Abstract
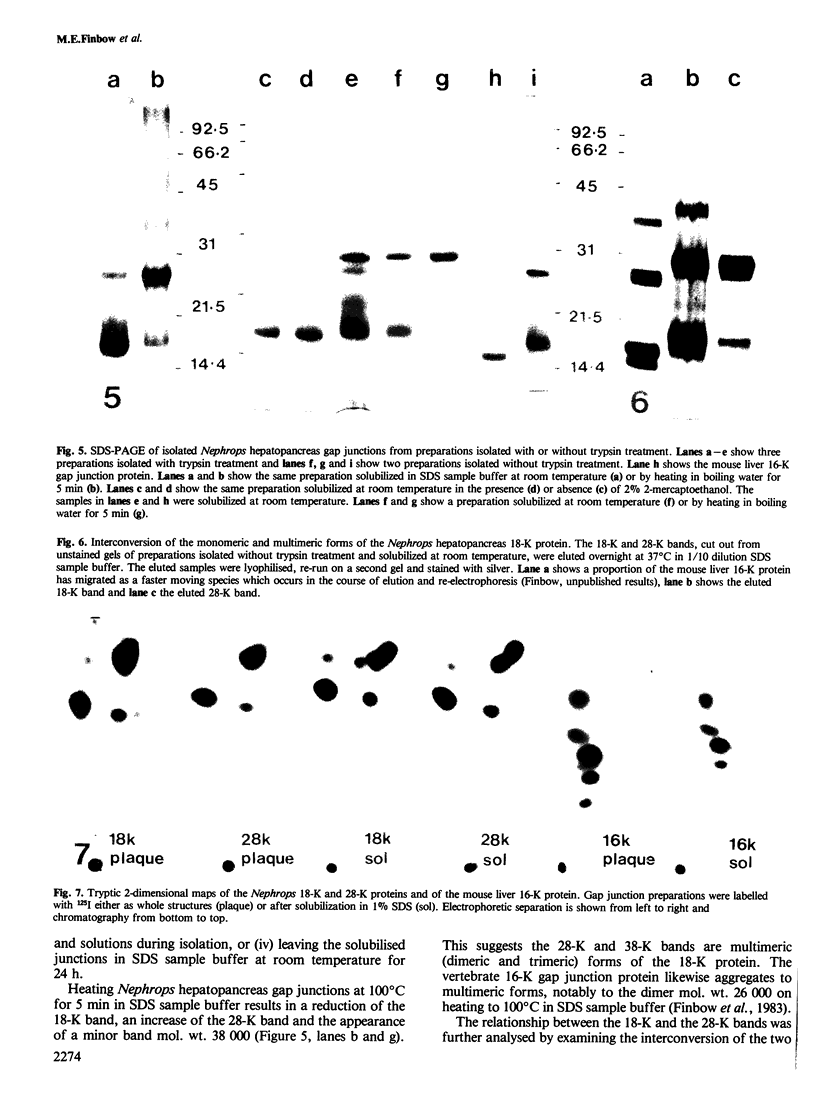
Gap junctions have been isolated from the hepatopancreas of the crustacean arthropod, Nephrops norvegicus (Norway lobster). SDS-PAGE of these preparations shows two major protein bands, mol. wt. 18 000 (18 K) and mol. wt. 28 000 (28 K). The 18-K and 28-K proteins are interconvertible, cannot be distinguished by two dimensional tryptic and chymotryptic peptide mapping, and therefore appear to be different (most likely monomeric and dimeric) forms of the same protein. The protein can also aggregate to higher multimeric forms mol. wt. 38 000 (presumed trimer), and mol. wt. 52 000 (presumed tetramer). The buoyant density of the isolated gap junctions in continuous potassium iodide gradients is 1.260 g/cm3. The junctions are progressively solubilized in increasing SDS concentrations, mostly between 0.1% and 0.2% SDS, and this is accompanied by the release of the 18-K and 28-K forms of the junctional protein. The Nephrops hepatopancreas 18-K junctional protein has antigenic determinants in common with the vertebrate 16-K junctional protein as shown by cross-reactivity with two different affinity purified antibody preparations. However, no detectable similarity can be seen between the major 125I-labelled tryptic and chymotrytpic peptides of the Nephrops hepatopancreas 18-K protein and the mouse liver 16-K protein.
Keywords: gap junctions, membrane proteins, arthropod junctions
Full text
PDF


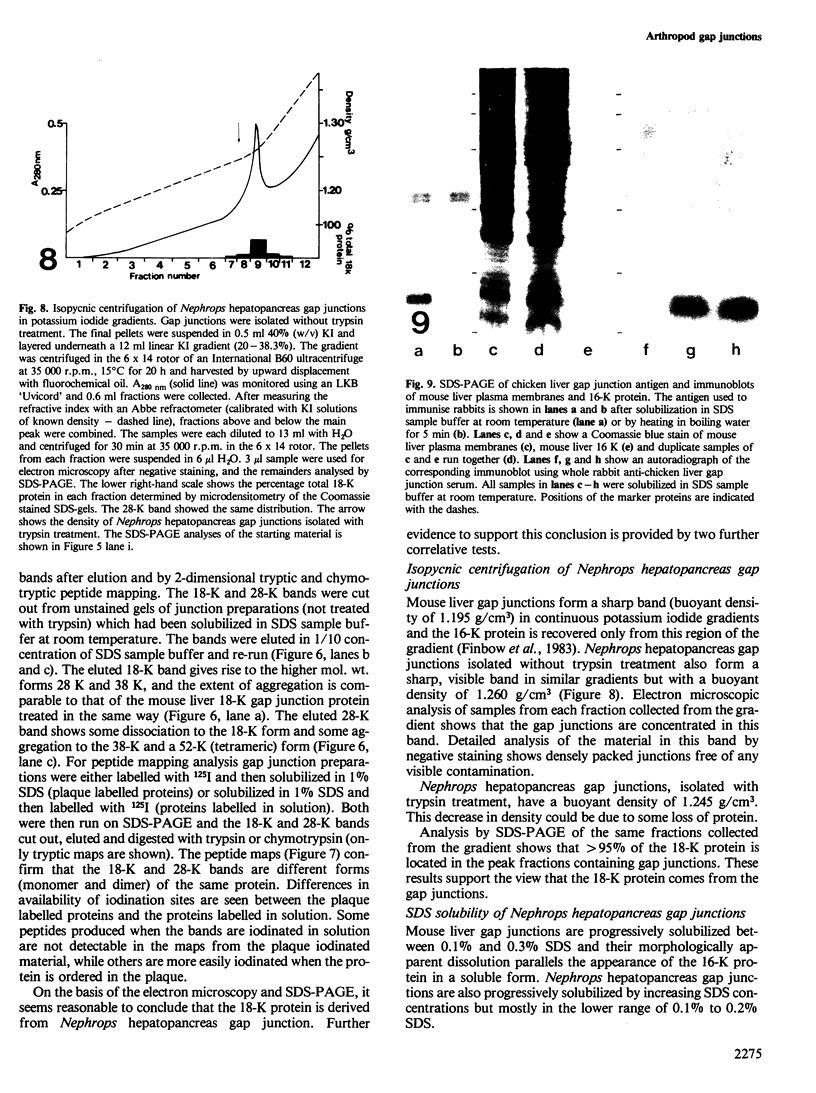



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caspar D. L., Goodenough D. A., Makowski L., Phillips W. C. Gap junction structures. I. Correlated electron microscopy and x-ray diffraction. J Cell Biol. 1977 Aug;74(2):605–628. doi: 10.1083/jcb.74.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunia I., Sen Ghosh C., Benedetti E. L., Zweers A., Bloemendal H. Isolation and protein pattern of eye lens fiber junctions. FEBS Lett. 1974 Sep 1;45(1):139–144. doi: 10.1016/0014-5793(74)80831-8. [DOI] [PubMed] [Google Scholar]
- Fallon R. F., Goodenough D. A. Five-hour half-life of mouse liver gap-junction protein. J Cell Biol. 1981 Aug;90(2):521–526. doi: 10.1083/jcb.90.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finbow M. E., Pitts J. D. Permeability of junctions between animal cells. Intercellular exchange of various metabolites and a vitamin-derived cofactor. Exp Cell Res. 1981 Jan;131(1):1–13. doi: 10.1016/0014-4827(81)90399-2. [DOI] [PubMed] [Google Scholar]
- Finbow M. E., Shuttleworth J., Hamilton A. E., Pitts J. D. Analysis of vertebrate gap junction protein. EMBO J. 1983;2(9):1479–1486. doi: 10.1002/j.1460-2075.1983.tb01611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagg-Newton J., Simpson I., Loewenstein W. R. Permeability of the cell-to-cell membrane channels in mammalian cell juncton. Science. 1979 Jul 27;205(4404):404–407. doi: 10.1126/science.377490. [DOI] [PubMed] [Google Scholar]
- Flower N. E. Septate and gap junctions between the epithelial cells of an invertebrate, the mollusc Cominella maculosa. J Ultrastruct Res. 1971 Nov;37(3):259–268. doi: 10.1016/s0022-5320(71)80123-5. [DOI] [PubMed] [Google Scholar]
- Gilula N. B., Reeves O. R., Steinbach A. Metabolic coupling, ionic coupling and cell contacts. Nature. 1972 Feb 4;235(5336):262–265. doi: 10.1038/235262a0. [DOI] [PubMed] [Google Scholar]
- Goodenough D. A. Lens gap junctions: a structural hypothesis for nonregulated low-resistance intercellular pathways. Invest Ophthalmol Vis Sci. 1979 Nov;18(11):1104–1122. [PubMed] [Google Scholar]
- Goodenough D. A., Revel J. P. A fine structural analysis of intercellular junctions in the mouse liver. J Cell Biol. 1970 May;45(2):272–290. doi: 10.1083/jcb.45.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D. A., Stoeckenius W. The isolation of mouse hepatocyte gap junctions. Preliminary chemical characterization and x-ray diffraction. J Cell Biol. 1972 Sep;54(3):646–656. doi: 10.1083/jcb.54.3.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. R., Noirot-Timothée C., Noirot C. Isolation and characterization of invertebrate smooth septate junctions. J Cell Sci. 1983 Jul;62:351–370. doi: 10.1242/jcs.62.1.351. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hertzberg E. L., Gilula N. B. Isolation and characterization of gap junctions from rat liver. J Biol Chem. 1979 Mar 25;254(6):2138–2147. [PubMed] [Google Scholar]
- Kensler R. W., Goodenough D. A. Isolation of mouse myocardial gap junctions. J Cell Biol. 1980 Sep;86(3):755–764. doi: 10.1083/jcb.86.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane N. J., Swales L. S. Dispersal of junctional particles, not internalization, during the in vivo disappearance of gap junctions. Cell. 1980 Mar;19(3):579–586. doi: 10.1016/s0092-8674(80)80034-1. [DOI] [PubMed] [Google Scholar]
- Lawrence T. S., Beers W. H., Gilula N. B. Transmission of hormonal stimulation by cell-to-cell communication. Nature. 1978 Apr 6;272(5653):501–506. doi: 10.1038/272501a0. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication and the control of growth. Biochim Biophys Acta. 1979 Feb 4;560(1):1–65. doi: 10.1016/0304-419x(79)90002-7. [DOI] [PubMed] [Google Scholar]
- Moor R. M., Smith M. W., Dawson R. M. Measurement of intercellular coupling between oocytes and cumulus cells using intracellular markers. Exp Cell Res. 1980 Mar;126(1):15–29. doi: 10.1016/0014-4827(80)90466-8. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Peracchia C. Low resistance junctions in crayfish. I. Two arrays of globules in junctional membranes. J Cell Biol. 1973 Apr;57(1):66–76. doi: 10.1083/jcb.57.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts J. D., Simms J. W. Permeability of junctions between animal cells. Intercellular transfer of nucleotides but not of macromolecules. Exp Cell Res. 1977 Jan;104(1):153–163. doi: 10.1016/0014-4827(77)90078-7. [DOI] [PubMed] [Google Scholar]
- Revel J. P., Karnovsky M. J. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967 Jun;33(3):C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. D. Membrane structure. J Cell Biol. 1981 Dec;91(3 Pt 2):189s–204s. doi: 10.1083/jcb.91.3.189s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan J. D., Finbow M. E., Pitts J. D. Metabolic interactions between animal cells through permeable intercellular junctions. Exp Cell Res. 1979 Oct 1;123(1):111–117. doi: 10.1016/0014-4827(79)90427-0. [DOI] [PubMed] [Google Scholar]
- Simpson I., Rose B., Loewenstein W. R. Size limit of molecules permeating the junctional membrane channels. Science. 1977 Jan 21;195(4275):294–296. doi: 10.1126/science.831276. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin P. N., Ennis P. D. Calcium-mediated changes in gap junction structure: evidence from the low angle X-ray pattern. J Cell Biol. 1983 Nov;97(5 Pt 1):1459–1466. doi: 10.1083/jcb.97.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin P. N., Zampighi G. Structure of the junction between communicating cells. Nature. 1980 Feb 7;283(5747):545–549. doi: 10.1038/283545a0. [DOI] [PubMed] [Google Scholar]
- Warner A. E., Lawrence P. A. Permeability of gap junctions at the segmental border in insect epidermis. Cell. 1982 Feb;28(2):243–252. doi: 10.1016/0092-8674(82)90342-7. [DOI] [PubMed] [Google Scholar]
- Weir M. P., Lo C. W. Gap junctional communication compartments in the Drosophila wing disk. Proc Natl Acad Sci U S A. 1982 May;79(10):3232–3235. doi: 10.1073/pnas.79.10.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]