Abstract
The conformational transition of the core domain of HIV-1 gp41 from a prehairpin intermediate to a six-helix bundle is responsible for virus–cell fusion. Several inhibitors which target the N-heptad repeat helical coiled-coil trimer that is fully accessible in the prehairpin intermediate have been designed. One such inhibitor is the peptide C34 derived from the C-heptad repeat of gp41 that forms the exterior of the six-helix bundle. Here, using a variety of biophysical techniques, including dye tagging, size-exclusion chromatography combined with multiangle light scattering, double electron–electron resonance EPR spectroscopy, and circular dichroism, we investigate the binding of C34 to two six-helix bundle mimetics comprising N- and C-heptad repeats either without (coreSP) or with (coreS) a short spacer connecting the two. In the case of coreSP, C34 directly exchanges with the C-heptad repeat. For coreS, up to two molecules of C34 bind the six-helix bundle via displacement of the C-heptad repeat. These results suggest that fusion inhibitors such as C34 can target a continuum of transitioning conformational states from the prehairpin intermediate to the six-helix bundle prior to the occurrence of irreversible fusion of viral and target cell membranes.
Graphical abstract
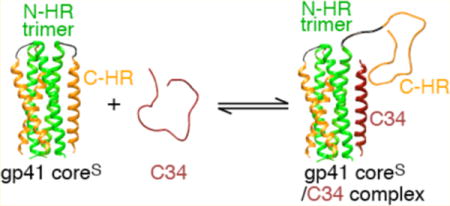
The entry of HIV-1 into target cells is mediated by the surface envelope (Env) glyproteins gp120 and gp41.1 The initial event involves binding of CD4 and the chemokine coreceptor on the target cell to gp120 on the surface of the virus, followed by a series of conformational changes in gp120 and gp41 that ultimately result in fusion of the viral and cell membranes.2–7 Early steps in this process have been visualized by crystallography and cryo-electron microscopy of a cleaved HIV-1 Env trimer, thought to represent an activated state of gp120 or gp41.8,9 The gp41 component in these structures is in a prefusion state, approximating the prehairpin intermediate,4,10–12 in which the trimeric coiled-coil N-heptad repeat (N-HR, residues 543–582) and the C-terminal heptad repeat (C-HR, residues 625–662) do not interact with one another, and the C- and N-termini of gp41 bridge the viral and target cell membranes, respectively. Further conformational changes in gp41 result in the formation of a six-helix bundle in which the N-HR trimeric helical coiled coil is surrounded by three C-HR helices packed as antiparallel helices into hydrophobic grooves,13–16 thereby bringing the viral and target cell membranes into direct contact with one another.4,17,18
Previous work showed that HIV-1 fusion can be blocked by targeting the N-HR and C-HR in the prehairpin intermediate.10,19–26 Inhibitors directed against the trimeric N-HR helical coiled-coil27 include peptides derived from the C-HR10,19 (such as C34 and T20) and antibodies that directly bind to the N-HR trimer,22,28–41 as well as a peptide [N36Mut(e,g)] derived from the N-HR that sequesters the N-HR of gp41 into inactive heterotrimers.42,43 The temporal window for inhibitors directed against the N-HR trimer of gp41 is similar with a half-life of 20–25 min post-CD4 engagement.31,43
In the series of monoclonal antibodies generated in our laboratory by selection against N-HR trimer mimetics,29–33 we made the interesting discovery that these antibodies not only bound prehairpin intermediate mimetics in which two or more N-HR helices of the trimer are fully exposed, but also bound directly to six-helix bundle mimetics.44 Further, neutralization activity was far better correlated to affinity for the six-helix bundle than for the prehairpin intermediate.44 Unexpectedly, binding of these neutralizing antibodies to the six-helix bundle did not occur via displacement of the C-HR helices, as might have been predicted on the basis of crystal structures with prehairpin intermediate mimetics,32,33 but was mapped to an epitope formed by a relatively small hydrophobic pocket on the N-HR that is exposed in the context of the six-helix bundle.44 The equilibrium dissociation constants (Kdiss) for binding of C34 to a prehairpin mimetic 5-helix, a single-chain construct lacking the last C-HR helix, as well as for six-helix bundle formation upon mixing N-HR and C-HR peptides, range from 0.3 to 2 μM, as determined by isothermal titration calorimetry.45–47 Thus, there appears to be a discrepancy between Kdiss values for the binding of C34 to prehairpin intermediate mimetics in vitro and IC50 values ranging from 4 to 70 nM, depending upon HIV-1 strain, for inhibition of HIV-1 fusion in cell-based assays.17,48 Here we investigate the interaction of C-HR-derived peptide C34 with two six-helix bundle domain constructs of gp41 differing in whether the N-HR and C-HR regions are covalently linked to one another and show that, in contrast to the case for N-HR-directed monoclonal antibodies, binding occurs in both instances via direct displacement of the C-HR helices. We show that in the case of the six-helix bundle construct in which the N-HR is linked to the CH-R by a six-residue spacer sequence (coreS), instead of the full-length, 42-residue, immune-dominant linker (IL) sequence, only two of the three C-HR helices are displaced by C34 with a Kdiss of ~ 1 μM.
MATERIALS AND METHODS
gp41 Constructs
The gp41 analogues used in this study are depicted schematically in Figure 1 and comprise a single-chain six-helix bundle (6-helix),21 a single-chain five-helix bundle (5-helix),21 coreS,16 coreSP,44 C34,13 and T20.19 DNA inserts were cloned in pET11a or pET15 vectors between NdeI/BamHI and NcoI/BamHI sites, respectively. To facilitate the isolation of recombinant C34-Cys (628–662) bearing the E662C substitution, a modified coreS construct (N37-SGLV-PRGS-C34) was created by exchanging the L6 spacer sequence to encompass a thrombin site between the N-HR (N37) and C-HR (C34) regions. CoreSP-Cys, which bears the same C-terminal E662C substitution as C34-Cys, was engineered from the coreSP template44 by QuikChange mutagenesis (Agilent Technologies, Santa Clara, CA). DNA sequences were verified by sequencing. T20 was obtained from the NIH Reference Reagent Program. Chemically synthesized C34 (Ac-C-HR628–661-NH2) was purchased from Commonwealth Bio-technologies, Inc. (Richmond, VA).
Figure 1.
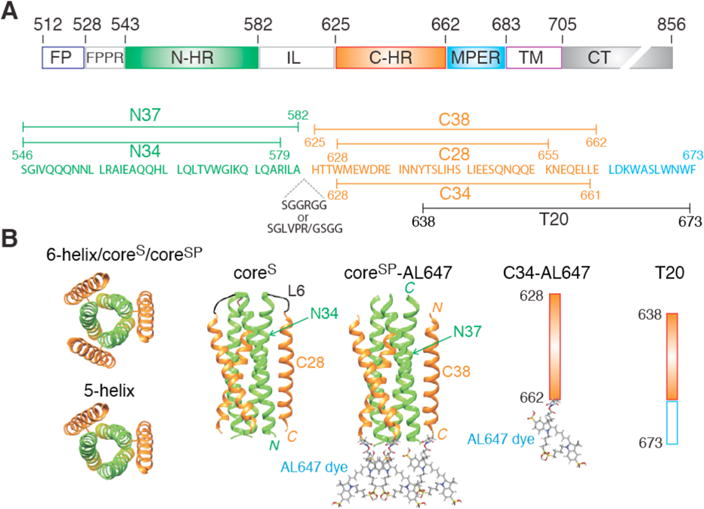
Domain organization and gp41 constructs used in this work. (A) Domain organization of HIV-1 gp41. Abbreviations: FP, fusion peptide; FPPR, fusion peptide proximal region; N-HR, N-heptad repeat; IL, immune-dominant linker; C-HR, C-heptad repeat; MPER, membrane proximal external region; TM, transmembrane region; CT, intraviral C-terminal domain. The numbering of residues corresponds to their positions in the HIV-1 Env sequence. (B) Constructs used in this work. The coordinates of the six-helix bundle formed by an internal trimer of N-HR helical repeats surrounded by three C-HR helical repeats are taken from ref 16 (Protein Data Bank entry 1SZT). 6-helix and 5-helix are single-chain constructs differing by the presence and absence, respectively, of the C-terminal C-HR helix. CoreS is a homotrimer in which the N-HR and C-HR helices of each subunit are connected by a six-residue linker (SGGRGG). In the coreSP six-helix bundle construct, the N-HR and C-HR helices are not connected by a linker. Sites of labeling of coreSP and the C34 peptide with the Alexafluor 647 dye (AL647) are indicated.
Protein Purification, Folding, and Labeling with Alexafluor 647 Dye
Escherichia coli BL21(DE3) bearing the appropriate plasmid was grown in Luria-Bertani medium and induced for expression at an A600 of 0.7 for 4 h. All expressed gp41 constructs invariably accumulate in the insoluble fraction. After isolating the insoluble fraction, the constructs were fractionated on size-exclusion Superdex-200 or -75 columns (GE Healthcare, Piscataway, NJ) under denaturing conditions followed by reverse-phase high-performance liquid chromatography as described previously.49 6-helix, 5-helix, coreS, and coreSP were folded by dialysis against 50 mM sodium formate (pH 3) followed by buffer exchange with 10 mM Tris-HCl (pH 7.6) and 150 mM NaCl (buffer A), concentrated to ~ 2 mg/mL, and stored.
CoreSP-Cys was subjected to fractionation on a Superdex-75 column in buffer A containing 2 mM tris(2-carboxyethyl)-phosphine hydrochloride (TCEP) (Sigma) to keep the cysteine residues reduced. Peak fractions were pooled, concentrated, and stored at −20 °C prior to labeling. An aliquot of coreSP-Cys was reacted with a 2–3-fold molar excess of Alexafluor 647 (AL647) C2-maleimide (Life Technologies) for ~ 2.5 h. The reaction was terminated by the addition of 2-mercaptoethanol (Sigma) to a final concentration of 10 mM, and the mixture was incubated for 10 min, followed by fractionation on a Superdex-75 column in buffer A to remove the unreacted dye. Peak fractions corresponding to coreSP-AL647 were pooled, concentrated, and stored. Recombinant C34-Cys was labeled with excess AL647 C2-maleimide in 6 M guanidine hydrochloride and 25 mM Tris-HCl at pH 8 for 2.5 h, purified on a Superdex-30 column to remove excess dye followed by anion-exchange (mono Q 5/50 GL, GE Healthcare) chromatography to remove the unlabeled C34-Cys, concentrated, and stored.
Theoretical masses of the proteins used are as follows: 6-helix, 29470 Da; 5-helix, 24459 Da; coreS, 8284 Da (per subunit); coreSP, 5216 Da for the N-HR (GSHM-N37-SGLVPR) and 4975 Da for the C-HR (GSGG-C38); recombinant C34-Cys, 4610 Da; synthetic C34, 4290 Da; and T20, 4492 Da. Recombinant C34-Cys contains four nonnative residues, GSGG, at the N-terminus. The composition of purified proteins and the extent of AL647 labeling were verified by electrospray ionization mass spectrometry (ESI-MS). All experiments were conducted in 10 mM Tris-HCl (pH 7.6) and 150 mM NaCl (buffer A) at room temperature unless stated otherwise.
Native Polyacrylamide Gel Electrophoresis (native-PAGE), Size-Exclusion Chromatography (SEC), and SEC with Multiangle Light Scattering (SEC–MALS)
Samples were mixed to give a final concentration of 10 μM coreS or coreSP trimer and an increasing molar ratio from 1- to 10-fold for C34 or T20 as indicated below the gel panels. Following incubation for 30 min at room temperature, the samples were subjected to electrophoresis on 20% homogeneous PhastGels (GE Healthcare) using 4 μL for each six-lane applicator and native buffer strips. Gels were stained in PhastGel Blue R, destained, and digitized.
Samples for evaluating the displacement of C38-AL647 from coreSP-AL647 and concomitant binding of C34 were mixed to a final volume of 100 μL/injection, and incubated for at least 30 min, followed by application to a Superdex-75 column pre-equilibrated and run in buffer A at a flow rate of 0.5 mL/min.
Molecular masses were estimated by analytical SEC with in-line MALS (DAWN Heleos-II, Wyatt Technology Inc., Santa Barbara, CA), refractive index (Optilab T-rEX, Wyatt Technology Inc.), and UV (Waters 2487, Waters Corp., Milford, MA) detectors. Typically, the protein (150–200 μg in 100 μL) either by itself or mixed with a 5-fold molar excess of C34 was applied to a pre-equilibrated Superdex-75 column (1.0 cm × 30 cm, GE Healthcare) and eluted at a flow rate of 0.5 mL/min in buffer A. Samples when mixed with C34 peptide were incubated for at least 30 min prior to injection. Molecular masses were calculated using Astra version 6.1 provided with the instrument.
Circular Dichroism
CD spectra were recorded in buffer A at 20 °C on a JASCO J-810 spectropolarimeter using Spectra Manager software and a 0.1 cm path-length flat cell. Scans of coreS and coreSP were taken in the absence and presence of a 5-fold molar excess of C34. The α-helical content was determined using CDNN.50
Defining a Method for Estimating the Binding Affinity of C34 for the gp41 Six-Helix Bundle
SEC coupled with monitoring of the distribution of the AL647 specific absorbance at 609 and 650 nm and protein absorbance at 280 nm was used to quantify the interaction of C34 with coreS and coreSP. The absorption spectrum of AL647 (and specifically the ratio of absorbance at 609 to 650 nm) is responsive to the intermolecular proximity of dye molecules (see Figure S1A). As association of two or more C34-AL647 peptides with coreS or coreSP results in a decrease in the absorbance at 650 nm and a corresponding increase at 609 nm, we used the sum of the two absorbance values to measure the total C34-AL647 eluting in each peak. Figure S1B shows the dependence of this sum with an increasing level of C34-AL647 with each injection. The total absorbance (for unbound C34-AL647 and its 1:1 and 1:2 complexes) matches the absorbance predicted for the amount of C34-AL647 added in each injection and thus validates the SEC/spectroscopic method used for quantitation.
C34-AL647 (1.4–33.8 μM) was titrated against a constant coreS trimer concentration of 2.33 μM in a total reaction/injection volume of 100 μL. After mixing, samples were equilibrated for more than 1 h prior to injection onto a Superdex-75 column (1 cm × 30 cm) equilibrated and run in buffer A at a flow rate of 0.7 mL/min. Areas for C34-AL647, free and bound to coreS, were determined by integration of the peaks monitored at 280, 609, and 650 nm using PeakFit version 4.12 (Systat Software Inc., San Jose, CA). The area measured at 280 nm and the sum of areas measured at 609 and 650 nm were used for subsequent calculations. Fitting of the experimental titration data to the relevant equilibrium binding models was conducted numerically using the program FACSIMILE.51
DEER Analysis
Cysteine residues were introduced at the N- and C-termini of coreS (constructs termed N-Cys and C-Cys, respectively) by QuikChange mutagenesis. Deuteration and MTSL labeling of these two constructs were conducted as described previously52 and verified by ESI-MS. Data were collected on 50 μM (in subunits) N-Cys or C-Cys coreS samples in 10 mM Tris-HCl (pH 7.6) and 150 mM NaCl (buffer A) dissolved in a 30:70 mixture of d8-glycerol and 99.99% D2O in the absence or presence of a 1.2-fold molar excess (per coreS subunit) of C34. All DEER53 data were collected at Q-band (33.8 GHz) on a Bruker E-580 spectrometer equipped with a 150 W traveling wave tube amplifier and a model ER5107D2 resonator. All experiments employed 8 ns pump (ELDOR) π pulses, 12 ns π/2 and 24 ns π observe pulses, a 95 MHz frequency difference between pump and observe pulses, and a 3.0 ms shot repetition time. The pump frequency was centered at the field spectrum maximum. The 400 ns half-echo periods of the first echo were incremented eight times in 16 ns increments to average 2H modulation. The pump pulse was incremented in 16 ns steps for the C34-bound C-Cys coreS sample. All other experiments utilized 8 ns pump pulse increments. All data were collected at 50 K. Samples were placed in 1.1 mm internal diameter quartz tubes (Wilmad WG-221T-RB) and flash-frozen in liquid N2. Total data collection times varied from ~ 3 to ~ 19 h. A 30–34 ns window was used for echo integration. On the basis of additional data collected for an experiment employing a shorter second echo period (not shown), data collected during the last 3 μs of the second echo period were deemed to be distorted for the C34-bound C-Cys coreS sample, possibly because of a “2 + 1” echo that results from excitation overlap of the pump and observe pulses. Therefore, data collected 3 μs prior to the end of the second echo period were not fitted for the C34-bound C-Cys coreS sample as indicated in Figure 5B. P(r) curves shown in Figure 5 were generated by Tikhonov Regularization in DeerAnalysis2013.54 Ghost Suppression55 for three spins was utilized in all fits. The regularization parameter, α, was determined by examination of the relevant L-curves (α = 10 in all cases). A dimension of 3.0 was used for all exponential background corrections.
Figure 5.
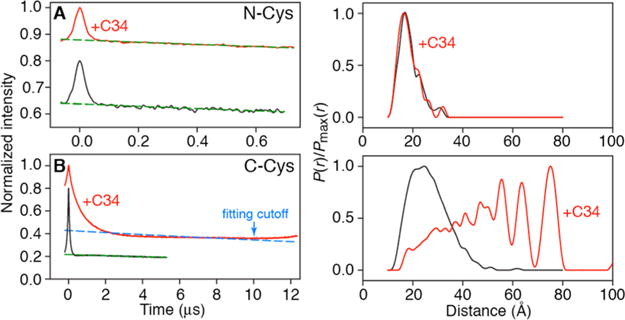
DEER EPR of N- and C-Cys nitroxide-labeled fully deuterated coreS constructs in the absence and presence of C34 peptide. Raw DEER data for N- and C-Cys MTSL deueterated coreS are shown in the left-hand graphs of panels A and B, respectively. Red and black curves represent data acquired with and without C34 peptide, respectively. Dashed dark green curves are the exponential background functions employed to separate the random intermolecular dipolar couplings from the desired intramolecular dipolar couplings. The results of the DeerAnalysis2013 Tikhonov Regularization fits54 of the background-corrected data (see Figure S5) are shown in the right-hand graphs. It should be noted that the broad array of long (as much as 75 Å) spin–spin distances for C-Cys coreS in the presence of C34 makes background correction of the raw dipolar evolution data challenging and reduces the accuracy of the modeled P(r) distance distribution for this system (red curve in the left graph of panel B), such that relative peak intensities and peak positions can vary by as much as 30% and 2 Å, respectively, depending upon how the baseline subtraction is done. This, however, does not affect the conclusion that binding of two C34 molecules to coreS results in concomitant displacement of two C28 C-HRs from the internal N-HR trimer of coreS and that the displaced C28 C-HRs adopt a wide range of random-coil conformations.
RESULTS AND DISCUSSION
Definition of Constructs and Peptides
Three constructs derived from the ectodomain of gp41 (Figure 1A), 6-helix, coreS, and coreSP, assemble to form a six-helix bundle (Figure 1B) that represents the fusogenic/postfusogenic state of gp41. The single-chain six-helix construct consists of three tandem repeats of the N-HR543–582-(L5)-C-HR625–662 segment connected by the five-residue spacer GSSGG; the L5 five-residue spacer (GGSGG) connects the N-HR and C-HR domains instead of the native immune-dominant linker (IL) domain of gp41. 5-helix is a truncated variant of 6-helix without the last C-terminal C-HR region and represents a prehairpin mimetic in which two neighboring N-HR helices are exposed. CoreS is a native six-helix bundle model comprising a trimer of three polypeptide chains, each bearing the arrangement N34546–579-(L6)-C28628–655 (Figure 1); L6 is a six-residue spacer (SGGRGG). CoreSP is composed of six peptides, three each of N36546–582 and C38625–662. Both coreS and coreSP are highly thermostable with melting temperatures of 80 and 68 °C, respectively (Figure S2). Peptides C34 and T20 span residues 628–661 and 638–673, respectively, of gp41. CoreSP bearing Alexafluor 647 dyes at the three C-termini of C38625–662 is termed coreSP-AL647, and C34 with the AL647 dye at its C-terminus is termed C34-AL647.
Binding of C34 to Single-Chain Six-Helix and Five-Helix Constructs
All experiments were conducted under nearly physiological conditions in 10 mM Tris-HCl buffer (pH 7.6) and 150 mM NaCl, ideal for stable trimerization of gp41,56 as well as for direct comparison with our earlier binding studies of bivalent and single-chain antibodies directed to the N-HR region of gp41 model proteins.44 6-helix and 5-helix serve as good models for assessing the binding of the exogenously added C34 peptide by SEC–MALS. In the case of 6-helix, addition of a 5-fold excess of C34 results in the appearance of a small shoulder to the left of the major 6-helix peak that can be attributed to weak binding of C34 as a 1:1 complex (Figure 2A), presumably via displacement of the C-terminal C-HR region (see below). In the case of 5-helix that lacks the last C-HR region, C34 binds with a 1:1 stoichiometry with a mass increase that clearly corresponds to the expected mass of C34 (Figure 2B).
Figure 2.
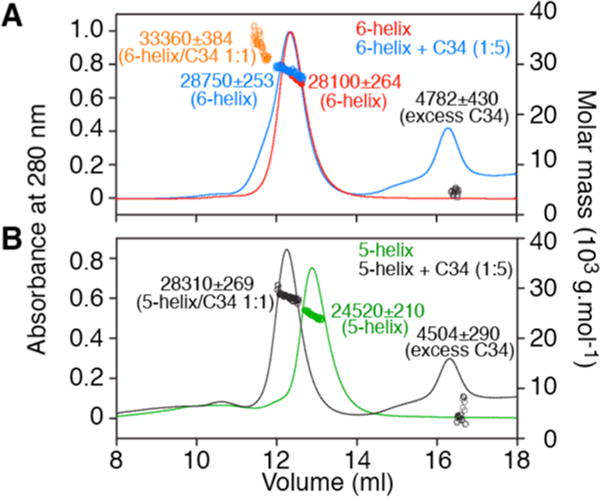
Molecular mass estimation by SEC–MALS under native conditions of 6-helix and 5-helix constructs in the presence or absence of excess C34 peptide. (A) Injection of 200 μg of 6-helix alone (red) and with a 5-fold molar excess of C34 (blue). (B) Injection of 200 μg of 5-helix alone (green) and with a 5-molar excess of C34 (black). Observed masses and compositions are indicated beside the peaks. Experiments were conducted in 10 mM Tris-HCl (pH 7.6) and 150 mM NaCl.
Binding of C34 to the Native-like gp41 Mimetic, CoreS
Studies for assessing binding of C34 to gp41 constructs containing the FPPR or MPER flanking region at the N- or C-terminus, respectively, of the coreS domain (Figure 1A) are not feasible because addition of detergent [e.g., dodecylphosphocholine (DPC)], which is essential to maintain the solubility of such longer constructs, dissociates the coreS trimer into monomers, even at pH 6, with no NMR-observable intramolecular contacts between the N-HR and CH-R regions of the monomer.49,57 Individual NH-R and CH-R peptides also associate with DPC.49 CoreS and coreSP, on the other hand, are highly soluble under nearly physiological conditions, permitting a variety of analyses as described below in the absence of DPC.
SEC–MALS shows that addition of excess C34 to coreS results in the formation of a complex with a binding stoichiometry of one coreS trimer to two C34 peptides with no dissociation of the coreS trimer (Figure 3A). SEC–MALS of coreSP, which lacks the linker connecting the N-HR and C-HR regions, was used to assess whether binding of C34 occurs via displacement of the C-HR region. The appearance of a peak corresponding to the C38 peptide of coreSP upon addition of excess C34, coupled to the expected small reduction in the molecular weight of the “coreSP” peak, demonstrates that binding of C34 to coreSP is accompanied by displacement of C38. This result is confirmed by size-exclusion chromatography of coreSP bearing the Alexafluor 647 dye at the C-terminus of each C38 peptide that shows that addition of C34 results in the appearance of a C38-AL647 peak (Figure 3C,D).
Figure 3.
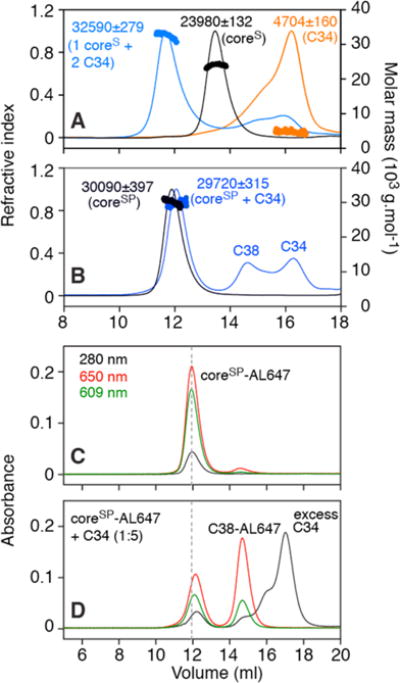
Size-exclusion chromatography elution profiles and mass analysis under native conditions of coreS, coreSP, and their complexes with C34. (A) SEC–MALS of the coreS trimer in the presence of a 5-fold molar excess of C34 (blue trace). Experimental masses and compositions are indicated beside the peaks. The peak for the complex is consistent with the binding of two C34 molecules to one coreS trimer. Control elution profiles (200 μg/100 μL injection) of coreS and C34 are colored black and orange, respectively. (B) SEC–MALS of coreSP (six-helix bundle assembled with individual N-HR and C-HR peptides) mixed with a 5-fold molar excess of C34 (blue trace). Observed peaks corresponding to C-HR peptide (C38, residues 625–662) and a complex of coreSP with C34 are consistent with displacement of C38 from coreSP by added excess C34. The elution profile and estimated mass of coreSP (control) are colored black. (C) Retention of coreSP labeled with AL647 matches the elution profile of unlabeled coreSP shown in panel B. (D) Displacement of AL647-labeled C38 by added C34 (5-fold molar excess) is consistent with data shown in panel B. Note that the relative extinction coefficients at 609 and 650 nm of AL647 differ depending on the proximity of the dyes to one another (elaborated in the text and Figure S1). Note also that two separate columns were used for SEC–MALS (panels A and B, flow rate of 0.5 mL/min) and absorbance measured at three wavelengths (panels C and D, flow rate of 0.7 mL/min); as a result, the retention volume for free C34 is slightly larger for the latter than the former. Experiments were conducted in 10 mM Tris-HCl (pH 7.6) and 150 mM NaCl.
Determination of the Apparent Binding Affinity of C34 to CoreS and CoreSP
Titration of C34 (0.05–0.1 mM) into 5–10 μM coreS in the experimental buffer [10 mM Tris-HCl (pH 7.6) and 150 mM NaCl] at 25 °C did not yield a heat signature in ITC experiments conducted in a Microcal iTC200 instrument. This result resembles our earlier observation of no heat response upon titration of coreS with a neutralizing antibody that exhibits tight binding to coreS as revealed by native band-shift assays.44 We therefore made use of C34 labeled with the Alexafluor 647 dye [C34-AL647 (Figure 1B)] to quantify the binding of C34 to coreS and coreSP.
CoreS was incubated with increasing concentrations of C34-AL647, and the resulting mixtures were analyzed by size-exclusion chromatography. As the relative absorbances at 609 and 650 nm differ depending on the intermolecular proximity of the dye molecules to one another (see Figure S1), the sum of the absorbances at these wavelengths was used to quantify the dye. The sum of peak areas at 609 and 650 nm for C34-AL647 free and in complex with coreS corresponds to the total concentration of C34-AL647 added to each sample, thereby validating this approach (Figure S1B). The sequential formation of 1:1 and 1:2 coreS–C34-AL647 complexes with increasing C34-AL647 concentrations, measured at three wavelengths, shows a decrease in absorbance at 280 nm of the peak with an elution volume of 13.7 mL corresponding to free coreS concomitant with the appearance of a 1:1 complex at ~ 12.5 mL, and subsequent appearance of a 1:2 complex (~ 12.0 mL) (Figure S3). A similar experiment in which C34-AL647 was added to coreSP shows a less pronounced shift in the 280 nm absorbance corresponding to complex formation such that complexes with C34 are not readily distinguishable from coreSP based on their elution volumes (Figure S4).
Dissociation of the coreS trimer at ambient temperature in 10 mM Tris-HCl (pH 7.6) and 150 mM NaCl is expected to be <0.25 μM based on estimating the mass as a function of the decreasing concentration (from 2 to 0.25 μM) by composition gradient–MALS analysis, the detection limit for coreS (data not shown). As both coreS and coreSP elute as stable trimers at an injected concentration of 2.33 μM with no discernible dissociation within the time scale of their elution and associated dilution by size-exclusion chromatography (~ 10-fold), even for coreSP that is assembled as peptides, we conclude that any exchange between components in the equilibrium mixture established prior to injection into the column is slow on the time scale of the experiment and therefore no dilution factors or re-equilibration during the course of elution on the column needs be considered during the analysis of the equilibrium binding data.
Because the SEC–MALS and band-shift assays provide no evidence of the existence of a 1:3 coreS–(C34)3 complex, the peak areas as a function of added C34 were analyzed in terms of formation of 1:1 and 1:2 complexes:
| (1) |
while for coreSP, (N37)3(C38)3, the data were analyzed in terms of three successive exchange reactions:
| (2) |
The summed peak areas at 609 and 650 nm are directly proportional to the concentration of bound C34 (cf. Figure S1), and thus, the measured peak area609+650 for coreS is given by
| (3) |
and for coreSP by
| (4) |
where S is a scale factor whose value is optimized during nonlinear least-squares minimization. In the case of coreS, we were also able to make use of the 280 nm data as the 1:1 and 1:2 complexes can be distinguished from free coreS (see Figure S3B). The combined area for the 1:1 and 1:2 peaks is then given by
| (5) |
where λ is the ratio of the extinction coefficients at 280 nm for the 1:2 to 1:1 complexes. λ has a value of 1.188 determined from the ε280 values, calculated from amino acid sequence, of 66460 and 78950 M−1 cm−1 for the 1:1 and 1:2 complexes, respectively.58 The resulting best fits to the experimental data are shown in panels A and B of Figure 4 for coreS and coreSP, respectively. The data show no evidence of any cooperativity: for coreS, K1 = K2 = (1.0 ± 0.1) × 106 M−1, and for coreSP, K1 = K2 = K3 = 0.9 ± 0.1.
Figure 4.
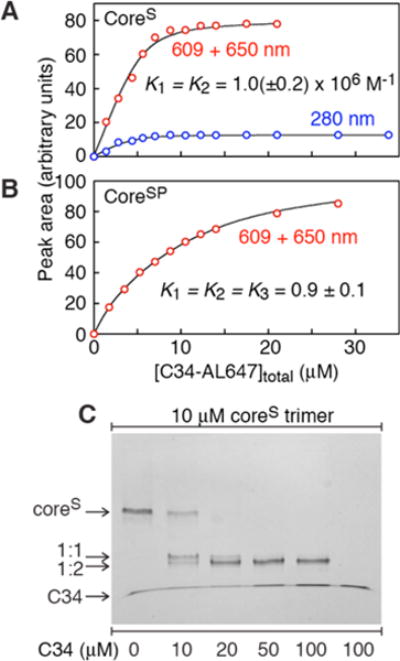
Characterization of the binding of C34 to coreS and coreSP. Fits (black lines) to the experimental data (red circles for the peak area at 609 + 650 nm, blue circles for the peak area at 280 nm) obtained by mixing 2.33 μM (A) coreS trimer and (B) coreSP (as a six-helix bundle) with increasing concentrations of C34-AL647 followed by size-exclusion chromatography and quantification (see Figures S3 and S4, respectively). For experimental details and data analysis, see the text. (C) Band shifts upon addition of increasing concentrations of C34 to a constant amount of coreS trimer analyzed by 20% homogeneous native-PAGE. Experiments were conducted in 10 mM Tris-HCl (pH 7.6) and 150 mM NaCl.
The value of 0.9 for the equilibrium constant for the exchange reaction of C34 with C38 in the case of coreSP is expected because one would predict that the affinity of C38 for the N-HR (N37)3 trimer would be only minimally larger than for C34.
The value of 106 M−1 for the equilibrium association constant for the binding of C34 to coreS is also reasonable given that binding of C34 to coreS requires displacement of the linked C28 C-HR from N34. The likely reason that a third molecule of C34 cannot bind to coreS is presumably due to the fact the resulting displaced C28 C-HRs are still covalently linked to the N-HR via a five-residue linker, and hence, the effective local concentration of C28 not in contact with the internal N-HR trimer, (N34)3, is extremely high.
Native-PAGE of complexes also indicates that even at 3:1 and 3:2 coreS:C34 ratios (Figure 4C) a significant amount of complex comprising two C34 molecules bound to one coreS trimer is formed in a manner consistent with the binding data. The migration of free C34, which runs at the dye front, is retarded when it is in complex with coreS. In accordance, the band doublet observed in Figure 4C (lane 2) likely corresponds to 1:1 and 1:2 complexes of coreS with C34, with the 1:2 complex migrating slightly faster than the 1:1 complex.
Conformation of the C-HR of CoreS Displaced by C34
To address the state of the displaced C28 C-HR of coreS upon C34 binding, we conducted EPR pulsed double electron–electron resonance (DEER) and CD measurements that provide distance (between nitroxide spin-labels) and secondary structure information, respectively.
Fully deuterated coreS constructs with nitroxide spin-labels added either at the N-terminus of the N-HR (construct N-Cys coreS) or at the C-terminus of the C-HR (construct C-Cys coreS) were employed. By significantly increasing the spin-label phase memory relaxation time, deuteration abolishes the dependence of the P(r) distance distribution on the length of the second echo period in the DEER experiment.52
Addition of C34 to N-Cys coreS has a negligible effect on either the raw DEER dipolar evolution data (Figure 5A, left graph) or the derived P(r) distance distributions [obtained by Tikhonov regularization (Figure 5A, right graph)] that reflect the short intersubunit distances (<20 Å) between the nitroxide spin-labels in the trimer (see also Figure S5). One can therefore conclude that the N-HR helical trimer of coreS is essentially unperturbed upon binding C34 and concomitant displacement of the C28 C-HR.
In the case of the C-Cys coreS, however, a very large increase in the average distance between electron spins upon addition of C34 is immediately apparent from inspection of the raw DEER dipolar evolution data (Figure 5B, left graph), ranging from 20 to 75 Å (red curve in the right graph of Figure 5B), indicating that the displaced C28 C-HRs attached to the N34 N-HR by a five-residue linker adopt a wide variety of presumably random-coil conformations.
CD spectra of C34, and coreS and coreSP in the absence and presence of C34, are shown in Figure 6. Binding of C34 to the N-HR (N37)3 trimer of coreSP with concomitant dissociation of C38 results in no change in helicity (Figure 6B), as expected because the number of residues in contact with the N-HR (N37)3 timer is predicted to be similar for C34 and C3813,14 and free C38 is a random coil. In the case of coreS, however, binding of two C34 molecules together with displacement of two C28 C-HR chains results in an ~ 18% increase in helicity (Figure 6A), corresponding to an additional 30 residues of the trimer in a helical conformation (6 × 3 from the N34 N-HR trimer and 2 × 6 from two molecules of C34 bound).
Figure 6.
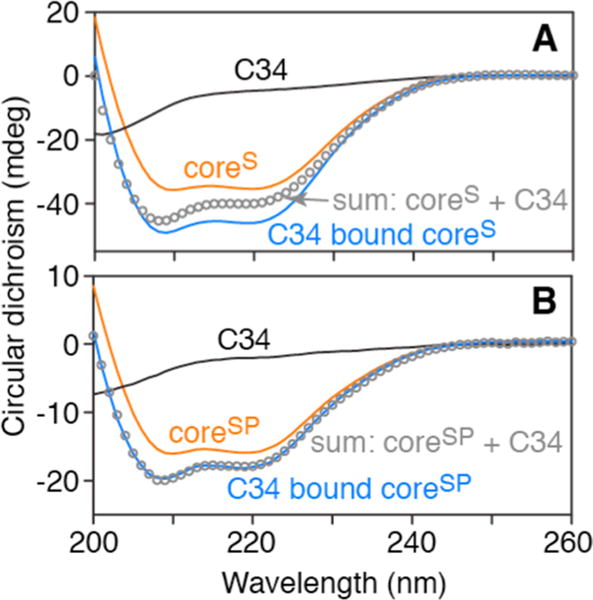
CD analysis of coreS and coreSP in the absence and presence of a 5-fold molar excess of C34. CD spectra of (A) coreS (7 μM as a trimer) and (B) coreSP (2.5 μM as a trimer, N-HR and C-HR peptides calculated as 1 unit) in the absence (orange line) and presence (blue line) of a 5-fold excess of C34. C34 alone (black line) shows no helical signature. The gray circles are the sums of the CD spectra of coreS or coreSP and C34.
T20 Does Not Bind to CoreS
The T20 peptide (also known as Enfuviritide or Fuzeon) comprising residues 638–673 of gp41 is a drug in clinical use as an inhibitor of HIV-1 fusion.59–61 Although T20 overlaps a major part of the C34 sequence, it lacks residues 628–637 of C34 at its N-terminus but extends 11 residues into the MPER domain at its C-terminus (Figure 1). The partial sequence overlap of T20 with C34 had suggested a similar mode of interaction with gp41 analogues. However, we were unable to detect binding of T20 to coreS (Figure 7A) or 5-helix (Figure 7B) under conditions where binding of C34 is clearly evident (Figures 4C and 7B), suggesting a different mode of action with regard to inhibition of HIV-1 fusion.62 This is supported by previous findings that binding of T20 to 5-helix is 6 orders of magnitude weaker than that of C37, a peptide similar to C34 but with a three-residue N-terminal extension,63 and that enhanced membrane interactions of T20 via the C-terminal WNWF sequence are an essential determinant of T20 potency.64–66 As T20 could not be fractionated on a Superdex-75 column in the current experimental buffer because of nonspecific interactions with the column matrix, similar analysis as described for the displacement of labeled C38-AL647 from coreSP-AL647 by C34 (Figure 3C,D) could not be conducted by adding T20 to the coreSP-AL647 trimer.
Figure 7.
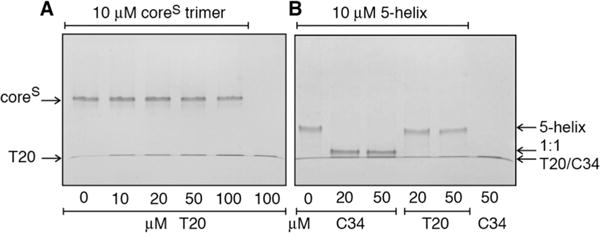
Assessment of binding of the T20 peptide to (A) coreS and (B) 5-helix by native-PAGE. Binding of C34 to 5-helix is shown in panel B as a positive control with a 1:1 stoichiometry of binding (see Figure 2).
CONCLUDING REMARKS
Using a variety of biophysical techniques, we have shown that the C34 HIV-1 fusion inhibitory peptide can bind not only to prehairpin intermediate mimetics of gp41 in which the N-HR trimer is fully solvent-exposed but also to the fusogenic/postfusogenic six-helix bundle conformation. In contrast to monoclonal antibodies targeted against the N-HR trimer that are also capable of binding to six-helix bundle mimetics via a small exposed hydrophobic pocket formed by the N-HR helices,44 binding of C34 occurs via complete displacement of the external C-HR helices. These results may relate to the conclusions of Markosyan et al.17 that the time window of C34 fusion inhibitory activity can extend from the point at which the prehairpin intermediate of gp41 becomes accessible through the formation of late prebundle intermediates and labile pore formation, but not after irreversible formation of the six-helix bundle required for robust pore formation and enlargement. The coreS model may represent a conformational state in trimer stability among a continuum of states similar to a late prebundle because coreS permits displacement of the C-HR by C34 and thus provides a basis for exploring the binding of C34 in the absence of DPC and possibly of the binding of T20 to longer gp41 mimetics that span either the FPPR or MPER regions, or both, in membrane-mimicking environments. Additionally, the method described here for monitoring binding to the six-helix bundle of the gp41 ectodomain by displacement of dye labeled C38 C-HR may prove to be useful for facile screening of compounds with properties similar to those of C34.
Supplementary Material
Acknowledgments
We thank Jane M. Sayer, Julien Roche, and Ad Bax for helpful discussions and Annie Aniana for technical assistance. T20 was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. We acknowledge use of the National Institute of Diabetes and Digestive and Kidney Diseases Advanced Mass Spectrometry Core Facility.
Funding
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, and the Intramural AIDS-Targeted Program of the Office of the Director, National Institutes of Health (to G.M.C.).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.bio-chem.5b01021.
Five supplementary figures pertaining to the properties of the AL647 dye, thermal melting, additional size-exclusion chromatography profiles, and EPR data (PDF)
Notes
The authors declare no competing financial interest.
References
- 1.Freed EO, Martin MA. The role of human immunodeficiency virus type 1 envelope glycoproteins in virus infection. J Biol Chem. 1995;270:23883–23886. doi: 10.1074/jbc.270.41.23883. [DOI] [PubMed] [Google Scholar]
- 2.Moore JP, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 3.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 4.Eckert DM, Kim PS. Mechanisms of viral membrane fusion and its inhibition. Annu Rev Biochem. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- 5.Gallo SA, Finnegan CM, Viard M, Raviv Y, Dimitrov A, Rawat SS, Puri A, Durell S, Blumenthal R. The HIV Env-mediated fusion reaction. Biochim Biophys Acta, Biomembr. 2003;1614:36–50. doi: 10.1016/s0005-2736(03)00161-5. [DOI] [PubMed] [Google Scholar]
- 6.Miyauchi K, Kim Y, Latinovic O, Morozov V, Melikyan GB. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell. 2009;137:433–444. doi: 10.1016/j.cell.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumenthal R, Durell S, Viard M. HIV entry and envelope glycoprotein-mediated fusion. J Biol Chem. 2012;287:40841–40849. doi: 10.1074/jbc.R112.406272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Julien JP, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, Klasse PJ, Burton DR, Sanders RW, Moore JP, Ward AB, Wilson IA. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science. 2013;342:1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyumkis D, Julien JP, de Val N, Cupo A, Potter CS, Klasse PJ, Burton DR, Sanders RW, Moore JP, Carragher B, Wilson IA, Ward AB. Cryo-EM structure of a fully glycosylated soluble cleaved HIV-1 envelope trimer. Science. 2013;342:1484–1490. doi: 10.1126/science.1245627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuta RA, Wild CT, Weng Y, Weiss CD. Capture of an early fusion-active conformation of HIV-1 gp41. Nat Struct Biol. 1998;5:276–279. doi: 10.1038/nsb0498-276. [DOI] [PubMed] [Google Scholar]
- 11.Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 12.Root MJ, Steger HK. HIV-1 gp41 as a target for viral entry inhibition. Curr Pharm Des. 2004;10:1805–1825. doi: 10.2174/1381612043384448. [DOI] [PubMed] [Google Scholar]
- 13.Chan DC, Fass D, Berger JM, Kim PS. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 14.Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 15.Caffrey M, Cai M, Kaufman J, Stahl SJ, Wingfield PT, Covell DG, Gronenborn AM, Clore GM. Three-dimensional solution structure of the 44 kDa ectodomain of SIV gp41. EMBO J. 1998;17:4572–4584. doi: 10.1093/emboj/17.16.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan K, Liu J, Wang J, Shen S, Lu M. Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc Natl Acad Sci U S A. 1997;94:12303–12308. doi: 10.1073/pnas.94.23.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markosyan RM, Cohen FS, Melikyan GB. HIV-1 envelope proteins complete their folding into six-helix bundles immediately after fusion pore formation. Mol Biol Cell. 2003;14:926–938. doi: 10.1091/mbc.E02-09-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melikyan GB. Common principles and intermediates of viral protein-mediated fusion: the HIV-1 paradigm. Retrovirology. 2008;5:111. doi: 10.1186/1742-4690-5-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wild CT, Shugars DC, Greenwell TK, McDanal CB, Matthews TJ. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci U S A. 1994;91:9770–9774. doi: 10.1073/pnas.91.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louis JM, Bewley CA, Clore GM. Design and properties of NCCG-gp41, a chimeric gp41 molecule with nanomolar HIV fusion inhibitory activity. J Biol Chem. 2001;276:29485–29489. doi: 10.1074/jbc.C100317200. [DOI] [PubMed] [Google Scholar]
- 21.Root MJ, Kay MS, Kim PS. Protein design of an HIV-1 entry inhibitor. Science. 2001;291:884–888. doi: 10.1126/science.1057453. [DOI] [PubMed] [Google Scholar]
- 22.Louis JM, Nesheiwat I, Chang L, Clore GM, Bewley CA. Covalent trimers of the internal N-terminal trimeric coiled-coil of gp41 and antibodies directed against them are potent inhibitors of HIV envelope-mediated cell fusion. J Biol Chem. 2003;278:20278–20285. doi: 10.1074/jbc.M301627200. [DOI] [PubMed] [Google Scholar]
- 23.Kilgore NR, Salzwedel K, Reddick M, Allaway GP, Wild CT. Direct evidence that C-peptide inhibitors of human immunodeficiency virus type 1 entry bind to the gp41 N-helical domain in receptor-activated viral envelope. J Virol. 2003;77:7669–7672. doi: 10.1128/JVI.77.13.7669-7672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Root MJ, Hamer DH. Targeting therapeutics to an exposed and conserved binding element of the HIV-1 fusion protein. Proc Natl Acad Sci U S A. 2003;100:5016–5021. doi: 10.1073/pnas.0936926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steger HK, Root MJ. Kinetic dependence to HIV-1 entry inhibition. J Biol Chem. 2006;281:25813–25821. doi: 10.1074/jbc.M601457200. [DOI] [PubMed] [Google Scholar]
- 26.Welch BD, Francis JN, Redman JS, Paul S, Weinstock MT, Reeves JD, Lie YS, Whitby FG, Eckert DM, Hill CP, Root MJ, Kay MS. Design of a potent D-peptide HIV-1 entry inhibitor with a strong barrier to resistance. J Virol. 2010;84:11235–11244. doi: 10.1128/JVI.01339-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan DC, Chutkowski CT, Kim PS. Evidence that a prominent cavity in the coiled coil of HIV type 1 gp41 is an attractive drug target 2. Proc Natl Acad Sci U S A. 1998;95:15613–15617. doi: 10.1073/pnas.95.26.15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golding H, Zaitseva M, de Rosny E, King LR, Manischewitz J, Sidorov I, Gorny MK, Zolla-Pazner S, Dimitrov DS, Weiss CD. Dissection of human immunodeficiency virus type 1 entry with neutralizing antibodies to gp41 fusion intermediates. J Virol. 2002;76:6780–6790. doi: 10.1128/JVI.76.13.6780-6790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louis JM, Bewley CA, Gustchina E, Aniana A, Marius Clore G. Characterization and HIV-1 fusion inhibitory properties of monoclonal Fabs obtained from a human non-immune phage library selected against diverse epitopes of the ectodomain of HIV-1 gp41. J Mol Biol. 2005;353:945–951. doi: 10.1016/j.jmb.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 30.Gustchina E, Louis JM, Lam SN, Bewley CA, Clore GM. A monoclonal Fab derived from a human nonimmune phage library reveals a new epitope on gp41 and neutralizes diverse human immunodeficiency virus type 1 strains. J Virol. 2007;81:12946–12953. doi: 10.1128/JVI.01260-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gustchina E, Louis JM, Frisch C, Ylera F, Lechner A, Bewley CA, Clore GM. Affinity maturation by targeted diversification of the CDR-H2 loop of a monoclonal Fab derived from a synthetic naive human antibody library and directed against the internal trimeric coiled-coil of gp41 yields a set of Fabs with improved HIV-1 neutralization potency and breadth. Virology. 2009;393:112–119. doi: 10.1016/j.virol.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gustchina E, Li M, Louis JM, Anderson DE, Lloyd J, Frisch C, Bewley CA, Gustchina A, Wlodawer A, Clore GM. Structural basis of HIV-1 neutralization by affinity matured Fabs directed against the internal trimeric coiled-coil of gp41. PLoS Pathog. 2010;6:e1001182. doi: 10.1371/journal.ppat.1001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gustchina E, Li M, Ghirlando R, Schuck P, Louis JM, Pierson J, Rao P, Subramaniam S, Gustchina A, Clore GM, Wlodawer A. Complexes of neutralizing and non-neutralizing affinity matured fabs with a mimetic of the internal trimeric coiled-coil of HIV-1 gp41. PLoS One. 2013;8:e78187. doi: 10.1371/journal.pone.0078187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller MD, Geleziunas R, Bianchi E, Lennard S, Hrin R, Zhang H, Lu M, An Z, Ingallinella P, Finotto M, Mattu M, Finnefrock AC, Bramhill D, Cook J, Eckert DM, Hampton R, Patel M, Jarantow S, Joyce J, Ciliberto G, Cortese R, Lu P, Strohl W, Schleif W, McElhaugh M, Lane S, Lloyd C, Lowe D, Osbourn J, Vaughan T, Emini E, Barbato G, Kim PS, Hazuda DJ, Shiver JW, Pessi A. A human monoclonal antibody neutralizes diverse HIV-1 isolates by binding a critical gp41 epitope. Proc Natl Acad Sci U S A. 2005;102:14759–14764. doi: 10.1073/pnas.0506927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luftig MA, Mattu M, Di Giovine P, Geleziunas R, Hrin R, Barbato G, Bianchi E, Miller MD, Pessi A, Carfi A. Structural basis for HIV-1 neutralization by a gp41 fusion intermediate-directed antibody. Nat Struct Mol Biol. 2006;13:740–747. doi: 10.1038/nsmb1127. [DOI] [PubMed] [Google Scholar]
- 36.Nelson JD, Kinkead H, Brunel FM, Leaman D, Jensen R, Louis JM, Maruyama T, Bewley CA, Bowdish K, Clore GM, Dawson PE, Frederickson S, Mage RG, Richman DD, Burton DR, Zwick MB. Antibody elicited against the gp41 N-heptad repeat (NHR) coiled-coil can neutralize HIV-1 with modest potency but non-neutralizing antibodies also bind to NHR mimetics. Virology. 2008;377:170–183. doi: 10.1016/j.virol.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choudhry V, Zhang MY, Sidorov IA, Louis JM, Harris I, Dimitrov AS, Bouma P, Cham F, Choudhary A, Rybak SM, Fouts T, Montefiori DC, Broder CC, Quinnan GV, Jr, Dimitrov DS. Cross-reactive HIV-1 neutralizing monoclonal antibodies selected by screening of an immune human phage library against an envelope glycoprotein (gp140) isolated from a patient (R2) with broadly HIV-1 neutralizing antibodies. Virology. 2007;363:79–90. doi: 10.1016/j.virol.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang MY, Vu BK, Choudhary A, Lu H, Humbert M, Ong H, Alam M, Ruprecht RM, Quinnan G, Jiang S, Montefiori DC, Mascola JR, Broder CC, Haynes BF, Dimitrov DS. Cross-reactive human immunodeficiency virus type 1-neutralizing human monoclonal antibody that recognizes a novel conformational epitope on gp41 and lacks reactivity against self-antigens. J Virol. 2008;82:6869–6879. doi: 10.1128/JVI.00033-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eckert DM, Shi Y, Kim S, Welch BD, Kang E, Poff ES, Kay MS. Characterization of the steric defense of the HIV-1 gp41 N-trimer region. Protein Sci. 2008;17:2091–2100. doi: 10.1110/ps.038273.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabin C, Corti D, Buzon V, Seaman MS, Lutje Hulsik D, Hinz A, Vanzetta F, Agatic G, Silacci C, Mainetti L, Scarlatti G, Sallusto F, Weiss R, Lanzavecchia A, Weissenhorn W. Crystal structure and size-dependent neutralization properties of HK20, a human monoclonal antibody binding to the highly conserved heptad repeat 1 of gp41. PLoS Pathog. 2010;6:e1001195. doi: 10.1371/journal.ppat.1001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai RP, Hock M, Radzimanowski J, Tonks P, Hulsik DL, Effantin G, Seilly DJ, Dreja H, Kliche A, Wagner R, Barnett SW, Tumba N, Morris L, LaBranche CC, Montefiori DC, Seaman MS, Heeney JL, Weissenhorn W. A fusion intermediate gp41 immunogen elicits neutralizing antibodies to HIV-1. J Biol Chem. 2014;289:29912–29926. doi: 10.1074/jbc.M114.569566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bewley CA, Louis JM, Ghirlando R, Clore GM. Design of a novel peptide inhibitor of HIV fusion that disrupts the internal trimeric coiled-coil of gp41. J Biol Chem. 2002;277:14238–14245. doi: 10.1074/jbc.M201453200. [DOI] [PubMed] [Google Scholar]
- 43.Gustchina E, Bewley CA, Clore GM. Sequestering of the prehairpin intermediate of gp41 by peptide N36Mut(e,g) potentiates the human immunodeficiency virus type 1 neutralizing activity of monoclonal antibodies directed against the N-terminal helical repeat of gp41 1. J Virol. 2008;82:10032–10041. doi: 10.1128/JVI.01050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Louis JM, Aniana A, Lohith K, Sayer JM, Roche J, Bewley CA, Clore GM. Binding of HIV-1 gp41-directed neutralizing and non-neutralizing fragment antibody binding domain (Fab) and single chain variable fragment (ScFv) antibodies to the ectodomain of gp41 in the pre-hairpin and six-helix bundle conformations. PLoS One. 2014;9:e104683. doi: 10.1371/journal.pone.0104683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng Y, Zheng Q, Ketas TJ, Moore JP, Lu M. Protein design of a bacterially expressed HIV-1 gp41 fusion inhibitor. Biochemistry. 2007;46:4360–4369. doi: 10.1021/bi7001289. [DOI] [PubMed] [Google Scholar]
- 46.Chong H, Yao X, Sun J, Qiu Z, Zhang M, Waltersperger S, Wang M, Cui S, He Y. The M-T hook structure is critical for design of HIV-1 fusion inhibitors. J Biol Chem. 2012;287:34558–34568. doi: 10.1074/jbc.M112.390393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He Y, Liu S, Jing W, Lu H, Cai D, Chin DJ, Debnath AK, Kirchhoff F, Jiang S. Conserved residue Lys574 in the cavity of HIV-1 Gp41 coiled-coil domain is critical for six-helix bundle stability and virus entry. J Biol Chem. 2007;282:25631–25639. doi: 10.1074/jbc.M703781200. [DOI] [PubMed] [Google Scholar]
- 48.Gustchina E, Hummer G, Bewley CA, Clore GM. Differential inhibition of HIV-1 and SIV envelope-mediated cell fusion by C34 peptides derived from the C-terminal heptad repeat of gp41 from diverse strains of HIV-1, HIV-2, and SIV. J Med Chem. 2005;48:3036–3044. doi: 10.1021/jm049026h. [DOI] [PubMed] [Google Scholar]
- 49.Roche J, Louis JM, Aniana A, Ghirlando R, Bax A. Complete dissociation of the HIV-1 gp41 ectodomain and membrane proximal regions upon phospholipid binding. J Biomol NMR. 2015;61:235–248. doi: 10.1007/s10858-015-9900-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bohm G, Muhr R, Jaenicke R. Quantitative analysis of protein far UV circular dichroism spectra by neural networks. Protein Eng, Des Sel. 1992;5:191–195. doi: 10.1093/protein/5.3.191. [DOI] [PubMed] [Google Scholar]
- 51.Chance EMC, Jones IP, Kirby CR. Atomic Energy Research Establishment Report R8775. Her Majesty’s Stationery Office; London: 1979. FACSIMILE: A computer program for flow and chemistry simulation and general initial value problems. [Google Scholar]
- 52.Baber JL, Louis JM, Clore GM. Dependence of distance distributions derived from double electron-electron resonance pulsed EPR spectroscopy on pulse-sequence time. Angew Chem, Int Ed. 2015;54:5336–5339. doi: 10.1002/anie.201500640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pannier M, Veit S, Godt A, Jeschke G, Spiess HW. Dead-time free measurement of dipole-dipole interactions between electron spins. J Magn Reson. 2000;142:331–340. doi: 10.1006/jmre.1999.1944. [DOI] [PubMed] [Google Scholar]
- 54.Jeschke G, Chechik V, Ionita P, Godt A, Zimmermann H, Banham J, Timmel CR, Hilger D, Jung H. DeerAnalysis2006 - a comprehensive software package for analyzing pulsed ELDOR data. Appl Magn Reson. 2006;30:473–498. [Google Scholar]
- 55.von Hagens T, Polyhach Y, Sajid M, Godt A, Jeschke G. Suppression of ghost distances in multiple-spin double electron-electron resonance. Phys Chem Chem Phys. 2013;15:5854–5866. doi: 10.1039/c3cp44462g. [DOI] [PubMed] [Google Scholar]
- 56.Dai Z, Tao Y, Liu N, Brenowitz MD, Girvin ME, Lai JR. Conditional trimerization and lytic activity of HIV-1 gp41 variants containing the membrane-associated segments. Biochemistry. 2015;54:1589–1599. doi: 10.1021/bi501376f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roche J, Louis JM, Grishaev A, Ying J, Bax A. Dissociation of the trimeric gp41 ectodomain at the lipid-water interface suggests an active role in HIV-1 Env-mediated membrane fusion. Proc Natl Acad Sci U S A. 2014;111:3425–3430. doi: 10.1073/pnas.1401397111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anthis NJ, Clore GM. Sequence-specific determination of protein and peptide concentrations by absorbance at 205 nm. Protein Sci. 2013;22:851–858. doi: 10.1002/pro.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lalezari JP, Henry K, O’Hearn M, Montaner JS, Piliero PJ, Trottier B, Walmsley S, Cohen C, Kuritzkes DR, Eron JJ, Jr, Chung J, DeMasi R, Donatacci L, Drobnes C, Delehanty J, Salgo M. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N Engl J Med. 2003;348:2175–2185. doi: 10.1056/NEJMoa035026. [DOI] [PubMed] [Google Scholar]
- 60.Lazzarin A, Clotet B, Cooper D, Reynes J, Arasteh K, Nelson M, Katlama C, Stellbrink HJ, Delfraissy JF, Lange J, Huson L, DeMasi R, Wat C, Delehanty J, Drobnes C, Salgo M. Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. N Engl J Med. 2003;348:2186–2195. doi: 10.1056/NEJMoa035211. [DOI] [PubMed] [Google Scholar]
- 61.Matthews T, Salgo M, Greenberg M, Chung J, DeMasi R, Bolognesi D. Enfuvirtide: the first therapy to inhibit the entry of HIV-1 into host CD4 lymphocytes. Nat Rev Drug Discovery. 2004;3:215–225. doi: 10.1038/nrd1331. [DOI] [PubMed] [Google Scholar]
- 62.Liu S, Lu H, Niu J, Xu Y, Wu S, Jiang S. Different from the HIV fusion inhibitor C34, the anti-HIV drug Fuzeon (T-20) inhibits HIV-1 entry by targeting multiple sites in gp41 and gp120. J Biol Chem. 2005;280:11259–11273. doi: 10.1074/jbc.M411141200. [DOI] [PubMed] [Google Scholar]
- 63.Champagne K, Shishido A, Root MJ. Interactions of HIV-1 inhibitory peptide T20 with the gp41 N-HR coiled coil. J Biol Chem. 2009;284:3619–3627. doi: 10.1074/jbc.M809269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peisajovich SG, Gallo SA, Blumenthal R, Shai Y. C-terminal octylation rescues an inactive T20 mutant: implications for the mechanism of HIV/SIMIAN immunodeficiency virus-induced membrane fusion. J Biol Chem. 2003;278:21012–21017. doi: 10.1074/jbc.M212773200. [DOI] [PubMed] [Google Scholar]
- 65.Wexler-Cohen Y, Shai Y. Demonstrating the C-terminal boundary of the HIV 1 fusion conformation in a dynamic ongoing fusion process and implication for fusion inhibition. FASEB J. 2007;21:3677–3684. doi: 10.1096/fj.07-8582com. [DOI] [PubMed] [Google Scholar]
- 66.Ashkenazi A, Wexler-Cohen Y, Shai Y. Multifaceted action of Fuzeon as virus-cell membrane fusion inhibitor. Biochim Biophys Acta, Biomembr. 2011;1808:2352–2358. doi: 10.1016/j.bbamem.2011.06.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


