Abstract
Background
Identifying youth who may engage in future substance use could facilitate early identification of substance use disorder vulnerability. We aimed to identify biomarkers that predicted future substance use in psychiatrically un-well youth.
Methods
LASSO regression for variable selection was used to predict substance use 24.3 months after neuroimaging assessment in 73 behaviorally and emotionally dysregulated youth aged 13.9 (sd=2.0), 30 female, from three clinical sites in the Longitudinal Assessment of Manic Symptoms (LAMS) study. Predictor variables included neural activity during a reward task, cortical thickness, clinical, and demographic variables.
Results
Future substance use was associated with higher left middle prefrontal cortex activity, lower left ventral anterior insula activity, thicker caudal anterior cingulate cortex, higher depression and lower mania scores, not using antipsychotic medication, more parental stress, older age. This combination of variables explained 60.4% of the variance in future substance use, and accurately classified 83.6%.
Conclusions
These variables explained a large portion of the variance, were useful classifiers of future substance use, and showed the value of combining multiple domains to provide a comprehensive understanding of substance use development. This may be a step toward identifying neural measures that can identify future substance use disorder risk, and act as targets for therapeutic interventions.
Keywords: substance use, prediction, GLMNET, fMRI, LASSO, youth
Introduction
Sensation seeking increases during adolescence (Kandel and Logan, 1984, Steinberg et al., 2008) often at the expense of safer choices. Some risk-taking, for example, practicing difficult sporting maneuvers or applying to highly ranked schools or jobs, is beneficial to growth and survival. Other risks taken by youth, however, are associated with deleterious behaviors, such as substance use and substance use disorders. The propensity for risky behaviors, such as substance use, in youth may be related to reward circuitry development, specifically, reduced ventral striatal function and volume (Schneider et al., 2012); and a delay in the development of prefrontal cortical regions implicated in cognitive control alongside the emergence of increased dopaminergic activity in subcortical regions during puberty (Steinberg et al., 2008).
Reward circuitry comprises a widespread neural network, including ventral striatum, amygdala, and insula, and specific prefrontal cortical regions: ventrolateral prefrontal cortex (vlPFC;BA47), dorsal anterior cingulate (dACC; BA24/32), medial and middle prefrontal cortex (mPFCBA10). Reward circuitry related activity, along with sensation seeking personality traits and risk taking behaviors, characterized early onset drinking (Nees et al., 2012). In addition, on a naturalistic risk taking task, activity in bilateral insula, parietal, orbitofrontal, and motor cortices, as well as left anterior cingulate cortex, together were able to discriminate between making a risky or safe choice on the next trial with 67% accuracy (Helfinstein et al., 2014). Additionally, in adolescence, cortical maturation often corresponds with substance use onset (Shaw et al., 2008). Animal studies reported differential changes in cortical thickness in adolescent animals exposed to substances (Vetreno et al., 2016), while adolescent marijuana users showed reduced cortical thicknesses relative to non-users (Lopez-Larson et al., 2011). The extent to which measures of reward circuitry function and structure in youth predict future substance use, however, remains to be determined. Identifying in youth such predictors, alongside clinical and demographic predictors, would not only provide objective neural markers to identify risk of future substance use disorders, but would also provide targets to ultimately guide early intervention, treatment choice, and novel treatment developments.
Predicting clinical outcome from neuroimaging measures is a burgeoning field of research (Berkman and Falk, 2013). Measures of neural structure and function predicted response to psychotherapy, CBT, and psychotropic medications in adults and children with major depressive (MDD) and anxiety (AnxD) disorders (Forbes et al., 2010, Fu et al., 2013, Hum et al., 2013, Masten et al., 2011, McClure et al., 2007, Morgan et al., 2013, Pizzagalli, 2010, Shin et al., 2013). Additionally, in youth, future positive mood and energy dysregulation was predicted by a combination of reward circuitry functional connectivity, white matter structure and clinical scores, together explaining 28% of the variance in clinical outcome (Bertocci et al., 2016). The latter study in particular points to the feasibility of using a multimodal neuroimaging approach to identify markers of neural function that, in combination with clinical and demographic measures, can predict future behavioral outcomes in youth with psychiatric disorders. Large sample sizes, multimodal neuroimaging techniques, and statistical analyses that can evaluate large numbers of potential predictor variables are needed to fully examine the extent to which combinations of measures predict future outcomes in youth. LASSO (Least Absolute Shrinkage and Selection Operator) regression is one such statistical technique that has been adopted for use in genetic studies (Kohannim et al., 2012a, Kohannim et al., 2012b, Luo et al., 2015, Wang et al., 2015, Zemmour et al., 2015), and is gaining favor in clinical research (Bertocci et al., 2016, Christensen et al., 2014, Yan et al., 2015). This technique evaluates a large number of potential predictor variables, relative to the number of study participants, while minimizing model error and minimizing the risk of overfitting through cross validation.
The goal of the present study was to identify measures of reward circuitry function and cortical structural thickness that predicted future substance use in a large group of youth in the Longitudinal Assessment of Manic Symptoms (LAMS) study. LAMS is an ongoing multi-site study examining longitudinal relationships among the course of symptoms, outcomes, and neural mechanisms associated with different clinical trajectories in youth with symptoms characterized by behavioral and emotional dysregulation (Findling et al., 2010, Horwitz et al., 2010). We hypothesized that in LAMS youth, future substance use would be predicted by increased prefrontal-cortical-striatal reward circuitry activity and reduced whole brain cortical thickness. We also aimed to determine the proportion of future substance use predicted by neuroimaging measures, and to test the discriminatory power of identified predictors.
Methods
Participants
One hundred and thirty youth, recruited from the LAMS1 cohort of 707 youth for whom parents were seeking psychiatric assessment and treatment participated in the neuroimaging component of LAMS2. All 130 youth from LAMS1 entered LAMS2 with a variety of symptoms and diagnoses. Inclusion criteria for the LAMS1 cohort were: no outpatient treatment at a LAMS clinic in the last 12 months; 6-12 years of age; and without a sibling who was screened for LAMS1 (Findling et al., 2010). Families of eligible children completed the Parental General Behavior Inventory–10 Item Mania scale (PGBI-10M). Children who scored ≥12 on this scale, and an age-sex-matched group of those who scored <12, were invited to participate in LAMS1. The 130 youth in the LAMS2 neuroimaging component were selected to include approximately equal numbers of youth: 1.with high (≥12) versus low (<12) PGBI-10M scores; 2.who were older (≥13 years) versus younger (≤12 years); 3.who were male versus female (each site was age and sex matched for each PGBI-10M subgroup).
Exclusion criteria for participating in the LAMS2 neuroimaging component included systemic medical illnesses, neurological disorders, history of trauma with loss of consciousness, use of non-psychotropic central nervous system effecting medications, IQ<70 assessed by the Wechsler Abbreviated Scale of Intelligence (WASI), positive drug and/or alcohol screen on scan day, significant visual disturbance, inability to communicate in English, autistic spectrum disorders/developmental delays, pregnancy, claustrophobia, and metal in the body.
Parents/guardians and youth provided written informed consent and assent, respectively, after receiving a complete study description.
The final sample included 73 LAMS youth (Age:M=13.91, SD=2.00, Range=9.89-17.71; 30 females; Table 1). 57 LAMS youth were excluded for behavioral data loss (n=5), excessive movement during neuroimaging acquisition (n=33), or cortical thickness processing problems (n=19; inability to read the pixelated data, mislabeled parcellations, non-symmetric colors, or missing cortical regions). Included youth were older, had higher IQ, and higher SES relative to excluded youth (Table 1).
Table 1.
Demographic information, clinical variables, and current medication usage (Mean ± Standard Deviation or Proportion) describing the total LAMS sample and comparing LAMS participants included versus excluded from neuroimaging.
| Total LAMS Imaging Sample n=130 M(SD/Range) or Proportion | Included Participants n=73 M(SD) or Proportion | Excluded Participants n=57 M(SD/Range) or Proportion | Statistic Comparing Included vs Excluded Participants | ||
|---|---|---|---|---|---|
| Demographic Information | p value | ||||
| Age (years) | 13.54(2.04/9.89-17.71) | 13.92(2.0) | 13.06(2.0) | t128=-2.4 | .018* |
| IQ | 100.56(16.35/70-140) | 105.44(17.3) | 94.32(12.7) | t127.6=-4.23 | <0.001* |
| SES (maternal education) | χ2=12.86 | <0.001* | |||
| No/some HS | 8/130 | 0/73 | 8/57 | ||
| GED or HS Diploma | 35/130 | 15/73 | 20/57 | ||
| Some post HS | 29/130 | 19/73 | 10/57 | ||
| Associate's Degree | 34/130 | 21/73 | 13/57 | ||
| Bachelor's Degree or higher | 24/130 | 18/73 | 6/57 | ||
| Sex (females) | 48/130 | 30/73 | 18/57 | χ2=0.87 | .351 |
| Clinical Measures | |||||
| CALS | 18.09(13.77/0-62) | 17.30(13.0) | 19.15(14.8) | t126=0.75 | .456 |
| PGBI-10M | 6.15(6.17/ 0-24) | 5.96(6.0) | 6.39(6.4) | t127=0.39 | .695 |
| KDRS | 3.85(4.68/0-24) | 4.16(4.9) | 3.44(4.4) | t126=-0.87 | .385 |
| KMRS | 4.41(6.77/0-31) | 4.44(6.9) | 4.38(6.7) | t126=0.05 | .963 |
| SCARED | 11.64(11.47/0-53) | 10.93(10.8) | 12.59(12.4) | t125=0.81 | .422 |
| Current Medication Use | |||||
| Antidepressant | 20/130 | 11/73 | 9/57 | χ2=0.00 | 1.0 |
| Antipsychotic | 27/130 | 20/73 | 7/57 | χ2=3.57 | .059 |
| Mood | 11/130 | 8/73 | 3/57 | χ2=0.71 | .401 |
| Stabilizer | |||||
| Non-stimulant | 11/130 | 6/73 | 5/57 | χ2=.00 | 1.0 |
| Stimulant | 49/130 | 29/73 | 20/57 | χ2=0.13 | .720 |
Abbreviations: *=significant at p<05; CALS=Child Affect Lability Scale (parent rating); GED=general education development test; HS=high school; IQ=intelligence quotient Wechsler Intelligence test; KDRS=Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Present Episode Depression Rating Scale; KMRS=Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Mania Rating Scale; M=mean; p=p value; PGBI-10M=Parent General Behavior Inventory 10 Item Mania Scale; SCARED=Screen for Child Anxiety Related Emotional Disorders (child rating); SD=standard deviation; SES=socio-economic status; Statistical comparisons between included and excluded participants, t=t-statistic value; χ2=chi-squared statistic value.
Reward Task
Reward-related neural activity measures were acquired using a well-validated card guessing task with a reward component (Bebko et al., 2014, Forbes et al., 2009); supplemental materials).
Neuroimaging Data Analysis
fMRI data were collected on a 1) 3T Siemens Verio MRI scanner at Case Western Reserve University, 2) 3T Philips Achieva X-series MRI scanner at Cincinnati Children's Hospital, and 3) 3T Siemens Trio MRI scanner at University of Pittsburgh. We preprocessed and analyzed fMRI data using Statistical Parametric Mapping software (SPM8 http://www.fil.ion.ucl.ac.uk/spm). An axial 3D magnetization prepared rapid gradient echo (MPRAGE) sequence (192 axial slices 1 mm thick; flip angle=9°; field of view=256×192 mm; TR=2300 msec; TE=3.93 msec; matrix=256×192) acquired T1-weighted volumetric anatomical images covering the whole brain. A reverse interleaved gradient echo planar imaging (EPI) sequence (38 axial slices 3.1 mm thick; flip angle=90°; field of view=205 mm; TR=2000 msec; TE=28 msec; matrix=64×64) acquired T2-weighted BOLD images covering the whole cerebrum and most of the cerebellum. Preprocessing involved realignment, coregistration, segmentation, normalization into a standard stereotactic space (Montreal Neurologic Institute, MNI; http://www.bic.mni.mcgill.ca), and spatial smoothing using a Gaussian kernel (FWHM: 8mm). A two level random-effects procedure was used to conduct region of interest (ROI) analyses. At the first level we constructed whole brain statistical maps to evaluate the win>control and loss>control contrasts. Movement parameters obtained from the realignment stage of preprocessing served as covariates of no interest.
A single anatomically-defined, bilateral ROI mask containing reward-related regions (Caseras et al., 2013, Nusslock et al., 2012) from the WFU PickAtlas (Maldjian et al., 2003) was used to avoid conducting multiple statistical tests over individual ROIs: dACC (BA24/32), mPFC (BA10), OFC (BA11), VLPFC (BA47), amygdala, insula, and VS (bilateral spheres centered on ±9, 9,-8; radius=8mm based on meta-analyses (Di Martino et al., 2008, Postuma and Dagher, 2006)). Using a one-sample t-test, we extracted significant activity to win>control and loss>control (voxelwise p<.001, corrected with a 3D cluster forming threshold of p<.05 (http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html) over the entire ROI. Means of significant clusters were extracted using the MarsBaR (Brett et al., 2002) toolbox in SPM.
Additionally, we examined gray matter structure across the whole brain as in other neuroimaging studies examining relationships between cortical thickness and risky behavior (Lopez-Larson et al., 2011); supplemental materials). Structural thicknesses was calculated using the freely available Freesurfer (Fischl, 2012) software. An unbiased within-subject template space and image were created. Next, skull stripping, Talairach transformation and atlas registration were completed. Finally, generation of spherical surface maps and parcellations with common information from the within-subject template was performed. The quality of surface reconstruction and segmentation was visually assessed. Each structure was extracted and adjusted for individual mean whole-brain thickness.
Clinical Assessments
On or near scan day, parents/guardians completed the PGBI-10M to assess their child's behavioral and emotional dysregulation severity (Youngstrom et al., 2005, Youngstrom et al., 2008), and the Children's Affect Lability Scale (CALS) to assess their child's affective regulation (Gerson et al., 1996). On scan day, parents and LAMS youth completed the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Mania Rating Scale (KMRS) (Axelson et al., 2003) and Depression Rating Scale (KDRS) (Kaufman et al., 1997) to assess hypo/mania and depressive symptom severity, respectively. LAMS youth also completed the Screen for Child Anxiety Related Emotional Disorders (SCARED) on scan day to assess anxiety symptoms over the last 6 months (Birmaher et al., 1997).
Substance Use Measure
To assess substance use at scan day and post fMRI scan [mean follow-up days:741 (sd:181.41)], questions concerning substance use from the KSADS (Kaufman et al., 1997), the Child and Adolescent Symptom Inventory (CASI) (Lavigne et al., 2009), and age appropriate versions of the Centers for Disease Control and Prevention's Youth Risk Behavior Survey (YRBS) [Middle school:10-12 years of age; 2005 version; High school:13-17 years of age; 2003 version; Adult:18-22 years of age; 2010 version] (www.cdc.gov/yrbs) were used. A report of substance use (more than a few sips of alcohol and/or any illicit drug use) on any of these measures put the participant into the substance user group.
Data Analytic Plan
The outcome measure used in this analysis was yes/no lifetime substance use. Of the 73 youth, 36 reported substance use 24 months post-scan. Clinical predictor variables on or near scan day included positive mood and energy dysregulation (PGBI-10M score), depressive symptoms, manic symptoms, anxious symptoms, and affective lability, diagnoses (ADHD, bipolar spectrum disorder, MDD, disruptive behavior disorder, and AnxD), medication status (taking versus not taking each psychotropic medication class: stimulant, non-stimulant ADHD, mood stabilizer, antipsychotic, and antidepressant medications). Demographic variables included age, IQ, and sex. Baseline measures of maternal education, parental life-stress (number of stressful events related to child's illness), and parental living arrangement (living with a new partner or alone) were also included as predictors (Kokkevi et al., 2007a, Kokkevi et al., 2007b). Neuroimaging predictor variables included the above BOLD measures to win>control and loss>control and the above whole brain gray matter cortical thickness variables. We additionally included scan site, and days between scan and follow up as predictor variables.
Given that our outcome variable was dichotomous and there were more predictor variables than observations, we used LASSO regression analysis with binomial family (logistic LASSO regression) for variable selection and reduction using the freely available GLMNET package in R (Friedman et al., 2014). LASSO is a modified form of least squares regression that penalizes complex models with a regularization parameter (λ) (Tibshirani, 1996). This penalization method shrinks coefficients toward zero, and eliminates unimportant terms entirely (Friedman et al., 2014, Friedman et al., 2010, Tibshirani, 1996) thus minimizing prediction error, reducing the chances of overfitting through cross validation (CV), and enforcing sparsity (Tibshirani, 1996).
GLMNET approximates the loglikelihood and then uses coordinate descent algorithm (Revolutionary Analytics, 2013, Wu and Lange, 2008) computed along a regularization path (an inner weighted least squares loop) to optimize the penalized loglikelihood. Coefficients are stabilized by coordinate descent (optimization of each parameter separately, holding all others fixed). Regularization adds constraints to a problem to avoid over-fitting. Regularization in GLMNET for a binomial regression is performed by producing the path of tuning parameter (λ) along the range of included variables, thus identifying the optimal λ (http://web.stanford.edu/∼hastie/glmnet/glmnet_alpha.html). GLMNET then uses CV to compute the mean CV error for each penalty term to guard against Type III errors (testing hypotheses suggested by the data). We used a k=10 fold CV approach.
A test statistic or p-value for LASSO that has a simple and exact asymptotic null distribution is still under development (Lockhart et al., 2014). We thus provide three other measures that are meaningful for data inference: 1) rate ratio (exponentiated coefficients) of the nonzero coefficients identified in the LASSO model; 2) Cox & Snell R-square for variance in future substance use explained by the model; 3) classification table results (cutoff =.1) from a hierarchical logistic regression analysis in SPSS, using the eight predictor variables identified from the LASSO model.
Post hoc sensitivity analysis
Of the 36 LAMS youth who at 24 months post-scan reported substance use, 15 also reported using substances at or prior to the scan. To test the importance of the combination of predictor variables derived from the LASSO, we examined the classification table from the logistic regression analysis after removing the 15 youth with substance use at scan. Additionally, to identify the nonzero variables related to future substance use only, we performed a new LASSO analysis, removing these 15 youth and including all of the original p=108 predictor variables.
Scan Site Signal Variability Reduction
We reduced signal variability between scan sites in two ways. First, we monitored signal-to-noise (SNR) monthly to ensure scanner stability over time with a Biomedical Informatics Research Network (fBIRN) phantom at each scan site (http://www.birncommunity.org). Second, we used scan site as a covariate in the LASSO models.
Results
Neuroimaging Results
LAMS youth showed significant activation to the win>control contrast in bilateral dACC (BA32) (MNI: -3,20, 46 and 3,20,46), left middle prefrontal cortex (mPFC; BA10) (MNI: -39,47,1 and -39,50,16), and bilateral ventral anterior insula MNI : 33,23,-5 and -48,17,1); and to the loss>control contrast, in bilateral dACC (BA32) (MNI: -9,8,52; 3,20,46; and 9,29,31) and ventral anterior insula (MNI: 30,20,-8 and -33,20,7) (voxelwise p<.001, clusterwise corrected p<.05, Table 2).
Table 2.
Reward-related activity in 73 LAMS youth.
| Contrast | MNI Coordinates | Statistic | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Region | BA | side | k | x | y | z | Test Statistic(df) | p |
| Win>control activity | ||||||||
| dACC | 32 | L | 17 | -3 | 20 | 46 | t(72)=6.49 | 0.001 |
| dACC | 32 | R | 40 | 3 | 20 | 46 | t(72) = 6.31 | 0.001 |
| insula | R | 105 | 33 | 23 | -5 | t(72) = 5.61 | 0.001 | |
| insula | L | 80 | -48 | 17 | 1 | t(72)=4.70 | 0.001 | |
| mPFC | 10 | L | 25 | -39 | 47 | 1 | t(72) = 5.60 | 0.001 |
| mPFC | 10 | L | 11 | -39 | 50 | 16 | t(72) = 5.34 | 0.001 |
| Loss>control | ||||||||
| dACC activity | 32 | L | 27 | -9 | 8 | 52 | t(72) = 5.53 | 0.001 |
| dACC | 32 | R | 25 | 3 | 20 | 46 | t(72) = 5.42 | 0.001 |
| dACC | 32 | R | 11 | 9 | 29 | 31 | t(72)=4.64 | 0.001 |
| insula | R | 39 | 30 | 20 | -8 | t(72)=4.54 | 0.001 | |
| insula | L | 40 | -33 | 20 | 7 | t(72)=4.06 | 0.001 | |
Region of interest analyses using voxelwise p<0.001 and cluster corrected p<0.05. Table rows represent the peak voxel within the specified region. Abbreviations: BA=Brodmann area; dACC=dorsal anterior cingulate cortex; df=degrees of freedom; k=cluster size in voxels; MNI=Montreal Neurological Institute coordinates; mPFC= middle prefrontal cortex; p=uncorrected voxelwise probability value; t=t-test statistical value; vlPFC=ventrolateral prefrontal cortex.
LASSO Results
Eight predictors together minimized mean squared error, enforced sparsity, (Friedman et al., 2014) and optimized model fit (Figure 1 and supplement). These eight predictors and the direction of the relationships were as follows.
Figure 1. LASSO plots generated in GLMNET.
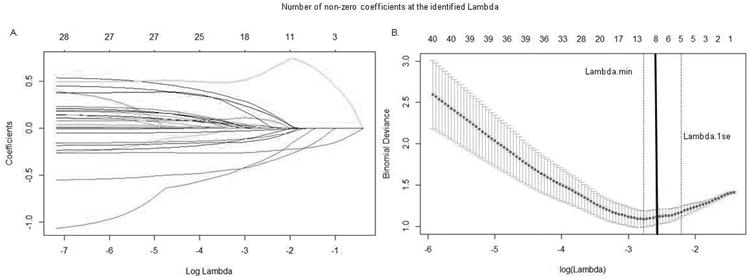
A. Plot of variable fit. Each curve corresponds to an independent variable in the full model prior to optimization. Curves indicate the path of each variable coefficient as λ varies. B. Plot of non-zero variable fit after cross validation. Representation of the 10-fold cross validation performed in GLMNET using LASSO which evaluates the error associated with each lambda. Lambda.min corresponds to the λ which minimizes mean squared error. Lambda.1se corresponds to the λ that is one standard error from the lambda.min. Solid black line corresponds to the optimal lambda selected due to significantly improved model fit over the Lambda.min and Lamba.1se based on chi square residual deviance comparisons ( supplemental).
Substance use 24 months post-scan was predicted by greater left middle prefrontal cortical activity to win, lower left ventral anterior insula activity to loss, and thicker caudal anterior cingulate cortex. In addition, older youth, higher depression scores, lower mania (KMRS) scores, more parental stressful events and not being on an antipsychotic medication at scan predicted future substance use (Table 3).
Table 3.
Nonzero coefficients generated from GLMNET using a LASSO regression with binomial family model. Exponentiated coefficient is the rate ratio change in the dependent variable (future substance use) corresponding to one unit change in the predictor variable.
| Variable | LASSO derived Exponentiated coefficient |
|---|---|
| Antipsychotic medication | .35 |
| Age | 1.20 |
| Depression scale | 1.07 |
| Left middle prefrontal cortex to win>control | 1.75 |
| Parental stress at baseline | 1.05 |
| Mania scale | .98 |
| Left ventral anterior insula activity to loss>control | 0.83 |
| Left caudal anterior cingulate thickness | 1.39 |
The full model explained 60.4% of the variance in future substance use. Hierarchical logistic regression showed that left middle prefrontal cortical and left ventral anterior insula activity, together with left caudal anterior cingulate cortical thickness, explained 14.4% of future substance use variance over and above the clinical and demographic variables (45.7%; depression and mania scores, parental stress, age, and antipsychotic medication use). Additionally, cutoff≤.1 from the logistic regression classification table correctly predicted 36/36 of future substance users and misidentified 12/37 of non-users as future substance users, correctly identifying 61/73 participants (83.6%).
Post hoc sensitivity analysis
After removing the 15 youth who reported substance use at scan, the model remained significant and the Cox & Snell R-square effect size increased from 0.6 to 0.63. The classification table using the eight non-zero predictor variables identified above (cutoff<.1) correctly predicted 21/21 future substance users and misidentified 6/37 non-users as future substance users (Cox & Snell=.631).
Additionally, in a new LASSO regression analysis including only the 58 youth who were not using substances at scan time, nonzero predictors of substance use were similar to the main analysis. Nonzero predictors were depression score, antipsychotic medication, parental stress at baseline, left middle prefrontal cortical activity to win, and right insula thickness. Notably absent variables in this post hoc LASSO analysis that may be driven by substance use prior to scan but were predictive of eventual use (see post hoc classification results above) included left caudal anterior cingulate thickness, left ventral anterior insula activity to loss, and mania scores.
Discussion
Our goal was to assess the ability of neuroimaging measures of reward circuitry activity and cortical thickness to predict future substance use in psychiatrically-unwell youth. We used LASSO regression, along with cross-validation, an approach that penalizes complex models with a regularization parameter and identifies the parameter that minimizes error, rendering unimportant coefficients as zero. Our LASSO analysis showed that engaging in substance use 24.3 months post-scan was predicted by a combination of neural activity to win and loss, cortical structure, and clinical and demographic characteristics. These findings explained 60.4% of the variance in substance use 24.3 months after neuroimaging assessment. Furthermore, neuroimaging measures incrementally predicted 14.7% of the variance, i.e., approximately a quarter of the explained variance, in this outcome measure. All eight predictor measures correctly classified 100% of youth who would use substances 24 months later, while misidentifying only 32% of non-users as future users. Including all identified nonzero variables in a logistic regression analysis, both with and without the 15 current users, successfully identified all future substance users 24 months post-scan.
In humans, the middle prefrontal cortex has been shown to be activated both by cognitively demanding tasks, e.g., working memory, and reward, and may subserve the higher cognitive aspects of reward value processing and related, goal-directed behaviors (Pochon et al., 2002). Our present finding of elevated left middle prefrontal cortical activity to reward in youth may thus reflect undue attention to, and higher order processing of, reward obtained during the task, which, in turn, may predispose to risk-taking behaviors, such as substance use. The left lateralization of our finding may reflect the role of the left hemisphere in approach related behaviors (Davidson, 1992, Davidson et al., 1990)(Figure 2).
Figure 2. Comparisons of neural measures of substance users and non-users 24.3 months post-scan and representation of the region on an average brain image.
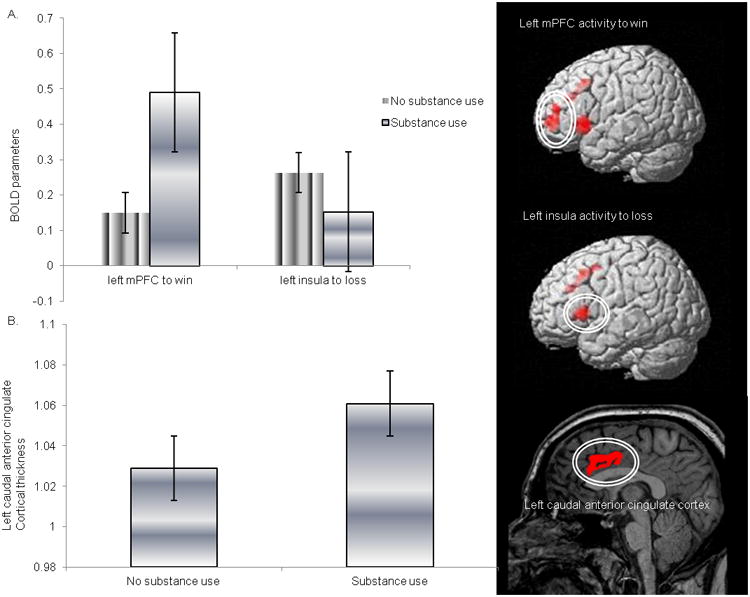
A. Reward-related left mPFC and left ventral anterior insula activity. B. Left caudal anterior cingulate thickness between the two groups (representative image). Thickness variables were adjusted for individual mean cortical thickness. Bars represent the standard error.
We showed that lower ventral anterior left insula activity to loss>control predicted more substance use in the future, although this was no longer the case after excluding the 15 youth who were using substances at scan. Subdivisions of the insula have been shown to have distinct patterns of functional connectivity (Deen et al., 2011). The ventral anterior insula is functionally connected to the anterior cingulate cortex and may have role in the processing of emotion (Deen et al., 2011). Our finding that lower left ventral anterior insula activity to loss predicted future substance use may thus suggest that reduced perception of emotion during loss may have a role in the development of risky behavior in youth. In support of this, in abstinent drug users, insula activity was reported during decision-making (Stewart et al., 2014a, Stewart et al., 2014b), while attenuation of bilateral insula activity was shown to predict relapse after one year among abstinent methamphetamine dependent youth (Gowin et al., 2014). Furthermore, individuals with insula lesions placed higher bets and showed less sensitivity to odds compared with controls (Clark et al., 2008). In healthy individuals, however, greater insula activity was associated with the safer choice during performance of a risky stock market decision-making paradigm (Kuhnen and Knutson, 2005). The above findings, taken together with our finding that lower left ventral anterior insula activity to loss may have been associated with substance use at scan, may thus suggest that LAMS youth who engaged in substance use may have perceived less emotion and, as a result, may have been less sensitive to the risks involved, and consequent losses sustained, when making decisions during the card number guessing task.
We also showed that greater right insula thickness predicted future substance use in the 58 youth who were not using substances at scan. Animal studies suggest normative thinning of subcortical and cingulate regions with age (Vetreno et al., 2016). Furthermore, the right insula is implicated in conscious awareness of interoception (Naqvi and Bechara, 2009). Our finding regarding right insula thickness may thus suggest that abnormal neurodevelopment of this region (ie., reduced pruning) may predispose to abnormally heightened awareness of interoceptive processes that, in turn, may have a deleterious impact on decision-making, but this needs further study.
Other studies have shown that neuroimaging measures may predict future substance use (Becker et al., 2015), although, in contrast to our findings, a previous report indicated that measures of neural activity may be less important predictors of risky behaviors than other factors in youth. This study reported that a factor consisting of insula, putamen, caudate nucleus, amygdala, cerebellar vermis, and prefrontal cortex activity, when combined with a personality factor and a genetic factor, was the least important factor in predicting drinking in adolescence (Heinrich et al., 2016). The fact that a significant proportion of the variance in future substance use was predicted by neuroimaging measures in our study, however, highlights a need for future studies to further examine the role of neuroimaging measures as predictors of risky behaviors in youth.
We additionally showed that greater cortical thickness in the caudal anterior cingulate cortex predicted future substance use, but not after excluding the 15 youth who used substances at scan. In young adults, left caudal anterior cingulate cortex was thicker in binge drinkers relative to light drinkers (Mashhoon et al., 2014). Additionally, normative cingulate cortical thinning was not observed in animals exposed to ethanol (Vetreno et al., 2016). Thus, similar to the left insula activity to loss finding above, greater anterior cingulate cortical thickness may be a marker of current substance use. More studies are needed to better understand this structural finding.
Non-neuroimaging variables also predicted future substance use. Consistent with the literature, older participants (Grant and Dawson, 1997, Kandel and Logan, 1984) and youth with higher depression scores (Deykin et al., 1987, Grigsby et al., 2016) more often reported future substance use. Youth not prescribed an antipsychotic medication at time of neuroimaging assessment were also more likely to use substances in the future, likely reflecting the moderating effect of these medications on psychotic and risk-taking behaviors. Intriguingly, youth with lower mania scores were also more likely to report future substance use. This may reflect the fact that youth with lower mania scores were less likely to be taking antipsychotic medication (p=.006), and thus did not benefit from the moderating effect of antipsychotic medications behaviors. While we do not suggest that youth be prescribed antipsychotic medication as a measure to reduce risk of future substance use, our findings do suggest that common patterns of neural activity may be associated with psychotic symptoms and substance use. This warrants further study. Finally, increased parental stress due to child's illness, predicted future substance use in youth. This accords with research showing that parental psychological distress is associated with emotional and conduct problems in children (Amrock and Weitzman, 2014, Reeb et al., 2015). Our findings thus add to present understanding of the role that parental stress and related behaviors may have on child behavior long-term, and suggest that these factors may be used to identify those high risk families most in need of intervention.
Limitations of the present study included the inability to assess the contribution of pubertal development and other psychosocial factors that show associations with substance use, such as sibling and peer substance use and parental monitoring (Kokkevi et al., 2007a, Kokkevi et al., 2007b), as they were not measured at scan time. Although the age of greatest risk for substance use was not yet reached by some youth in our sample, a larger portion of the LAMS sample report substance use than is expected from the general population (Abuse, 2014). As the children in the LAMS sample are, and have been, behaviorally and emotionally dysregulated for at least five years and for as many as ten years, and are at risk for a myriad of psychiatric disorders, it is, perhaps not unexpected that they engage in substance use at a higher rate than we see in healthy children. Finally, this analysis was designed post hoc and we therefore were not able to control for substance use at the initial scan visit. Additionally we suspect that some of the misidentification as a substance user may, in fact, be due to the subjective account of substance use by participants. Although the statistical methods utilized here (LASSO with cross-validation) do well at identifying predictors, the estimates may shrink, and error rates for classification of users may be higher, in new, independent samples.
We believe this is the first study to use functional and structural neuroimaging measures to predict future substance use in youth. Specifically, we show that approximately a quarter of the explained variance in future substance use was predicted by neuroimaging measures, especially measures of reward circuitry function. Furthermore, the high discriminative ability to identify future substance use in youth highlights the utility of using a combination of neuroimaging, clinical and demographic measures to help identify those youth most at risk of future substance use. This is an important step toward identifying neurobiological measures characterizing youth at risk of substance use, and provides promising neural targets for the development of novel future therapeutic interventions.
Supplementary Material
Acknowledgments
Supported by the National Institute of Mental Health grants 2R01 MH73953-09A1 (Birmaher and Phillips, University of Pittsburgh), 2R01 MH73816-09A1 (Holland, Children's Hospital Medical Center), 2R01 MH73967-09A1 (Findling, Case Western Reserve University), and 2R01 MH73801-09A1 (Fristad, Ohio State University), and the Pittsburgh Foundation (Phillips).
Dr. Arnold has received research funding from Curemark, Forest, Lilly, Neuropharm, Novartis, Noven, Shire, Supernus, and YoungLiving (as well as NIH and Autism Speaks) and has consulted with or been on advisory boards for Arbor, Gowlings, Ironshore, Neuropharm, Novartis, Noven, Organon, Otsuka, Pfizer, Roche, Seaside Therapeutics, Sigma Tau, Shire, Tris Pharma, and Waypoint and received travel support from Noven.
Dr. Birmaher has or will receive royalties from for publications from Random House, Inc (New hope for children and teens with bipolar disorder) and Lippincott Williams & Wilkins (Treating Child and Adolescent Depression). He is employed by the University of Pittsburgh and the University of Pittsburgh Medical Center and receives research funding from NIMH.
Dr. Frazier has received federal funding or research support from, acted as a consultant to, received travel support from, and/or received a speaker's honorarium from the Simons Foundation, Ingalls Foundation, Forest Laboratories, Ecoeos, IntegraGen, Shire Development, Bristol-Myers Squibb, National Institutes of Health, and the Brain and Behavior Research Foundation.
Dr. Findling receives or has received research support, acted as a consultant and/or served on a speaker's bureau for Alcobra, American Academy of Child & Adolescent Psychiatry, American Physician Institute, American Psychiatric Press, Bracket, CogCubed, Cognition Group, Coronado Biosciences, Dana Foundation, Elsevier, Forest, Guilford Press, Ironshore, Johns Hopkins University Press, Jubilant Clinsys, KemPharm, Lundbeck, Merck, NIH, Neurim, Novartis, Otsuka, Oxford University Press, Pfizer, Physicians Postgraduate Press, Purdue, Rhodes Pharmaceuticals, Roche, Sage, Shire, Sunovion, Supernus Pharmaceuticals, Transcept Pharmaceuticals, Tris, Validus, and WebMD.
Dr. Fristad receives royalties from Guilford Press, American Psychiatric Press, and CFPSI.
Dr. Kowatch is a consultant for Forest Pharmaceutical and the REACH Foundation. He is employed by the Ohio State Wexner Medical Center.
Dr. Phillips is a consultant for Roche Pharmaceuticals.
Dr. Sunshine receives research support from Siemens Healthcare.
Dr. Youngstrom has consulted with Pearson, Western Psychological Services, Janssen, Lundbeck and Otsuka about assessment, as well as having grant support from the NIH.
Footnotes
Disclosures: Almeida, Axelson, Bebko, Bertocci, Bonar, Diwadkar, Forbes, Gill, Holland, Horwitz, Iyengar, Perlman, Schirda, Travis, and Versace report no competing interests.
References
- Abuse S. Mental Health Services Administration (2013) Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. No. NSDUH Series H-46, HHS Publication No.(SMA) 13–4795. [Google Scholar]
- Amrock SM, Weitzman M. Parental psychological distress and children's mental health: results of a national survey. Academic Pediatrics. 2014;14:375–81. doi: 10.1016/j.acap.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Axelson D, Birmaher BJ, Brent D, Wassick S, Hoover C, Bridge J, Ryan N. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. Journal of Child Adolescent Psychopharmacology. 2003;13:463–70. doi: 10.1089/104454603322724850. [DOI] [PubMed] [Google Scholar]
- Bebko G, Bertocci MA, Fournier JC, Hinze AK, Bonar L, Almeida JR, Perlman SB, Versace A, Schirda C, Travis M, Gill MK, Demeter C, Diwadkar VA, Ciuffetelli G, Rodriguez E, Olino T, Forbes E, Sunshine JL, Holland SK, Kowatch RA, Birmaher B, Axelson D, Horwitz SM, Arnold LE, Fristad MA, Youngstrom EA, Findling RL, Phillips ML. Parsing dimensional vs diagnostic category-related patterns of reward circuitry function in behaviorally and emotionally dysregulated youth in the Longitudinal Assessment of Manic Symptoms study. Journal of the American Medical Association Psychiatry. 2014;71:71–80. doi: 10.1001/jamapsychiatry.2013.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ET, Falk EB. Beyond Brain Mapping Using Neural Measures to Predict Real-World Outcomes. Current Directions in Psychological Science. 2013;22:45–50. doi: 10.1177/0963721412469394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertocci MA, Bebko G, Versace A, Fournier JC, Iyengar S, Olino T, Bonar L, Almeida JRC, Perlman SB, Schirda C, Travis MJ, Gill MK, Diwadkar VA, Forbes EE, Sunshine JL, Holland SK, Kowatch RA, Birmaher B, Axelson D, Horwitz SM, Frazier TW, Arnold LE, Fristad MA, Youngstrom EA, Findling RL, Phillips ML. Predicting clinical outcome from reward circuitry function and white matter structure in behaviorally and emotionally dysregulated youth. Molecular Psychiatry. 2016 doi: 10.1038/mp.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:545–53. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Romain Valabregue JBP. Presented at the 8th International Conference on Functional Mapping of the Human Brain. Sendai, Japan: 2002. Region of interest analysis using an SPM toolbox. [Google Scholar]
- Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: differences between bipolar I and II disorders. American Journal of Psychiatry. 2013;170:533–41. doi: 10.1176/appi.ajp.2012.12020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JA, Zoetmulder M, Koch H, Frandsen R, Arvastson L, Christensen SR, Jennum P, Sorensen HB. Data-driven modeling of sleep EEG and EOG reveals characteristics indicative of pre-Parkinson's and Parkinson's disease. Journal of Neuroscience Methods. 2014;235:262–76. doi: 10.1016/j.jneumeth.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131:1311–22. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition. 1992;20:125–51. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Ekman P, Saron CD, Senulis JA, Friesen WV. Approach-withdrawal and cerebral asymmetry: emotional expression and brain physiology: I. Journal of Personality and Social Psychololgy. 1990;58:330–41. [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cerebral Cortex. 2011;21:1498–506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deykin EY, Levy JC, Wells V. Adolescent depression, alcohol and drug abuse. American Journal of Public Health. 1987;77:178–182. doi: 10.2105/ajph.77.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cerebral Cortex. 2008;18:2735–47. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Findling RL, Youngstrom EA, Fristad MA, Birmaher B, Kowatch RA, Arnold LE, Frazier TW, Axelson D, Ryan N, Demeter C, Gill MK, Fields B, Depew J, Kennedy SM, Marsh L, Rowles BM, Horwitz SM. Characteristics of children with elevated symptoms of mania: the Longitudinal Assessment of Manic Symptoms (LAMS) Study. The Journal of Clinical Psychiatry. 2010;71:1664–1672. doi: 10.4088/JCP.09m05859yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. NeuroImage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Olino TM, Ryan ND, Birmaher B, Axelson D, Moyles DL, Dahl RE. Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cognitive, Affective, and Behavioral Neuroscience. 2010;10:107–18. doi: 10.3758/CABN.10.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Hastie T, Simon N, Tibshirani R. GLMNET CRAN 2014 [Google Scholar]
- Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. Journal of Statistical Software. 2010;33:1. [PMC free article] [PubMed] [Google Scholar]
- Fu CH, Steiner H, Costafreda SG. Predictive neural biomarkers of clinical response in depression: a meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiology of Disease. 2013;52:75–83. doi: 10.1016/j.nbd.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Gerson AC, Gerring JP, Freund L, Joshi PT, Capozzoli J, Brady K, Denckla MB. The Children's Affective Lability Scale: a psychometric evaluation of reliability. Psychiatry Research. 1996;65:189–98. doi: 10.1016/s0165-1781(96)02851-x. [DOI] [PubMed] [Google Scholar]
- Gowin JL, Harle KM, Stewart JL, Wittmann M, Tapert SF, Paulus MP. Attenuated insular processing during risk predicts relapse in early abstinent methamphetamine-dependent individuals. Neuropsychopharmacology. 2014;39:1379–87. doi: 10.1038/npp.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grigsby TJ, Forster M, Unger JB, Sussman S. Predictors of alcohol-related negative consequences in adolescents: A systematic review of the literature and implications for future research. Journal of Adolescence. 2016;48:18–35. doi: 10.1016/j.adolescence.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich A, Muller KU, Banaschewski T, Barker GJ, Bokde AL, Bromberg U, Buchel C, Conrod P, Fauth-Buhler M, Papadopoulos D, Gallinat J, Garavan H, Gowland P, Heinz A, Ittermann B, Mann K, Martinot JL, Paus T, Pausova Z, Smolka M, Strohle A, Rietschel M, Flor H, Schumann G, Nees F. Prediction of alcohol drinking in adolescents: Personality-traits, behavior, brain responses, and genetic variations in the context of reward sensitivity. Biological Psychology. 2016;118:79–87. doi: 10.1016/j.biopsycho.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Helfinstein SM, Schonberg T, Congdon E, Karlsgodt KH, Mumford JA, Sabb FW, Cannon TD, London ED, Bilder RM, Poldrack RA. Predicting risky choices from brain activity patterns. Procedings of the National Academy of Sciences of the United States of America. 2014;111:2470–5. doi: 10.1073/pnas.1321728111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz SM, Demeter C, Pagano ME, Youngstrom EA, Fristad MA, Arnold LE, Birmaher B, Gill MK, Axelson D, Kowatch RA. Longitudinal Assessment of Manic Symptoms (LAMS) Study: background, design and initial screening results. The Journal of Clinical Psychiatry. 2010;71:1511. doi: 10.4088/JCP.09m05835yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hum KM, Manassis K, Lewis MD. Neurophysiological Markers That Predict and Track Treatment Outcomes in Childhood Anxiety. Journal of Abnormal Child Psychology. 2013:1–13. doi: 10.1007/s10802-013-9755-7. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Logan JA. Patterns of drug use from adolescence to young adulthood: I. Periods of risk for initiation, continued use, and discontinuation. American Journal of Public Health. 1984;74:660–6. doi: 10.2105/ajph.74.7.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kohannim O, Hibar DP, Jahanshad N, Stein JL, Hua X, Toga AW, Jack CR, Jr, Weiner MW, Thompson PM. Predicting Temporal Lobe Volume on Mri from Genotypes Using L(1)-L(2) Regularized Regression. Procedings Institute of Eelectrical and Electronics Engineers International Symposium Biomedical Imaging. 2012a:1160–1163. doi: 10.1109/ISBI.2012.6235766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohannim O, Hibar DP, Stein JL, Jahanshad N, Hua X, Rajagopalan P, Toga AW, Jack CR, Jr, Weiner MW, de Zubicaray GI, McMahon KL, Hansell NK, Martin NG, Wright MJ, Thompson PM. Discovery and Replication of Gene Influences on Brain Structure Using LASSO Regression. Frontiers in Neuroscience. 2012b;6 doi: 10.3389/fnins.2012.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkevi A, Richardson C, Florescu S, Kuzman M, Stergar E. Psychosocial correlates of substance use in adolescence: a cross-national study in six European countries. Drug and Alcohol Dependence. 2007a;86:67–74. doi: 10.1016/j.drugalcdep.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Kokkevi AE, Arapaki AA, Richardson C, Florescu S, Kuzman M, Stergar E. Further investigation of psychological and environmental correlates of substance use in adolescence in six European countries. Drug and Alcohol Dependence. 2007b;88:308–12. doi: 10.1016/j.drugalcdep.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–70. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Lavigne JV, Cromley T, Sprafkin J, Gadow KD. The Child and Adolescent Symptom Inventory-Progress Monitor: a brief Diagnostic and Statistical Manual of Mental Disorders, 4th edition-referenced parent-report scale for children and adolescents. Journal of Child and Adolescent Psychopharmacology. 2009;19:241–52. doi: 10.1089/cap.2008.052. [DOI] [PubMed] [Google Scholar]
- Lockhart R, Taylor J, Tibshirani RJ, Tibshirani R. A significance test for the lasso. 2014:413–468. doi: 10.1214/13-AOS1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, Yurgelun-Todd D. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behavioural Brain Research. 2011;220:164–172. doi: 10.1016/j.bbr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, McShan D, Kong F, Schipper M, Haken RT. TH-AB-304-07: A Two-Stage Signature-Based Data Fusion Mechanism to Predict Radiation Pneumonitis in Patients with Non-Small-Cell Lung Cancer (NSCLC) Medical Physics. 2015;42:4926122. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mashhoon Y, Czerkawski C, Crowley DJ, Cohen-Gilbert JE, Sneider JT, Silveri MM. Binge alcohol consumption in emerging adults: anterior cingulate cortical “thinness” is associated with alcohol use patterns. Alcoholism: Clinical and Experimental Research. 2014;38:1955–64. doi: 10.1111/acer.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, McNealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: a marker of adolescents' risk for depression. Development and Psychopathology. 2011;23:283–92. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EB, Adler A, Monk CS, Cameron J, Smith S, Nelson EE, Leibenluft E, Ernst M, Pine DS. fMRI predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology. 2007;191:97–105. doi: 10.1007/s00213-006-0542-9. [DOI] [PubMed] [Google Scholar]
- Morgan JK, Olino TM, McMakin DL, Ryan ND, Forbes EE. Neural response to reward as a predictor of increases in depressive symptoms in adolescence. Neurobiology of Disease. 2013;52:66–74. doi: 10.1016/j.nbd.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends in Neurosciences. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nees F, Tzschoppe J, Patrick CJ, Vollstadt-Klein S, Steiner S, Poustka L, Banaschewski T, Barker GJ, Buchel C, Conrod PJ, Garavan H, Heinz A, Gallinat J, Lathrop M, Mann K, Artiges E, Paus T, Poline JB, Robbins TW, Rietschel M, Smolka MN, Spanagel R, Struve M, Loth E, Schumann G, Flor H. Determinants of early alcohol use in healthy adolescents: the differential contribution of neuroimaging and psychological factors. Neuropsychopharmacology. 2012;37:986–95. doi: 10.1038/npp.2011.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Almeida JR, Forbes EE, Versace A, Frank E, LaBarbara EJ, Klein CR, Phillips ML. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar disorders. 2012;14:249–260. doi: 10.1111/j.1399-5618.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2010;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, Le Bihan D, Dubois B. The neural system that bridges reward and cognition in humans: An fMRI study. Proceedings of the National Academy of Sciences. 2002;99:5669–5674. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cerebral Cortex. 2006;16:1508–21. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Reeb BT, Wu EY, Martin MJ, Gelardi KL, Shirley Chan SY, Conger KJ. Long-term Effects of Fathers' Depressed Mood on Youth Internalizing Symptoms in Early Adulthood. Journal of Research on Adolescence. 2015;25:151–162. doi: 10.1111/jora.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revolutionary Analytics. Trevor hastie presents glmnet: lasso and elastic-net regularization in R 2013 [Google Scholar]
- Schneider S, Peters J, Bromberg U, Brassen S, Miedl SF, Banaschewski T, Barker GJ, Conrod P, Flor H, Garavan H, Heinz A, Ittermann B, Lathrop M, Loth E, Mann K, Martinot JL, Nees F, Paus T, Rietschel M, Robbins TW, Smolka MN, Spanagel R, Strohle A, Struve M, Schumann G, Buchel C. Risk taking and the adolescent reward system: a potential common link to substance abuse. American Journal of Psychiatry. 2012;169:39–46. doi: 10.1176/appi.ajp.2011.11030489. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. The Journal of Neuroscience. 2008;28:3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Davis FC, VanElzakker MB, Dahlgren MK, Dubois SJ. Neuroimaging predictors of treatment response in anxiety disorders. Biology of Mood & Anxiety Disorders. 2013;3:15. doi: 10.1186/2045-5380-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Developmental Psycholology. 2008;44:1764–78. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Connolly CG, May AC, Tapert SF, Wittmann M, Paulus MP. Striatum and insula dysfunction during reinforcement learning differentiates abstinent and relapsed methamphetamine-dependent individuals. Addiction. 2014a;109:460–71. doi: 10.1111/add.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, May AC, Poppa T, Davenport PW, Tapert SF, Paulus MP. You are the danger: attenuated insula response in methamphetamine users during aversive interoceptive decision-making. Drug and Alcohol Dependence. 2014b;142:110–9. doi: 10.1016/j.drugalcdep.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani R. Regression Shrinkage and Selection via the Lasso. Journal of the Royal Statistical Society Series B (Methodological) 1996;58:267–288. [Google Scholar]
- Vetreno RP, Yaxley R, Paniagua B, Johnson GA, Crews FT. Adult rat cortical thickness changes across age and following adolescent intermittent ethanol treatment. Addiction Biology. 2016 doi: 10.1111/adb.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Xu W, Liu Y. Integrating full spectrum of sequence features into predicting functional microRNA-mRNA interactions. Bioinformatics. 2015;30 doi: 10.1093/bioinformatics/btv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TT, Lange K. Coordinate decent algorithms for lasso penalized regression. The Annals of Applied Statistics. 2008;2:224–244. [Google Scholar]
- Yan S, Tsurumi A, Que YA, Ryan CM, Bandyopadhaya A, Morgan AA, Flaherty PJ, Tompkins RG, Rahme LG. Prediction of multiple infections after severe burn trauma: a prospective cohort study. Annals of Surgery. 2015;261:781–92. doi: 10.1097/SLA.0000000000000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstrom E, Meyers O, Demeter C, Youngstrom J, Morello L, Piiparinen R, Feeny N, Calabrese JR, Findling RL. Comparing diagnostic checklists for pediatric bipolar disorder in academic and community mental health settings. Bipolar Disorders. 2005;7:507–517. doi: 10.1111/j.1399-5618.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- Youngstrom EA, Frazier TW, Demeter C, Calabrese JR, Findling RL. Developing a Ten Item Mania Scale from the Parent General Behavior Inventory for Children and Adolescents. The Journal of Clinical Psychiatry. 2008;69:831. doi: 10.4088/jcp.v69n0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemmour C, Bertucci F, Finetti P, Chetrit B, Birnbaum D, Filleron T, Boher JM. Prediction of early breast cancer metastasis from DNA microarray data using high-dimensional cox regression models. Cancer Informatics. 2015;14:129–38. doi: 10.4137/CIN.S17284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


