Abstract
Proper regulation of the balance between hematopoietic stem cell (HSC) proliferation, self-renewal, and differentiation is necessary to maintain hematopoiesis throughout life. The Wnt family of ligands has been implicated as critical regulators of these processes through a network of signaling pathways. Previously, we have demonstrated that the Wnt5a ligand can induce HSC quiescence through a noncanonical Wnt pathway, resulting in an increased ability to reconstitute hematopoiesis. In this study, we tested the hypothesis that the Ryk protein, a Wnt ligand receptor that can bind the Wnt5a ligand, regulated the response of HSCs to Wnt5a. We observed that inhibiting Ryk blocked the ability of Wnt5a to induce HSC quiescence and enhance short-term and long-term hematopoietic repopulation. We found that Wnt5a suppressed production of reactive oxygen species, a known inducer of HSC proliferation. The ability of Wnt5a to inhibit ROS production was also regulated by Ryk. From these data, we propose that Wnt5a regulates HSC quiescence and hematopoietic repopulation through the Ryk receptor and that this process is mediated by suppression of reactive oxygen species.
Keywords: Hematopoietic Stem Cells, Stem Cell Transplantation, Cellular Proliferation, Cell Biology
Introduction
Hematopoietic stem cells (HSCs) are a rare population of cells responsible for life-long regeneration of mature blood cells through initiating the process of hematopoiesis [1]. The daily turnover rate of hematopoietic cells is estimated be on the order of 1012 in humans. Molecular mechanisms that control the processes of HSC proliferation, self-renewal, and differentiation ensure that a sufficient number of HSCs are present to sustain hematopoiesis throughout life. Defects in these mechanisms are implicated in the development of bone marrow failure syndromes and hematologic malignancies [2]. Furthermore, the HSC is the only cell capable of regenerating hematopoiesis and thus making possible the clinical application of bone marrow transplantation. In this context, the HSCs must rapidly expand and reestablish durable hematopoiesis without compromising long-term function. Thus, understanding the mechanisms that regulate HSC fate has extensive clinical implications.
The Wnt proteins are an evolutionarily conserved family of soluble glycoproteins that regulate numerous physiologic processes, including cell fate decisions of adult and embryonic stem cells [3]. Wnt ligands can activate several distinct signaling pathways, classified as “canonical” versus “noncanonical.” Activation of the canonical Wnt pathway results in the translocation of the β-catenin protein to the nucleus where it acts as a transcriptional coactivator. The family of noncanonical Wnt pathways are a diverse compilation that use alternative secondary messengers besides β-catenin, such as intracellular calcium and c-Jun N-terminal kinase [4, 5]. Both types of pathways have a significant role in hematopoiesis as the Wnt3a, Wnt4, and Wnt16 ligands activate canonical and noncanonical Wnt pathways to regulate HSC self-renewal and proliferation, apoptosis, and development [6–8] [4, 5]. We and others demonstrated that Wnt5a activates noncanonical Wnt signaling to promote the ability of mouse and human HSCs to reconstitute hematopoiesis [9–11]. In adult bone marrow, the Wnt5a ligand is expressed in HSCs and progenitors, B-lymphocytes, and bone marrow stromal cells [12, 13]. We observed that Wnt5a suppressed proliferation of HSCs and maintained them in a quiescent state [9]. Approximately 70% of adult HSCs reside within the quiescent G0 phase of the cell cycle until they are called upon to either maintain the HSC pool through self-renewal or differentiate into mature blood cells [14]. Maintenance of HSC quiescence is critical for long-term function as excessive proliferation of HSCs is associated with a decreased capacity to sustain hematopoiesis over an extended period of time (reviewed in [15]). Recently, Sugimura et al. [11] also observed that Wnt5a-mediated signaling regulates HSC quiescence. Buckley et al. [16] demonstrated that inhibiting Wnt5a in long-term bone marrow cultures resulted in decreased numbers of HSCs over time, implicating Wnt5a in long-term HSC maintenance.
The activation of a particular Wnt pathway depends in part on the presence of specific receptors and coreceptors. These include, but are not limited to, members of the Frizzled family, low-density lipoprotein receptor-related protein (Lrp) 5 and 6, receptor tyrosine kinase-like orphan receptors (Ror) 2, Flamingo, and related to receptor tyrosine kinase (Ryk) [11, 17–19]. Ryk is a Wnt ligand receptor that is present in multiple cell types, including hematopoietic progenitors [20–23]. Ryk can act as a receptor or coreceptor and has been linked to several Wnt signaling pathways. Ryk can bind to multiple Wnt ligands, including Wnt5a [24]. Ryk−/− mice die shortly after birth due to respiratory complications associated with a severe cleft palate and exhibit defects in limb morphology similar to Wnt5a−/− mice [25].
There are few reports on the function of the Ryk receptor in hematopoiesis. Forsberg et al. [22] observed decreased Ryk mRNA levels in mobilized, proliferating HSCs compared to steady state. De Graaf et al. [23] studied hypomorphic Plt4 mice, which exhibit increased HSC proliferation compared to wild type, and observed a similar correlation between decreased Ryk transcription and increased HSC proliferation. In this study, we observed that HSCs and progenitors (HSPCs) expressing Ryk exhibited a reduced rate of cell proliferation compared to cells expressing low or no Ryk. These data suggested a role for Ryk in regulating HSC proliferation. We hypothesized that the ability of Wnt5a to induce HSC quiescence was regulated through the Ryk receptor. To test this hypothesis, we cultured murine HSCs and progenitors under serum-free conditions in the presence of recombinant Wnt5a and a neutralizing antibody against the Ryk receptor. We observed that blocking Ryk had a substantial effect on the ability of Wnt5a to induce quiescence and enhance hematopoietic repopulation. Furthermore, we observed that Wnt5a was able to suppress production of reactive oxygen species (ROS) in a Ryk-dependent manner, suggesting that regulation of ROS is one mechanism by which Wnt5a governs HSC function. Together, our data point to a model in which the Ryk receptor regulates the ability of Wnt5a to induce quiescence and promote hematopoietic repopulation by HSCs.
Materials and Methods
Mice
Animals were housed in sterile conditions in the Roswell Park Cancer Institute vivarium. B6.SJL-Ptprca Pepcb/BoyJ (CD45.1) and C57BL/6 (CD45.2) mice were purchased from Jackson Laboratories (Bar Harbor, ME, http://www.jax.org). C57BL/6-Tg (UBC-GFP) 30Scha/J mice were kindly provided by Dr. James Thompson (Roswell Park Cancer Institute, Buffalo, NY). For specific experiments, mice were injected with 150 mg/kg 5-fluorouracil (5-FU) (Sigma Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) or 0.2 mL EZ-Link Sulfo-NHS-LC-LC-biotin (Thermo Scientific, Rockford, IL, http://www.thermoscientific.com) at a concentration of 5 mg/mL in 0.9% saline [26].
Isolation and Culture of HSPC
For all experiments, lineage−, Sca-1+, c-kit+ (LSK) and LSK, CD150+, CD48− cells were isolated as previously described [9]. Sorted LSK cells were cultured in serum-free StemSpan media (Stem Cell Technologies, Vancouver, BC, Canada, http://www.stemcell.com) supplemented with 25 ng/mL stem cell factor (SCF) and fms-like tyrosine kinase-3 ligand (Flt3L). Sorted and LSK, CD150+, CD48− cells were cultured in serumfree StemSpan media (supplemented with 25 ng/mL SCF and thrombopoietin (TPO). All cytokines were obtained from Peprotech (Rocky Hill, NJ, http://www.peprotech.com). Sorted cells were incubated overnight before reseeding at 5 × 104 cells (LSK) or 1.5 × 104 cells (LSK, CD150+, CD48−) per milliliter in fresh media. Recombinant Wnt5a (R&D Systems, Minneapolis, MN, http://www.abgent.com) and anti-Ryk antibody (clone RB1491, Abgent, San Diego, CA) were added at 250 ng/mL and 1 μg/mL, respectively. Media were changed at 48 hours. For specific experiments, cells were treated with 0.1 mM buthionine sulfoximine (BSO) (Sigma Aldrich).
Flow Cytometry Analysis of Bone Marrow Cells
Ryk Expression in Bone Marrow Cells
Mouse bone marrow cells were stained with fluorescein isothiocyanate-conjugated anti-mouse lineage markers (CD4 (clone GK1.5), CD8a (53-6.7), B220 (RA3–6B2), Gr-1 (RB6–8C5), CD11b (M1/70), Ter119 (Ter-119), PE-Cy7-conjugated anti-Sca-1 (D7), allophycocyanin (APC)-eFluor780-conjugated anti-C-kit (2B8), PE-conjugated anti-CD150 (mShad), Pacific Blue-conjugated anti-Endoglin (MJ7/18), APC-conjugated anti-CD41 (eBioMWReg30), PerCp-Cy5.5 anti-CD16/32 (93), and biotinylated anti-Ryk (R and D Systems) and incubated on ice for 15 minutes. All antibodies except for anti-Ryk were from eBioscience (San Diego, CA, http://www.ebioscience.com). Cells were subsequently stained with eFluor710-conjugated streptavidin. For this and subsequent experiments, live cells were determined by staining cells with 4′,6-diamidino-2-phenylindole (DAPI); live cells were defined as DAPI-negative.
Cell Cycle
Bone marrow cells were analyzed for cell cycle status as described under Wilson et al. [14]. For analysis of bone marrow from mice injected with 5-FU, cell surface markers were the same as described with the following exceptions: anti-CD11b was removed from the lineage panel and anti-c-kit and anti-CD150 were replaced by PerCp-Cy5.5 conjugated CD48 (HM48-1, BioLegend, San Diego, CA, http://www.biolegend.com). Cell cycle phases were determined by staining cells with Hoechst 33343 (Sigma Aldrich) and APC-conjugated anti-Ki-67 (SolA15, eBioscience) as previously described [14].
Proliferation Kinetics
Bone marrow cells were analyzed for retention of biotin labeling as previously described [26]. Cell surface markers used for analysis were identical to those used for cell cycle analysis with the following exceptions: biotinylated Ryk was replaced with sheep anti-mouse Ryk (AF4649, R&D Systems) with Alexa-Fluor 546-conugated donkey anti-sheep IgG acting as a secondary antibody (Invitrogen, Carlsbad, CA, http://www.invitrogen.com). APC-conjugated streptavidin (eBioscience) was used to detect biotin-labeled cells.
ROS
Bone marrow cells were stained as described above with the exception that APC-conjugated antilineage markers were used. After cell surface staining, bone marrow cells were resuspended in phosphate buffered saline (PBS) and incubated for 30 minutes with 5.0 μM chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (Invitrogen) at 37°C and immediately analyzed by flow cytometry.
Flow Cytometry Analysis of Cell Cycle and ROS in Cultured Cells
Cell Cycle
After 72 hours of seeding, cells were resuspended in PBS. An equal volume of 2% formaldehyde in PBS was slowly added while vortexing for a final concentration of 1% fixative and incubated for 1 hour on ice. Cells were resuspended in 0.9% NaCl saline solution and incubated on ice for 30 minutes. After incubation, Triton-X 100 (final concentration 0.5%) was added along with Hoechst 33343 (final concentration 3 μg/mL) and incubated overnight at 4°C. Cells were then stained with APC-conjugated anti-Ki-67 antibody for 30 minutes at room temperature prior to analysis by flow cytometry.
ROS
Cells were analyzed by flow cytometry as described above.
In Vitro Hematopoietic Colony Assay
C57BL/6 LSK cells were cultured for 4 days as described. For in vitro analysis of hematopoietic progenitors, cultured LSK cells were seeded in Methocult GF M3434 methylcellulose media (Stem Cell Technologies). Colony forming units-granulocyte, macrophage were scored after 7 days in culture.
Hematopoietic Repopulation Assay
C57BL/6 LSK and LSK, CD150+, CD48− cells were cultured for 4 days under the described conditions. For transplants involving cultured LSK cells, 10,000 cultured cells were injected retro-orbitally into lethally irradiated (10.5 cGy) B6.SJL mice along with 5 × 105 C57BL/6-Tg (UBC-GFP) 30Scha/J whole bone marrow cells. For transplants involving cultured LSK, CD150+, CD48− cells, 1,000 cultured cells were injected along with the same number of UBC-GFP bone marrow cells.
To measure hematopoietic repopulation, peripheral blood cells were harvested 4 and 16 weeks post-transplant and stained with APC-conjugated anti-CD3 (1452C11), PerCp-Cy5.5-conjugated anti-B220, APC-eFluor780 conjugated anti-Gr-1, PE-Cy7 conjugated anti-CD11b, Pacific Blue conjugated anti-CD45.1 (A20), Alexa Fluor 700-conjugated anti-CD45.2 (104). All antibodies were from eBioscience. Engraftment of cultured cells was defined as greater than 1% CD45.2+, green fluorescent protein GFP− of total nucleated live blood cells.
Data Analysis and Statistics
Flow cytometry was analyzed using FlowJo version 9.5. For all experiments p values were determined using paired t test or Fisher’s exact probability test.
Results
Expression of Ryk in HSPCs
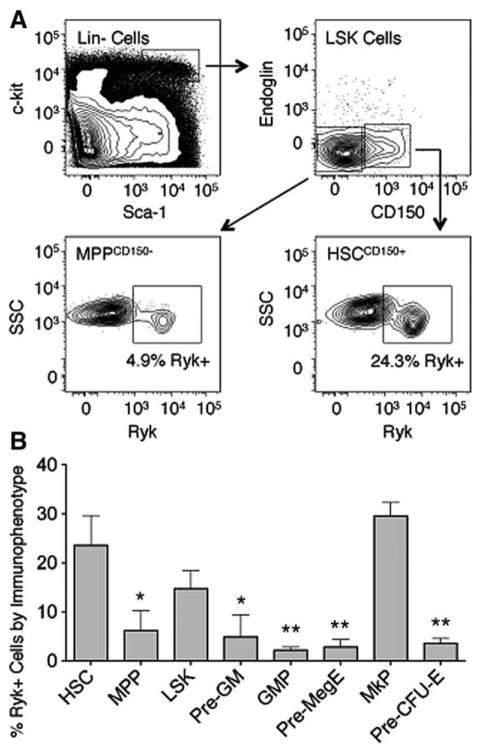
We determined the pattern of Ryk expression is adult mouse bone marrow HSPCs using flow cytometry based on the criteria established in Pronk et al. [27]. On average, 23.6% of cells that expressed the HSC immunophenotype (LSK, CD150+) expressed (Ryk+) (Fig. 1A, 1B). This percentage declined 3.8-fold in the transition from HSCs to multipotent progenitors (MPP, LSK, CD150−) (p<.05). Most of the lineage-restricted progenitors exhibited a similar decline in the percentage of Ryk+ cells. The single exception to this was in the megakaryocyte progenitor (MkP) population. On average, 29.6% of MkP cells were Ryk+, a level comparable to that found in the HSC population. These results are similar to those reported in Forsberg et al. and De Graaf et al. indicating that Ryk mRNA levels are lower in MPPs compared to HSCs and indicate a general trend that Ryk protein levels decline as HSCs differentiate into committed progenitor cells [22, 23].
Figure 1.
Analysis of Ryk levels in adult bone marrow cells. (A): Representative flow cytometry analysis of Ryk in adult mouse bone marrow progenitor populations. (Top Left): LSK bone marrow cells (black box). (Top Right): CD150+ and CD150− cells in the LSK population. (Bottom Left): Ryk+ cells in the MPP CD150− population. (Bottom Right): Ryk+ cells in the HSC CD150+ population. Regions were drawn based on fluorescence minus one control. (B): Average percentage of Ryk+ cells among different hematopoietic stem and progenitor populations (n=3). Progenitor populations were defined based on the phenotypic criteria in Pronk et al. [27]. Error bars represent SD. p values were generated using paired t tests to compare the percentage of Ryk+ progenitors to Ryk+ HSCs (*, p<.05; **, p<.01). Abbreviations: GMP, granulocyte-macrophage progenitor; HSC, hematopoietic stem cell; LSK, lin−, Sca-1+, c-kit+; MPP, multipotent progenitor; MkP, megakaryocyte progenitor; Pre-GM, pre-granulocyte-macrophage progenitor; Pre-MegE, pre-megakaryocyte, erythroid progenitor; Pre-CFU-E, pre-colony forming unit, erythroid; Ryk, related to receptor tyrosine kinase; SSC, side scatter.
Expression of Ryk in Proliferating HSPCs
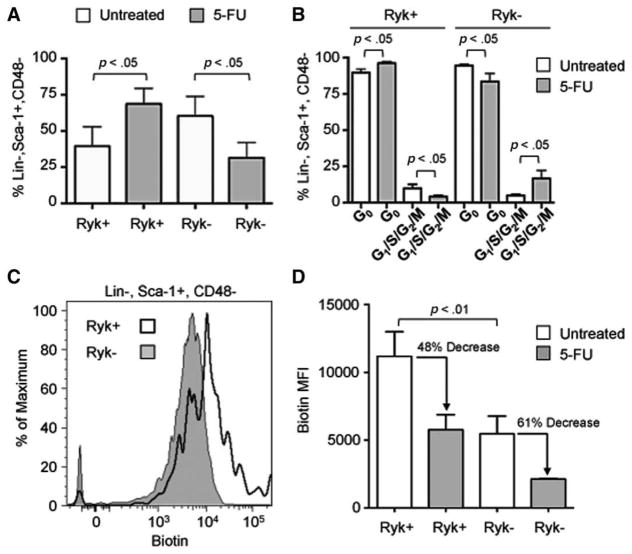
We determined whether Ryk expression was associated with changes in proliferative status of HSCPs. To induce proliferation, we injected mice with 150 mg/kg 5-FU and analyzed Ryk levels and cell cycle status after 4 days. For this study, lin, Sca-1, and CD48 markers are used to detect different progenitor populations as treatment with 5-FU alters c-kit levels [28, 29]. After 5-FU treatment, there was a significant 1.7-fold increase in the percentage of Ryk+ lin−, Sca-1+, CD48− cells compared to a 1.9-fold decrease in the percentage of Ryk− lin−, Sca-1+, CD48− cells (p<.05) (Fig. 2A).
Figure 2.
Response of Ryk-expressing hematopoietic stem cells and progenitors to 5-FU. Mice were injected with 150 mg/kg of 5-FU and their bone marrow cells were analyzed by flow cytometry 4 days later. (A): Average percentage of lin−, Sca-1+, CD48− cells that are Ryk+ or Ryk− in untreated mice (control, white bars) or treated with 5-FU (5-FU, gray bars) (n=3). Ryk+ and Ryk− populations were determined based on fluorescence minus one controls. (B): Average percentage of Ryk+ and Ryk−, lin−, Sca-1+, CD48− cells in G0, and G1/S/G2/M phases of the cell cycle in untreated mice (control, white bars) or treated with 5-FU (5-FU, gray bars) (n=3). G0 cells were defined as 2n DNA, Ki-67−; G1 cells as 2n DNA, Ki67+, and S/G2/M as >2n DNA, Ki-67+. (C): Representative flow cytometry analysis of biotin labeled Ryk+ (white histogram) and Ryk−, lin−, Sca-1+, CD48− cells. Mice were injected with 1 mg biotin and their bone marrow cells were analyzed following a 7-day chase period. (D): Average MFI of biotin in Ryk+ and Ryk−, lin−, Sca-1+, CD48− cells in untreated mice (white bars) and in mice treated with 5-FU (gray bars) (n=3). Mice were injected with 5-FU at day 3 of the 7-day chase period. For all panels, error bars represent SD and p values were determined using paired t test. Abbreviations: 5-FU, 5-flurouracil; MFI, median fluorescent intensity; Ryk, related to receptor tyrosine kinase.
We next analyzed cell cycle status of lin−, Sca-1+, CD48− cells after 5-FU treatment. In untreated animals, there was no difference in the cell cycle distribution of lin−, Sca-1+, CD48− cells based on Ryk expression (Fig. 2B). However, after 5-FU treatment, there was a significant increase in the percentage of Ryk+, lin−, Sca-1+, CD48− cells in G0 and a significant decrease in the percentage of Ryk+, lin−, Sca-1+, CD48− cells in G1/S/G2/M compared to control (p<.01) (Fig. 2B). We observed the reciprocal effect for Ryk− lin−, Sca-1+, CD48− cells. Thus, after 5-FU treatment, Ryk+, lin−, Sca-1+, CD48− cells were more likely to reside in G0 and Ryk+, lin−, Sca-1+, CD48− were more likely to be actively cycling compared to steady state.
To determine the kinetics of cell proliferation, we performed a pulse-chase experiment in which we injected mice with a single dose of biotin, which labels the membrane proteins of bone marrow cells [26]. Subsequent cell divisions reduce the amount of labeled proteins on daughter cells. This method is comparable to bromodeoxyuridine (BrdU) incorporation, but unlike BrdU, biotin neither requires nor activates cell proliferation prior to labeling [14]. Three days after the biotin pulse, we injected mice with 150 mg/kg 5-FU and then analyzed biotin labeling in lin−, Sca-1+, CD48− cells 4 days later (a chase period of 7 days total). In untreated animals, Ryk+, lin−, Sca-1+, CD48− cells exhibited a significant increase in biotin labeling compared to Ryk−, lin−, Sca-1+, CD48−, indicating that these cells underwent fewer cell divisions during the chase period (p<.01) (Fig. 2C, 2D). As expected, treatment with 5-FU stimulated cell division as there were significant decreases in biotin labeling in Ryk+ and Ryk−, lin−, Sca-1+, CD48− after 5-FU treatment compared to control (Fig. 2D). The percentage decrease in biotin labeling was similar between Ryk+ (48%) and Ryk+, lin−, Sca-1+, CD48− cells (61%), suggesting that treatment with 5-FU resulted in equivalent proliferation rates between Ryk+ and Ryk− cells during the chase period.
Ryk Regulates the Wnt5a-Mediated Suppression of HSPC Proliferation
These data suggested an association between Ryk and proliferation of HPSCs. Based on our previous observations that Wnt5a, a known ligand of Ryk, can suppress proliferation of LSK cells, we tested the hypothesis that the Ryk receptor regulates the response of HSCPs to Wnt5a. We cultured LSK cells ex vivo for 4 days in serum-fee media supplemented with 25 ng/mL SCF and Flt3L. During this period, we treated cells twice with 250 ng/mL recombinant mouse Wnt5a (rWnt5a) and 1 μg/mL neutralizing anti-Ryk antibody (α-Ryk) [30].
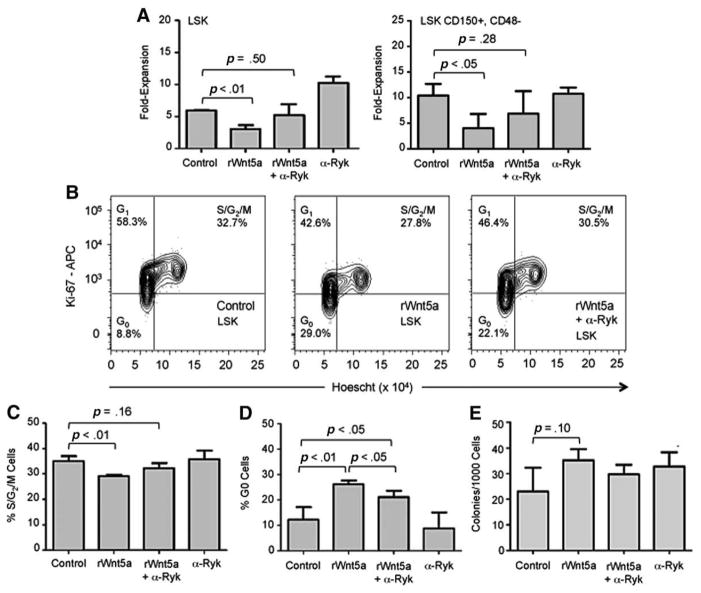
As expected based on previous results, rWnt5a inhibited expansion by LSK cells 1.8-fold compared to control (p<.01; Fig. 3A). Cotreatment with α-Ryk and rWnt5a did not result in a significant decrease in cell expansion compared to control. α-Ryk treatment alone resulted in a significant 1.7-fold increase in cell expansion (p<.01) compared to control. rWnt5a also inhibited expansion of LSK, CD150+, CD48− cells by 2.6-fold compared to control (p<.05, Fig. 3A, cultures supplemented by SCF and 25 ng/mL TPO instead of Flt3L). These cells comprise a more purified HSC population compared to LSK cells [29]. Similarly, cotreatment with α-Ryk blocked the ability of rWnt5a to suppress expansion of LSK, CD150+, CD48− cells. Unlike LSK cells, α-Ryk treatment alone did not inhibit cell expansion.
Figure 3.
Effect of inhibiting Ryk on Wnt5a-mediated proliferation of LSK cells. LSK and LSK, CD150+, CD48− cells were cultured as described under Materials and Methods section. (A): Average fold-expansion of LSK (left) and LSK, CD150+, CD48− cells (right) after 4 days in culture with rWnt5a and anti-Ryk antibody (α-Ryk) (n=3). (B): Representative flow cytometry analysis of cell cycle status of LSK cells cultured for 3 days under control conditions (top), with rWnt5a (middle), and with rWnt5a+α-Ryk (bottom). Percentages of cells in G0, G1, and S/G2/M are displayed in their respective gates. (C): Average percentage of LSK cells in S/G2/M phases of the cell cycle after 3 days in culture (n=3). (D): Average percentage of LSK cells in G0 after 3 days in culture (n=3). (E): Average colony forming units-granulocyte, macrophage frequency in LSK cells after 4 days in culture (n=3). For all panels, error bars represent SD and p values were determined using paired t test. Abbreviations: APC, allophycocyanin; LSK, lin−, Sca-1+, c-kit+; Ryk, related to receptor tyrosine kinase; rWnt5a, recombinant Wnt5a.
Previously, we demonstrated that rWnt5a does not prevent LSK cells from proceeding through mitosis and thus the difference in cell expansion is not due to a population of undivided cells in any of the treatments [9]. In this same study, Wnt5a suppressed cell expansion of LSK cells expressing high levels of the antiapoptotic Bcl2 gene, indicating that increased apoptosis is also unlikely to be responsible for the decrease in cell numbers.
rWnt5a inhibits cell cycling and promotes quiescence of LSK cells [9]. To test whether Ryk regulates rWnt5a-mediated induction of quiescence, we determined the cell cycle profiles of LSK cells cultured with rWnt5a and α-Ryk (Fig. 3B). rWnt5a treatment resulted in a 1.2-fold decrease in the percentage of cells in the S/G2/M phases of the cell cycle (p<.01) (Fig. 3C). Cotreatment with α-Ryk and rWnt5a did not have a significant effect compared to control (p=.16). rWnt5a treatment significantly increased the percentage of mitotically quiescent G0 cells by 2.3-fold compared to control (p<.01; Fig. 3D). Cotreatment with α-Ryk and rWnt5a also resulted in a significant increase in the percentage of G0 cells compared to control (p<.05). However, treatment with α-Ryk and rWnt5a also reduced the percentage of quiescent cells compared to rWnt5a alone (p<.05) indicating that α-Ryk was able to partially block the effect of rWnt5a on quiescence. Together, these data indicate that inhibiting Ryk blocks the effects of rWnt5a on cell proliferation. There were no differences in the ability of cultured LSK cells to form hematopoietic colonies, indicating that treatment with rWnt5a or α-Ryk did not impact differentiation of LSK cells (Fig. 3E).
Ryk Regulates the Wnt5a-Mediated Enhancement of Hematopoietic Repopulation
Previously, we observed that the ability of rWnt5a to suppress proliferation of LSK cells correlated with an ability to enhance hematopoietic repopulation. Thus, we tested the hypothesis that blocking Ryk inhibited the ability of rWnt5a to enhance short-term and long-term hematopoietic repopulation.
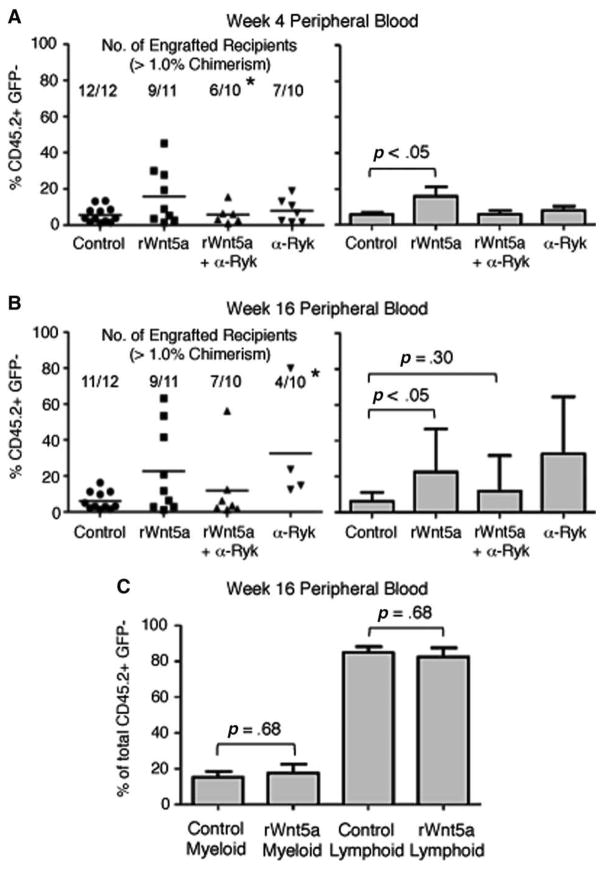
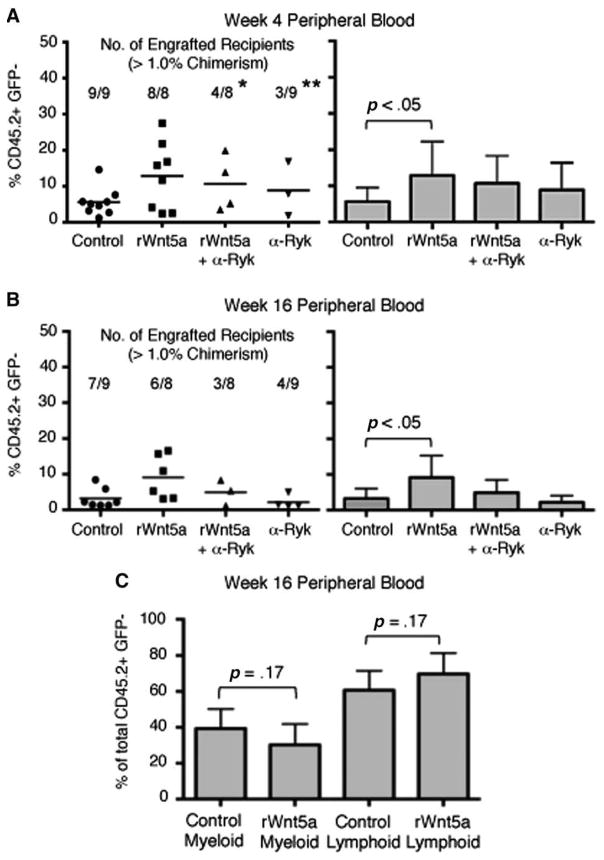
We cultured C57BL/6 LSK cells for 4 days under the conditions previously described and transplanted 1 × 104 cultured cells along with 5 × 105 bone marrow cells from C57BL/6-Tg (UBC-GFP) 30Scha/J mice (a wild-type GFP transgenic line) into lethally irradiated B6.SJL recipients [31]. Four weeks after transplant, we analyzed recipients’ peripheral blood for evidence of short-term hematopoietic repopulation by cultured LSK cells (CD45.2+, GFP−). We defined successful engraftment as greater than 1% chimerism. As expected, treatment of LSK cells with rWnt5a significantly increased their contribution to short-term hematopoiesis by 2.8-fold compared to control (p<.05, Fig. 4A). A comparable increase was not observed when LSK cells were cotreated with rWnt5a and α-Ryk.
Figure 4.
Effect of inhibiting Ryk on Wnt5a-mediated hematopoietic repopulation by ex vivo expanded lin−, Sca-1+, c-kit+ (LSK) cells. (A, Left): Raw data of hematopoietic chimerism of peripheral blood cells 4 weeks after transplant of cultured LSK cells. Each point represents a single recipient. Average percent contribution by cultured cells (% CD45.2+, GFP−) for each condition is marked with a horizontal bar. The number of engrafted recipients (defined as >1% chimerism) and the total number of transplanted recipients are shown for each condition. (A, Right): Bar graph showing average percentage of hematopoietic chimerism of peripheral blood cells. Only recipients with >1% chimerism are included. Group sizes are control, n=12; rWnt5a, n=9; rWnt5a+α-Ryk, n=6; α-Ryk, n=7. Data are pooled from two independent experiments. (B, Left): Raw data of hematopoietic chimerism of peripheral blood cells 16 weeks after transplant of cultured LSK cells. Average percent contribution by cultured cells (% CD45.2+, GFP−) for each condition is marked with a horizontal bar. The number of engrafted recipients (defined as >1% chimerism) and the total number of transplanted recipients are shown for each condition. (B, Right): Bar graph showing average percentage of hematopoietic chimerism of peripheral blood cells. Group sizes are control, n=11; rWnt5a, n=9; rWnt5a+α-Ryk, n=7; α-Ryk, n=4. Only recipients with >1% chimerism are included. Data are pooled from two independent experiments. (C): Average percentage of CD45.2+, GFP− peripheral blood cells of myeloid (left) or lymphoid lineage (right) in recipients 16 weeks after transplantation of cells cultured under control conditions or with rWnt5a. Myeloid engraftment is the percentage of CD45.2+, GFP− cells that are positive for the cell surface markers Gr-1, CD11b, and Ter-119. Lymphoid engraftment is the percentage of CD45.2+, GFP− cells that are positive for the cell surface markers CD3 and B220. For all panels, error bars represent SD. Due to differences in sample size between groups of engrafted recipients, we performed paired t tests between control versus rWnt5a for short- and long-term repopulation and control versus rWnt5a+α-Ryk for long-term repopulation. Statistical analysis of engraftment frequencies between control groups and other experimental groups were determined using Fisher’s Exact Probability test (*, p<.05). Abbreviations: GFP, green fluorescent protein; rWnt5a, recombinant Wnt5a, Ryk, related to receptor tyrosine kinase.
To determine the effect of blocking Ryk on long-term hematopoietic repopulation, we analyzed recipients’ peripheral blood after 16 weeks. Treatment of LSK cells with rWnt5a significantly increased their contribution to long-term hematopoiesis by 3.7-fold, (p<.05, Fig. 4B). In contrast, cotreatment with α-Ryk and rWnt5a did not increase long-term hematopoiesis compared to control (p=.30). Treatment with α-Ryk alone decreased the frequency of long-term engraftment compared to control (p<.05) (Fig. 4B). We did not observe that treatment with rWnt5a skewed subsequent differentiation toward myeloid or lymphoid lineage (Fig. 4C).
We then determined the effect of rWnt5a treatment on hematopoietic repopulation by a more homogenous HSC population by culturing LSK, CD150+, CD48− cells for 4 days under serum-free conditions and performing bone marrow transplant assays. Similar to its effect on LSK cells, rWnt5a treatment significantly increased the contribution to short-term and long-term hematopoiesis (p<.05; Fig. 5A, 5B). Treatment with α-Ryk, either alone or in combination with Wnt5a, decreased the frequency of successful short-term engraftment compared to control (Fig. 5A). Treatment with rWnt5a did not skew hematopoietic repopulation toward the myeloid or lymphoid lineage (Fig. 5C). Together, these data indicate that inhibiting Ryk ex vivo is sufficient to block the ability of rWnt5a to promote hematopoietic repopulation.
Figure 5.
Effect of inhibiting Ryk on Wnt5a-mediated hematopoietic repopulation by ex vivo expanded lin−, Sca-1+, c-kit+ (LSK), CD150+, CD48− cells. (A, Left): Raw data of hematopoietic chimerism of peripheral blood cells 4 weeks after transplant of cultured LSK, CD150+, CD48− cells. Each point represents a single recipient. Average percent contribution by cultured cells (% CD45.2+, GFP−) for each condition is marked with a horizontal bar. The number of engrafted recipients (defined as >1% chimerism) and the total number of transplanted recipients are shown for each condition. (A, Right): Bar graph showing average percentage of hematopoietic chimerism of peripheral blood cells. Only recipients with >1% chimerism are included. Group sizes are control, n=9; rWnt5a, n=8; rWnt5a+α-Ryk, n=4; α-Ryk, n=3. Data are pooled from two independent experiments. (B, Left): Raw data of hematopoietic chimerism of peripheral blood cells 16 weeks after transplant of cultured LSK, CD150+, CD48− cells. Average percent contribution by cultured cells (% CD45.2+, GFP−) for each condition is marked with a horizontal bar. The number of engrafted recipients (defined as >1% chimerism) and the total number of transplanted recipients are shown for each condition. (B, Right): Bar graph showing average percentage of hematopoietic chimerism of peripheral blood cells. Group sizes are control, n=7; rWnt5a, n=6; rWnt5a+α-Ryk, n=3; α-Ryk, n=4. Only recipients with >1% chimerism are included. Data are pooled from two independent experiments. (C): Average percentage of CD45.2+, GFP− peripheral blood cells of myeloid (left) or lymphoid lineage (right) in recipients 16 weeks after transplantation of cells cultured under control conditions or with rWnt5a. Myeloid engraftment is the percentage of CD45.2+, GFP− cells that are positive for the cell surface markers Gr-1, CD11b, and Ter-119. Lymphoid engraftment is the percentage of CD45.2+, GFP− cells that are positive for the cell surface markers CD3 and B220. For all panels, error bars represent SD and p values between control versus rWnt5a groups were determined using paired t test. Statistical analysis of engraftment frequencies between control groups and other experimental groups were determined using Fisher’s exact probability test (*, p<.05; **, p<.01). Abbreviations: GFP, green fluorescent protein; Ryk, related to receptor tyrosine kinase; rWnt5a, recombinant Wnt5a.
Wnt5a Inhibits Production of ROS
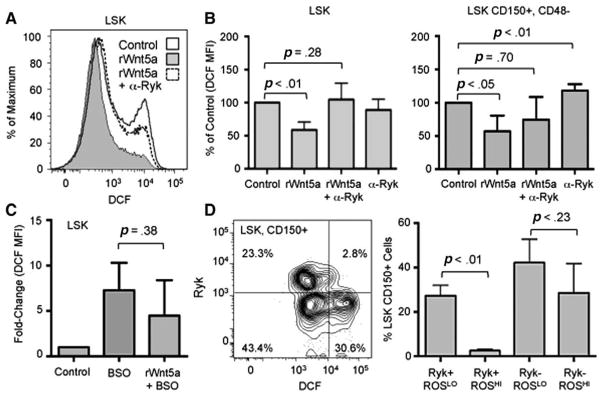
To identify a mechanism that can link the effects of rWnt5a on repopulating ability and proliferation of HSPCs, we focused on molecular regulators that exhibit a similar inverse association between proliferation and long-term HSC function. One such regulator is ROS. Increased production of ROS in HSCs in vivo and ex vivo is associated with increased proliferation and decreased quiescence (e.g., [32–34]). We cultured LSK and LSK, CD150+, CD48− cells with rWnt5a and α-Ryk cells under serum-free conditions for 4 days, stained the cells with the general oxidative stress probe chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, and measured the total amount of ROS using flow cytometry (Fig. 6A). rWnt5a treatment resulted in a 1.6-fold and 1.8-fold decrease in ROS levels in LSK and LSK, CD150+, CD48− cells, respectively, compared to control (p<.05, Fig. 6B). Cotreatment with α-Ryk and rWnt5a did not result in a significant decrease in ROS levels compared to control (p=.28). Treatment of LSK, CD150+, CD48− cells with α-Ryk alone resulted in a significant increase in ROS levels (p<.01).
Figure 6.
Effect of Wnt5a on ROS levels in LSK cells. (A): Representative flow cytometry analysis of ROS levels in LSK cells cultured for 4 days under control (solid line, white histogram) conditions, with rWnt5a (solid line, gray histogram), or with rWnt5a and anti-Ryk antibody (α-Ryk, dotted line, white histogram). To detect ROS, cells were stained with DCF as described under Materials and Methods section. (B): Average fold-changes of ROS levels in LSK (left) and LSK, CD150+, CD48− cells cultured for 4 days with rWnt5a and α-Ryk (n=3). For each individual experiment, the median DCF MFI of LSK cells cultured under control conditions was normalized to 100%. (C): Average fold-changes of ROS levels in LSK cells cultured for 4 days under control conditions, with 0.1 mM BSO, or with BSO and rWnt5a (n=3). For each individual experiment, the median DCF MFI of LSK cells cultured under control conditions was normalized to 1. (D, Left): Representative flow cytometry analysis of ROS and Ryk expression levels in LSK, CD150+ bone marrow cells. (Right): Average percentage of LSK, CD150+ cells detected within Ryk+, ROSLO, Ryk+, ROSHI, Ryk−, ROSLO, and Ryk−, ROSHI populations (n=3). For all experiments, error bars represent SD and p values were generated by paired t test. Abbreviations: BSO, buthionine sulfoximine; DCF, chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate; LSK, lin−, Sca-1+, c-kit+; MFI, median fluorescence intensity; ROS, reactive oxygen species; Ryk, related to receptor tyrosine kinase; rWnt5a, recombinant Wnt5a.
We tested whether Wnt5a is sufficient to reduce pharmacologic induction of ROS production by treating LSK cells with BSO, which increases ROS levels by depleting intracellular glutathione. We were unable to detect a significant difference in ROS levels between cells treated with BSO versus BSO and rWnt5a (Fig. 6C). These data indicate that Wnt5a can inhibit endogenous ROS levels.
Using the same analysis performed in Figure 1B, we compared the ROS levels of Ryk+ and Ryk− LSK, CD150+ cells in vivo. This population could be separated into four distinct populations based on ROS (ROSLO and ROSHI) and Ryk (Ryk+ and Ryk−) levels (Fig. 6D). ROSLO cells are enriched for long-term repopulating ability [35]. We observed significant 11-fold increase in the percentage of Ryk+ LSK, CD150+ cells that were ROSLO compared to ROSHI (p<.01). In contrast, there was no difference between the percentages of Ryk− LSK, CD150+ cells that were ROSLO compared to ROSHI. These data indicate that Ryk expression is associated with decreased levels of ROS in phenotypic HSCs in vivo.
Discussion
In this study, we demonstrate a novel function of the Ryk receptor in regulating the response of HSCs to Wnt5a. Previously, we demonstrated that Wnt5a activates noncanonical Wnt pathways in LSK cells to maintain them in a quiescent state and enhance their ability to repopulate hematopoiesis [9]. Similarly, Wnt5a enhances the repopulating ability of human cord blood cells [10]. Loss of function studies demonstrated that Wnt5a-mediated noncanonical Wnt signaling was necessary to maintain HSC quiescence and long-term function in vivo [11, 16]. These studies indicate that Wnt5a and its cognate noncanonical signaling pathways are significant regulators of HSC function.
The mechanisms by which Wnt5a regulates HSC repopulation are still unclear. We hypothesized that the Ryk receptor regulated Wnt5a-mediated effects on HSC proliferation and hematopoietic repopulation. This hypothesis was based on studies demonstrating that increased Ryk transcription was associated with decreased HSC proliferation [22, 23]. Our data indicate that this decrease is due to prolonged transit of Ryk+ HSPCs through the cell cycle rather than a preference for the quiescent state. Increased Ryk levels are not sufficient to inhibit proliferation during hematopoietic stress as Ryk+ and Ryk− HSPCs proliferation increased at a similar rate after treatment with 5-FU. However, Ryk+ HSPCs were less likely than Ryk− HSPCs to be actively cycling. One explanation is that Ryk+ HSPCs return to a quiescent state more rapidly, an effect similar to how Hmgb3 deficiency promotes quiescence of HSCs following 5-FU treatment [36]. The differences in proliferation rate at steady state may cause Ryk+ HSPCs to be less sensitive to the initial effects of 5-FU, which would explain the relative increase in Ryk+ HSPCs following treatment.
These observations support our hypothesis that Ryk activity is associated with HSPC proliferation. The requirement of Ryk for complete Wnt5a-mediated suppression of HSPC proliferation and induction of quiescence further supports this model. Induction of quiescence may be one mechanism by which the interaction between Wnt5a and Ryk regulates hematopoietic repopulation; quiescent HSCs are more efficient at engrafting and reconstituting hematopoiesis than their actively cycling counterparts [37, 38].
However, inhibition of Ryk was sufficient to suppress the frequency and contribution to hematopoietic repopulation even though the anti-Ryk antibody alone had no effect on quiescence. One explanation is that the interaction between Wnt5a and Ryk inhibits HSC differentiation in addition to proliferation, resulting in increased hematopoietic repopulation. The decline in the percentage of Ryk+ cells as HSCs differentiate into progenitors (with megakaryocyte progenitors the exception) supports an association between decreased Ryk activity and hematopoietic differentiation. Since the progenitor populations are largely Ryk−, this could explain how neither Wnt5a treatment nor inhibition of Ryk altered the frequency of hematopoietic progenitors in colony assays.
Ryk can act as an independent receptor or as a coreceptor in combination with Frizzled receptors [20, 39, 40]. Sugimura et al. [11] indicated that Wnt5a can exert its biological effects through the Frizzled 8 receptor and Flamingo coreceptor. Thus, one explanation is that Wnt5a induces quiescence through a single receptor complex that incorporates Ryk in addition to Frizzled 8 and Flamingo. However, based on our observations that blocking Ryk only partially inhibited the effect of Wnt5a on quiescence, it is equally possible that Wnt5a regulates HSC quiescence through distinct receptor complexes. Ryk binds to Wnt ligands through an N-terminal WIF domain, originally described in the Wnt-inhibitory-factor-1 protein [20, 41]. To date, no other types of ligands have been observed binding to a WIF domain. Thus, it is unlikely that our observations are due to the Ryk receptor binding a non-Wnt ligand.
We demonstrated that Wnt5a inhibits endogenous production of ROS and that inhibiting Ryk blocked this ability of Wnt5a. Inhibition of Ryk in purified HSCs was sufficient to increase ROS levels. These in vitro data, in combination with our in vivo observation that higher expression of Ryk in phenotypic HSCs is associated with lower ROS levels, support a model in which Wnt5a regulates ROS production through the Ryk receptor. Excessive ROS production has been implicated as a causal factor in decreased HSC quiescence and long-term HSC function [33–35]. Conversely, suppression of ROS production enhances the ability of mouse and human HSCs to regenerate hematopoiesis [32, 42]. Thus, suppression of ROS production may be one mechanism by which Wnt5a promotes HSC quiescence and repopulating ability.
In addition to suppressing ROS production, Wnt5a antagonizes activation of the canonical Wnt pathway and the nuclear factor of activated T-cells-1 (NFAT1) transcription factor in HSCs [9, 11]. Both of these pathways are implicated in Wnt5a-mediated regulation of HSC quiescence and repopulating activity. Increase in ROS levels can lead to increased canonical Wnt signaling and vice versa. [43, 44]. Suppression of ROS levels can inhibit activation of NFAT family members [45, 46]. Thus, Wnt5a may regulate other signal transduction pathways in HSCs through modulating ROS production. However, the effects of Wnt5a on ROS production, canonical Wnt signaling, and NFAT activation may represent distinct molecular mechanisms.
Recently, Schaap-Oziemlak et al [47] found that ex vivo treatment of purified HSCs with Wnt5a was insufficient to maintain long-term hematopoietic repopulation and may even inhibit it. Part of the discrepancy between these data and our results may be due to experimental conditions, especially with regards to the dosage of recombinant Wnt5a. Luis et al. [48] demonstrated that canonical Wnt signaling regulates HSC fate in a dose-dependent manner and it is possible that Wnt5a-mediated signaling has a similar dose-dependent effect.
Conclusion
We propose a model in which Wnt5a maintains HSCs within a quiescent state through the Ryk receptor. The Wnt5a-Ryk signaling axis may regulate quiescence through several distinct mechanisms, one of which is suppressing formation of ROS. Through these mechanisms, the functional interaction between Wnt5a and Ryk results in enhanced hematopoietic repopulation by HSCs. Based on our model, we predict that suppression of Ryk would stimulate production of ROS and HSC proliferation and that activation of Ryk would inhibit production of ROS and HSC proliferation. Our model also predicts that targeting the Wnt5a-Ryk interaction could alter the response of HSCs to myeloablative agents such as 5-FU. Thus, strategies for modulating the activity of the Ryk receptor could be novel approaches for manipulating HSC function ex vivo or in vivo.
Acknowledgments
We thank Dawn Barnas, Craig Jones, Kitty de Jong, Aimee Stablewski, and Earl Timm for technical assistance and the Laboratory Animal and Flow Cytometry Resources at Roswell Park Cancer Institute, which are supported by Cancer Center Support Grant P30 CA016056; Drs. William Burhans, Joseph Lau, and Philip McCarthy for careful reading of the manuscript. B.J.P. and M.J.N. were supported by the Roswell Park Alliance Foundation, the National Blood Foundation, and by institutional funds provided by Roswell Park Cancer Institute.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Author Contributions
B.J.P: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript; M.J.N: conception and design, financial support, data analysis and interpretation, manuscript writing, and final approval of manuscript.
References
- 1.Kondo M, Wagers A, Manz M, et al. Biology of hematopoietic stem cells and progenitors: Implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 2.Warr MR, Pietras EM, Passegué E. Mechanisms controlling hematopoietic stem cell functions during normal hematopoiesis and hematological malignancies. Wiley Interdiscip Rev Syst Biol Med. 2011;3:681–701. doi: 10.1002/wsbm.145. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slusarski DC, Yang-Snyder J, Busa WB, et al. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev Biol. 1997;182:114–120. doi: 10.1006/dbio.1996.8463. [DOI] [PubMed] [Google Scholar]
- 5.Boutros M, Paricio N, Strutt DI, et al. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- 6.Willert K, Brown JD, Danenberg E, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 7.Heinonen KM, Vanegas JR, Lew D, et al. Wnt4 enhances murine hematopoietic progenitor cell expansion through a planar cell polarity-like pathway. Plos One. 2011;6:e19279. doi: 10.1371/journal.pone.0019279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clements WK, Kim AD, Ong KG, et al. A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature. 2011;474:220–224. doi: 10.1038/nature10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemeth MJ, Topol L, Anderson SM, et al. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci USA. 2007;104:15436–15441. doi: 10.1073/pnas.0704747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murdoch B, Chadwick K, Martin M, et al. Wnt-5A augments repopulating capacity and primitive hematopoietic development of human blood stem cells in vivo. Proc Natl Acad Sci USA. 2003;100:3422–3427. doi: 10.1073/pnas.0130233100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugimura R, He XC, Venkatraman A, et al. Noncanonical wnt signaling maintains hematopoietic stem cells in the niche. Cell. 2012;150:351–365. doi: 10.1016/j.cell.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang H, Chen Q, Coles A, et al. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell. 2003;4:349–360. doi: 10.1016/s1535-6108(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 13.Reya T, O’Riordan M, Okamura R, et al. Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity. 2000;13:15–24. doi: 10.1016/s1074-7613(00)00004-2. [DOI] [PubMed] [Google Scholar]
- 14.Wilson A, Laurenti E, Oser G, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 15.Li J. Quiescence regulators for hematopoietic stem cell. Exp Hematol. 2011;39:511–520. doi: 10.1016/j.exphem.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Buckley SM, Ulloa-Montoya F, Abts D, et al. Maintenance of HSC by Wnt5a secreting AGM-derived stromal cell line. Exp Hematol. 2011;39:114–123. doi: 10.1016/j.exphem.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhanot P, Brink M, Samos C, et al. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 18.Tamai K, Semenov M, Kato Y, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 19.Oishi I, Suzuki H, Onishi N, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 20.Lu W, Yamamoto V, Ortega B, et al. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 21.Simoneaux DK, Fletcher FA, Jurecic R, et al. The receptor tyrosine kinase-related gene (ryk) demonstrates lineage and stage-specific expression in hematopoietic cells. J Immunol. 1995;154:1157–1166. [PubMed] [Google Scholar]
- 22.Forsberg EC, Passegué E, Prohaska SS, et al. Molecular signatures of quiescent, mobilized and leukemia-initiating hematopoietic stem cells. Plos One. 2010;5:e8785. doi: 10.1371/journal.pone.0008785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Graaf CA, Kauppi M, Baldwin T, et al. Regulation of hematopoietic stem cells by their mature progeny. Proc Natl Acad Sci USA. 2010;107:21689–21694. doi: 10.1073/pnas.1016166108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keeble TR, Halford MM, Seaman C, et al. The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J Neurosci. 2006;26:5840–5848. doi: 10.1523/JNEUROSCI.1175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halford MM, Armes J, Buchert M, et al. Ryk-deficient mice exhibit craniofacial defects associated with perturbed Eph receptor crosstalk. Nat Genet. 2000;25:414–418. doi: 10.1038/78099. [DOI] [PubMed] [Google Scholar]
- 26.Nygren JM, Bryder D. A novel assay to trace proliferation history in vivo reveals that enhanced divisional kinetics accompany loss of hematopoietic stem cell self-renewal. Plos One. 2008;3:e3710. doi: 10.1371/journal.pone.0003710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pronk CJH, Rossi DJ, Månsson R, et al. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell. 2007;1:428–442. doi: 10.1016/j.stem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Randall T, Weissman I. Phenotypic and functional changes induced at the clonal level in hematopoietic stem cells after 5-fluorouracil treatment. Blood. 1997;89:3596–3606. [PubMed] [Google Scholar]
- 29.Kiel M, Yilmaz O, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 30.Miyashita T, Koda M, Kitajo K, et al. Wnt-Ryk signaling mediates axon growth inhibition and limits functional recovery after spinal cord injury. J Neurotrauma. 2009;26:955–964. doi: 10.1089/neu.2008.0776. [DOI] [PubMed] [Google Scholar]
- 31.Schaefer B, Schaefer M, Kappler J, et al. Observation of antigen-dependent CD8+ T-cell/dendritic cell interactions in vivo. Cell Immunol. 2001;214:110–122. doi: 10.1006/cimm.2001.1895. [DOI] [PubMed] [Google Scholar]
- 32.Ito K, Hirao A, Arai F, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 33.Tothova Z, Kollipara R, Huntly BJ, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Ito K, Hirao A, Arai F, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 35.Jang Y-Y, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110:3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nemeth MJ, Kirby MR, Bodine DM. Hmgb3 regulates the balance between hematopoietic stem cell self-renewal and differentiation. Proc Natl Acad Sci USA. 2006;103:13783–13788. doi: 10.1073/pnas.0604006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Passegue E, Wagers A, Giuriato S, et al. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med. 2005;202:1599–1611. doi: 10.1084/jem.20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowie M, McKnight K, Kent D, et al. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J Clin Invest. 2006;116:2808–2816. doi: 10.1172/JCI28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitt AM, Shi J, Wolf AM, et al. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature. 2006;439:31–37. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- 40.Lin S, Baye LM, Westfall TA, et al. Wnt5b-Ryk pathway provides directional signals to regulate gastrulation movement. J Cell Biol. 2010;190:263–278. doi: 10.1083/jcb.200912128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsieh JC, Kodjabachian L, Rebbert ML, et al. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- 42.Yahata T, Takanashi T, Muguruma Y, et al. Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood. 2011;118:2941–2950. doi: 10.1182/blood-2011-01-330050. [DOI] [PubMed] [Google Scholar]
- 43.Yoon JC, Ng A, Kim BH, et al. Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev. 2010;24:1507–1518. doi: 10.1101/gad.1924910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Funato Y, Michiue T, Asashima M, et al. The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nat Cell Biol. 2006;8:501–508. doi: 10.1038/ncb1405. [DOI] [PubMed] [Google Scholar]
- 45.Lin H, Sue Y-M, Chou Y, et al. Activation of a nuclear factor of activated T-lymphocyte-3 (NFAT3) by oxidative stress in carboplatin-mediated renal apoptosis. Br J Pharmacol. 2010;161:1661–1676. doi: 10.1111/j.1476-5381.2010.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Huang B, Shi X, et al. Involvement of hydrogen peroxide in asbestos-induced NFAT activation. Mol Cell Biochem. 2002;234–235:161–168. [PubMed] [Google Scholar]
- 47.Schaap-Oziemlak AM, Schouteden S, Khurana S, et al. Wnt5a does not support hematopoiesis in stroma-free, serum-free cultures. Plos One. 2013;8:e53669. doi: 10.1371/journal.pone.0053669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luis TC, Naber BAE, Roozen PPC, et al. Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell. 2011;9:345–356. doi: 10.1016/j.stem.2011.07.017. [DOI] [PubMed] [Google Scholar]