Abstract
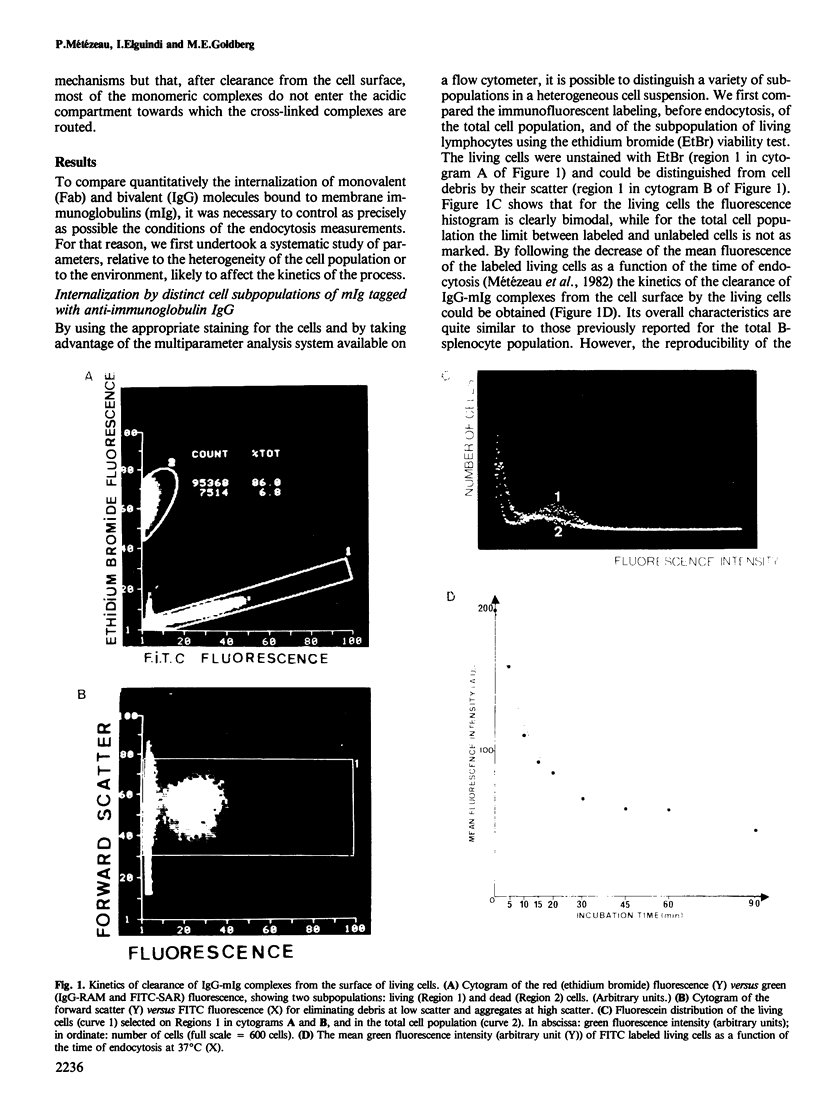
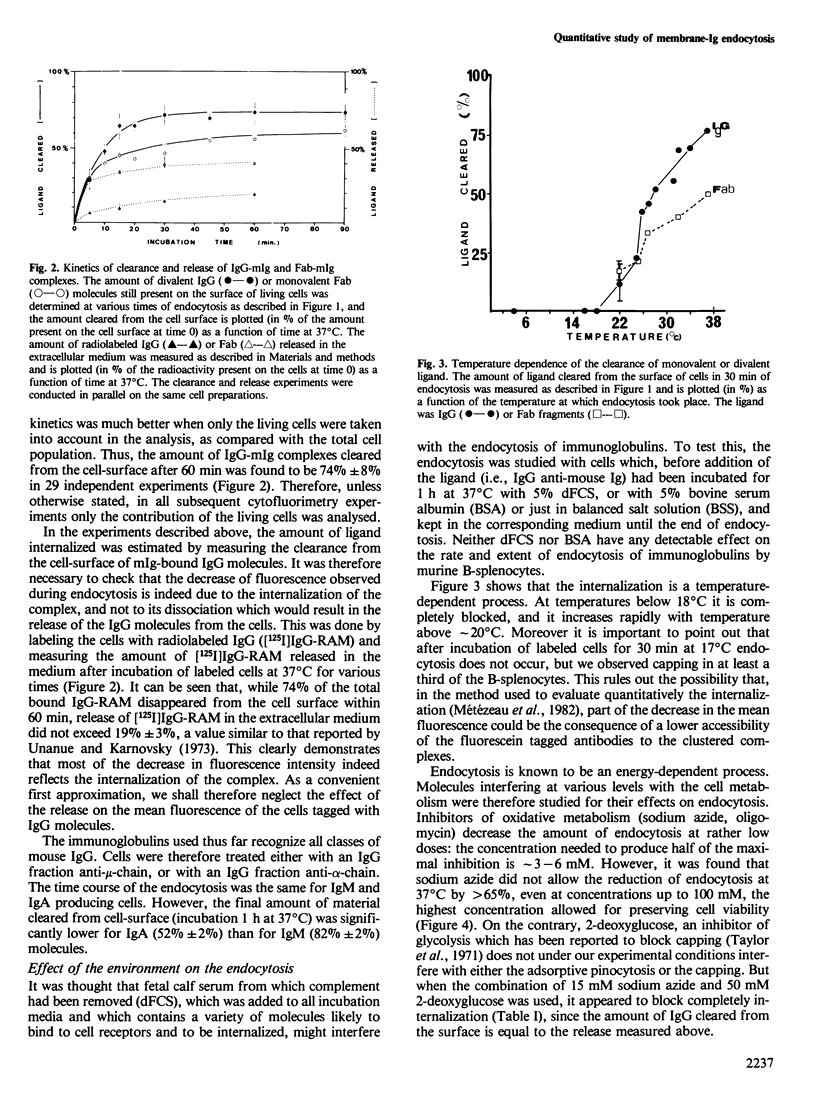
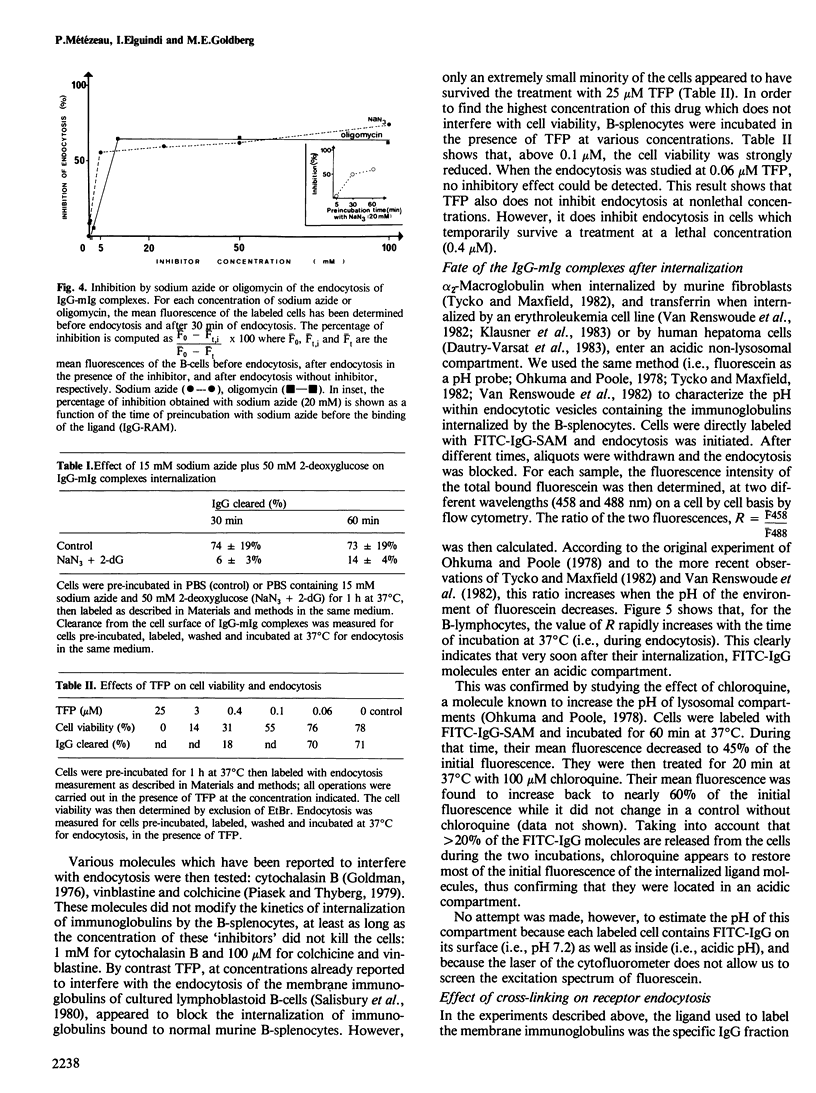
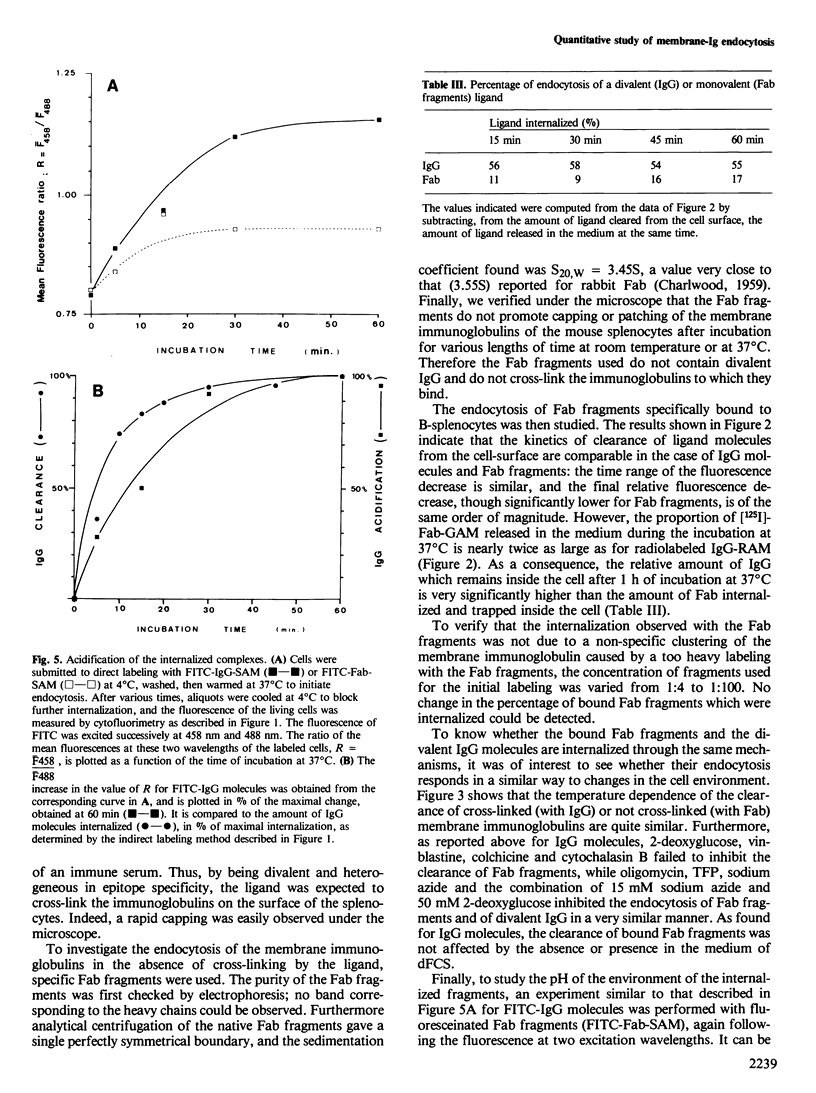
A quantitative analysis by flow cytometry of the rate and extent of endocytosis of ligands bound to the membrane immunoglobulins of mouse B-splenocytes is reported. The temperature dependence and the response to inhibitors of oxidative metabolism are described. Inhibitors of the cytoskeleton (cytochalasin B, vinblastine and colchicine) and of calmodulin (trifluoperazine) do not interfere with endocytosis at non-lethal doses. Similarly fetal calf serum does not modify the rate and extent of the process. Endocytosis occurs in a similar time range, but to a lesser extent, when the ligand is monovalent than when it cross-links the membrane immunoglobulins. Finally, it is shown that, within minutes after its internalization, the divalent ligand is found in an acidic environment, while the monovalent ligand is not. These results, in agreement with the model of receptor recycling, suggest that the divalent ligand-receptor complex is indeed internalized and captured in an acidic environment while the monovalent ligand-receptor complex is internalized and probably rapidly recycled back to the cell surface.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Goldstein J. L., Brown M. S. Localization of low density lipoprotein receptors on plasma membrane of normal human fibroblasts and their absence in cells from a familial hypercholesterolemia homozygote. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2434–2438. doi: 10.1073/pnas.73.7.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besterman J. M., Low R. B. Endocytosis: a review of mechanisms and plasma membrane dynamics. Biochem J. 1983 Jan 15;210(1):1–13. doi: 10.1042/bj2100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn B. Flow cytometry: a novel approach for the quantitative analysis of receptor--ligand interactions on surfaces of living cells. Mol Cell Endocrinol. 1980 Oct;20(1):1–15. doi: 10.1016/0303-7207(80)90090-8. [DOI] [PubMed] [Google Scholar]
- CHARLWOOD P. A. Ultracentrifugal examination of digestion products from rabbit gamma-globulin. Biochem J. 1959 Sep;73:126–127. [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Goldstein J. L., Anderson R. G., Brown M. S., Orci L. Binding and internalization of 125I-LDL in normal and mutant human fibroblasts. A quantitative autoradiographic study. Exp Cell Res. 1979 Jun;121(1):135–142. doi: 10.1016/0014-4827(79)90453-1. [DOI] [PubMed] [Google Scholar]
- Connolly D. T., Townsend R. R., Kawaguchi K., Bell W. R., Lee Y. C. Binding and endocytosis of cluster glycosides by rabbit hepatocytes. Evidence for a short-circuit pathway that does not lead to degradation. J Biol Chem. 1982 Jan 25;257(2):939–945. [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Kaneko I., Thomson G. G. Membrane distribution and adsorptive endocytosis by C3b receptors on human polymorphonuclear leukocytes. J Exp Med. 1981 Jun 1;153(6):1615–1628. doi: 10.1084/jem.153.6.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. The effect of cytochalasin B and colchicine on concanavalin A induced vacuolation in mouse peritoneal macrophages. Exp Cell Res. 1976 May;99(2):385–394. doi: 10.1016/0014-4827(76)90596-6. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Fan J. Y., Orci L. Receptor mediated endocytosis of polypeptide hormones: mechanism and significance. Metabolism. 1982 Jul;31(7):664–669. doi: 10.1016/0026-0495(82)90196-2. [DOI] [PubMed] [Google Scholar]
- Goud B., Antoine J. C. Emergence of a surface immunoglobulin recycling process during B lymphocyte differentiation. J Cell Biol. 1984 Apr;98(4):1238–1246. doi: 10.1083/jcb.98.4.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. J., Unanue E. R. Mapping and migration of lymphocyte surface macromolecules. Fed Proc. 1973 Jan;32(1):55–59. [PubMed] [Google Scholar]
- Klausner R. D., Ashwell G., van Renswoude J., Harford J. B., Bridges K. R. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2263–2266. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loor F., Forni L., Pernis B. The dynamic state of the lymphocyte membrane. Factors affecting the distribution and turnover of surface immunoglobulins. Eur J Immunol. 1972 Jun;2(3):203–212. doi: 10.1002/eji.1830020304. [DOI] [PubMed] [Google Scholar]
- Mellman I., Plutner H. Internalization and degradation of macrophage Fc receptors bound to polyvalent immune complexes. J Cell Biol. 1984 Apr;98(4):1170–1177. doi: 10.1083/jcb.98.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Plutner H., Ukkonen P. Internalization and rapid recycling of macrophage Fc receptors tagged with monovalent antireceptor antibody: possible role of a prelysosomal compartment. J Cell Biol. 1984 Apr;98(4):1163–1169. doi: 10.1083/jcb.98.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metezeau P., Djavadi-Ohaniance L., Goldberg M. E. The kinetics and homogeneity of endocytosis of a receptor-bound ligand in a heterogeneous cell population studied by flow cytofluorometry. J Histochem Cytochem. 1982 Apr;30(4):359–363. doi: 10.1177/30.4.7061830. [DOI] [PubMed] [Google Scholar]
- Natowicz M., Hallett D. W., Frier C., Chi M., Schlesinger P. H., Baenziger J. U. Recognition and receptor-mediated uptake of phosphorylated high mannose-type oligosaccharides by cultured human fibroblasts. J Cell Biol. 1983 Mar;96(3):915–919. doi: 10.1083/jcb.96.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D. R., Bryan V. M., Oi V. T., Herzenberg L. A. Antigen-specific identification and cloning of hybridomas with a fluorescence-activated cell sorter. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1962–1966. doi: 10.1073/pnas.76.4.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Receptor-mediated endocytosis of hormones in cultured cells. Annu Rev Physiol. 1981;43:239–250. doi: 10.1146/annurev.ph.43.030181.001323. [DOI] [PubMed] [Google Scholar]
- Piasek A., Thyberg J. Effects of colchicine on endocytosis and cellular inactivation of horseradish peroxidase in cultured chondrocytes. J Cell Biol. 1979 May;81(2):426–437. doi: 10.1083/jcb.81.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald R. Distribution of immunoglobulin G receptors in the small intestine of the young rat. J Cell Biol. 1980 Apr;85(1):18–32. doi: 10.1083/jcb.85.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury J. L., Condeelis J. S., Maihle N. J., Satir P. Receptor-mediated endocytosis by clathrin-coated vesicles: evidence for a dynamic pathway. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):733–741. doi: 10.1101/sqb.1982.046.01.070. [DOI] [PubMed] [Google Scholar]
- Salisbury J. L., Condeelis J. S., Satir P. Role of coated vesicles, microfilaments, and calmodulin in receptor-mediated endocytosis by cultured B lymphoblastoid cells. J Cell Biol. 1980 Oct;87(1):132–141. doi: 10.1083/jcb.87.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. Lateral and rotational diffusion of EGF-receptor complex: relationship to receptor-mediated endocytosis. Biopolymers. 1983 Jan;22(1):347–353. doi: 10.1002/bip.360220145. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone D. K., Xie X. S., Racker E. An ATP-driven proton pump in clathrin-coated vesicles. J Biol Chem. 1983 Apr 10;258(7):4059–4062. [PubMed] [Google Scholar]
- Tycko B., Maxfield F. R. Rapid acidification of endocytic vesicles containing alpha 2-macroglobulin. Cell. 1982 Mar;28(3):643–651. doi: 10.1016/0092-8674(82)90219-7. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Karnovsky M. J. Redistribution and fate of Ig complexes on surface of B lymphocytes: functional implications and mechanisms. Transplant Rev. 1973;14:184–210. doi: 10.1111/j.1600-065x.1973.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Perkins W. D., Karnovsky M. J. Ligand-induced movement of lymphocyte membrane macromolecules. I. Analysis by immunofluorescence and ultrastructural radioautography. J Exp Med. 1972 Oct 1;136(4):885–906. doi: 10.1084/jem.136.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Petris S., Raff M. C. Normal distribution, patching and capping of lymphocyte surface immunoglobulin studied by electron microscopy. Nat New Biol. 1973 Feb 28;241(113):257–259. doi: 10.1038/newbio241257a0. [DOI] [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]




