Abstract
Undergraduate students learn about mammalian cell culture applications in introductory biology courses. However, laboratory modules are rarely designed to provide hands-on experience with mammalian cells or teach cell culture techniques, such as trypsinization and cell counting. Students are more likely to learn about cell culture using bacteria or yeast, as they are typically easier to grow, culture, and manipulate given the equipment, tools, and environment of most undergraduate biology laboratories. In contrast, the utilization of mammalian cells requires a dedicated biological safety cabinet and rigorous antiseptic techniques. For this reason, we have devised a laboratory module and method herein that familiarizes students with common cell culture procedures, without the use of a sterile hood or large cell culture facility. Students design and perform a time-efficient inquiry-based cell viability experiment using HeLa cells and tools that are readily available in an undergraduate biology laboratory. Students will become familiar with common techniques such as trypsinizing cells, cell counting with a hemocytometer, performing serial dilutions, and determining cell viability using trypan blue dye. Additionally, students will work with graphing software to analyze their data and think critically about the mechanism of death on a cellular level. Two different adaptations of this inquiry-based lab are presented—one for non-biology majors and one for biology majors. Overall, these laboratories aim to expose students to mammalian cell culture and basic techniques and help them to conceptualize their application in scientific research.
INTRODUCTION
Background
One of the most important model systems for researchers in numerous fields of biology and medicine is the culture of mammalian cells or, more specifically, the growth and dispersal of cells derived from animal tissues, with an appropriate surface, proper nutrients, and a suitable environment. While the culture of cells and tissues has been in practice since the late 1800s, it was not until the early 1950s, with the remarkable growth of cells biopsied from the cervical cancer of Mrs. Henrietta Lacks, that the practice began to yield significant discoveries. The successful culture of Mrs. Henrietta Lacks’s cells, now called HeLa cells, effectively revolutionized biological and medical research, enabling countless cellular, molecular, and therapeutic breakthroughs, including the discovery of the first effective polio vaccine (1,2). Today, the HeLa cell line has yielded more than 60,000 publications and has been instrumental in several Nobel prize-winning discoveries (3,4). Moreover, the publication of the book The Immortal Life of Henrietta Lacks in 2011 generated popular interest in human cell culture and techniques, even prompting many high schools to assign the book as required reading (5). Despite the now widespread use of mammalian cell culture as a model for biological research, many undergraduate biology students are not exposed to the technique until upper level classes or even graduate level research. The reason for the lack of mammalian cell culture-based laboratory modules is the large and expensive equipment requirements and rigorous sterile-technique training that are not well suited to a large classroom environment. It is for this reason that we have devised a straightforward approach to teaching common mammalian cell culture techniques to undergraduate students in an introductory biology class. The approach can be adapted to both biology major and nonmajor classrooms with a few simple changes.
In this paper, we describe how HeLa cells were used as a mammalian model to teach undergraduates about cell culture techniques including cell manipulation, trypsinizing cells, counting, and determining cell viability. Three different adaptations of this inquiry-based lab are presented—one for non-biology majors, one for biology majors, and one for honors biology majors. Overall, this approach allows biology students to acquire and develop skills useful to them in subsequent experiences in cell culture laboratories while gaining an introductory understanding of how scientists use mammalian cells in research. Furthermore, we find that this laboratory stimulates reflection and discussions related to biomedical ethics and consent in scientific research.
Intended audience/Prerequisite knowledge
This laboratory exercise is intended for undergraduate students in introductory biology courses, with details provided to tailor the experience to students in a general biology education class (nonmajors) or biology majors. This laboratory explores concepts related to mammalian cell culture, the effects of different compounds on cell viability, and cell manipulation. For students in the general biology education class, this also dovetails into an introduction to the history of cell culture and the contribution of Mrs. Henrietta Lacks, as well as the ethical implications of this research. Biology major students will be expected to select and design their experimental conditions, set up serial dilutions, and employ appropriate controls in order to study mammalian cell viability. Therefore, prior to the beginning of the laboratory period, students should be familiar with terms such as HeLa cells, trypsin, trypan blue, and cell viability and have been introduced to the concept of experimental design. Prior pipetting practice, sterile technique, and acquaintance with a compound microscope are encouraged.
Learning time
General education biology (nonmajors)
Students were given a reading assignment by Rodríguez-Hernández et al. to complete before the lab period, introducing the historical background of cell culture as well as its application in research (1). To complete the laboratory in its entirety takes one full lab period of approximately two hours, since the cell treatment option has been limited to pre-selected temperatures. It is helpful if students are already familiar with the compound microscope; however this is not a requirement. Incubation times can be used to provide students with more information on the history of HeLa cells and to discuss the ethics surrounding their initial collection and use in research today. Students are expected to collect all of their data within the allotted two hours, but they will conduct their analysis and generate figures following the lab period.
Biology majors
To complete the laboratory in its entirety will take one lab period of approximately three hours, provided that students have already been introduced to dilution calculations and are comfortable with the use of disposable bulb pipettes, micropipettes, and compound microscopes. Prior to the lab, students should be given a list of potential compounds and treatments from which to choose so that they may study and research the cellular mechanism by which their condition causes cell death. A list of potential treatments and suggested ranges is listed in Table 1. This inquiry-based learning lab will help the students develop a hypothesis related to cell viability and the dosage of their compound of interest. Students are expected to collect all of their data within the three hours, but they will generate their figures, methods, and results sections outside of the given lab time.
TABLE 1.
HeLa cell treatment options and suggested ranges.
| Treatment | Low | Medium | High |
|---|---|---|---|
| Temperature | 4ºC | 37ºC | 42ºC |
| pH | 2 | 7 | 12 |
| NaCl | 0 M | 0.1 Ma | 0.5 M |
| KCl | 0 M | 0.1 Ma | 0.5 M |
| NaF | 0 M | 0.1 Ma | 0.5 M |
| Ethanol | 0% | 25% | 50% |
Treatments are expressed in final concentrations. All solutions were prepared in 1 × PBS.
Concentration based on the approximate amount of NaCl present in PBS.
PBS = phosphate-buffered saline.
Learning objectives
Upon completion of this lab, students will be able to:
Perform the basics of cell culture technique, including trypsinizing cells and cell counting, and determine cell viability using trypan blue exclusion.
Utilize dilution math and graphs to analyze cell viability data.
Create a scientific figure that conveys cell viability data.
PROCEDURE
Materials
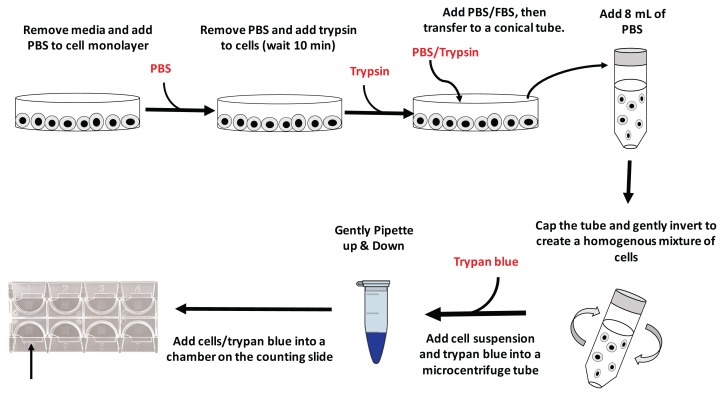
A graphic outline of the procedure is shown in Figure 1. Special materials include the following:
FIGURE 1.
A schematic outlining the experimental procedure to count cells. PBS = phosphate-buffered saline; FBS = fetal bovine serum.
HeLa cells (ATCC, CCL-2)
minimum essential medium (GIBCO DMEM High Glucose, Pyruvate)
0.05% (w/v) trypsin-EDTA solution (GIBCO, 25-300-054)
fetal bovine serum (GIBCO, 26-140-087)
phosphate buffered saline (GIBCO, 10-010-023)
trypan blue solution, 0.4% (GIBCO, 15-250-061)
traditional hemocytometer or a Glasstic Slide 10 with Counting Grids (Kova International, 87144) – one per pair of students
Standard materials for the laboratory preparation include
tissue culture-treated T-75 flask (to expand the cells)
serological pipettes
centrifuge tubes
tissue culture-treated culture dishes (Fisher Scientific, 12-565-94)
bulb pipettes
P20 pipettes and tips
microcentrifuge tubes
15 mL centrifuge tubes
Faculty or laboratory personnel should conduct the initial HeLa cell growth, stock freezing, and dish preparation in a cell culture hood; however, all experiments described herein can be conducted in a standard undergraduate biology laboratory. Cell counts are collected using a compound light microscope fitted with 10×, 20×, and 40× objectives. Materials do not have to be sterile as cells will be discarded once the short experiments have been completed. A water bath to warm reagents to 37ºC (ideally proximal to the student work area) is recommended, but not required. Detailed instructions and ordering information can be found in the student/instructor guides (Appendices 1, 3, and 5).
Student instructions
HeLa cell washing and dissociation
Students were provided with a petri dish of HeLa cells at 80 to 90% confluence. Culture media was removed with a disposable bulb pipette and cells were rinsed by adding 5 mL of 1 × PBS and gently rocking the plate. After 1 min, the PBS was removed using a bulb pipette, and 1 mL of a 0.05% trypsin-EDTA solution was added. The plate was gently rocked back and forth to ensure that the trypsin entirely covered the cells. After 10 minutes of periodic rocking, 1 mL of 1% FBS (in 1×PBS) was added to the plate and mixed gently with the cell/trypsin mixture in order to stop the enzymatic digestion by trypsin. Finally, the 2 mL mixture of cells and media was transferred from the culture dish to a sterile 15-mL conical centrifuge tube and gently mixed with an additional 8 mL of pre-warmed 1 × PBS.
HeLa cell counting
Once the cells were dispersed, 20 μL of cell suspension was transferred to a microcentrifuge tube; 20 μL of trypan blue was then added and mixed gently with a pipette; 20 μL of cell/trypan blue suspension was then loaded into a cell counting chamber and placed under the microscope for counting.
HeLa cell viability—general biology education
To investigate HeLa cell viability, nonmajor students were directed to split an equal volume of their cells into nine microfuge tubes, and incubate three replicates each at temperatures of 4°C, 37°C, and 42°C, respectively. After 30 minutes, cells should be counted via the trypan blue method described above and data should be recorded for subsequent analysis.
HeLa cell viability—biology majors
Biology majors were instructed to pick a chemical or treatment from a list provided. Table 1 provides some examples of treatments and suggested ranges. For example, students can elect to treat their cells with various concentrations of ethanol ranging from 0 to 25%. To provide a more inquiry-based laboratory, students can also be given general instructions to determine their own treatments and/or determine three diverse treatment conditions. Based on cell concentrations determined in the previous step, biology majors should then be able to calculate how to dilute their cells to a concentration of approximately 4 × 104 cells/mL using PBS as the diluent. To run three replicates each for three treatments and a negative control (untreated sample), 500 μL of the 4 × 104 cells/mL cell suspension should be pipetted into 12 microcentrifuge tubes. After treating with the appropriate chemical or condition, cells should be counted via the trypan blue method described above. For students selecting a drug or chemical, the treatment should increase the total volume by no more than 500 μL to avoid low cell counts. Also, it is important that the final volume be consistent for all treatments. For example, if the student adds 100 μL of compound solution at the highest concentration to the cell suspension, the control should receive 100 μL of 1 × PBS and other experimental treatments should have a total of 100 μL added (compound solution + 1 × PBS).
Faculty instructions
Faculty should be familiar with techniques involving basic cell culture and manipulation, including passaging and freezing of cells (to maintain HeLa cell stocks). For the chemical or drug selection, we allowed biology majors to do the math to determine their treatment concentration. However, to simplify the exercise, we provided stock solutions (for example 50% ethanol in PBS). Faculty should familiarize themselves with basic cell counting techniques, including the equations needed to determine cell concentrations using hemocytometers or cell counting chambers, as they can differ significantly. Preferred software for graphing is Microsoft Excel; however, Google Sheets can also be used to perform the analysis. To keep costs to a minimum, each lab student pair or group was only given one plate of cells and the minimum reagents needed to perform these experiments. A detailed preparation guide for instructors, including ordering information for a disposable and easy-to-use hemocytometer, is provided in Appendix 5.
Suggestions for determining student learning
Both biology nonmajors and majors were instructed to generate a scientific figure and a write-up detailing their initial hypothesis and indicating whether their data supported their hypothesis and why they thought this was the case. If no differences were found between treatments, students were asked to explain what might have occurred to produce these results. In addition, biology majors were required to include methods and results sections to accompany their scientific figure, to include error bars, and to perform a Student’s t-test to determine whether their results were significant.
Sample data—general biology education
In this laboratory, we determine the effects of diverse temperatures on the viability of HeLa cells. A sample of cells was taken from each treatment, mixed with an equal volume of trypan blue and pipetted into a hemocytometer or cell counting slide. Using a compound microscope, students were able to differentiate the number of viable (excluded trypan blue) and nonviable (took up trypan blue) cells from each of the three replicates at three different treatment temperatures. For Glasstic Slide 10 with Counting Grids, cell viability was then determined using the following equation:
On average, students counted cells in a total of 18 squares out of a possible 81 to get a live cell concentration estimate. Figures 2A and 2B show representative student figures generated from the data collected in this experiment. Additional samples of student work can be found in Appendix 2. The rubric used by instructors to grade students’ work is provided in Table 2, and the average student scores on the lab deliverable are provided in Figure 4.
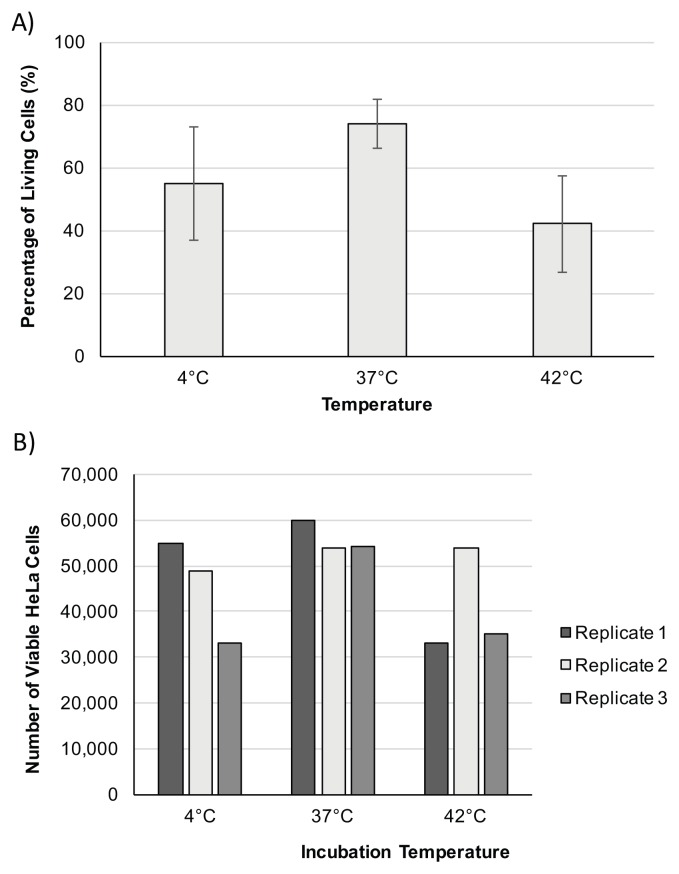
FIGURE 2.
Data from non-biology majors representing HeLa cell viability based on temperature. Student viability counts are shown from the incubation of HeLa cells at 4°C, 37°C, and 42°C. A) Representative data from a student pair that graphed average cell counts. Standard deviation is indicated as error bars where n=3 for all treatments. B) Representative data from a student pair that graphed individual cell counts for each treatment.
TABLE 2.
Rubric for assessment of student deliverables.
| Criteria | Points |
|---|---|
| Correct type of graph | 1 |
| Axes are labeled appropriately (including units) | 1 |
| Axis units and ranges are appropriate | 1 |
| Reasonable data values | 1 |
| Appropriate statistics in graph (e.g., error bars or p values) | 2 |
| Data categories are clear (in legend or key) | 1 |
| Figure legend is clear and well written | 2 |
| Descriptive figure title in figure legend | 1 |
| TOTAL POSSIBLE SCORE | 10 |
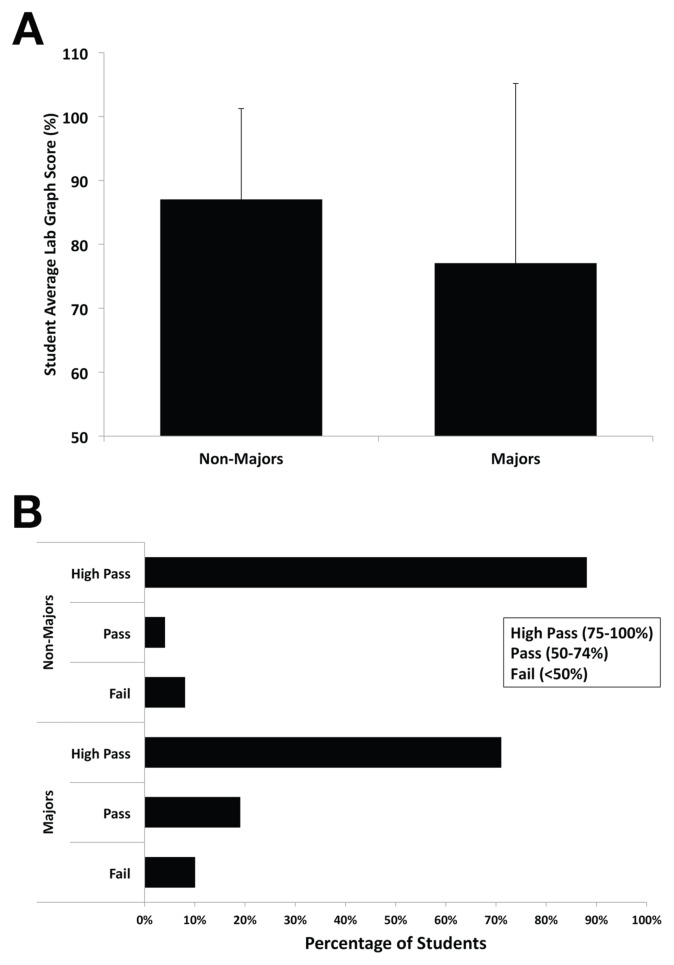
FIGURE 4.
Student scores on lab deliverables suggest learning objectives were met. Student lab graphs were graded by instructors using the assessment rubric described above (Table 2). The values shown correspond to students taught in the fall of 2016 (n > 100). A) Student average scores for both Biology majors and nonmajors. Standard deviation is indicated as error bars. B) Student scores as a percentage of students attaining a failing, passing, or high passing score.
Sample data—biology majors
In the modified version of the lab for biology majors, we examined the effect of various treatments on the viability of HeLa cells. As outlined above, cell viability was determined using trypan and a designated cell counting chamber. Figures 3A and 3B show representative student figures generated from cells treated with diverse concentrations of ethanol and NaCl, respectively. The rubric used by instructors to grade students’ work is provided in Table 2 and the average student scores on the lab deliverable are provided in Figure 4.
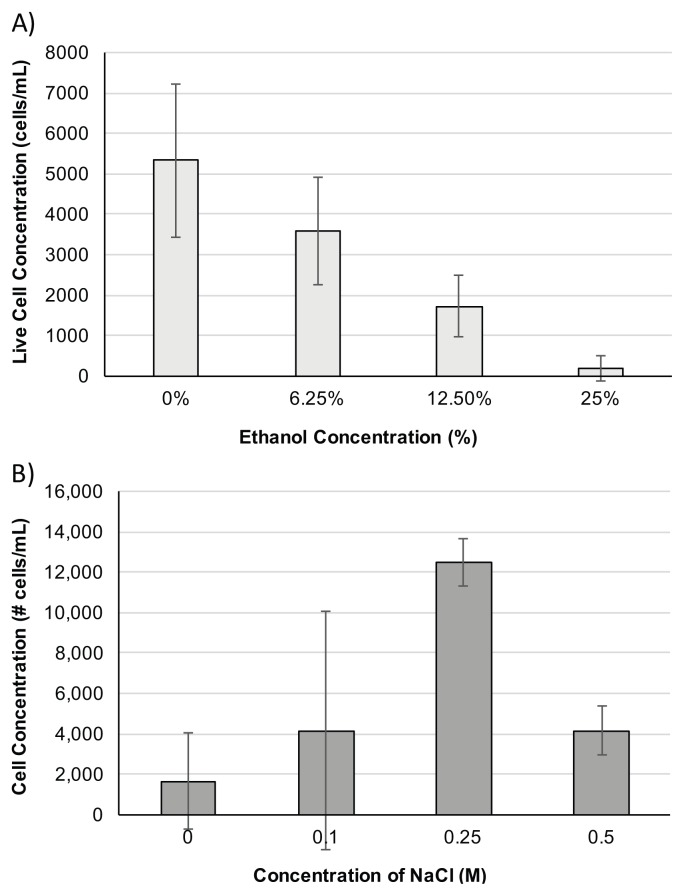
FIGURE 3.
Data from biology majors representing HeLa cell viability based on diverse treatments. A) Student viability data from the incubation of HeLa cells with ethanol concentrations ranging from 0 to 25%. Standard deviation is indicated as error bars where n=3 for all treatments. B) Student viability data from the incubation of HeLa cells with NaCl concentrations ranging from 0 to 0.5 M. Standard deviation is indicated as error bars where n=2 for all treatments.
Safety issues
Before the first day of lab, students were required to complete a reading and watch a series of third-party videos on lab safety. After completing these assignments, compliant with the ASM Guidelines for Biosafety in Teaching Laboratories (specifically those for working with BSL-2 materials like HeLa cells), students were required to sign a lab safety agreement confirming that they would abide by these guidelines (6). For instructors to be sure students understood the key points of lab safety, students completed a quiz on lab safety and were required to obtain a passing grade on this assessment before continuing to engage in lab activities. On the first day of lab, lab instructors reviewed the highlights of lab safety and students were given the opportunity to ask additional questions or share any concerns. In the weeks leading to the experiments described in this manuscript involving HeLa cells (BSL-2), students were required to demonstrate they had the skills required to work with BSL-2 materials using a BSL-1 organism, the budding yeast Saccharomyces cerevisiae. In these experiments, students tested the ability of yeast to survive UV treatment or to grow in different carbon sources. In this way, students demonstrated that they could safely and appropriately handle BSL-2 organisms such as HeLa cells. Students working with HeLa cells (BSL-2) and chemical reagents must wear lab coats, gloves, closed-toed shoes, and protective eyewear during the experiment. Trypan blue stain can cause irritation to the eyes, skin, and respiratory system if protective wear is not utilized. Disposal of BSL-2 materials and reagents should be contained to appropriate biohazard containers within the laboratory. Waste should be autoclaved or bleached before disposal. In accordance with ASM guidelines, students should maintain a clean workspace (ethanol spray bottle should be provided), wear gloves, lab coats, and safety goggles, as well as thoroughly wash their hands before entering and exiting each laboratory session (6).
DISCUSSION
Field testing
This activity was implemented in fall 2015, spring 2016, summer 2016, and fall 2016 in general biology courses at High Point University. A total of 595 students experienced this inquiry-based laboratory activity.
Evidence of student learning—general education biology
A student-generated cell viability graph and analysis are included in Appendix 2 as an example of student learning. It is clear from these data that students were able to successfully manipulate, treat, count, and assess cell viability. Further, students were able to thoughtfully consider the data and relate it to their own knowledge of human cells by highlighting the increased viability observed at 37°C. In their post-lab reflection surveys, students indicated that they learned about the importance of precision and accuracy in scientific work in the lab, as well as the technical skills acquired including trypsinization and cell manipulation. Students also indicated that they enjoyed how “hands on and active it was” and that they were “actually using human cells” in the lab. Students tended to dislike the length of the lab, which took a full two hours of active engagement. A detailed summary of student reflections is included in the preparation guide for instructors provided in Appendix 5.
Evidence of student learning—biology majors
A cell viability chart and analysis produced by a biology major is included in Appendix 4. The figure, legends, and axes demonstrate that students were able to successfully design and implement their experiment, as well as present their data in a way that facilitates meaningful analysis. The students were also capable of including the correct control (0% ethanol) to determine the effect of their treatment by comparison. It is clear from this data that students were able to convey their findings in a way that allowed them to then draw conclusions based on the given treatment. In their post-lab reflection surveys, students indicated how much they enjoyed being able to select their individual treatments and “design an experiment,” as well as the ability to work with and visualize human cells. In contrast to the nonmajors students, more biology majors tended to dislike counting the cells, a process they found monotonous, and disliked “waiting for the cells to finish incubating.”
Evidence of student learning—lab deliverables
To demonstrate what they had learned, biology majors and nonmajors were required to construct a scientific figure with their data to convey their findings. The rubric used by instructors to grade students’ work is provided in Table 2, and the average student scores on the lab deliverable are provided in Figure 4. The items assessed on student deliverables were designed to help the instructors determine whether the students had been able to meet the first three learning objectives of the laboratory module (Table 2). A passing student average score suggests the majority of students met these first three learning objectives (Fig. 4).
Possible modifications
The purpose of these experiments is to introduce students to the basic principles of cell culture, determine the effects of various treatments on cell viability, and analyze the data using a data analysis software package. As mentioned, a possible modification for a nonmajors biology course could be restricting treatment types to temperature only. This would avoid much of the math associated with dosing and allow for the lab to be completed in a shorter period (see Appendix 3 for non-biology major student lab handout/guide). Another extension, if equipment and resources are available, is for students to engage in a longer multi-week project, which could be suitable for an honors track. For example, students could study the effects of a nicotine treatment on HeLa cells (100 nM up to 100 μM), or a similar substance, for at least 12 hours and up to two days, thereby studying the effects of both concentration and time. In addition to assessing cell survival rates, students could also isolate RNA, and utilize RT-PCR to examine alterations in the expression levels of candidate proliferation genes. Students could be asked not only to test a hypothesis generated based on the effects of low to moderate levels of nicotine exposure and the influence of incubation time on cell viability and the expression of candidate proliferation genes, but to determine whether HeLa cells express their gene of interest and to examine whether their candidate genes change in expression levels as a result of nicotine exposure.
SUPPLEMENTAL MATERIALS
ACKNOWLEDGMENTS
This experiment was utilized in fall 2015, spring 2016, summer 2016, and fall 2016 in general biology courses at High Point University, and the authors appreciate the efforts of the students. Funding was supplied by the Department of Biology at High Point University and a Roberta Williams Laboratory Teaching Initiative Grant from the Association for Biology Laboratory Education to V.A. Segarra. The authors declare that there are no conflicts of interest.
Footnotes
Supplemental materials available at http://asmscience.org/jmbe
REFERENCES
- 1.Rodríguez-Hernández CO, Torres-Garcia SE, Olvera-Sandoval C, Ramirez-Castillo FY, Muro AL, Avelar-Gonzalez FJ. Cell culture: history, development and prospects. Int J Curr Res Acad Rev. 2014;2:188–200. [Google Scholar]
- 2.del Carpio A. The good, the bad, and the HeLa. Berkley Sci Rev. 2014. Spring. http://berkeleysciencereview.com/article/good-bad-hela/
- 3.Masters JR. HeLa cells 50 years on: the good, the bad and the ugly. Nat Rev Cancer. 2002;2:315–319. doi: 10.1038/nrc775. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314:111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- 5.Skloot R. The immortal life of Henrietta Lacks. Random House; New York, NY: 2010. [Google Scholar]
- 6.Emmert E. Biosafety guidelines for handling microorganisms in the teaching laboratory: development and rationale. J Microbiol Biol Educ. 2013;14(1):78–83. doi: 10.1128/jmbe.v14i1.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.