Abstract
We evaluated the incidence of all-cause and malaria-specific clinic visits during follow-up of a recent trial of iron therapy. In the main trial, Ugandan children 6–59 months with smear-confirmed malaria and iron deficiency [zinc protoporphyrin (ZPP > = 80 μmol/mol heme)] were treated for malaria and randomized to start a 27-day course of oral iron concurrently with (immediate group) or 28 days after (delayed group) antimalarial treatment. All children were followed for the same 56-day period starting at the time of antimalarial treatment (Day 0) and underwent passive and active surveillance for malaria and other morbidity for the entire follow-up period. All ill children were examined and treated by the study physician. In this secondary analysis of morbidity data from the main trial, we report that although the incidence of malaria-specific visits did not differ between the groups, children in the immediate group had a higher incidence rate ratio of all-cause sick-child visits to the clinic during the follow-up period (Incidence Rate Ratio (IRR) immediate/delayed = 1.76; 95%CI: 1.05–3.03, p = 0.033). Although these findings need to be tested in a larger trial powered for malaria-specific morbidity, these preliminary results suggest that delaying iron by 28 days in children with coexisting malaria and iron deficiency is associated with a reduced risk of subsequent all-cause illness.
Introduction
Malaria is endemic in many regions where iron deficiency is prevalent. The conditions frequently co-occur in the same child. The World Health Organization standard-of-care regimen for treating children with malaria and iron deficiency is to give antimalarial treatment and iron therapy concurrently [1,2]. However, studies following this regimen have reported unresolved anemia and increased risk of infection [3–5].
Increased concentrations of the hepatic protein hepcidin that accompany a malaria episode may explain the findings of unresolved anemia. Hepcidin rises with malaria infection, impairing intestinal iron absorption and release of iron from reticuloendothelial stores [6]. Because elevated hepcidin normalizes approximately four weeks after antimalarial treatment [7], oral iron given before this time may not be well absorbed or utilized. In a recent randomized trial, we demonstrated with iron stable isotopes that starting iron 28 days after, rather than concurrently with, antimalarial treatment in children with malaria and iron deficiency was associated with a two-fold increase of iron incorporation into hemoglobin [8]. However, iron status was equivalent between the groups at the end of the 56-day follow up period, thus demonstrating no clear, short-term benefit or harm of delaying iron on iron status.
Whether a 28-day delay in the start of iron therapy might affect subsequent morbidity is unknown. Iron that is better absorbed would result in less iron trapped in the intestine and available to pathogenic bacteria. Alternatively, better iron absorption may result in more iron-rich blood cells, shown in vitro to be preferred by P. falciparum [9]. Either scenario could increase infection risk.
To determine whether delaying iron until 28 days after antimalarial treatment in children with coexisting iron deficiency and malaria is associated with a difference in the risk of subsequent illness compared to the standard of care concurrent iron therapy, we analyzed morbidity data from our previous trial to investigate the frequency and incidence of physician-diagnosed episodes of illness over the 56-day follow-up period.
Subjects and methods
Study population
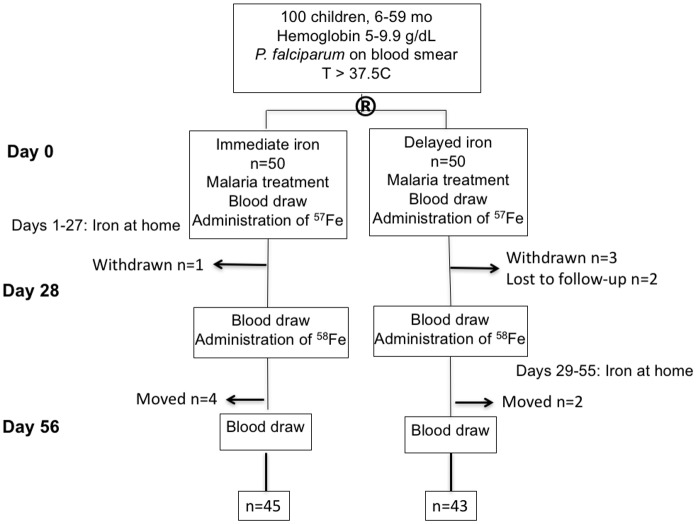
As described previously [8], 100 children 6–59 months old with malaria (positive Giemsa smear and T>37.5°C) and hemoglobin 5.0–9.9 g/dL were enrolled in a randomized trial of iron therapy at Mulago Hospital in Kampala, Uganda (Fig 1). Children were treated for malaria with parenteral artesunate and also given a 3-day course of oral artemether-lumefantrine. If children had a zinc protoporphyin (ZPP) concentration > = 80 μmol/mol heme, they were randomized either to begin a 27-day iron therapy regimen (2 mg/kg/day as liquid oral ferrous sulfate) concurrently with antimalarial treatment on Day 0 (immediate group, n = 50) or 28 days later on Day 28 (delayed group, n = 50). Children were given an insecticide-treated bednet and were followed for 56 days (Day 0 –Day 56).
Fig 1. Consort diagram from original study [8].
During the 56-day follow-up period, all children were under active and passive surveillance for illness. In addition to scheduled clinic visit on Days 28 and 56, home visits were made to each enrolled child’s home by study staff on Days 14 and 42. Any ill child was brought to the study clinic for assessment and treatment. Additionally, all caregivers were instructed to contact the study office in the event of illness that occurred between study visits. Mobile phone airtime was reimbursed for these calls, and transportation to and from the study clinic was provided directly or reimbursed. All medical examination and treatment was provided free of charge. All ill children, whether identified during a home visit or self-referred, were given a physical examination by a study medical officer in the Paediatric Acute Care Unit at Mulago Hospital.
Diagnoses for common illnesses were made according to Mulago Hospital guidelines, including: 1) Uncomplicated malaria: positive Giemsa smear or Rapid Diagnostic Test (RDT), with fever or history of fever, in absence of any of the World Health Organization’s Clinical Signs of Severe Malaria [10]; 2) Severe malaria: positive Giemsa smear or RDT, fever or history of fever, concurrent with one or more clinical signs of severe malaria, including severe anemia, prostration, cerebral malaria, repeated seizures or symptoms like persistent vomiting, high temperature (>39.5°C), or tea-colored urine; 3) Upper respiratory tract infection (URTI): runny nose, cough with or without fever with normal examination chest findings; 4) Lower respiratory tract infection (LRTI): cough, history of fever or fever and examination findings of either respiratory rate that was high for age or crepitations; 5) Otitis media: ear infection; 6) Conjunctivitis: red eyes and discharge; 7) Gastroenteritis: diarrhea (more than 3 loose motions/day) as main symptom, with or without fever or vomiting; 8) Unspecified fevers: fever, negative malaria test, normal complete blood counts with no focus of investigations; 8) Chicken pox: vesicles characteristic of chicken pox (varicella).
For any visit to the study clinic, the findings from the child’s clinical exam, the primary diagnosis and any additional diagnoses were recorded on a sick child visit form and entered into the study’s database.
Ethical considerations
Caregivers of all enrolled children provided written informed consent. The study was approved by the Institutional Review Board of the University of Minnesota, the School of Medicine Research Ethics Committee at Makerere University, the Uganda National Council of Science and Technology, and the National Drug Authority of Uganda.
Statistical methods
The original trial was powered to compare the primary outcome of percentage iron incorporation into red blood cells between the immediate vs. delayed groups [8]. This secondary analysis compared the immediate and delayed groups according to the 56-day period prevalence and incidence rate of all-cause morbidity and malaria-specific morbidity, as recorded by sick-child visits to the study clinic. A visit was considered a “malaria visit” if a diagnosis of uncomplicated or severe malaria was made as primary or other diagnosis. For both the all-cause and malaria-specific outcomes, period prevalence was calculated in each group by dividing the number of children with at least one sick visit to the study clinic in the follow-up period by the number of children randomized to the group. We compared the period prevalence between groups using Pearson’s chi-square test. Incidence rates for all-cause and malaria-specific visits were calculated in each group by dividing the total number of sick visits in each group by the number of person-weeks. We compared groups according to incidence rates using Poisson regression with the log link and over-dispersion, which estimated incidence rate ratios. Secondary analyses adjusted these ratios for age, sex, baseline malaria parasite density, baseline hemoglobin, and baseline height-for-age z-score, with each secondary analysis considering one adjuster. Baseline characteristics were compared between the treatment groups using t-tests (age, z-scores) or Wilcoxon rank sum test (malaria parasite density) for continuous outcomes and chi-square for categorical outcomes (sex). Analyses used SPSS version 22.0 (IBM Corp., Armonk, NY), STATA version 12.1 (College Station, TX), and JMP version 12.0 Pro (SAS Institute Inc., Cary, NC).
Results
The groups did not differ with regard to age, sex, hemoglobin concentration, anthropometry, or malaria parasite density at baseline (Table 1). During the 56-day follow-up period, forty-one children had at least one sick-child visit (Table 2). During the 56-day follow-up period, 28 children (15 immediate, 13 delayed) had one sick visit, 12 children (9 immediate, 3 delayed) had two sick visits, and one child in the immediate group had three sick visits. The mean (range) time to first event was 24.1 (5–54) days in the immediate group and 24.0 days (8–56) days in the delayed group.
Table 1. Baseline characteristics of Ugandan children with malaria and anemia by study group1.
| Immediate | Delayed | p-value | |
|---|---|---|---|
| n | 50 | 50 | |
| Age, years ±SD2 | 2.2 ± 1.2 | 2.2 ± 1.1 | 0.793 |
| Sex, male, n (%) | 30 (60%) | 27 (54%) | 0.624 |
| Hemoglobin, g/dL2 | 8.0 (1.5) | 8.0 (1.5) | 0.82 |
| Height-for-age z-score, ±SD 2,5 | -1.4 ±1.1 | -1.3 ± 1.0 | 0.57 |
| HAZ < -2, n (%) | 11 (22.9) | 10 (21.7) | 0.89 |
| Weight-for-height z-score, ±SD2,5 | -0.85 ± 1.2 | -0.78 ±1.1 | 0.76 |
| WHZ < -2, n (%) | 8 (16.7) | 5 (10.8) | 0.42 |
| Weight-for-age z-score, ±SD2, 6 | -1.5 ± 1.1 | -0.78 ± 1.1 | 0.59 |
| WAZ < -2, n (%) | 19 (38.0) | 17 (34.7) | 0.73 |
| Malaria parasite density, parasites/μL, [IQR]7 | 46,700 (4600; 111,000) | 31,300 (1240; 94,100) | 0.408 |
1First published in [8];
2Values are means ± SDs
3T-test comparing immediate vs. delayed groups (all means)
4Chi-square p (all proportions);
5Immediate, n = 48; Delayed, n = 46;
6Delayed, n = 49;
7Values are medians [IQRs]; Immediate, n = 46; Delayed, n = 43;
8Wilcoxon rank sum comparing immediate vs. delayed groups.
HAZ: Height-for-age Z-score; WAZ: Weight-for-age Z-score; WHZ: Weight-for-height Z-score
Table 2. Incidence of all-cause and malaria-specific illness1.
| Immediate | Delayed | p2 | |
|---|---|---|---|
| All-cause illness visit | |||
| Period prevalence3 | 25/50 | 16/50 | 0.07 |
| Incidence rate4 | 36/378 | 19/351 | 0.03 |
| Malaria visit | |||
| Period prevalence | 12/50 | 8/50 | 0.32 |
| Incidence rate | 13/378 | 9/351 | 0.49 |
1As assessed by sick-child visits to hospital clinic in the 56-day follow-up period of a recent iron therapy trial that compared the effect on iron status outcomes of iron started concurrently with vs. 28 days after antimalarial treatment in 100 Ugandan children 6–59 months old with malaria and anemia;
2P-value from chi-square for period prevalence and Poisson regression for incidence rate;
3Period prevalence = children with at least one sick visit in the follow-up period/children enrolled at beginning of study;
4Incidence rate = total number of sick visits in follow-up period/person weeks.
The period prevalence, or number of children with at least one illness in the follow-up period divided by the number of children enrolled, did not differ significantly between the groups (p = 0.07, Table 2). However, the incidence rate of all-cause sick-child visits, which accounted for multiple visits per child, was significantly greater among children in the immediate iron group compared to the delayed iron group [Incidence Rate Ratio (IRR) immediate/delayed = 1.76; 95% CI: 1.05–3.03, p = 0.033, Table 2]. For visits in which a malaria diagnosis (uncomplicated or severe) was made, the group comparisons for period prevalence and incidence rate did not reach statistical significance [IRR immediate/delayed = 1.34; 95% CI: 0.59–3.19, p = 0.49]. Adjusting for age, sex, malaria parasite density, hemoglobin, or height-for-age z-score did not significantly change the estimated IRR for either sick child visits or malaria-specific visits (S1 and S2 Tables).
The most frequent diagnoses were upper respiratory tract infection (n = 23), malaria (uncomplicated or severe, n = 22), and gastroenteritis (n = 14, Table 3). The number of children with the most frequently diagnosed illnesses did not differ between groups (immediate vs. delayed; URTI: 12 vs 9, p = 0.46; malaria: 12 vs. 8, p = 0.32; gastroenteritis: 9 vs. 5, p = 0.25). The number of children admitted to the hospital because of their illness also did not differ between groups (8 immediate vs. 6 delayed; p = 0.56). The most common cause of hospitalization was severe malaria (8 children total: 5 immediate vs. 3 delayed, p = 0.72). One child in the immediate group was hospitalized twice for severe malaria.
Table 3. Diagnoses of Ugandan children in immediate or delayed iron study1.
| Primary Diagnosis | Secondary Diagnosis | Other diagnosis | |
|---|---|---|---|
| URTI | 16 | 7 | |
| Uncomplicated malaria | 6 | 4 | 3 |
| Severe malaria | 9 | ||
| Gastroenteritis | 10 | 4 | |
| LRTI | 9 | 3 | |
| Skin rash | 1 | 1 | 1 |
| Chicken pox | 1 | ||
| Unspecified fever | 1 | 3 | |
| Otitis Media | 1 | 1 | |
| Conjunctivitis | 1 | ||
| Other | 1 | 5 | 1 |
1Numbers represent number of times the indicated diagnosis was made as primary, secondary, or other diagnosis for entire study sample and for all visits over the course of the 56-day follow-up period after 100 Ugandan children were treated for malaria and then given a 27-day supply of iron that started immediately or after 28 days.
Discussion
We did a secondary analysis of morbidity outcomes in a recent randomized trial of iron treatment in which children began a 27-day course of an oral iron supplement concurrently with or 28 days after antimalarial treatment. Our primary finding was that children in the delayed-iron group had lower incidence of all-cause sick-child visits to the study clinic in the 56-day follow-up period compared to children in the immediate-iron group, despite ending the 56-day period with equivalent iron status [8].
Three recent Cochrane reviews [11–13] found no increased risk of malaria with iron supplementation if malaria prevention and management strategies are in place. We also did not find a significant difference between the groups in malaria-specific morbidity, although our study was not powered for this outcome. Similar to a recent study of iron-fortified micronutrient sprinkles in Pakistan, we did find iron to be linked with an increased risk of illnesses other than malaria, including respiratory illness [14].
It is unclear why the incidence of all-cause sick-child visits was greater in our study's immediate group. Perhaps lower absorption of iron at the time of supplementation, which we observed in the immediate iron group [8], translated into more unabsorbed iron in the gut. Unabsorbed iron may shift the gut microbiome in favor of enteropathogenic bacteria, rather than beneficial barrier bifidobacteria [15]. Such a pathogenic shift with iron was observed in Kenyan children receiving iron-fortified food products [15–16] and was associated with more frequent diarrheal illness. Associations between pathogenic shifts in the gut microbiome and exacerbation of respiratory infections have also been described [17–18].
Limitations to this secondary analysis include its small sample size and resulting insufficient power for malaria outcomes. Further, it is important to note that nine children in the immediate group as compared to three children in the delayed group had two episodes of illness. Thus, the difference in incidence rates of all-cause illness between the groups was driven largely by these six children. Although these data are preliminary, they suggest that delaying iron by 28 days after antimalarial treatment is associated with a reduction in all-cause morbidity while not harming iron status. Longer-term studies powered for iron status, morbidity, and neurocognitive developmental outcomes are needed to verify these findings and to determine the safest and most effective management options for children with malaria and iron deficiency.
Supporting information
1IRR for all sick-child visits adjusted for age, sex, malaria parasite density, hemoglobin, or height-for-age z-score; 2Standard deviation of the adjuster. HAZ = Height-for-age Z-score, IRR Incidence Rate Ratio.
(DOCX)
1IRR for malaria-specific visits adjusted for age, sex, malaria parasite density, hemoglobin, or height-for-age z-score; 2Standard deviation of the adjuster. HAZ = Height-for-age Z-score, IRR Incidence Rate Ratio.
(DOCX)
(XLSX)
(DOCX)
Acknowledgments
The authors would like to acknowledge the study caregivers and patients as well as the study physicians, Dr. Denis Muyaka and Dr. Ahmed Ddungu, and study coordinator Ms. Doreen Bitwayi.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development grant 1R03HD074262; and National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114 (KL2 Award S Cusick).
References
- 1.Conclusions and recommendations of the WHO Consultation on prevention and control of iron deficiency in infants and young children in malaria-endemic areas. Food Nutr Bull. 2007;28(4 Suppl);S621–7. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organziation e-library of Evidence for Nutrition Actions. Accessed at: http://www.who.int/elena/titles/guidance_summaries/micronutrientpowder_infants/en/.
- 3.Desai MR, Dhar R, Rosen DH, Kariuki SK, Shi YP, Kager PA, Ter Kuile FO. Randomized, controlled trial of daily iron supplementation and intermittent sulfadoxine-pyramethamine for the treatment of mild childhood anemia in western Kenya. J Infect Dis. 2003;187(4):658–66. doi: 10.1086/367986 [DOI] [PubMed] [Google Scholar]
- 4.Nwanyanwu OC, Ziba C, Kazembe PN, Gamadzi G, Gandwe J, Redd SC. The effect of oral iron therapy during treatment for Plasmodium falciparum malaria with sulphadoxine-pyramethamine on Malawian children under 5 years of age. Ann Trop Med Parasitol. December 1996;90(6):589–595. [DOI] [PubMed] [Google Scholar]
- 5.Verhoef H, West CE, Nzyuko SM, de Vogel S, van der Valk R, Wanga MA, Kuijsten A, Veenemans J, Kok FJ. Intermittent administration of iron and sulfadoxine-pyramethamine to control anaemia in Kenyan children: a randomised controlled trial. Lancet. 2002;360(9337):908–14. doi: 10.1016/S0140-6736(02)11027-0 [DOI] [PubMed] [Google Scholar]
- 6.Howard CT, McKakpo US, Quakyi IA, Bosompem KM, Addison EA, Sun K, Sullivan D, Semba RD. Relationship of hepcidin with parasitemia and anemia among patients with uncomplicated malaria in Ghana. Am J Trop Med Hyg. 2007. 77(4):623–6. [PubMed] [Google Scholar]
- 7.de Mast Q, Nadjm B, Reyburn H, Kemna EH, Amos B, Lassrakkers CM, Silalye S, Verhoef H, Sauerwein RW, Swinkels D. Assessment of Urinary Concentrations of Hepcidin Provides Novel Insight into Disturbances in Iron Homeostasis during Malarial Infection. 2009. J Infect Dis; 199 (2): 253–262. doi: 10.1086/595790 [DOI] [PubMed] [Google Scholar]
- 8.Cusick SE, Opoka RO, Abrams SA, John CC, Georgieff MK, Mupere E. Delaying Iron Therapy until 28 Days after Antimalarial Treatment Is Associated with Greater Iron Incorporation and Equivalent Hematologic Recovery after 56 Days in Children: A Randomized Controlled Trial. J Nutr. 2016;146(9):1769–74. doi: 10.3945/jn.116.233239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark M, Morgan MG, Fulford A. Host iron status and iron supplementation mediate susceptibility to erythrocytic stage Plasmodium falciparum. Nature Communications. 2014. 5, Article number: 4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Management of Severe Malaria: A Practical Handbook. 3rd ed 2012. Accessed at: http://apps.who.int/iris/bitstream/10665/79317/1/9789241548526_eng.pdf. [Google Scholar]
- 11.Ojukwu JU, Okebe JU, Yahav D, Paul M. (2009). Oral iron supplementation for preventing or treating anaemia among children in malaria-endemic areas. Cochrane Database Syst Rev, 2009 (3). [DOI] [PubMed] [Google Scholar]
- 12.Okebe JU, Yahav D, Shbita R, Paul M. (2011). Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst Rev, 2011 (10). [DOI] [PubMed] [Google Scholar]
- 13.Neuberger A, Okebe J, Yahav D, Paul M. (2016) Oral iron supplements for children in malaria-endemic areas. Cochrane Database of Syst Rev, 2016(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soofi S, Cousens S, Iqbal SP, Akhund T, Khan J, Ahmed I, Zaidi AK, Bhutta ZA. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomized trial. Lancet. 2013. July 6; 382 (9886):29–40. doi: 10.1016/S0140-6736(13)60437-7 [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann MB, Chassard C, Rohner F, N’goran EK, Nindjin C, Dostal A, Utzinger J, Ghattas H, Lacroix C, Hurrell RF. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d’Ivoire. Am J Clin Nutr. 2010. December; 92(6):1406–15. doi: 10.3945/ajcn.110.004564 [DOI] [PubMed] [Google Scholar]
- 16.Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A, Boekhorst J, Timmerman HM, Swinkels DW, Tjalsma H, Njenga J, Mwangi A, Kvalsvig J, Lacroix C, Zimmermann MB. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut. 2015. May; 64(5);731–42. doi: 10.1136/gutjnl-2014-307720 [DOI] [PubMed] [Google Scholar]
- 17.Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJ, de Boer JD, Hoogendijk AJ, de Beer R, de Vos A, Belzer C, de Vos WM, van der Poll T, Wiersinga WJ. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016. April;65(4):575–83. doi: 10.1136/gutjnl-2015-309728 Epub 2015 Oct 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsland Benjamin J., Trompette Aurélien, and Gollwitzer Eva S.. The Gut—Lung Axis in Respiratory Disease. Annals of the American Thoracic Society, Vol. 12, No. Supplement 2(2015), pp. S150–S156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1IRR for all sick-child visits adjusted for age, sex, malaria parasite density, hemoglobin, or height-for-age z-score; 2Standard deviation of the adjuster. HAZ = Height-for-age Z-score, IRR Incidence Rate Ratio.
(DOCX)
1IRR for malaria-specific visits adjusted for age, sex, malaria parasite density, hemoglobin, or height-for-age z-score; 2Standard deviation of the adjuster. HAZ = Height-for-age Z-score, IRR Incidence Rate Ratio.
(DOCX)
(XLSX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.