates that substantial functional differences exist as well. Outstanding challenges for the future include experimentally determining the target recognition requirements and biochemical properties of Piwi, as well as establishing structures of Piwi bound engages with target RNAs.
Figure 4. Comparison of Argonaute and Piwi structures.
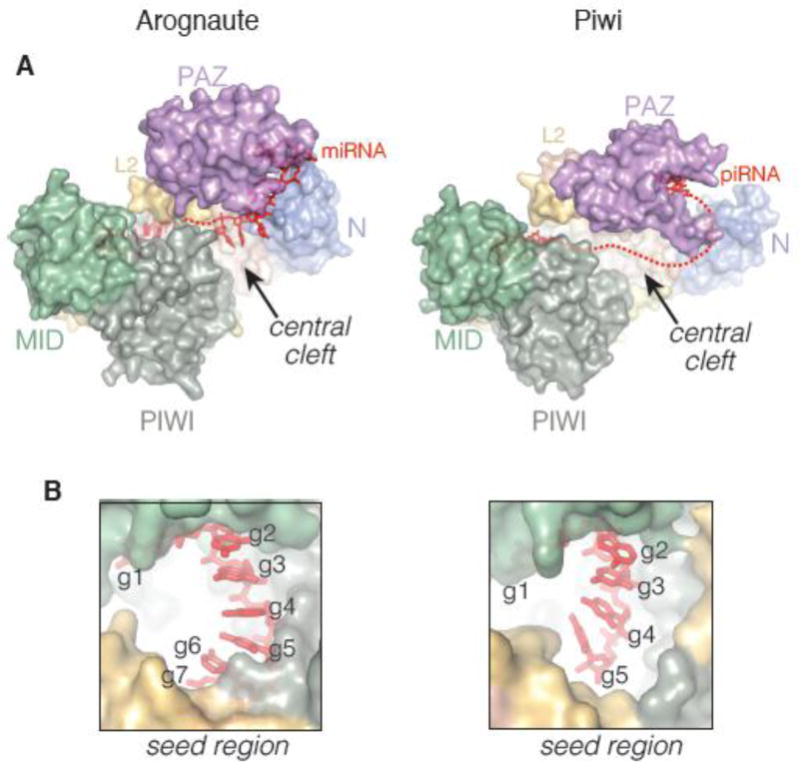
A. Surface representation of human Ago2 (PDB 4W5N, right) alongside silkworm Piwi (PDB 5GUH, left). Both proteins share a common domain architecture. However, differences in the positions of the N and PAZ domains results in a substantially wider and more open central cleft in Piwi. B. The Piwi seed region is less constrained and ordered prior to target binding than the Argonaute seed region.
Outstanding questions surrounding targeting mechanisms
Although many advances in understanding targeting mechanisms by Argonaute have been made, major questions remain unanswered. Argonaute clearly reshapes the binding properties of its small RNA guide [6], but the mechanisms underlying these changes remain poorly defined. For example, helix-7 in Ago2 has been suggested to play a role in modulating seed-pairing [69], but this idea has have yet to be tested. Additionally, although only a small fraction of predicted miRNA target sites contain complementarity outside of the seed region [15, 77, 137], a growing body of literature shows that guide-target interactions beyond the seed play an important role in miRNA target recognition in vivo [157, 162–167]. The structural basis for these interactions, and the functional differences between members of the same miRNA family, remain undefined. Similarly, how Ago2 accommodates extensive guide-target pairing during siRNA targeting remains speculation based on analogy to prokaryotic Argonaute structures, which employ DNA guides to seek DNA targets [119, 168], and thus may or may not accurately reflect siRNA-targeting mechanisms in humans (see below). Finally, the structure of silkworm Piwi shows that the seed region of piRNAs is less pre-organized than the seed miRNA bound to Ago2 [67], suggesting that targeting mechanisms and target recognition requirements of piRNAs may be substantially different than their miRNA cousins. The functional manifestations of these structural differences remain to be determined.
Mechanisms of silencing
Endonucleolytic cleavage of target RNAs is the most direct mechanism of silencing by Argonaute proteins. Alternatively, some Argonautes have evolved to recruit additional silencing factors to identified targets and thereby promote mRNA decay or even induce modifications to chromatin structure.
Endonucleolytic cleavage
The first crystal structure of a full-length Argonaute from Pyrococcus furiosus revealed that the PIWI domain is a structural relative of ribonuclease H (RNase H) [66]. This discovery enabled identification of Argonaute as the endonucleolytic “slicer” responsible for silencing by RNAi [3]. RNase H recognizes DNA-RNA heteroduplexes and uses a conserved catalytic tetrad (DEDD, or Asp-Glu-Asp-Asp) for two-metal hydrolysis of the RNA backbone [169]. However, in initial structures of prokaryotic Argonautes as well as a fungal MID-PIWI domain, only three catalytic residues were identified, leading to the hypothesis that Argonautes possess a catalytic DDX (X: Asp or His) triad [170]. A subsequent structure of yeast Argonaute, however, revealed a fourth catalytic glutamate residue, thereby completing the active site tetrad [64]. These residues coordinate two divalent cations, and, similarly to their RNase H counterparts, utilize two-metal hydrolysis for cleavage of the target RNA (Figure 3A). Argonautes cleave the RNA backbone between nucleotides 10 and 11 (t10 and t11, paired to guide positions 10 and 11) [2].
Figure 3. Structural features of human Ago2.

A. Close up view of the slicer active site in Ago2 reveals a magnesium ion (yellow-orange sphere) coordinated to Ago2 and 4 water molecules (blue spheres). Comparison with T. thermophilus (purple) indicates the magnesium ion is in an inactive position prior to target binding (right panel). B. Tryptophan-binding pockets in the Ago2 PIWI domain. C. Positions of post-translations modifications mapped onto the Ago2 crystal structure. Phosphoration sites are indicated in green. Prolyl hydroxylation site indicted in blue.
A series of structures of T. thermophilus Ago in complex with a guide-target duplex allow for molecular visualization of Argonaute’s catalytic cycle [63, 68, 119, 171]. These groundbreaking contributions had a profound impact on shaping modern understanding of Argonaute function [172]. Under low magnesium conditions, a single magnesium ion was observed, coordinated by the scissile phosphate and two active site residues. It was hypothesized that this initial ion assists nucleophilic attack. Increased magnesium concentration promoted binding of the second ion, which likely facilitates departure of the cleaved phosphate. The coordinated active site closely resembles bacterial RNase H, supporting the shared use of a two-metal-ion hydrolysis mechanism.
In T. thermophilus, extensive pairing induces a conformational shift in which the fourth catalytic residue, a glutamate, inserts into the active site, thereby ensuring specificity of cleavage [64, 171]. All current structures of eukaryotic Argonautes, however, have the equivalent glutamate positioned in the active site irrespective of pairing status, and therefore, whether or not this mechanism of endonucleolytic specificity functions in eukaryotes remains unclear [64, 65, 69, 83]. A structure of human Ago2 in complex with a seed-matched target revealed that a magnesium coordinated in the active site was shifted ~1.5 Å away from the position of the corresponding ion in T. thermophilus Ago (Figure 3A). It is therefore likely that chelating to the scissile phosphate induces a shift in the magnesium into the catalytically active position. The position of the duplex surrounding the scissile phosphate as well as its orientation within the central cleft could influence coordination geometry substantially, thereby providing a mechanism by which target pairing is sensed and specificity of cleavage is maintained. Indeed, structures and domain-swapping experiments with the catalytically inactive mammalian Ago homologs revealed that small structural elements in both the PIWI and N domains substantially contribute to silencing, likely through accurate positioning of the duplex relative to the active site [83, 141, 173].
miRNA-mediated translational repression and mRNA decay
Targets of human miRNAs only very rarely contain enough complementarity to promote efficient cleavage by Ago2 [105]. Instead, the vast majority of miRNA targets are silenced by translational repression, deadenylation, and degradation [174–179], via recruitment of additional silencing factors to the target mRNA by Argonaute [180]. Chief among these silencing factors are members of the GW182 (TNRC6A, B and C in humans) protein family [181–184]. GW182 proteins are proposed to act as flexible structural scaffolds that bridge interactions between Argonaute and downstream effectors [180]. These downstream effectors include the cytoplasmic deadenylase complexes PAN2–PAN3 [185, 186] and CCR4–NOT [187–189], which in turn interacts with the translational repressor and decapping activator DDX6 [190, 191]. The massive complex responsible for mediating repression by miRNAs is termed miRISC [100].
Association of Argonaute and GW182, and thereby nucleation of the miRNA-induced silencing complex, has been shown to be mediated through a multitude of tryptophans located in the N-terminus of GW182 [192–198]. A structure of hAgo2 in complex with tryptophan revealed two putative trp-binding pockets located in the PIWI domain [65] (Figure 3B). Mutational studies show that disrupting areas surrounding these pockets leads to loss of GW182 association, indicating that these pockets likely act as a recognition interface for the tryptophans located in the ABD [199–202].
Unanswered questions surrounding miRNA-mediated repression
Although many of the interactions between individual miRISC components have been mapped at the molecular and structural level [190, 191, 202–210], the assembly process and overall structure of the intact complex remains unknown. GW182 proteins and Argonaute are also known to associate in cytoplasmic foci termed GW-bodies (GWBs) [211, 212]. GWBs appear to be related to P-bodies, cytoplasmic foci that often contain mRNAs not being actively translated and mRNA decay factors [213]. However, disruption of P-bodies in Drosophila S2 cells did not inhibit miRNA-mediated silencing [195, 214]. Therefore, the function of GWBs, if any, in miRNA-mediated silencing processes remains to be established.
MicroRNA turnover
MiRNAs are typically exceedingly stable in the cell, largely due to their sequestration within Argonaute [30, 85, 86, 215, 216]. Indeed, miRNAs bound within Argonaute are largely resistant to nuclease degradation in vitro [217]. Despite their observed longevity, certain biological contexts prompt rapid turnover of some miRNAs in a sequence specific fashion. Some herpes viruses, for example, down regulate host miR-27 upon infection [218–221]. Additionally, some miRNAs are known to endogenously undergo active turnover during situations wherein rapid changes in gene expression are necessary, such as light-dark transitions in retinal cells [222], progression through the cell cycle [223], and T cell differentiation [224]. Neuronal miRNAs are also significantly less stable than those in other, less dynamically regulated cell types [222, 225, 226].
Notably, only specific miRNAs are regulated in the contexts listed above, rather than global regulation of the miRNA pool. Available evidence indicates that sequence specificity is achieved by a process termed target-directed miRNA degradation, or TDMD. In TDMD, binding of certain targets promotes miRNA degradation [85, 108, 219, 220, 227, 228]. TDMD requires specialized targets that evade silencing by miRNAs and instead act as regulators themselves. Indeed, specific base-pairing patterns between the guide and target, which likely influence the resulting complex formed with Argonaute, are required to license a miRNA for turnover [227, 229, 230]. In addition to seed complementarity, such targets also tend to contain strong pairing to the miRNA 3′ end, as well as central mismatches. Moreover, canonical, seed-paired targets cannot induce TDMD and, in fact, do not compromise degradation induced by highly complementary targets [227, 230]. Upon target binding, degradation follows a two-step process in which non-templated nucleotides are added to the miRNA 3′ end (termed “tailing”), followed by 3′–5′ exonucleolytic trimming and, ultimately, removal from Argonaute [108]. These processes occur on the protein and do not result in co-degradation of the target [227].
Unknowns in small RNA decay
The structure of Argonaute immediately reveals that the process of trimming and tailing presents a challenge, as typically, the miRNA 3′ end is bound within the PAZ domain and therefore protected from terminal modifications [65, 69, 83, 141]. Accordingly, binding of a target with extensive 3′ pairing has been suggested to expose the 3′ end in a conformation that leaves it susceptible to attack by modifying enzymes [108]. However, the manner by which TDMD targets facilitate 3′ end exposure remains to be structurally explored. Additionally, the enzymes responsible for trimming and tailing are obscure and it is not clear how many different enzymes act upon the miRNA 3′ end in different cellular contexts. Finally, although non-coding viral RNAs have been shown to promote TDMD [218–221], a natural cellular RNA dedicated to this function has yet to be identified.
Regulation of Argonaute
Alongside turnover of miRNAs, Argonaute itself is regulated by a variety of post-translational modifications (Figure 3C). Phosphorylation of hAgo2 at S387, mediated by either MAPK-activated protein kinase-2 or Akt3, leads to increased localization in P-bodies, a reduction in slicing activity, and an increase in translational repression activity [231, 232]. During the inflammatory response, Tyr-529 on hAgo2 has also been shown to be phosphorylated by an unidentified kinase, resulting in reduced binding to miRNAs [233, 234]. Tyr-529 is located within the 5′-binding pocket and forms a hydrogen bond with the 5′-phosphate of the guide RNA. Therefore, phosphorylation would likely preclude guide RNA binding, thereby explaining this observation. Hypoxic induction of EGFR results in phosphorylation at Tyr-393, which was proposed to inhibit binding of Dicer and loading of precursor duplexes [235]. Recently, CRISPR-Cas9 screening has revealed that hAgo2 undergoes a phosphorylation cycle involving several highly-conserved residues (S824–S834), mediated by the kinase CSNK1A1 and the ANKRD52-PPP6C phosphatase complex [236]. The phosphorylated residues reside on a mobile element, termed the “eukaryotic insertion”, which resides adjacent to the miRNA seed region [201], suggesting they may contribute to interactions with target RNAs. Indeed, analysis of these phosphorylation events suggested that phosphorylation reduces target association. In the absence of phosphorylation, human Ago2 associates with a larger range of target RNAs at steady state, leading to a model wherein phosphorylation promotes timely disassociation of Argonaute from targets, thereby enabling miRISC to function as a multiple-turnover machine [85, 236].
Several studies have also identified additional post-translational modifications that may modulate Argonaute activity. Both cellular stress and viral infection have been shown to induce PARP13-mediated poly-ADP-ribosylation of human Ago2, resulting in repression of miRNA-mediated silencing activities [237, 238]. Prolyl 4-hydroxylation of human Ago2 by type I collagen prolyl-4-hydroxylase has also been implicated in facilitating stability and activity of the protein [239, 240] (Figure 3C).
Lastly, it has been suggested that Argonaute turnover is initiated by ubiquitylation, mediated by TRIM71, an E3 ubiquitin ligase [241]. However, TRIM71 was subsequently shown to directly repress translation of specific mRNAs [242] without influencing Ago2 stability [242–244]. Therefore, the mechanism for regulation of Argonaute ubiquitylation remains to be clarified. Empty Argonautes, unbound to a guide RNA, have also been found to be largely unstable and to undergo autophagy- [245, 246] or proteasome-mediated turnover [247], allowing the cell to coordinate levels of Argonaute protein with miRNA biogenesis.
Open questions surrounding Argonaute regulation
The field of Argonaute regulation is relatively new and unexplored, with many fundamental questions currently unanswered. The mechanism by which Tyr-529, which normally resides buried at the interface between the MID and PIWI domains, is recognized and phosphorylated is a compelling structural mystery. Additionally, although cellular data strongly support the phosphorylation cycle of miRNA targeting [236], the effect of eukaryotic insertion phosphorylation on target affinity has yet to be directly measured. Finally, and perhaps most importantly, how different modifications impact the interaction of Argonaute with other silencing factors and contribute to the overall structure and assembly of miRISC remains an open question.
Acknowledgments
We are grateful for the critical reading of this manuscript by Luca F.R. Gebert. JSG is a Predoctoral Fellow of the American Heart Association. JSG and IJM are supported by NIH grants GM104475, CA201861, and GM115649.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hammond SM, Bernstein E, Beach D, Hannon GJ. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature. 2000;404:293–6. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- 2.Rivas FV, Tolia NH, Song JJ, Aragon JP, Liu J, Hannon GJ, et al. Purified Argonaute2 and an siRNA form recombinant human RISC. Nature structural & molecular biology. 2005;12:340–9. doi: 10.1038/nsmb918. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–41. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 4.Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Molecular cell. 2004;15:185–97. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Martinez J, Patkaniowska A, Urlaub H, Luhrmann R, Tuschl T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell. 2002;110:563–74. doi: 10.1016/s0092-8674(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 6.Salomon WE, Jolly SM, Moore MJ, Zamore PD, Serebrov V. Single-Molecule Imaging Reveals that Argonaute Reshapes the Binding Properties of Its Nucleic Acid Guides. Cell. 2015;162:84–95. doi: 10.1016/j.cell.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chivukula RR, Shi G, Acharya A, Mills EW, Zeitels LR, Anandam JL, et al. An Essential Mesenchymal Function for miR-143/145 in Intestinal Epithelial Regeneration. Cell. 2014;157:1104–16. doi: 10.1016/j.cell.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xin M, Olson EN, Bassel-Duby R. Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nature reviews Molecular cell biology. 2013;14:529–41. doi: 10.1038/nrm3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasuwa H, Ueda J, Ikawa M, Okabe M. miR-200b and miR-429 function in mouse ovulation and are essential for female fertility. Science. 2013;341:71–3. doi: 10.1126/science.1237999. [DOI] [PubMed] [Google Scholar]
- 10.Coolen M, Bally-Cuif L. MicroRNAs in brain development and physiology. Current Opinion in Neurobiology. 2009;19:461–70. doi: 10.1016/j.conb.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 12.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–62. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 13.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome research. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic acids research. 2014;42:D68–73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4 doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lujambio A, Lowe SW. The microcosmos of cancer. Nature. 2012;482:347–55. doi: 10.1038/nature10888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. The EMBO journal. 2002;21:4663–70. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, et al. MicroRNA genes are transcribed by RNA polymerase II. The EMBO journal. 2004;23:4051–60. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai X, Hagedorn CH, Cullen BR. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA (New York, NY) 2004;10:1957–66. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–9. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 21.Han J, Lee Y, Yeom KH, Nam JW, Heo I, Rhee JK, et al. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell. 2006;125:887–901. doi: 10.1016/j.cell.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 22.Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432:231–5. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 23.Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, et al. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–40. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 24.Landthaler M, Yalcin A, Tuschl T. The human DiGeorge syndrome critical region gene 8 and Its D. melanogaster homolog are required for miRNA biogenesis. Current biology: CB. 2004;14:2162–7. doi: 10.1016/j.cub.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN. The Drosha-DGCR8 complex in primary microRNA processing. Genes & development. 2004;18:3016–27. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes & development. 2003;17:3011–6. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303:95–8. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 28.Bohnsack MT, Czaplinski K, Gorlich D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA. 2004;10:185–91. doi: 10.1261/rna.5167604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123:631–40. doi: 10.1016/j.cell.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 30.Hutvagner G, McLachlan J, Pasquinelli AE, Balint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–8. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 31.Ketting RF, Fischer SE, Bernstein E, Sijen T, Hannon GJ, Plasterk RH. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes & development. 2001;15:2654–9. doi: 10.1101/gad.927801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matranga C, Tomari Y, Shin C, Bartel DP, Zamore PD. Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell. 2005;123:607–20. doi: 10.1016/j.cell.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 33.Rand TA, Petersen S, Du F, Wang X. Argonaute2 cleaves the anti-guide strand of siRNA during RISC activation. Cell. 2005;123:621–9. doi: 10.1016/j.cell.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 34.Leuschner PJ, Ameres SL, Kueng S, Martinez J. Cleavage of the siRNA passenger strand during RISC assembly in human cells. EMBO reports. 2006;7:314–20. doi: 10.1038/sj.embor.7400637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–6. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 36.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 37.Ding SW, Voinnet O. Antiviral immunity directed by small RNAs. Cell. 2007;130:413–26. doi: 10.1016/j.cell.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung WJ, Okamura K, Martin R, Lai EC. Endogenous RNA interference provides a somatic defense against Drosophila transposons. Current biology: CB. 2008;18:795–802. doi: 10.1016/j.cub.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Czech B, Malone CD, Zhou R, Stark A, Schlingeheyde C, Dus M, et al. An endogenous small interfering RNA pathway in Drosophila. Nature. 2008;453:798–802. doi: 10.1038/nature07007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghildiyal M, Seitz H, Horwich MD, Li C, Du T, Lee S, et al. Endogenous siRNAs derived from transposons and mRNAs in Drosophila somatic cells. Science (New York, NY) 2008;320:1077–81. doi: 10.1126/science.1157396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawamura Y, Saito K, Kin T, Ono Y, Asai K, Sunohara T, et al. Drosophila endogenous small RNAs bind to Argonaute 2 in somatic cells. Nature. 2008;453:793–7. doi: 10.1038/nature06938. [DOI] [PubMed] [Google Scholar]
- 42.Maillard PV, Ciaudo C, Marchais A, Li Y, Jay F, Ding SW, et al. Antiviral RNA Interference in Mammalian Cells. Science (New York, NY) 2013;342:235–8. doi: 10.1126/science.1241930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheloufi S, Dos Santos CO, Chong MM, Hannon GJ. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature. 2010;465:584–9. doi: 10.1038/nature09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cifuentes D, Xue H, Taylor DW, Patnode H, Mishima Y, Cheloufi S, et al. A novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science. 2010;328:1694–8. doi: 10.1126/science.1190809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 46.Czech B, Hannon GJ. One Loop to Rule Them All: The Ping-Pong Cycle and piRNA-Guided Silencing. Trends in biochemical sciences. 2016;41:324–37. doi: 10.1016/j.tibs.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vagin VV, Sigova A, Li C, Seitz H, Gvozdev V, Zamore PD. A distinct small RNA pathway silences selfish genetic elements in the germline. Science. 2006;313:320–4. doi: 10.1126/science.1129333. [DOI] [PubMed] [Google Scholar]
- 48.Aravin A, Gaidatzis D, Pfeffer S, Lagos-Quintana M, Landgraf P, Iovino N, et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006;442:203–7. doi: 10.1038/nature04916. [DOI] [PubMed] [Google Scholar]
- 49.Girard A, Sachidanandam R, Hannon GJ, Carmell MA. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature. 2006;442:199–202. doi: 10.1038/nature04917. [DOI] [PubMed] [Google Scholar]
- 50.Lau NC, Seto AG, Kim J, Kuramochi-Miyagawa S, Nakano T, Bartel DP, et al. Characterization of the piRNA complex from rat testes. Science. 2006;313:363–7. doi: 10.1126/science.1130164. [DOI] [PubMed] [Google Scholar]
- 51.Watanabe T, Tomizawa S, Mitsuya K, Totoki Y, Yamamoto Y, Kuramochi-Miyagawa S, et al. Role for piRNAs and noncoding RNA in de novo DNA methylation of the imprinted mouse Rasgrf1 locus. Science. 2011;332:848–52. doi: 10.1126/science.1203919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, et al. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128:1089–103. doi: 10.1016/j.cell.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 53.Li XZ, Roy CK, Dong X, Bolcun-Filas E, Wang J, Han BW, et al. An ancient transcription factor initiates the burst of piRNA production during early meiosis in mouse testes. Molecular cell. 2013;50:67–81. doi: 10.1016/j.molcel.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han BW, Wang W, Li C, Weng Z, Zamore PD. Noncoding RNA. piRNA-guided transposon cleavage initiates Zucchini-dependent, phased piRNA production. Science. 2015;348:817–21. doi: 10.1126/science.aaa1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ipsaro JJ, Haase AD, Knott SR, Joshua-Tor L, Hannon GJ. The structural biochemistry of Zucchini implicates it as a nuclease in piRNA biogenesis. Nature. 2012;491:279–83. doi: 10.1038/nature11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohn F, Handler D, Brennecke J. piRNA-guided slicing specifies transcripts for Zucchini-dependent, phased piRNA biogenesis. Science. 2015;348:812–7. doi: 10.1126/science.aaa1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishimasu H, Ishizu H, Saito K, Fukuhara S, Kamatani MK, Bonnefond L, et al. Structure and function of Zucchini endoribonuclease in piRNA biogenesis. Nature. 2012;491:284–7. doi: 10.1038/nature11509. [DOI] [PubMed] [Google Scholar]
- 58.Izumi N, Shoji K, Sakaguchi Y, Honda S, Kirino Y, Suzuki T, et al. Identification and Functional Analysis of the Pre-piRNA 3′ Trimmer in Silkworms. Cell. 2016;164:962–73. doi: 10.1016/j.cell.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gunawardane LS, Saito K, Nishida KM, Miyoshi K, Kawamura Y, Nagami T, et al. A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila. Science. 2007;315:1587–90. doi: 10.1126/science.1140494. [DOI] [PubMed] [Google Scholar]
- 60.Rouget C, Papin C, Boureux A, Meunier AC, Franco B, Robine N, et al. Maternal mRNA deadenylation and decay by the piRNA pathway in the early Drosophila embryo. Nature. 2010;467:1128–32. doi: 10.1038/nature09465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gou LT, Dai P, Yang JH, Xue Y, Hu YP, Zhou Y, et al. Pachytene piRNAs instruct massive mRNA elimination during late spermiogenesis. Cell Res. 2014;24:680–700. doi: 10.1038/cr.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pratt AJ, MacRae IJ. The RNA-induced silencing complex: a versatile gene-silencing machine. The Journal of biological chemistry. 2009;284:17897–901. doi: 10.1074/jbc.R900012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y, Juranek S, Li H, Sheng G, Tuschl T, Patel DJ. Structure of an argonaute silencing complex with a seed-containing guide DNA and target RNA duplex. Nature. 2008;456:921–6. doi: 10.1038/nature07666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakanishi K, Weinberg DE, Bartel DP, Patel DJ. Structure of yeast Argonaute with guide RNA. Nature. 2012;486:368–74. doi: 10.1038/nature11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schirle NT, MacRae IJ. The crystal structure of human Argonaute2. Science. 2012;336:1037–40. doi: 10.1126/science.1221551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song JJ, Smith SK, Hannon GJ, Joshua-Tor L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–7. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- 67.Matsumoto N, Nishimasu H, Sakakibara K, Nishida KM, Hirano T, Ishitani R, et al. Crystal Structure of Silkworm PIWI-Clade Argonaute Siwi Bound to piRNA. Cell. 2016;167:484–97 e9. doi: 10.1016/j.cell.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Sheng G, Juranek S, Tuschl T, Patel DJ. Structure of the guide-strand-containing argonaute silencing complex. Nature. 2008;456:209–13. doi: 10.1038/nature07315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schirle NT, Sheu-Gruttadauria J, MacRae IJ. Structural basis for microRNA targeting. Science. 2014;346:608–13. doi: 10.1126/science.1258040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parker JS, Roe SM, Barford D. Structural insights into mRNA recognition from a PIWI domain-siRNA guide complex. Nature. 2005;434:663–6. doi: 10.1038/nature03462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma JB, Yuan YR, Meister G, Pei Y, Tuschl T, Patel DJ. Structural basis for 5′-end-specific recognition of guide RNA by the A. fulgidus Piwi protein. Nature. 2005;434:666–70. doi: 10.1038/nature03514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 73.Frank F, Sonenberg N, Nagar B. Structural basis for 5′-nucleotide base-specific recognition of guide RNA by human AGO2. Nature. 2010;465:818–22. doi: 10.1038/nature09039. [DOI] [PubMed] [Google Scholar]
- 74.Khvorova A, Reynolds A, Jayasena SD. Functional siRNAs and miRNAs exhibit strand bias. Cell. 2003;115:209–16. doi: 10.1016/s0092-8674(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 75.Schwarz DS, Hutvagner G, Du T, Xu Z, Aronin N, Zamore PD. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115:199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- 76.Frank F, Hauver J, Sonenberg N, Nagar B. Arabidopsis Argonaute MID domains use their nucleotide specificity loop to sort small RNAs. The EMBO journal. 2012;31:3588–95. doi: 10.1038/emboj.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–98. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 79.Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 80.Doench JG, Petersen CP, Sharp PA. siRNAs can function as miRNAs. Genes & development. 2003;17:438–42. doi: 10.1101/gad.1064703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lai EC. Micro RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. Nature genetics. 2002;30:363–4. doi: 10.1038/ng865. [DOI] [PubMed] [Google Scholar]
- 82.Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, et al. Combinatorial microRNA target predictions. Nature genetics. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 83.Nakanishi K, Ascano M, Gogakos T, Ishibe-Murakami S, Serganov AA, Briskin D, et al. Eukaryote-specific insertion elements control human ARGONAUTE slicer activity. Cell Rep. 2013;3:1893–900. doi: 10.1016/j.celrep.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma JB, Ye K, Patel DJ. Structural basis for overhang-specific small interfering RNA recognition by the PAZ domain. Nature. 2004;429:318–22. doi: 10.1038/nature02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baccarini A, Chauhan H, Gardner TJ, Jayaprakash AD, Sachidanandam R, Brown BD. Kinetic analysis reveals the fate of a microRNA following target regulation in mammalian cells. Current biology: CB. 2011;21:369–76. doi: 10.1016/j.cub.2011.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–9. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 87.Hill DA, Ivanovich J, Priest JR, Gurnett CA, Dehner LP, Desruisseau D, et al. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325:965. doi: 10.1126/science.1174334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heravi-Moussavi A, Anglesio MS, Cheng SW, Senz J, Yang W, Prentice L, et al. Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. The New England journal of medicine. 2012;366:234–42. doi: 10.1056/NEJMoa1102903. [DOI] [PubMed] [Google Scholar]
- 89.Foulkes WD, Priest JR, Duchaine TF. DICER1: mutations, microRNAs and mechanisms. Nature reviews Cancer. 2014 doi: 10.1038/nrc3802. [DOI] [PubMed] [Google Scholar]
- 90.Wu MK, Sabbaghian N, Xu B, Addidou-Kalucki S, Bernard C, Zou D, et al. Biallelic DICER1 mutations occur in Wilms tumours. The Journal of pathology. 2013;230:154–64. doi: 10.1002/path.4196. [DOI] [PubMed] [Google Scholar]
- 91.Doros LA, Rossi CT, Yang J, Field A, Williams GM, Messinger Y, et al. DICER1 mutations in childhood cystic nephroma and its relationship to DICER1-renal sarcoma. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2014;27:1267–80. doi: 10.1038/modpathol.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Kock L, Sabbaghian N, Plourde F, Srivastava A, Weber E, Bouron-Dal Soglio D, et al. Pituitary blastoma: a pathognomonic feature of germ-line DICER1 mutations. Acta neuropathologica. 2014;128:111–22. doi: 10.1007/s00401-014-1285-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Slade I, Bacchelli C, Davies H, Murray A, Abbaszadeh F, Hanks S, et al. DICER1 syndrome: clarifying the diagnosis, clinical features and management implications of a pleiotropic tumour predisposition syndrome. Journal of medical genetics. 2011;48:273–8. doi: 10.1136/jmg.2010.083790. [DOI] [PubMed] [Google Scholar]
- 94.Foulkes WD, Bahubeshi A, Hamel N, Pasini B, Asioli S, Baynam G, et al. Extending the phenotypes associated with DICER1 mutations. Human mutation. 2011;32:1381–4. doi: 10.1002/humu.21600. [DOI] [PubMed] [Google Scholar]
- 95.Wegert J, Ishaque N, Vardapour R, Georg C, Gu Z, Bieg M, et al. Mutations in the SIX1/2 pathway and the DROSHA/DGCR8 miRNA microprocessor complex underlie high-risk blastemal type Wilms tumors. Cancer Cell. 2015;27:298–311. doi: 10.1016/j.ccell.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 96.Torrezan GT, Ferreira EN, Nakahata AM, Barros BD, Castro MT, Correa BR, et al. Recurrent somatic mutation in DROSHA induces microRNA profile changes in Wilms tumour. Nature communications. 2014;5:4039. doi: 10.1038/ncomms5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kobayashi H, Tomari Y. RISC assembly: Coordination between small RNAs and Argonaute proteins. Biochim Biophys Acta. 2016;1859:71–81. doi: 10.1016/j.bbagrm.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 98.Tomari Y, Du T, Zamore PD. Sorting of Drosophila small silencing RNAs. Cell. 2007;130:299–308. doi: 10.1016/j.cell.2007.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Forstemann K, Horwich MD, Wee L, Tomari Y, Zamore PD. Drosophila microRNAs are sorted into functionally distinct argonaute complexes after production by dicer-1. Cell. 2007;130:287–97. doi: 10.1016/j.cell.2007.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, et al. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- 101.Pham JW, Pellino JL, Lee YS, Carthew RW, Sontheimer EJ. A Dicer-2-dependent 80s complex cleaves targeted mRNAs during RNAi in Drosophila. Cell. 2004;117:83–94. doi: 10.1016/s0092-8674(04)00258-2. [DOI] [PubMed] [Google Scholar]
- 102.Zhou R, Hotta I, Denli AM, Hong P, Perrimon N, Hannon GJ. Comparative analysis of argonaute-dependent small RNA pathways in Drosophila. Molecular cell. 2008;32:592–9. doi: 10.1016/j.molcel.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Czech B, Zhou R, Erlich Y, Brennecke J, Binari R, Villalta C, et al. Hierarchical rules for Argonaute loading in Drosophila. Molecular cell. 2009;36:445–56. doi: 10.1016/j.molcel.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoda M, Kawamata T, Paroo Z, Ye X, Iwasaki S, Liu Q, et al. ATP-dependent human RISC assembly pathways. Nature structural & molecular biology. 2010;17:17–23. doi: 10.1038/nsmb.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–6. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- 106.Tomari Y, Matranga C, Haley B, Martinez N, Zamore PD. A protein sensor for siRNA asymmetry. Science. 2004;306:1377–80. doi: 10.1126/science.1102755. [DOI] [PubMed] [Google Scholar]
- 107.Liu X, Jiang F, Kalidas S, Smith D, Liu Q. Dicer-2 and R2D2 coordinately bind siRNA to promote assembly of the siRISC complexes. RNA (New York, NY) 2006;12:1514–20. doi: 10.1261/rna.101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ameres SL, Horwich MD, Hung JH, Xu J, Ghildiyal M, Weng Z, et al. Target RNA-directed trimming and tailing of small silencing RNAs. Science. 2010;328:1534–9. doi: 10.1126/science.1187058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marques JT, Kim K, Wu PH, Alleyne TM, Jafari N, Carthew RW. Loqs and R2D2 act sequentially in the siRNA pathway in Drosophila. Nature structural & molecular biology. 2010;17:24–30. doi: 10.1038/nsmb.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Okamura K, Robine N, Liu Y, Liu Q, Lai EC. R2D2 organizes small regulatory RNA pathways in Drosophila. Molecular and cellular biology. 2011;31:884–96. doi: 10.1128/MCB.01141-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iwasaki S, Sasaki HM, Sakaguchi Y, Suzuki T, Tadakuma H, Tomari Y. Defining fundamental steps in the assembly of the Drosophila RNAi enzyme complex. Nature. 2015;521:533–6. doi: 10.1038/nature14254. [DOI] [PubMed] [Google Scholar]
- 112.Nishida KM, Miyoshi K, Ogino A, Miyoshi T, Siomi H, Siomi MC. Roles of R2D2, a Cytoplasmic D2 Body Component, in the Endogenous siRNA Pathway in Drosophila. Molecular cell. 2013 doi: 10.1016/j.molcel.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 113.Kawamata T, Seitz H, Tomari Y. Structural determinants of miRNAs for RISC loading and slicer-independent unwinding. Nature structural & molecular biology. 2009;16:953–60. doi: 10.1038/nsmb.1630. [DOI] [PubMed] [Google Scholar]
- 114.Betancur JG, Tomari Y. Dicer is dispensable for asymmetric RISC loading in mammals. RNA. 2012;18:24–30. doi: 10.1261/rna.029785.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Murchison EP, Partridge JF, Tam OH, Cheloufi S, Hannon GJ. Characterization of Dicer-deficient murine embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12135–40. doi: 10.1073/pnas.0505479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Suzuki HI, Katsura A, Yasuda T, Ueno T, Mano H, Sugimoto K, et al. Small-RNA asymmetry is directly driven by mammalian Argonautes. Nature structural & molecular biology. 2015;22:512–21. doi: 10.1038/nsmb.3050. [DOI] [PubMed] [Google Scholar]
- 117.Noland CL, Doudna JA. Multiple sensors ensure guide strand selection in human RNAi pathways. RNA. 2013;19:639–48. doi: 10.1261/rna.037424.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kwak PB, Tomari Y. The N domain of Argonaute drives duplex unwinding during RISC assembly. Nature structural & molecular biology. 2012 doi: 10.1038/nsmb.2232. [DOI] [PubMed] [Google Scholar]
- 119.Wang Y, Juranek S, Li H, Sheng G, Wardle GS, Tuschl T, et al. Nucleation, propagation and cleavage of target RNAs in Ago silencing complexes. Nature. 2009;461:754–61. doi: 10.1038/nature08434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu Y, Ye X, Jiang F, Liang C, Chen D, Peng J, et al. C3PO, an endoribonuclease that promotes RNAi by facilitating RISC activation. Science. 2009;325:750–3. doi: 10.1126/science.1176325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ye X, Huang N, Liu Y, Paroo Z, Huerta C, Li P, et al. Structure of C3PO and mechanism of human RISC activation. Nature structural & molecular biology. 2011 doi: 10.1038/nsmb.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wee LM, Flores-Jasso CF, Salomon WE, Zamore PD. Argonaute divides its RNA guide into domains with distinct functions and RNA-binding properties. Cell. 2012;151:1055–67. doi: 10.1016/j.cell.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kawamata T, Yoda M, Tomari Y. Multilayer checkpoints for microRNA authenticity during RISC assembly. EMBO reports. 2011;12:944–9. doi: 10.1038/embor.2011.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gu S, Jin L, Huang Y, Zhang F, Kay MA. Slicing-Independent RISC Activation Requires the Argonaute PAZ Domain. Current biology: CB. 2012 doi: 10.1016/j.cub.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nykanen A, Haley B, Zamore PD. ATP requirements and small interfering RNA structure in the RNA interference pathway. Cell. 2001;107:309–21. doi: 10.1016/s0092-8674(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 126.Iki T, Yoshikawa M, Nishikiori M, Jaudal MC, Matsumoto-Yokoyama E, Mitsuhara I, et al. In vitro assembly of plant RNA-induced silencing complexes facilitated by molecular chaperone HSP90. Molecular cell. 2010;39:282–91. doi: 10.1016/j.molcel.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 127.Iwasaki S, Kobayashi M, Yoda M, Sakaguchi Y, Katsuma S, Suzuki T, et al. Hsc70/Hsp90 chaperone machinery mediates ATP-dependent RISC loading of small RNA duplexes. Molecular cell. 2010;39:292–9. doi: 10.1016/j.molcel.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 128.Smibert P, Yang JS, Azzam G, Liu JL, Lai EC. Homeostatic control of Argonaute stability by microRNA availability. Nature structural & molecular biology. 2013;20:789–95. doi: 10.1038/nsmb.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Suzuki HI, Katsura A, Yasuda T, Ueno T, Mano H, Sugimoto K, et al. Small-RNA asymmetry is directly driven by mammalian Argonautes. Nature structural & molecular biology. 2015;22:512–21. doi: 10.1038/nsmb.3050. [DOI] [PubMed] [Google Scholar]
- 130.Olivieri D, Senti KA, Subramanian S, Sachidanandam R, Brennecke J. The cochaperone shutdown defines a group of biogenesis factors essential for all piRNA populations in Drosophila. Molecular cell. 2012;47:954–69. doi: 10.1016/j.molcel.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xiol J, Cora E, Koglgruber R, Chuma S, Subramanian S, Hosokawa M, et al. A role for Fkbp6 and the chaperone machinery in piRNA amplification and transposon silencing. Molecular cell. 2012;47:970–9. doi: 10.1016/j.molcel.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 132.Izumi N, Kawaoka S, Yasuhara S, Suzuki Y, Sugano S, Katsuma S, et al. Hsp90 facilitates accurate loading of precursor piRNAs into PIWI proteins. RNA. 2013;19:896–901. doi: 10.1261/rna.037200.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Specchia V, Piacentini L, Tritto P, Fanti L, D’Alessandro R, Palumbo G, et al. Hsp90 prevents phenotypic variation by suppressing the mutagenic activity of transposons. Nature. 2010;463:662–5. doi: 10.1038/nature08739. [DOI] [PubMed] [Google Scholar]
- 134.Gangaraju VK, Yin H, Weiner MM, Wang J, Huang XA, Lin H. Drosophila Piwi functions in Hsp90-mediated suppression of phenotypic variation. Nature genetics. 2011;43:153–8. doi: 10.1038/ng.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yao C, Sasaki HM, Ueda T, Tomari Y, Tadakuma H. Single-Molecule Analysis of the Target Cleavage Reaction by the Drosophila RNAi Enzyme Complex. Molecular cell. 2015;59:125–32. doi: 10.1016/j.molcel.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 136.Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–94. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 137.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Molecular cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Parker JS, Parizotto EA, Wang M, Roe SM, Barford D. Enhancement of the seed-target recognition step in RNA silencing by a PIWI/MID domain protein. Molecular cell. 2009;33:204–14. doi: 10.1016/j.molcel.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Filipowicz W. RNAi: the nuts and bolts of the RISC machine. Cell. 2005;122:17–20. doi: 10.1016/j.cell.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 140.Tomari Y, Zamore PD. Perspective: machines for RNAi. Genes & development. 2005;19:517–29. doi: 10.1101/gad.1284105. [DOI] [PubMed] [Google Scholar]
- 141.Faehnle CR, Elkayam E, Haase AD, Hannon GJ, Joshua-Tor L. The making of a slicer: activation of human Argonaute-1. Cell Rep. 2013;3:1901–9. doi: 10.1016/j.celrep.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Willkomm S, Oellig CA, Zander A, Restle T, Keegan R, Grohmann D, et al. Structural and mechanistic insights into an archaeal DNA-guided Argonaute protein. Nat Microbiol. 2017;2:17035. doi: 10.1038/nmicrobiol.2017.35. [DOI] [PubMed] [Google Scholar]
- 143.Kaya E, Doxzen KW, Knoll KR, Wilson RC, Strutt SC, Kranzusch PJ, et al. A bacterial Argonaute with noncanonical guide RNA specificity. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:4057–62. doi: 10.1073/pnas.1524385113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chandradoss SD, Schirle NT, Szczepaniak M, MacRae IJ, Joo C. A Dynamic Search Process Underlies MicroRNA Targeting. Cell. 2015;162:96–107. doi: 10.1016/j.cell.2015.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ragunathan K, Liu C, Ha T. RecA filament sliding on DNA facilitates homology search. eLife. 2012;1:e00067. doi: 10.7554/eLife.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature. 2014;507:62–7. doi: 10.1038/nature13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Klein M, Chandradoss SD, Depken M, Joo C. Why Argonaute is needed to make microRNA target search fast and reliable. Semin Cell Dev Biol. 2016 doi: 10.1016/j.semcdb.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 148.Ameres SL, Martinez J, Schroeder R. Molecular basis for target RNA recognition and cleavage by human RISC. Cell. 2007;130:101–12. doi: 10.1016/j.cell.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 149.Hammar P, Leroy P, Mahmutovic A, Marklund EG, Berg OG, Elf J. The <em>lac</em> Repressor Displays Facilitated Diffusion in Living Cells. Science (New York, NY) 2012;336:1595–8. doi: 10.1126/science.1221648. [DOI] [PubMed] [Google Scholar]
- 150.Stratmann SA, Morrone SR, van Oijen AM, Sohn J. The innate immune sensor IFI16 recognizes foreign DNA in the nucleus by scanning along the duplex. eLife. 2015;4:e11721. doi: 10.7554/eLife.11721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Leith JS, Tafvizi A, Huang F, Uspal WE, Doyle PS, Fersht AR, et al. Sequence-dependent sliding kinetics of p53. Proceedings of the National Academy of Sciences. 2012;109:16552–7. doi: 10.1073/pnas.1120452109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Blainey PC, Luo G, Kou SC, Mangel WF, Verdine GL, Bagchi B, et al. Nonspecifically bound proteins spin while diffusing along DNA. Nature structural & molecular biology. 2009;16:1224–9. doi: 10.1038/nsmb.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gorman J, Wang F, Redding S, Plys AJ, Fazio T, Wind S, et al. Single-molecule imaging reveals target-search mechanisms during DNA mismatch repair. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E3074–83. doi: 10.1073/pnas.1211364109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Graneli A, Yeykal CC, Robertson RB, Greene EC. Long-distance lateral diffusion of human Rad51 on double-stranded DNA. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1221–6. doi: 10.1073/pnas.0508366103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bailey S, Eliason WK, Steitz TA. The crystal structure of the Thermus aquaticus DnaB helicase monomer. Nucleic acids research. 2007;35:4728–36. doi: 10.1093/nar/gkm507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Broderick JA, Salomon WE, Ryder SP, Aronin N, Zamore PD. Argonaute protein identity and pairing geometry determine cooperativity in mammalian RNA silencing. RNA. 2011;17:1858–69. doi: 10.1261/rna.2778911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Grosswendt S, Filipchyk A, Manzano M, Klironomos F, Schilling M, Herzog M, et al. Unambiguous Identification of miRNA:Target Site Interactions by Different Types of Ligation Reactions. Molecular cell. 2014;54:1042–54. doi: 10.1016/j.molcel.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schirle NT, Sheu-Gruttadauria J, Chandradoss SD, Joo C, MacRae IJ. Water-mediated recognition of t1-adenosine anchors Argonaute2 to microRNA targets. Elife. 2015;4 doi: 10.7554/eLife.07646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Haley B, Zamore PD. Kinetic analysis of the RNAi enzyme complex. Nature structural & molecular biology. 2004;11:599–606. doi: 10.1038/nsmb780. [DOI] [PubMed] [Google Scholar]
- 160.Goh WS, Falciatori I, Tam OH, Burgess R, Meikar O, Kotaja N, et al. piRNA-directed cleavage of meiotic transcripts regulates spermatogenesis. Genes & development. 2015;29:1032–44. doi: 10.1101/gad.260455.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nishida KM, Iwasaki YW, Murota Y, Nagao A, Mannen T, Kato Y, et al. Respective functions of two distinct Siwi complexes assembled during PIWI-interacting RNA biogenesis in Bombyx germ cells. Cell Rep. 2015;10:193–203. doi: 10.1016/j.celrep.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 162.Helwak A, Kudla G, Dudnakova T, Tollervey D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell. 2013;153:654–65. doi: 10.1016/j.cell.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Boudreau RL, Jiang P, Gilmore BL, Spengler RM, Tirabassi R, Nelson JA, et al. Transcriptome-wide discovery of microRNA binding sites in human brain. Neuron. 2014;81:294–305. doi: 10.1016/j.neuron.2013.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang X. Composition of seed sequence is a major determinant of microRNA targeting patterns. Bioinformatics. 2014;30:1377–83. doi: 10.1093/bioinformatics/btu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Broughton JP, Lovci MT, Huang JL, Yeo GW, Pasquinelli AE. Pairing beyond the Seed Supports MicroRNA Targeting Specificity. Molecular cell. 2016;64:320–33. doi: 10.1016/j.molcel.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460:479–86. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chi SW, Hannon GJ, Darnell RB. An alternative mode of microRNA target recognition. Nature structural & molecular biology. 2012;19:321–7. doi: 10.1038/nsmb.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Swarts DC, Jore MM, Westra ER, Zhu Y, Janssen JH, Snijders AP, et al. DNA-guided DNA interference by a prokaryotic Argonaute. Nature. 2014;507:258–61. doi: 10.1038/nature12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nowotny M, Gaidamakov SA, Crouch RJ, Yang W. Crystal structures of RNase H bound to an RNA/DNA hybrid: substrate specificity and metal-dependent catalysis. Cell. 2005;121:1005–16. doi: 10.1016/j.cell.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 170.Song JJ, Joshua-Tor L. Argonaute and RNA–getting into the groove. Curr Opin Struct Biol. 2006;16:5–11. doi: 10.1016/j.sbi.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 171.Sheng G, Zhao H, Wang J, Rao Y, Tian W, Swarts DC, et al. Structure-based cleavage mechanism of Thermus thermophilus Argonaute DNA guide strand-mediated DNA target cleavage. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:652–7. doi: 10.1073/pnas.1321032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Swarts DC, Makarova K, Wang Y, Nakanishi K, Ketting RF, Koonin EV, et al. The evolutionary journey of Argonaute proteins. Nature structural & molecular biology. 2014;21:743–53. doi: 10.1038/nsmb.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hauptmann J, Dueck A, Harlander S, Pfaff J, Merkl R, Meister G. Turning catalytically inactive human Argonaute proteins into active slicer enzymes. Nature structural & molecular biology. 2013;20:814–7. doi: 10.1038/nsmb.2577. [DOI] [PubMed] [Google Scholar]
- 174.Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, et al. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–63. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 175.Bazzini AA, Lee MT, Giraldez AJ. Ribosome profiling shows that miR-430 reduces translation before causing mRNA decay in zebrafish. Science. 2012;336:233–7. doi: 10.1126/science.1215704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Djuranovic S, Nahvi A, Green R. miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science. 2012;336:237–40. doi: 10.1126/science.1215691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–40. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–73. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 179.Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 180.Jonas S, Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nature reviews Genetics. 2015;16:421–33. doi: 10.1038/nrg3965. [DOI] [PubMed] [Google Scholar]
- 181.Meister G, Landthaler M, Peters L, Chen PY, Urlaub H, Luhrmann R, et al. Identification of novel argonaute-associated proteins. Current biology: CB. 2005;15:2149–55. doi: 10.1016/j.cub.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 182.Ding L, Spencer A, Morita K, Han M. The developmental timing regulator AIN-1 interacts with miRISCs and may target the argonaute protein ALG-1 to cytoplasmic P bodies in C. elegans. Molecular cell. 2005;19:437–47. doi: 10.1016/j.molcel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 183.Rehwinkel J, Behm-Ansmant I, Gatfield D, Izaurralde E. A crucial role for GW182 and the DCP1:DCP2 decapping complex in miRNA-mediated gene silencing. RNA. 2005;11:1640–7. doi: 10.1261/rna.2191905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu J, Rivas FV, Wohlschlegel J, Yates JR, 3rd, Parker R, Hannon GJ. A role for the P-body component GW182 in microRNA function. Nat Cell Biol. 2005;7:1261–6. doi: 10.1038/ncb1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chen CY, Zheng D, Xia Z, Shyu AB. Ago-TNRC6 triggers microRNA-mediated decay by promoting two deadenylation steps. Nature structural & molecular biology. 2009;16:1160–6. doi: 10.1038/nsmb.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Braun JE, Huntzinger E, Fauser M, Izaurralde E. GW182 proteins directly recruit cytoplasmic deadenylase complexes to miRNA targets. Molecular cell. 2011;44:120–33. doi: 10.1016/j.molcel.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 187.Behm-Ansmant I, Rehwinkel J, Doerks T, Stark A, Bork P, Izaurralde E. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes & development. 2006;20:1885–98. doi: 10.1101/gad.1424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chekulaeva M, Mathys H, Zipprich JT, Attig J, Colic M, Parker R, et al. miRNA repression involves GW182-mediated recruitment of CCR4-NOT through conserved W-containing motifs. Nature structural & molecular biology. 2011;18:1218–26. doi: 10.1038/nsmb.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fabian MR, Cieplak MK, Frank F, Morita M, Green J, Srikumar T, et al. miRNA-mediated deadenylation is orchestrated by GW182 through two conserved motifs that interact with CCR4-NOT. Nature structural & molecular biology. 2011;18:1211–7. doi: 10.1038/nsmb.2149. [DOI] [PubMed] [Google Scholar]
- 190.Chen Y, Boland A, Kuzuoglu-Ozturk D, Bawankar P, Loh B, Chang CT, et al. A DDX6-CNOT1 complex and W-binding pockets in CNOT9 reveal direct links between miRNA target recognition and silencing. Molecular cell. 2014;54:737–50. doi: 10.1016/j.molcel.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 191.Mathys H, Basquin J, Ozgur S, Czarnocki-Cieciura M, Bonneau F, Aartse A, et al. Structural and biochemical insights to the role of the CCR4-NOT complex and DDX6 ATPase in microRNA repression. Molecular cell. 2014;54:751–65. doi: 10.1016/j.molcel.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 192.Baillat D, Shiekhattar R. Functional dissection of the human TNRC6 (GW182-related) family of proteins. Molecular and cellular biology. 2009;29:4144–55. doi: 10.1128/MCB.00380-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Boland A, Huntzinger E, Schmidt S, Izaurralde E, Weichenrieder O. Crystal structure of the MID-PIWI lobe of a eukaryotic Argonaute protein. Proceedings of the National Academy of Sciences of the United States of America. 2011 doi: 10.1073/pnas.1103946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.El-Shami M, Pontier D, Lahmy S, Braun L, Picart C, Vega D, et al. Reiterated WG/GW motifs form functionally and evolutionarily conserved ARGONAUTE-binding platforms in RNAi-related components. Genes & development. 2007;21:2539–44. doi: 10.1101/gad.451207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Eulalio A, Helms S, Fritzsch C, Fauser M, Izaurralde E. A C-terminal silencing domain in GW182 is essential for miRNA function. RNA. 2009;15:1067–77. doi: 10.1261/rna.1605509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pfaff J, Hennig J, Herzog F, Aebersold R, Sattler M, Niessing D, et al. Structural features of Argonaute-GW182 protein interactions. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E3770–9. doi: 10.1073/pnas.1308510110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Takimoto K, Wakiyama M, Yokoyama S. Mammalian GW182 contains multiple Argonaute-binding sites and functions in microRNA-mediated translational repression. RNA. 2009;15:1078–89. doi: 10.1261/rna.1363109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Till S, Lejeune E, Thermann R, Bortfeld M, Hothorn M, Enderle D, et al. A conserved motif in Argonaute-interacting proteins mediates functional interactions through the Argonaute PIWI domain. Nature structural & molecular biology. 2007;14:897–903. doi: 10.1038/nsmb1302. [DOI] [PubMed] [Google Scholar]
- 199.Kuzuoglu-Ozturk D, Bhandari D, Huntzinger E, Fauser M, Helms S, Izaurralde E. miRISC and the CCR4-NOT complex silence mRNA targets independently of 43S ribosomal scanning. The EMBO journal. 2016;35:1186–203. doi: 10.15252/embj.201592901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Eulalio A, Huntzinger E, Izaurralde E. GW182 interaction with Argonaute is essential for miRNA-mediated translational repression and mRNA decay. Nature structural & molecular biology. 2008;15:346–53. doi: 10.1038/nsmb.1405. [DOI] [PubMed] [Google Scholar]
- 201.Boland A, Huntzinger E, Schmidt S, Izaurralde E, Weichenrieder O. Crystal structure of the MID-PIWI lobe of a eukaryotic Argonaute protein. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10466–71. doi: 10.1073/pnas.1103946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Till S, Lejeune E, Thermann R, Bortfeld M, Hothorn M, Enderle D, et al. A conserved motif in Argonaute-interacting proteins mediates functional interactions through the Argonaute PIWI domain. Nature structural & molecular biology. 2007;14:897–903. doi: 10.1038/nsmb1302. [DOI] [PubMed] [Google Scholar]
- 203.Christie M, Boland A, Huntzinger E, Weichenrieder O, Izaurralde E. Structure of the PAN3 pseudokinase reveals the basis for interactions with the PAN2 deadenylase and the GW182 proteins. Molecular cell. 2013;51:360–73. doi: 10.1016/j.molcel.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 204.Boland A, Chen Y, Raisch T, Jonas S, Kuzuoglu-Ozturk D, Wohlbold L, et al. Structure and assembly of the NOT module of the human CCR4-NOT complex. Nature structural & molecular biology. 2013;20:1289–97. doi: 10.1038/nsmb.2681. [DOI] [PubMed] [Google Scholar]
- 205.Jinek M, Fabian MR, Coyle SM, Sonenberg N, Doudna JA. Structural insights into the human GW182-PABC interaction in microRNA-mediated deadenylation. Nature structural & molecular biology. 2010;17:238–40. doi: 10.1038/nsmb.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wolf J, Valkov E, Allen MD, Meineke B, Gordiyenko Y, McLaughlin SH, et al. Structural basis for Pan3 binding to Pan2 and its function in mRNA recruitment and deadenylation. The EMBO journal. 2014;33:1514–26. doi: 10.15252/embj.201488373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Schafer IB, Rode M, Bonneau F, Schussler S, Conti E. The structure of the Pan2-Pan3 core complex reveals cross-talk between deadenylase and pseudokinase. Nature structural & molecular biology. 2014;21:591–8. doi: 10.1038/nsmb.2834. [DOI] [PubMed] [Google Scholar]
- 208.Jonas S, Christie M, Peter D, Bhandari D, Loh B, Huntzinger E, et al. An asymmetric PAN3 dimer recruits a single PAN2 exonuclease to mediate mRNA deadenylation and decay. Nature structural & molecular biology. 2014;21:599–608. doi: 10.1038/nsmb.2837. [DOI] [PubMed] [Google Scholar]
- 209.Petit AP, Wohlbold L, Bawankar P, Huntzinger E, Schmidt S, Izaurralde E, et al. The structural basis for the interaction between the CAF1 nuclease and the NOT1 scaffold of the human CCR4-NOT deadenylase complex. Nucleic acids research. 2012;40:11058–72. doi: 10.1093/nar/gks883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kozlov G, Safaee N, Rosenauer A, Gehring K. Structural basis of binding of P-body-associated proteins GW182 and ataxin-2 by the Mlle domain of poly(A)-binding protein. The Journal of biological chemistry. 2010;285:13599–606. doi: 10.1074/jbc.M109.089540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Eystathioy T, Chan EK, Tenenbaum SA, Keene JD, Griffith K, Fritzler MJ. A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles. Molecular biology of the cell. 2002;13:1338–51. doi: 10.1091/mbc.01-11-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Eystathioy T, Jakymiw A, Chan EK, Seraphin B, Cougot N, Fritzler MJ. The GW182 protein colocalizes with mRNA degradation associated proteins hDcp1 and hLSm4 in cytoplasmic GW bodies. RNA. 2003;9:1171–3. doi: 10.1261/rna.5810203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jagannath A, Wood MJ. Localization of double-stranded small interfering RNA to cytoplasmic processing bodies is Ago2 dependent and results in up-regulation of GW182 and Argonaute-2. Molecular biology of the cell. 2009;20:521–9. doi: 10.1091/mbc.E08-08-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Eulalio A, Behm-Ansmant I, Schweizer D, Izaurralde E. P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. Molecular and cellular biology. 2007;27:3970–81. doi: 10.1128/MCB.00128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gatfield D, Le Martelot G, Vejnar CE, Gerlach D, Schaad O, Fleury-Olela F, et al. Integration of microRNA miR-122 in hepatic circadian gene expression. Genes & development. 2009;23:1313–26. doi: 10.1101/gad.1781009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hwang HW, Wentzel EA, Mendell JT. A hexanucleotide element directs microRNA nuclear import. Science. 2007;315:97–100. doi: 10.1126/science.1136235. [DOI] [PubMed] [Google Scholar]
- 217.De N, Young L, Lau PW, Meisner NC, Morrissey DV, Macrae IJ. Highly Complementary Target RNAs Promote Release of Guide RNAs from Human Argonaute2. Molecular cell. 2013;50:344–55. doi: 10.1016/j.molcel.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cazalla D, Steitz JA. Down-regulation of a host microRNA by a viral noncoding RNA. Cold Spring Harbor symposia on quantitative biology. 2010;75:321–4. doi: 10.1101/sqb.2010.75.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Buck AH, Perot J, Chisholm MA, Kumar DS, Tuddenham L, Cognat V, et al. Post-transcriptional regulation of miR-27 in murine cytomegalovirus infection. RNA (New York, NY) 2010;16:307–15. doi: 10.1261/rna.1819210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Marcinowski L, Tanguy M, Krmpotic A, Rädle B, Lisnić VJ, Tuddenham L, et al. Degradation of Cellular miR-27 by a Novel, Highly Abundant Viral Transcript Is Important for Efficient Virus Replication In Vivo. PLOS Pathogens. 2012;8:e1002510. doi: 10.1371/journal.ppat.1002510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lee S, Song J, Kim S, Kim J, Hong Y, Kim Y, et al. Selective degradation of host MicroRNAs by an intergenic HCMV noncoding RNA accelerates virus production. Cell host & microbe. 2013;13:678–90. doi: 10.1016/j.chom.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 222.Krol J, Busskamp V, Markiewicz I, Stadler MB, Ribi S, Richter J, et al. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell. 2010;141:618–31. doi: 10.1016/j.cell.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 223.Rissland OS, Hong SJ, Bartel DP. MicroRNA destabilization enables dynamic regulation of the miR-16 family in response to cell-cycle changes. Molecular cell. 2011;43:993–1004. doi: 10.1016/j.molcel.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Monticelli S, Ansel KM, Xiao C, Socci ND, Krichevsky AM, Thai TH, et al. MicroRNA profiling of the murine hematopoietic system. Genome biology. 2005;6:R71. doi: 10.1186/gb-2005-6-8-r71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sethi P, Lukiw WJ. Micro-RNA abundance and stability in human brain: specific alterations in Alzheimer’s disease temporal lobe neocortex. Neuroscience letters. 2009;459:100–4. doi: 10.1016/j.neulet.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 226.Rajasethupathy P, Fiumara F, Sheridan R, Betel D, Puthanveettil SV, Russo JJ, et al. Characterization of small RNAs in Aplysia reveals a role for miR-124 in constraining synaptic plasticity through CREB. Neuron. 2009;63:803–17. doi: 10.1016/j.neuron.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.de la Mata M, Gaidatzis D, Vitanescu M, Stadler MB, Wentzel C, Scheiffele P, et al. Potent degradation of neuronal miRNAs induced by highly complementary targets. EMBO Rep. 2015;16:500–11. doi: 10.15252/embr.201540078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cazalla D, Yario T, Steitz JA. Down-regulation of a host microRNA by a Herpesvirus saimiri noncoding RNA. Science. 2010;328:1563–6. doi: 10.1126/science.1187197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Park JH, Shin SY, Shin C. Non-canonical targets destabilize microRNAs in human Argonautes. Nucleic acids research. 2017;45:1569–83. doi: 10.1093/nar/gkx029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Denzler R, McGeary SE, Title AC, Agarwal V, Bartel DP, Stoffel M. Impact of MicroRNA Levels, Target-Site Complementarity, and Cooperativity on Competing Endogenous RNA-Regulated Gene Expression. Molecular cell. 2016;64:565–79. doi: 10.1016/j.molcel.2016.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Horman SR, Janas MM, Litterst C, Wang B, Macrae IJ, Sever MJ, et al. Akt-Mediated Phosphorylation of Argonaute 2 Downregulates Cleavage and Upregulates Translational Repression of MicroRNA Targets. Molecular cell. 2013;50:356–67. doi: 10.1016/j.molcel.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zeng Y, Sankala H, Zhang X, Graves PR. Phosphorylation of Argonaute 2 at serine-387 facilitates its localization to processing bodies. The Biochemical journal. 2008;413:429–36. doi: 10.1042/BJ20080599. [DOI] [PubMed] [Google Scholar]
- 233.Rüdel S, Wang Y, Lenobel R, Körner R, Hsiao H-H, Urlaub H, et al. Phosphorylation of human Argonaute proteins affects small RNA binding. Nucleic acids research. 2011;39:2330–43. doi: 10.1093/nar/gkq1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mazumder A, Bose M, Chakraborty A, Chakrabarti S, Bhattacharyya SN. A transient reversal of miRNA - mediated repression controls macrophage activation. EMBO Reports. 2013;14:1008–16. doi: 10.1038/embor.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Shen J, Xia W, Khotskaya YB, Huo L, Nakanishi K, Lim SO, et al. EGFR modulates microRNA maturation in response to hypoxia through phosphorylation of AGO2. Nature. 2013;497:383–7. doi: 10.1038/nature12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Golden RJ, Chen B, Li T, Braun J, Manjunath H, Chen X, et al. An Argonaute phosphorylation cycle promotes microRNA-mediated silencing. Nature. 2017;542:197–202. doi: 10.1038/nature21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Leung AK, Vyas S, Rood JE, Bhutkar A, Sharp PA, Chang P. Poly(ADP-ribose) regulates stress responses and microRNA activity in the cytoplasm. Molecular cell. 2011;42:489–99. doi: 10.1016/j.molcel.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Seo GJ, Kincaid RP, Phanaksri T, Burke JM, Pare JM, Cox JE, et al. Reciprocal inhibition between intracellular antiviral signaling and the RNAi machinery in mammalian cells. Cell host & microbe. 2013;14:435–45. doi: 10.1016/j.chom.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Qi HH, Ongusaha PP, Myllyharju J, Cheng D, Pakkanen O, Shi Y, et al. Prolyl 4-hydroxylation regulates Argonaute 2 stability. Nature. 2008;455:421–4. doi: 10.1038/nature07186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wu C, So J, Davis-Dusenbery BN, Qi HH, Bloch DB, Shi Y, et al. Hypoxia potentiates microRNA-mediated gene silencing through posttranslational modification of Argonaute2. Molecular and cellular biology. 2011;31:4760–74. doi: 10.1128/MCB.05776-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rybak A, Fuchs H, Hadian K, Smirnova L, Wulczyn EA, Michel G, et al. The let-7 target gene mouse lin-41 is a stem cell specific E3 ubiquitin ligase for the miRNA pathway protein Ago2. Nature cell biology. 2009;11:1411–20. doi: 10.1038/ncb1987. [DOI] [PubMed] [Google Scholar]
- 242.Loedige I, Gaidatzis D, Sack R, Meister G, Filipowicz W. The mammalian TRIM-NHL protein TRIM71/LIN-41 is a repressor of mRNA function. Nucleic acids research. 2013;41:518–32. doi: 10.1093/nar/gks1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chang H-M, Martinez NJ, Thornton JE, Gregory RI. Trim71 cooperates with microRNAs to repress Cdkn1a expression and promote embryonic stem cell proliferation. Nature communications. 2012;3:923. doi: 10.1038/ncomms1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Chen J, Lai F, Niswander L. The ubiquitin ligase mLin41 temporally promotes neural progenitor cell maintenance through FGF signaling. Genes & development. 2012;26:803–15. doi: 10.1101/gad.187641.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Martinez NJ, Gregory RI. Argonaute2 expression is post-transcriptionally coupled to microRNA abundance. RNA (New York, NY) 2013;19:605–12. doi: 10.1261/rna.036434.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Gibbings D, Mostowy S, Jay F, Schwab Y, Cossart P, Voinnet O. Selective autophagy degrades DICER and AGO2 and regulates miRNA activity. Nature cell biology. 2012;14:1314–21. doi: 10.1038/ncb2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Smibert P, Yang J-S, Azzam G, Liu J-L, Lai EC. Homeostatic control of Argonaute stability by microRNA availability. Nature structural & molecular biology. 2013;20:789–95. doi: 10.1038/nsmb.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]


