Abstract
This review is focused on continuous IOP monitoring using telemetry systems in the nonhuman primate (NHP), presented in the context that IOP fluctuations at various timescales may be involved in glaucoma pathogenesis and progression. We use glaucoma as the primary framework to discuss how the dynamic nature of IOP might change with age, racial heritage, and disease in the context of glaucoma susceptibility and progression. We focus on the limited work that has been published in IOP telemetry in NHPs, as well as the emerging data and approaches. We review the ongoing efforts to measure continuous IOP, and the strengths, weaknesses and general pitfalls of the various approaches.
1. Introduction
Glaucoma is primarily a disease of aging (Gordon, Beiser et al. 2002, Leske, Heijl et al. 2003) and is one of the leading causes of blindness in the developed world (Quigley and Broman 2006). Lowering IOP is the only clinical treatment that has been shown to retard the onset and progression of glaucoma, but once damaged, the optic nerve head (ONH) is thought to be more susceptible to further glaucomatous progression even after clinical intervention has lowered mean IOP to ‘normal’ levels. IOP is a mechanical load, and ONH and scleral biomechanics are also thought to be centrally involved in glaucoma susceptibility, as well as disease onset and progression (Figure 1) (Zeimer and Ogura 1989, Burgoyne, Downs et al. 2005, Downs, Roberts et al. 2008, Sigal and Ethier 2009, Sigal, Roberts et al. 2010). In this review, we discuss the existing data and ongoing efforts to measure continuous IOP in nonhuman primates (NHP) and humans, and the strengths, weaknesses and general pitfalls of the various approaches used. We review continuous IOP data gathered using telemetry systems, presented in the framework that IOP fluctuations at various timescales may be involved in glaucoma pathogenesis. We also discuss how the dynamic nature of IOP is likely to change with age, racial heritage, and disease in the context of glaucoma susceptibility and progression.
Figure 1. IOP-related stress and strain are a constant presence within the ONH at all levels of IOP.
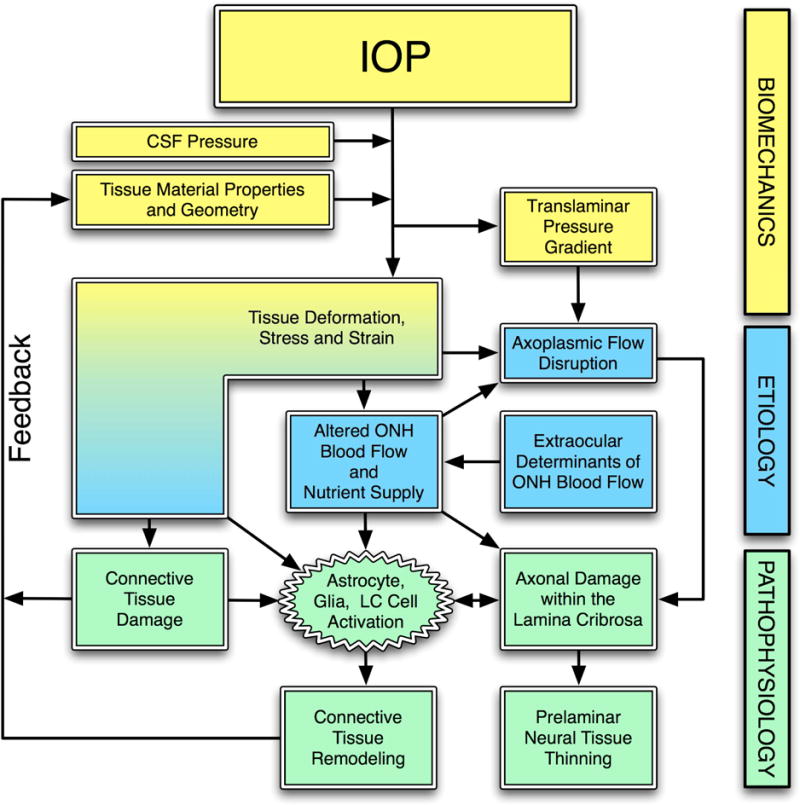
IOP and cerebrospinal fluid pressure act mechanically on the tissues of the eye, producing deformations, strain and stress within the tissues. These deformations depend on the eye-specific geometry and material properties of the individual eye. In a biomechanical paradigm, stress and strain alter blood flow and may also induce connective tissue damage directly (laminar beam yield), and drives a connective tissue remodeling process that alters the tissues’ geometry and mechanical response to loading. This feeds back directly onto the mechanical effects of IOP. Adapted from Sigal, Roberts, and Downs. (Sigal, Roberts et al. 2010)
2. The difference between high frequency and low frequency IOP fluctuations
There is a large and controversial literature surrounding the importance of low frequency fluctuations of clinically measured mean IOP in glaucoma (Sacca, Rolando et al. 1998, Bengtsson and Heijl 2005, Bengtsson, Leske et al. 2007, Medeiros, Weinreb et al. 2008). These studies all rely on snapshot measurements of IOP that report a mean baseline value at each time point, and those measurements are taken at relatively infrequent intervals (hourly at the most frequent). Recently however, there has been some interest in ocular pulse amplitude, or the fluctuation in IOP associated with the cardiac cycle, which can be measured by Dynamic Contour Tonometry or DCT (Hoffmann, Grus et al. 2004, Punjabi, Ho et al. 2006, Pourjavan, Boelle et al. 2007, Kotecha, White et al.). DCT provides continuous measurement of IOP, but only for a period of tens of seconds in which a patient can tolerate corneal contact without blinking or eye movement, which ironically are two of the most common sources of large high frequency IOP fluctuations according to telemetric IOP data collected from humans (Coleman and Trokel 1969) and NHPs (Downs, Burgoyne et al. 2011) as shown in Figure 2.
Figure 2. High- and Low-frequency IOP Fluctuation in the Human and NHP.
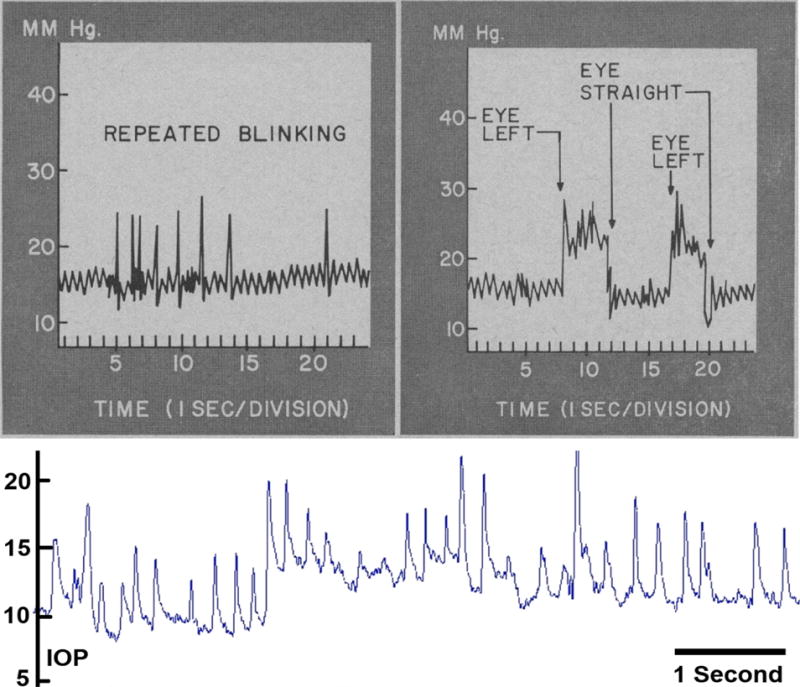
Top: Pressure recording of continuous IOP from an unrestrained awake patient with baseline mean IOP of ~16 mmHg and IOP fluctuations up to 10 and 14 mmHg associated with blinks and saccadic eye movements, respectively. Adapted from Coleman and Trokel (Coleman and Trokel 1969). Bottom: Screen capture of ~7 seconds of continuous telemetric IOP trace from an unrestrained awake NHP with baseline mean IOP of ~8–14 mmHg and IOP fluctuations up to 12 and 8 mmHg associated with blinks and saccadic eye movements, respectively. IOP fluctuations can be much larger and of longer duration, especially when the animal squints or is agitated or stressed. Adapted from Downs, et al. (Downs, Burgoyne et al. 2011).
3. What Is the True IOP In a Patient?
IOP is an important risk factor for glaucoma, and lowering IOP, even when IOP is in the normal range as defined epidemiologically, remains the only proven-effective treatment for the disease. However, our knowledge of the true character of IOP in humans or how it affects ocular tissues is limited in part by the lack of a continuous IOP monitoring technology for patients. Two telemetry devices have been extensively tested in humans. The first is based on a stretch-sensitive contact lens that measures corneal stretch presumably induced by changes in IOP (Mansouri and Shaarawy 2011). Contact lens-based devices such as the Triggerfish by Sensimed AG are not well tolerated for more than 24 hours and no calibration scheme has yet been devised, so these systems can presumably record when IOP is high or low (Mottet, Aptel et al. 2013) but cannot measure actual IOP in mmHg (Mansouri and Shaarawy 2011). Ocular discomfort has also been reported in patients fitted with contact lens-based systems (Mansouri and Shaarawy 2011), which may affect blinking rates and therefore alter quantification of high-frequency IOP fluctuations. The second device is a MEMS-based silicone-encapsulated ring that is implanted in the ciliary sulcus after lens replacement. The first trial in 4 patients showed that the device can detect a step in IOP due to massage, but the pressure readings were not consistent and drifted considerably over time compared to applanation tonometry readings (Koutsonas, Walter et al. 2015). Another similar device was implanted in one patient, and IOP was a reasonable match with applanation tonometry readings (Melki, Todani et al. 2014). These devices do not measure IOP continuously, as they measure IOP and transmit data only when a reader is placed in close proximity to the eye to inductively power the device. Also, placement of large intraocular devices in the sulcus risks dispersing pigment into the aqueous, which could indirectly elevate IOP by increasing conventional outflow resistance. This results in a lack of understanding of how IOP is involved in the underlying mechanisms of glaucoma development and progression.
Although clinical IOP-lowering remains the only proven method of preventing the onset and progression of glaucoma, the role of IOP in the development and progression of the disease is not well understood. This largely arises from the clinical observation that significant numbers of patients with normal IOPs develop glaucoma (normal or low-tension glaucoma), while other individuals with elevated IOP show no signs of the disease. This could mean that IOP (or some factor driven by IOP) is a primary causative factor in glaucoma, and IOP vulnerability varies between individuals. Another possibility is that clinical characterization of mean IOP using infrequent snapshot measurements fails to capture exposure to injurious IOP fluctuations that are partly driving the disease in these normotensive glaucoma patients, which contributes to the murkiness of the IOP-glaucoma relationship.
Recent data indicate that IOP fluctuates as much as 5 mmHg day-to-day and hour-to-hour, and 15 to 40 mmHg second-to-second when measured continuously via telemetry in unrestrained, awake NHPs (Downs, Burgoyne et al. 2011) (Figure 3). Aside from an acute study in a single patient eye (Coleman and Trokel 1969), very little is known about IOP fluctuations in humans and how the eye responds to those fluctuations, but IOP levels at all timescales have the potential to injure the retinal ganglion cell axons in the ONH (Cullen and LaPlaca 2006, Resta, Novelli et al. 2007).
Figure 3. IOP Fluctuates Throughout the Day in the NHP.
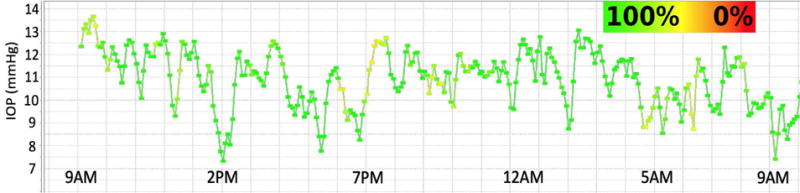
Plot of the 10-minute time-window average of 24 hours of continuous IOP showing low frequency IOP fluctuation from a typical NHP eye. Note that room lights were on from 6AM to 6PM daily. The color of the plot points and lines indicate how much data remained in each 10-minute window after post-hoc digital filtering of signal dropout and noise. Green indicates that 100% of the continuous IOP data were used in the 10-minute average IOP plotted in each point, and yellow indicates that 50% were eliminated due to signal dropout or noise. Note the fluctuations in IOP are substantial even when the high-frequency IOP spikes seen in Figure 2 are averaged out. Adapted from Downs, et al. (Downs, Burgoyne et al. 2011)
4. IOP Dynamics and Ocular Biomechanics
4.A. The relationship of age, racial heritage, and existing disease to the onset and progression of glaucoma
The results of several randomized prospective trials have identified risk factors associated with the development or progression of glaucoma (Gordon, Beiser et al. 2002, Leske, Heijl et al. 2003, Miglior, Pfeiffer et al. 2007). Across these studies, IOP, age, central corneal thickness, increased optic disc cupping, and African ancestry were independently associated with glaucomatous progression. Importantly, age and race (OHTS; univariate only) are the only risk factors other than IOP that are independently associated with the onset and progression of glaucoma across all of the major prospective clinical trials conducted over the past twenty years (2002, Leske, Heijl et al. 2003, Miglior, Pfeiffer et al. 2007, Musch, Gillespie et al. 2009). In addition, the degree of visual field loss (indicating the severity of existing glaucoma) was a risk factor for disease progression in all but one (Musch, Gillespie et al. 2009) of these large prospective trials.
In addition to data from prospective trials in glaucoma and ocular hypertension, every population-based survey conducted to date has demonstrated a strong relationship between the prevalence of glaucoma with advancing age (Rudnicka, Mt-Isa et al. 2006), despite almost all studies showing no changes in IOP with age (Klein, Klein et al. 1992, Nomura, Shimokata et al. 1999, Weih, Mukesh et al. 2001, Nomura, Ando et al. 2002, Rochtchina, Mitchell et al. 2002). Furthermore, while normal tension glaucoma is not uncommon within elderly populations and people of African ancestry (Chumbley and Brubaker 1976, Levene 1980), it is rarely seen in children or young adults (Geijssen 1991).
4.B. The effects of age, race, and glaucomatous damage on IOP fluctuations
IOP is a pressure and hence it is a mechanical load that must be borne by the ocular coats and ONH. IOP can cause glaucomatous damage even at statistically defined “normal” IOPs of less than 21 mmHg if a particular eye is unusually susceptible to IOP insult, regardless of its mechanism of action (Figure 1). IOP and ocular perfusion pressure (OPP) fluctuation could harm the tissues of the ONH in a similar manner as mean IOP or mean OPP. As with any solid structure, the degree of instantaneous deformation (strain) experienced by the ONH under a given level of stress (IOP) is dependent upon its 3D architecture and material properties (Downs, Roberts et al. 2008). The stress and strain in the corneoscleral shell and ONH is dependent on the forces applied (IOP), the geometry of the load bearing structure, and the material properties (stiffness) of the constituent tissues (Downs, Roberts et al. 2008). IOP fluctuations down to the millisecond may induce potentially pathological stresses and strains in the ONH.
Mean IOP has traditionally been thought of as the driver of biomechanical insult to the ONH in glaucoma, even when reported in terms of hourly fluctuations. It has not been truly appreciated, however, that the structural stiffness of the corneoscleral shell is also a strong determinant of the amplitude of IOP fluctuations that occur when the eye is perturbed (Liu and He 2009, Morris, Tang et al. 2013). The ocular coats act as a shock absorber, actively stretching to absorb energy from small perturbations in eye shape or volume, and thereby decreasing the IOP fluctuations associated with those perturbations. The stiffer the ocular coats, the larger the IOP fluctuations will be for an identical perturbation. These perturbations can be from internal sources such as systolic vascular filling (Kaufmann, Bachmann et al. 2006) or due to external forces such as saccades, eye rubs, and blinks (Coleman and Trokel 1969). As the ocular coats stiffen, the eye cannot damp these perturbations as easily by elastic expansion, which leads directly to higher IOP fluctuations at all levels of mean IOP. Thus, greater IOP fluctuation associated with stiffening of the corneoscleral shell and ONH that has been shown to occur with aging (Albon 2000, Girard, Suh et al. 2009, Knox Cartwright, Tyrer et al., Fazio, Grytz et al. 2014) in response to chronic exposure to elevated IOP (Downs, Suh et al. 2005, Girard, Suh et al. 2011), and in people of African heritage (Fazio, Grytz et al. 2014, Grytz, Fazio et al. 2014). These findings may have important clinical ramifications in that for a given mean IOP, the ocular coats are stiffer and IOP fluctuation is greater in the elderly, persons of African heritage, and/or those with a history of elevated IOP, which may account for some portion of the increased susceptibility to IOP-induced injury in these at-risk populations.
In addition to these longer-term causes of increased structural stiffness, the ocular coats are nonlinear in a material property sense, such that the coats stiffen as they become stretched when subjected to acutely elevated IOP (Girard, Suh et al. 2009). This IOP-related stiffening is an acute phenomenon, in that high-frequency IOP fluctuations have higher magnitudes when mean IOP is higher (Dastiridou, Ginis et al. 2009, Downs, Burgoyne et al. 2011) for identical perturbations (Figure 4), which may contribute to a higher risk of glaucoma in ostensibly ‘normotensive’ patients with high baseline mean IOP for some part of the day or night.
Figure 4. Ocular Pulse Amplitude Increases with Baseline IOP.
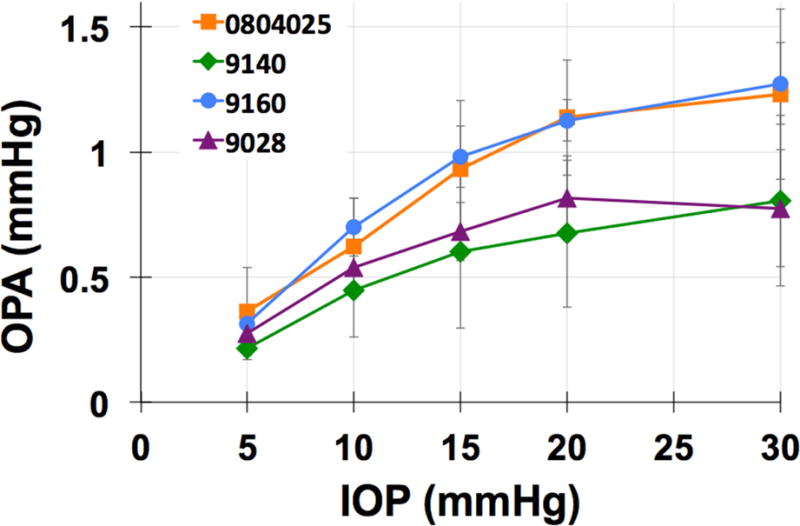
Top: The amplitude of IOP fluctuations associated with systolic vascular filling, known as ocular pulse amplitude or OPA, plotted as a function of baseline IOP in one eye of four NHPs. These data show that IOP fluctuations increase significantly in magnitude as IOP increases, presumably driven by the stretching and stiffening of the ocular coats with increasing IOP.
5. The Nycthemeral Rhythm and Repeatability of IOP Patterns
While mean IOP has been shown to have a nycthemeral rhythm in patients (Liu, Kripke et al. 1998, Liu, Kripke et al. 1999), clinical studies have shown that this pattern varies between individuals and is not robustly repeatable from day-to-day in the same patient (Realini, Khouri et al. 2006, Realini, Weinreb et al. 2010, Realini, Weinreb et al. 2011). While the nocturnal rise is IOP has been attributed to habitual supine sleeping position (Jorge, Ramoa-Marques et al. 2010), there is some evidence that the nocturnal rise is IOP is still detectable in the sitting position (Liu, Bouligny et al. 2003). The nycthemeral rhythm in NHPs is insignificant (Downs, Burgoyne et al. 2011), but this may be due to the fact that NHPs generally sleep sitting up. This is a limitation of the NHP model of glaucoma should nightly mean IOP elevations prove important in the disease. The daily pattern of mean IOP is not repeatable in NHPs, with IOPs ranging ~10 mmHg to 20 mmHg using hourly averages of continuous IOP measured in several days of the same week (Figure 5).
Figure 5. The Nycthemeral Rhythm and Variability of Mean IOP in the NHP as Measured with Continuous IOP Telemetry.
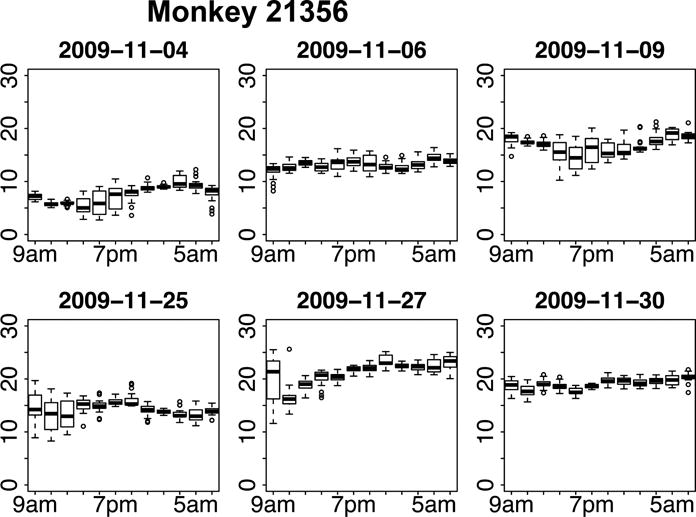
Plots of the two-hour time-window average distributions of IOP for six 24-hour periods in a single NHP eye. Note that room lights were on from 6AM to 6PM daily. The date is shown above each plot, and the each row represents three days in the same week. The box and whisker plots are shown wherein the central bar indicates the mean IOP in each two-hour segment, the extents of the box show the central 50% of the measurements, the whiskers indicate the 95% limits of the measurements in that time window, and circles indicate outliers. IOP in the NHP demonstrates no discernable nycthemeral rhythm, and shows a highly variable pattern and magnitude in different days. Adapted from Downs, et al. (Downs, Burgoyne et al. 2011)
6. IOP Telemetry in NHPs
To our knowledge, only one system has been successfully used to measure IOP continuously over extended periods in NHPs. This system was developed from fully implantable radiotelemetry systems that have been used to monitor physiologic pressures continuously in large animals (Figure 6). The benefits of the telemetry system from Konigsberg Instruments, Inc. (Pasadena, CA) are that it allows continuous monitoring of IOP at a 500 Hz measurement frequency for ~24 months, the pressure sensors have low drift of < 3 mmHg per month, the pressure transducer can be directly calibrated using anterior chamber cannulation and manometry, and the transducer is mounted in the orbital wall adjacent to the eye (Downs, Burgoyne et al. 2011) (Figure 6). The disadvantages of the Konigsberg system are the high cost, the difficulty of the implantation surgery, and the lack of a replaceable battery or inductive powering/charging.
Figure 6.
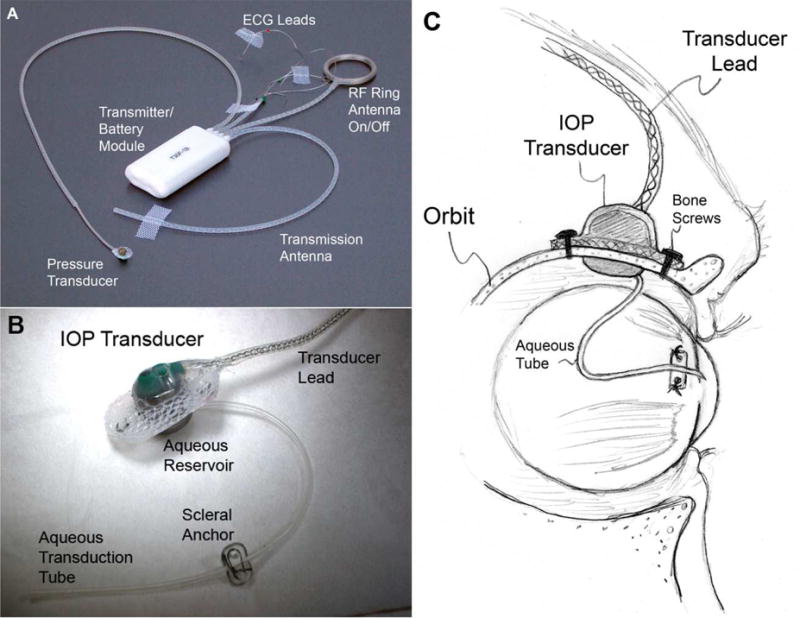
(A) Photograph of a typical T30F total implant system showing the battery/transmitter module, radio frequency ring antenna for on/off, transmission antenna, a pressure transducer, and two ECG electrodes plus ground; (B) Photograph of the extra-orbital surface of our custom IOP transducer housing that is secured within a ¼-inch hole in the lateral orbital wall with bone screws as shown in (C) A 23-gauge silicone tube delivers aqueous from the anterior chamber to a fluid reservoir on the intra-orbital side of the transducer (partially hidden from view in B); The tube (with appropriate slack to allow for eye movement) is trimmed, inserted into the anterior chamber, sutured to the sclera using the integral scleral tube anchor plate, and covered with a scleral patchgraft (not shown). Adapted from Downs, et al. (Downs, Burgoyne et al. 2011)
7. The Importance of Pressure Transducer Placement
Many commercial pressure telemetry systems use a fluid-filled, gel-tip catheter to transduce the pressure at the catheter tip to a remote pressure transducer included in the electronics/battery package. This system has been used in several studies in rabbits to characterize IOP in response to pharmacological agents (McLaren, Brubaker et al. 1996, McLaren, Bachman et al. 1999). The electronics/batter/transducer package is too large to be placed subcutaneously in the head however, and is generally placed under the skin in the nape of the neck or between the clavicles. This is a reasonable approach for rabbits, rodents, and other small animals whose eyes remain at about the same height as the pressure transducer during daily activity. For animals with a large head and flexible neck, small changes in head position change the height of the eye relative to a remote pressure transducer, which leads to large errors in the IOP measurement due to the hydrostatic pressure from fluid in the catheter (1.3 cm of height differential is approximately 1 mmHg of IOP measurement error). Hence for NHPs and other large animals, IOP transducers must be placed in the orbit or eye to minimize head position artifact.
8. A Look Ahead to IOP, Ocular Perfusion Pressure (OPP) and Intracranial/Cerebrospinal Pressure Telemetry Systems on the Horizon
Several systems in development hold promise for monitoring the physiologic pressures relevant to glaucoma: IOP, OPP, and cerebrospinal fluid measurement. We have enhanced our unilateral NHP telemetry system (Downs, Burgoyne et al. 2011) to measure continuous bilateral IOP, bilateral electro-oculogram, and aortic blood pressure (Figure 7). Using this enhanced system, OPP is calculated 500 times per second as: central retinal artery blood pressure – IOP. The central retinal artery systolic and diastolic pressures can be calibrated directly to the telemetric aortic systolic and diastolic pressures via ophthalmodynamometry, by visualizing the minimum IOPs at which the central retinal artery begins to flutter (diastolic) and fully collapse (systolic). We are beginning work on adding an intracranial pressure channel to this system, which would allow unprecedented monitoring of the physiologic pressures thought to be important in glaucoma. Ideally, pressure telemetry systems for glaucoma research should allow for high frequency sampling and near continuous monitoring of IOP, OPP, and cerebrospinal fluid pressure until we understand which component(s) of the these signals are important in disease pathogenesis and progression. To my knowledge, no current system including our own (no cerebrospinal fluid pressure measurement), meets all these requirements.
Figure 7.
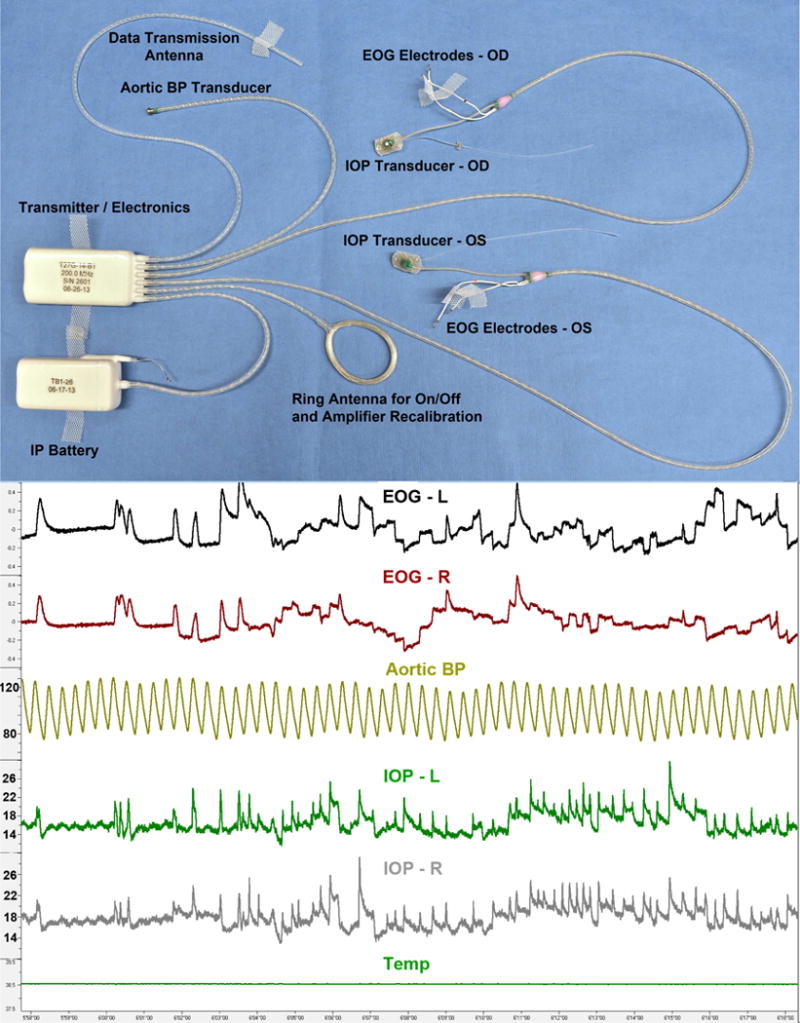
(Top) Photograph of a our enhanced ITS total implant system for continuous monitoring of bilateral IOP, bilateral electro-oculogram (EOG), aortic blood pressure, and body temperature; (Bottom) Screen capture of 20 seconds of data from a single, awake, unrestrained NHP, showing IOP fluctuations from ocular pulse amplitude, blinks, and saccades, which are very similar in fellow eyes and correlated with orbital muscle activity as captured by the EOG signals.
For rodents, rabbits and other small animals, new systems from DSI (Data Sciences International, St. Paul, MN), Emka Technologies (Paris, France), and TSE Systems (Chesterfield, MO) offer promising options for IOP, blood pressure, and cerebrospinal fluid measurement, although catheter placement and long-term stability in small eyes remains a challenge. Passaglia and colleagues are developing a new system based on a recent patent that would allow for both monitoring and control of IOP in rats (Passaglia 2014). This would be an exciting advance, as IOP is still highly variable in inducible, elevated IOP models of glaucoma. A system with which IOP could be held constant or cycling at the same fixed, elevated level in a cohort of animals would allow studies of other variables of interest that may influence eye-specific susceptibility to axon loss (e.g., ONH size, laminar thickness/stiffness, scleral stiffness, …etc.), and allow more sensitive testing of the efficacy of therapeutic agents and approaches.
9. Clinical Implications
Whether it is mean IOP and/or IOP fluctuations that drive glaucomatous pathogenesis, there is a wide spectrum of individual susceptibility to IOP-related glaucomatous vision loss. However, the biomechanical effects of both mean IOP and IOP fluctuations are likely to play a central role in the development and progression of the disease at all IOP levels. There are currently no science-based tools to predict at what level of IOP an individual ONH will be damaged. Eventually, knowing the relationship between mean IOP and OPP, IOP and OPP fluctuations, mechanical strain -driven remodeling, ONH blood flow, and astrocyte and axonal homeostasis will drive the clinical assessment of safe target IOP, although the technologies to assess many of these factors have yet to materialize. Studies of glaucoma progression in human patients using future IOP telemetry systems will play a critical role in elucidating the links between mean IOP, IOP fluctuations, ONH biomechanics, and the cellular, mechanical and vascular contributors to glaucoma pathogenesis and progression.
If high frequency IOP and/or OPP fluctuations or transients are found to independently contribute to glaucoma onset and/or progression, IOP fluctuation reduction would become a new therapeutic pathway for glaucoma treatment. There are currently no treatments in use that are specifically designed to lower IOP fluctuation, although some of the existing treatments designed to lower mean IOP could also be lowering IOP fluctuation. At least one potential treatment based on IOP fluctuation reduction has already been devised; Cascade Ophthalmics holds a patent on an implantable intraocular vessel filled with a compressible gas that would quickly expand and contract to attenuate high frequency IOP fluctuations (Connors 2009), but this company is no longer viable for want of definitive evidence that IOP and/or OPP fluctuation independently contribute to glaucoma.
Highlights of “IOP Telemetry in Nonhuman Primates”.
Nontechnical review focused on continuous IOP measurement in NHPs
Focus on glaucoma, and why continuous IOP measurement might be important in disease
Review of how ocular biomechanics influence IOP fluctuations and dynamics
Review the current systems and data on IOP telemetry in NHPs
Review the technical challenges and emerging approaches to IOP telemetry
Acknowledgments
The author would like to acknowledge Drs. Claude F. Burgoyne and Christopher A. Girkin, whose clinical and surgical expertise in glaucoma and assistance was critical in developing our unilateral and bilateral IOP telemetry system for NHPs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000;130(4):429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- The Advanced Glaucoma Intervention Study (AGIS): 12. Baseline risk factors for sustained loss of visual field and visual acuity in patients with advanced glaucoma. Am J Ophthalmol. 2002;134(4):499–512. doi: 10.1016/s0002-9394(02)01659-8. [DOI] [PubMed] [Google Scholar]
- Albon J. Age related compliance of the lamina cribrosa in human eyes. British Journal of Ophthalmology. 2000;84(3):318–323. doi: 10.1136/bjo.84.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson B, Heijl A. Diurnal IOP fluctuation: not an independent risk factor for glaucomatous visual field loss in high-risk ocular hypertension. Graefes Arch Clin Exp Ophthalmol. 2005;243(6):513–518. doi: 10.1007/s00417-004-1103-8. [DOI] [PubMed] [Google Scholar]
- Bengtsson B, Leske MC, Hyman L, Heijl A. Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial. Ophthalmology. 2007;114(2):205–209. doi: 10.1016/j.ophtha.2006.07.060. [DOI] [PubMed] [Google Scholar]
- Burgoyne CF, Downs JC, Bellezza AJ, Francis Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24(1):39–73. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Chumbley LC, Brubaker RF. Low-tension glaucoma. Am J Ophthalmol. 1976;81(6):761–767. doi: 10.1016/0002-9394(76)90359-7. [DOI] [PubMed] [Google Scholar]
- Coleman DJ, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Arch Ophthalmol. 1969;82(5):637–640. doi: 10.1001/archopht.1969.00990020633011. [DOI] [PubMed] [Google Scholar]
- Connors KGW,MA,US, Pintauro William L (Ft Lauderdale, FL, US), Wallin Sheila K (Carlsbad, CA, US), Kilcoyne John T (San Diego, CA, US), Cao Hung H (Corona, CA, US), Nguyen Khoi M (Murietta, CA, US), Yurek Matthew T (San Diego, CA, US) Implantable self-inflating attenuation device and method for treating ocular pressure spikes. United States, Cascade Ophthalmics, Inc.; Irvine, CA, US: 2009. [Google Scholar]
- Cullen DK, LaPlaca MC. Neuronal response to high rate shear deformation depends on heterogeneity of the local strain field. J Neurotrauma. 2006;23(9):1304–1319. doi: 10.1089/neu.2006.23.1304. [DOI] [PubMed] [Google Scholar]
- Dastiridou AI, Ginis HS, De Brouwere D, Tsilimbaris MK, Pallikaris IG. Ocular rigidity, ocular pulse amplitude, and pulsatile ocular blood flow: the effect of intraocular pressure. Invest Ophthalmol Vis Sci. 2009;50(12):5718–5722. doi: 10.1167/iovs.09-3760. [DOI] [PubMed] [Google Scholar]
- Downs JC, Burgoyne CF, Seigfreid WP, Reynaud JF, Strouthidis NG, Sallee V. 24-hour IOP telemetry in the nonhuman primate: implant system performance and initial characterization of IOP at multiple timescales. Invest Ophthalmol Vis Sci. 2011;52(10):7365–7375. doi: 10.1167/iovs.11-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JC, Roberts MD, Burgoyne CF. Mechanical environment of the optic nerve head in glaucoma. Optom Vis Sci. 2008;85(6):425–435. doi: 10.1097/OPX.0b013e31817841cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JC, Suh JK, Thomas KA, Bellezza AJ, Hart RT, Burgoyne CF. Viscoelastic material properties of the peripapillary sclera in normal and early-glaucoma monkey eyes. Invest Ophthalmol Vis Sci. 2005;46(2):540–546. doi: 10.1167/iovs.04-0114. [DOI] [PubMed] [Google Scholar]
- Fazio MA, Grytz R, Morris JS, Bruno L, Gardiner SK, Girkin CA, Downs JC. Age-related changes in human peripapillary scleral strain. Biomech Model Mechanobiol. 2014;13(3):551–563. doi: 10.1007/s10237-013-0517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio MA, Grytz R, Morris JS, Bruno L, Girkin CA, Downs JC. Human scleral structural stiffness increases more rapidly with age in donors of african descent compared to donors of European descent. Invest Ophthalmol Vis Sci. 2014;55(11):7189–7198. doi: 10.1167/iovs.14-14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijssen H. Studies on Normal-Pressure Glaucoma. Amsterdam: Kugler Publications; 1991. [Google Scholar]
- Girard MJ, Suh JK, Bottlang M, Burgoyne CF, Downs JC. Scleral biomechanics in the aging monkey eye. Invest Ophthalmol Vis Sci. 2009;50(11):5226–5237. doi: 10.1167/iovs.08-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard MJ, Suh JK, Bottlang M, Burgoyne CF, Downs JC. Biomechanical changes in the sclera of monkey eyes exposed to chronic IOP elevations. Invest Ophthalmol Vis Sci. 2011;52(8):5656–5669. doi: 10.1167/iovs.10-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Kass MA. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714–720. doi: 10.1001/archopht.120.6.714. discussion 829–730. [DOI] [PubMed] [Google Scholar]
- Grytz R, Fazio MA, Libertiaux V, Bruno L, Gardiner S, Girkin CA, Downs JC. Age- and race-related differences in human scleral material properties. Invest Ophthalmol Vis Sci. 2014;55(12):8163–8172. doi: 10.1167/iovs.14-14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann EM, Grus FH, Pfeiffer N. Intraocular pressure and ocular pulse amplitude using dynamic contour tonometry and contact lens tonometry. BMC Ophthalmol. 2004;4:4. doi: 10.1186/1471-2415-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge J, Ramoa-Marques R, Lourenco A, Silva S, Nascimento S, Queiros A, Gonzalez-Meijome JM. IOP variations in the sitting and supine positions. Journal of glaucoma. 2010;19(9):609–612. doi: 10.1097/IJG.0b013e3181ca7ca5. [DOI] [PubMed] [Google Scholar]
- Kaufmann C, Bachmann LM, Robert YC, Thiel MA. Ocular pulse amplitude in healthy subjects as measured by dynamic contour tonometry. Arch Ophthalmol. 2006;124(8):1104–1108. doi: 10.1001/archopht.124.8.1104. [DOI] [PubMed] [Google Scholar]
- Klein BE, Klein R, Linton KL. Intraocular pressure in an American community. The Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1992;33(7):2224–2228. [PubMed] [Google Scholar]
- Cartwright NE Knox, Tyrer JR, Marshall J. Age-related differences in the elasticity of the human cornea. Invest Ophthalmol Vis Sci. 2011;52(7):4324–4329. doi: 10.1167/iovs.09-4798. [DOI] [PubMed] [Google Scholar]
- Kotecha A, White E, Schlottmann PG, Garway-Heath DF. Intraocular pressure measurement precision with the Goldmann applanation, dynamic contour, and ocular response analyzer tonometers. Ophthalmology. 2010;117(4):730–737. doi: 10.1016/j.ophtha.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Koutsonas A, Walter P, Roessler G, Plange N. Implantation of a novel telemetric intraocular pressure sensor in patients with glaucoma (ARGOS study): 1-year results. Invest Ophthalmol Vis Sci. 2015;56(2):1063–1069. doi: 10.1167/iovs.14-14925. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121(1):48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E, G Early Manifest Glaucoma Trial Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121(1):48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- Levene RZ. Low tension glaucoma: a critical review and new material. Surv Ophthalmol. 1980;24(6):621–664. doi: 10.1016/0039-6257(80)90123-x. [DOI] [PubMed] [Google Scholar]
- Liu J, He X. Corneal stiffness affects IOP elevation during rapid volume change in the eye. Invest Ophthalmol Vis Sci. 2009;50(5):2224–2229. doi: 10.1167/iovs.08-2365. [DOI] [PubMed] [Google Scholar]
- Liu JH, Bouligny RP, Kripke DF, Weinreb RN. Nocturnal elevation of intraocular pressure is detectable in the sitting position. Invest Ophthalmol Vis Sci. 2003;44(10):4439–4442. doi: 10.1167/iovs.03-0349. [DOI] [PubMed] [Google Scholar]
- Liu JH, Kripke DF, Hoffman RE, Twa MD, Loving RT, Rex KM, Gupta N, Weinreb RN. Nocturnal elevation of intraocular pressure in young adults. Invest Ophthalmol Vis Sci. 1998;39(13):2707–2712. [PubMed] [Google Scholar]
- Liu JH, Kripke DF, Twa MD, Hoffman RE, Mansberger SL, Rex KM, Girkin CA, Weinreb RN. Twenty-four-hour pattern of intraocular pressure in the aging population. Invest Ophthalmol Vis Sci. 1999;40(12):2912–2917. [PubMed] [Google Scholar]
- Mansouri K, Shaarawy T. Continuous intraocular pressure monitoring with a wireless ocular telemetry sensor: initial clinical experience in patients with open angle glaucoma. The British journal of ophthalmology. 2011;95(5):627–629. doi: 10.1136/bjo.2010.192922. [DOI] [PubMed] [Google Scholar]
- McLaren JW, Bachman LA, Brubaker RF. Comparison of effects of topical ibopamine and epinephrine on the circadian rhythm of intraocular pressure of the rabbit eye as measured by telemetry. J Ocul Pharmacol Ther. 1999;15(2):107–116. doi: 10.1089/jop.1999.15.107. [DOI] [PubMed] [Google Scholar]
- McLaren JW, Brubaker RF, FitzSimon JS. Continuous measurement of intraocular pressure in rabbits by telemetry. Invest Ophthalmol Vis Sci. 1996;37(6):966–975. [PubMed] [Google Scholar]
- Medeiros FA, Weinreb RN, Zangwill LM, Alencar LM, Sample PA, Vasile C, Bowd C. Long-term intraocular pressure fluctuations and risk of conversion from ocular hypertension to glaucoma. Ophthalmology. 2008;115(6):934–940. doi: 10.1016/j.ophtha.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melki S, Todani A, Cherfan G. An implantable intraocular pressure transducer: initial safety outcomes. JAMA Ophthalmol. 2014;132(10):1221–1225. doi: 10.1001/jamaophthalmol.2014.1739. [DOI] [PubMed] [Google Scholar]
- Miglior S, Pfeiffer N, Torri V, Zeyen T, Cunha-Vaz J, Adamsons I. Predictive factors for open-angle glaucoma among patients with ocular hypertension in the European Glaucoma Prevention Study. Ophthalmology. 2007;114(1):3–9. doi: 10.1016/j.ophtha.2006.05.075. [DOI] [PubMed] [Google Scholar]
- Morris HJ, Tang J, Perez B Cruz, Pan X, Hart RT, Weber PA, Liu J. Correlation between biomechanical responses of posterior sclera and IOP elevations during micro intraocular volume change. Invest Ophthalmol Vis Sci. 2013;54(12):7215–7222. doi: 10.1167/iovs.13-12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottet B, Aptel F, Romanet JP, Hubanova R, Pepin JL, Chiquet C. 24-hour intraocular pressure rhythm in young healthy subjects evaluated with continuous monitoring using a contact lens sensor. JAMA Ophthalmol. 2013;131(12):1507–1516. doi: 10.1001/jamaophthalmol.2013.5297. [DOI] [PubMed] [Google Scholar]
- Musch DC, Gillespie BW, Lichter PR, Niziol LM, Janz NK. Visual field progression in the Collaborative Initial Glaucoma Treatment Study the impact of treatment and other baseline factors. Ophthalmology. 2009;116(2):200–207. doi: 10.1016/j.ophtha.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura H, Ando F, Niino N, Shimokata H, Miyake Y. The relationship between age and intraocular pressure in a Japanese population: the influence of central corneal thickness. Curr Eye Res. 2002;24(2):81–85. doi: 10.1076/ceyr.24.2.81.8161. [DOI] [PubMed] [Google Scholar]
- Nomura H, Shimokata H, Ando F, Miyake Y, Kuzuya F. Age-related changes in intraocular pressure in a large Japanese population: a cross-sectional and longitudinal study. Ophthalmology. 1999;106(10):2016–2022. doi: 10.1016/S0161-6420(99)90417-7. [DOI] [PubMed] [Google Scholar]
- Passaglia CL. Auto-Regulation System for Intraocular Pressure, Google Patents 2014 [Google Scholar]
- Pourjavan S, Boelle PY, Detry-Morel M, De Potter P. Physiological diurnal variability and characteristics of the ocular pulse amplitude (OPA) with the dynamic contour tonometer (DCT-Pascal) Int Ophthalmol. 2007;27(6):357–360. doi: 10.1007/s10792-007-9161-7. [DOI] [PubMed] [Google Scholar]
- Punjabi OS, Ho HK, Kniestedt C, Bostrom AG, Stamper RL, Lin SC. Intraocular pressure and ocular pulse amplitude comparisons in different types of glaucoma using dynamic contour tonometry. Curr Eye Res. 2006;31(10):851–862. doi: 10.1080/02713680600899887. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Realini AD, Khouri A, Amos-Realini J, Fechtner R. Does IOP Follow a Conserved Daily Rhythm? Invest Ophthalmol Vis Sci. 2006;47(5):4464. [Google Scholar]
- Realini T, Weinreb RN, Wisniewski S. Short-term repeatability of diurnal intraocular pressure patterns in glaucomatous individuals. Ophthalmology. 2011;118(1):47–51. doi: 10.1016/j.ophtha.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Realini T, Weinreb RN, Wisniewski SR. Diurnal intraocular pressure patterns are not repeatable in the short term in healthy individuals. Ophthalmology. 2010;117(9):1700–1704. doi: 10.1016/j.ophtha.2010.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resta V, Novelli E, Vozzi G, Scarpa C, Caleo M, Ahluwalia A, Solini A, Santini E, Parisi V, Di Virgilio F, Galli-Resta L. Acute retinal ganglion cell injury caused by intraocular pressure spikes is mediated by endogenous extracellular ATP. Eur J Neurosci. 2007;25(9):2741–2754. doi: 10.1111/j.1460-9568.2007.05528.x. [DOI] [PubMed] [Google Scholar]
- Rochtchina E, Mitchell P, Wang JJ. Relationship between age and intraocular pressure: the Blue Mountains Eye Study. Clin Experiment Ophthalmol. 2002;30(3):173–175. doi: 10.1046/j.1442-9071.2002.00519.x. [DOI] [PubMed] [Google Scholar]
- Rudnicka AR, Mt-Isa S, Owen CG, Cook DG, Ashby D. Variations in primary open-angle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis. Invest Ophthalmol Vis Sci. 2006;47(10):4254–4261. doi: 10.1167/iovs.06-0299. [DOI] [PubMed] [Google Scholar]
- Sacca SC, Rolando M, Marletta A, Macri A, Cerqueti P, Ciurlo G. Fluctuations of intraocular pressure during the day in open-angle glaucoma, normal-tension glaucoma and normal subjects. Ophthalmologica. 1998;212(2):115–119. doi: 10.1159/000027290. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Ethier CR. Biomechanics of the optic nerve head. Exp Eye Res. 2009;88(4):799–807. doi: 10.1016/j.exer.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Roberts MD, Girard MJA, Burgoyne CF, Downs JC. Chapter 20: Biomechanical Changes of the Optic Disc. In: Levin LA, Albert DM, editors. Ocular Disease: Mechanisms and Management. London: Elsevier; 2010. pp. 153–164. [Google Scholar]
- Weih LM, Mukesh BN, McCarty CA, Taylor HR. Association of demographic, familial, medical, and ocular factors with intraocular pressure. Arch Ophthalmol. 2001;119(6):875–880. doi: 10.1001/archopht.119.6.875. [DOI] [PubMed] [Google Scholar]
- Zeimer RC, Ogura Y. The relation between glaucomatous damage and optic nerve head mechanical compliance. Arch Ophthalmol. 1989;107(8):1232–1234. doi: 10.1001/archopht.1989.01070020298042. [DOI] [PubMed] [Google Scholar]


