Significance
Maize was initially domesticated in the Balsas region of Mexico ∼9,000 y ago, but it remains unclear when this globally important cultigen became a staple crop in the Americas. We demonstrate that highly productive maize varieties were present in Central America outside the natural distribution of ancestral teosinte populations [Zea mays subspecies (ssp.) parviglumis] by ∼4,340 calendar years B.P., and we hypothesize that reduced introgression with Z. mays ssp. parviglumis and Z. mays ssp. mexicana was instrumental in the development of more productive staple grain varieties.
Keywords: maize, teosinte, Central America, domestication, agriculture
Abstract
The first steps toward maize (Zea mays subspecies mays) domestication occurred in the Balsas region of Mexico by ∼9,000 calendar years B.P. (cal B.P.), but it remains unclear when maize was productive enough to be a staple grain in the Americas. Molecular and microbotanical data provide a partial picture of the timing and nature of morphological change, with genetic data indicating that alleles for some domestication traits were not yet fixed by 5,300 cal B.P. in the highlands of Mexico. Here, we report 88 radiocarbon dates on the botanical remains from El Gigante rockshelter (Honduras) to establish a Bayesian chronology over the past ∼11,000 y spanning the transition to maize-based food production. Botanical remains are remarkably well preserved and include over 10,000 maize macrofossils. We directly dated 37 maize cobs to establish the appearance and local change of maize at the site. Cobs are common in deposits dating between 4,340 and 4,020 cal B.P., and again between 2,350 and 980 cal B.P. The earliest cobs appear robustly domesticated, having 10–14 rows, suggesting strong selection for increased yield. The later cobs are comparable to these earliest ones, but show clear emergence of diverse traits, including increased cob width, rachis segment length, and cupule width. Our results indicate that domesticated landraces of maize productive enough to be a staple grain existed in Central America by 4,300 cal B.P.
The domestication and diversification of maize [Zea mays subspecies (ssp.) mays] during the past ∼9,000 y (1, 2) has resulted in landraces with remarkable genetic and morphological diversity adapted to a range of geographic constraints and climatic conditions (3–11). Fifty-nine extant landraces of maize are documented in Mexico alone (11), and morphological variability exists elsewhere in North, Central, and South America (4–6, 12). The known extant landraces represent only a fraction of the diversity that existed during the evolutionary history of this globally important domesticate; however, this diversity is presently threatened by the proliferation of genetically modified (GM) and improved hybrid varieties (13), as well as an associated loss of cultural knowledge and traditional farming practices (14). In the context of global climate change (15), germplasm in seed banks and macrofossils archived in archaeological deposits provide an important source of genetic information in the face of increasing environmental instability.
Genetic data indicate that maize was initially domesticated from the annual grass teosinte (Zea mays ssp. parviglumis) that grows today between 400 and 1,800 meters above sea level (masl) in the Balsas region of southwestern Mexico (6, 9, 16). Phytolith and starch evidence from archaeological sites in the Balsas region indicate the early use of maize by at least ∼8,700 calendar years B.P. (cal B.P.) (1, 2) and a widespread dispersal of this domesticate through the lowland neotropics soon after this time (17–20), an observation consistent with the earliest maize macrofossils in South America (6,775–6,500 cal B.P.) (5). The earliest known small, two-row distichous cobs from the highlands of Oaxaca, Mexico, date to 6,250 cal B.P. (21, 22) and share derived characteristics (i.e., rigid rachis, paired spikelets, perpendicular-oriented spikelets) with the earliest polystichous cobs from San Marcos Cave in highland Mexico’s Tehuacan Valley dating to between ∼5,300 and 5,000 cal B.P. (23). Changes in cob architecture evident at this time are consistent with early selection for increased grain accessibility and productivity. Genetic data indicate that some genes controlling for stem and inflorescence architecture were essentially modern, carrying domestic maize-type alleles, by 5,300–4,950 cal B.P. in the Tehuacan Valley, but that others controlling ear shattering and starch biosynthesis retained genetic variants comparable to ancestral teosinte populations (24, 25).
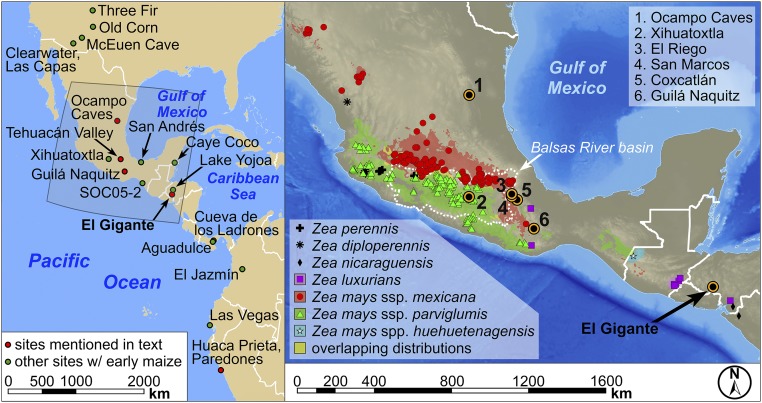
Less is known about the domestication, hybridization, local adaptation, and diversification of maize in the Americas after 4,950 cal B.P., as well as the tempo and character of subsequent change as farmers in Mesoamerica shifted to maize-based food production in the context of emerging state-level societies between ∼3,500 and 1,000 cal B.P. It remains unclear when maize became productive enough to be a staple grain crop, and the small size of the earliest maize cobs from Oaxaca and Tehuacan have led some to hypothesize early nongrain use as a green vegetable (26) or stalk sugar to produce alcoholic beverages (27). Changes in maize cob size in the Tehuacan Valley (28) suggest that the transition to maize as a staple grain crop unfolded gradually (26, 29). However, we know much less about this process in regions outside the known distribution of the two dominant teosinte subspecies in Mesoamerica, Z. mays ssp. parviglumis and Zea mays ssp. mexicana (hereafter referred to as parviglumis and mexicana; Fig. 1). Well-preserved maize cobs are exceedingly rare in the archaeological record beyond the Mexican highlands, and this fact has limited our ability to track both (i) the diversification process outside the heartland of maize domestication and (ii) whether a staple grain crop was first engineered outside the natural distribution of parviglumis and mexicana. A handful of maize cobs from dry caves (Ocampo) in the Tamaulipas region of northern Mexico (1,900 masl) suggest significant changes in cob size and row number by ∼4,400 cal B.P. (30). Genetic work on these cobs suggests that starch productivity and protein storage allele frequencies were similar to some modern varieties (31); however, the number of samples is limited, and it is unknown how much morphological and biochemical variability existed regionally.
Fig. 1.
Distribution of sites with early maize in the Americas and the observed and modeled distributions of teosinte in Mesoamerica and Central America (Zea mays ssp. parviglumis, mexicana, and huehuetenagensis; Zea perennis; Zea diploperennis; Zea luxurians; Zea nicaraguensis) (7, 32, 33). Modeled distributions of parviglumis (green) and mexicana (red) are from Hufford et al. (7).
Here, we report the morphological characteristics of 35 directly accelerator mass spectrometer (AMS) 14C-dated maize cobs from El Gigante rockshelter in western Honduras within the context of a Bayesian chronology for the site spanning the past 11,000 y. El Gigante falls well outside the known natural range of parviglumis (7, 32, 33), the progenitor wild relative of domesticated maize, and subspecies distribution modeling accounting for climatic change indicates that such has been the case throughout the past 10,000 y (7). This region is also outside the range of mexicana (33), a subspecies that is adapted to the dry and cool elevations above 1,600 m in the Mexican highlands. Genetic studies have demonstrated introgression between domesticated maize and mexicana in highland regions, and introgression is thought to confer an adaptive advantage to drier conditions (4, 34). Therefore, maize macrofossils from El Gigante provide an important point of comparison with assemblages from the Mexican highlands.
El Gigante Rockshelter
El Gigante is a large rockshelter located on the western escarpment of the Estanzuela River in the highlands of western Honduras (88.06° W, 14.22° N, 1,300 masl; Fig. 1). The shallow, ashy, and erodible soils in the region are derived from weathered Miocene- to Pliocene-aged volcanic tuffs and ignimbrites, and the hill slopes surrounding the cave support little potential for large-scale intensive agriculture. Deeper soils in the narrow valley bottoms below support more intensive forms of agriculture today and provide the most likely location where the prehistoric people who episodically used the rockshelter grew maize and other crops for subsistence. Two species of teosinte (Zea luxurians and Zea nicaraguensis) and a teosinte subspecies (Zea mays ssp. huehuetenagensis), henceforth nicaraguensis, luxurians, and huehuetenagensis, were recorded in low densities historically in Central America (32, 33), but the distribution of these species in the past is unknown. Huehuetenagensis has a limited distribution in western Guatemala today and is the only teosinte subspecies in the vicinity of El Gigante capable of hybridization with maize. Luxurians and nicaraguensis are more distantly related to Z. mays and highly adapted to flooded and coastal conditions along the Pacific coasts of Guatemala and Nicaragua (33).
El Gigante rockshelter is 42 m wide, 17 m deep, and 12 m high, and conditions inside the drip line favored the preservation of organic materials, including ∼10,000 carbonized and uncarbonized maize remains. Large excavation blocks (∼20 m2 in lateral extent; SI Appendix, Fig. S1) in the southwestern end of the shelter revealed stratified deposits extending 2.5 m below the surface and a complex history of deposition extending back to ∼11,000 cal B.P. (35). Nine stratigraphic units were identified: four noncultural strata (VI–IX) and five upper cultural strata (I–V) containing hearths, disturbed burial features, and pit features (SI Appendix, Fig. S2). Artifacts recovered from the upper portions of stratum VI were trampled into this culturally sterile stratum when it was first occupied. Nineteen radiocarbon dates were originally used to establish a provisional chronology for these deposits (35). As part of the original study, two maize cobs were directly AMS 14C-dated to 2,010 ± 40 (Beta-171701, 2,400–2,200 cal B.P.) and 2,280 ± 40 (Beta-159055, 2,100–1,030 cal B.P.).
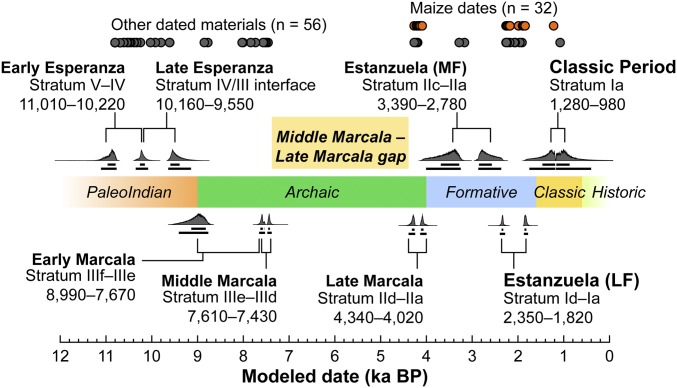
Here, we use 89 new AMS 14C dates derived from identified plant specimens and small fragments of wood charcoal (e.g., twigs) to establish a Bayesian chronological model for the rockshelter. Samples originated from hearth and pit features and include 37 maize cobs (Fig. 2 and SI Appendix, Tables S1 and S2). This model is based on an iterative analysis of AMS 14C dates, with each cultural layer treated as a phase. No ordering of dates in each layer is assumed, and the overall model is a sequence of phases and boundaries. Calibrations and modeling were done in OxCal 4.2.3 (36) using the IntCal13 calibration curve (37). Outliers (20 of 108 available 14C dates; SI Appendix, Table S3) were removed after reviewing the stratigraphy and provenience of the sampled materials. In most cases, these outliers were related to stratigraphic mixing or intrusive pit features most common in the uppermost stratum (stratum Ia). Eighty-eight 14C dates were ultimately used to constrain the stratigraphic model, and overall statistical agreement for the sequence is high (SI Appendix, Fig. S3 and Model CQL2 Code; Amodel = 102.4, Aoverall = 101.4) (36).
Fig. 2.
Sequenced radiocarbon chronology for El Gigante, displaying modeled occupational phases and the temporal frequency of dated maize and other samples (details are provided in SI Appendix, Fig. S3 and Table S2). During the highlighted lacunae between the Middle and Late Marcala phases, there is little evidence for site use.
Results indicate intermittent use of the rockshelter during the past 11,000 y. Eight primary phases of occupation were identified, with a large hiatus between ∼7,430 and 4,340 cal B.P. This hiatus does not correspond to a sterile cultural level and is only visible in the radiocarbon record. Only three of the 20 outlying dates removed from the model fall within this interval (more specifically, within the range of 7,400–7,000 cal B.P.). Although they belong to no discernible phase due to inconsistent stratigraphic positioning, these dates suggest a longer, if more ephemeral, Middle Archaic occupation than is indicated by the model and an ∼2,700 y hiatus in rockshelter use during the Middle Holocene.
Four phases of occupation were identified between 11,010 and 7,430 cal B.P. The earliest cultural deposits identified (Early Esperanza phase) occur in strata IV and V. These deposits started accumulating between 11,110 and 10,770 cal B.P. and terminated between 10,360 and 10,080 cal B.P. A separate distribution of radiocarbon dates taken from the interface of strata III–IV enabled identification of a Late Esperanza occupation, with these deposits accumulating between 10,160 and 9,550 cal B.P. Both Early and Late Esperanza phase deposits contain high concentrations of deer bone and flaked stone tools. Important plants identified in these early levels include agave (Agave), avocado (Persea), hog plum (Spondius), mamey sapote (Pouteria), mesquite bean (Prosopis), and acorns (Quercus). Wide-stemmed projectile points, some with basal notches or flutes, also occur in these early deposits (35). These distinctive points were absent in the subsequent phases of occupation between 8,990 and 7,670 cal B.P. (Early Marcala, stratum IIIe–IIIf) and between 7,610 and 7,430 cal B.P. (Middle Marcala, stratum IIId–IIIe). Deer bone is less common in Early and Middle Marcala strata, and there is a relative increase in smaller game, including turtles, birds, snails, and crabs. Soursop (Annona), hackberry (Celtis), squash (Cucurbita), and gourd (Lagenaria) were added to this list of diverse agroforest products in the Marcala phase. Overall, these data are consistent with dietary expansion in the context of an apparent decline in the use of large game (35).
Four additional phases of occupation occur between 4,340 and 980 cal B.P. after a long hiatus in rockshelter use (between 7,430 and 4,340 cal B.P.). The earliest of these deposits dates between 4,340 and 4,020 cal B.P. (Late Marcala, stratum IIa–IId) and is limited in extent to the central excavation block (units 1, 3, 6, 18, and 19). Maize first appears in these deposits and is commingled with materials similar to the materials found in earlier Marcala phase deposits (e.g., tree fruits, maguey quids). No ceramics were present in these Late Marcala deposits, which are followed by an ∼600-y hiatus during the Terminal Archaic/Early Formative interface (4,020–3,390 cal B.P.). Ceramics appear sometime after ∼3,390 cal B.P. during the subsequent Estanzuela Middle Formative phase. Radiocarbon dating of the Middle-Late Formative transition is complicated by a lack of Middle Formative dates, as well as the Halstatt Plateau calibration curve anomaly (2,750–2,350 cal B.P.). Model results are constrained to either side of the anomaly (SI Appendix, Fig. S4), concealing what is likely an uninterrupted transition occurring at ∼2,500 cal B.P. The most substantial deposits date between 2,350 and 1,820 cal B.P. (Estanzuela Late Formative phase, stratum Ia–Ic) and contain large quantities of maize found in association with ground stone milling equipment typical of Mesoamerican farmers. Beans (Phaseolus spp.) also appear for the first time in the Late Formative period assemblage. Following an ∼500-y gap, two later radiocarbon dates (1,280–980 cal B.P.) suggest that the use of the cave extended at least until the Classic period. Deposits dating later than 2,350 cal B.P. contain a diverse range of well-preserved materials (e.g., woven mats, a deer-skin bag) that are contemporary with two agricultural villages known for the region (El Pelón and Los Gentiles). Looting activity, mostly directed at burials dating to the Late Formative or Classic period, has rendered stratum Ia–Ic highly disturbed in many places.
El Gigante Maize Cob Morphology
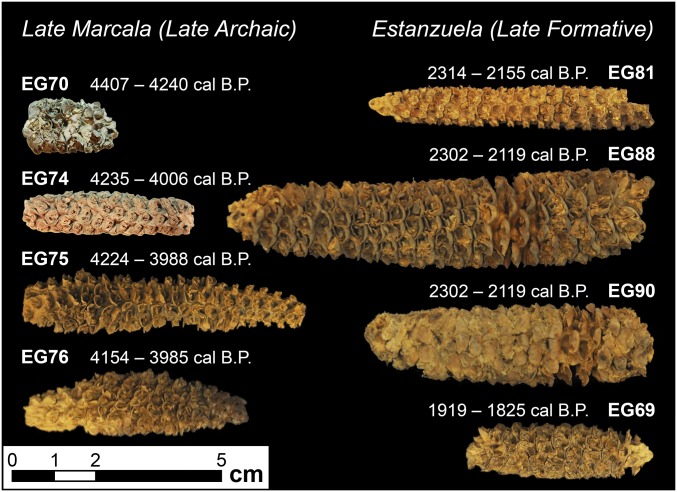
The earliest cob analyzed in the assemblage (UCIAMS-128441/EG 70) occurs in the preceramic Late Marcala phase (stratum IId) and was directly AMS 14C-dated to 4,340–4,160 cal B.P. It has 12 rows with paired spikelets at the base of each long, deep cupule and exhibits glume induration and thickening consistent with relatively recent genetic contributions from teosinte in the time line of domestication (22). Nine additional cobs also occur in stratum II (a–d) and were directly AMS 14C-dated to between 4,280 and 4,020 cal B.P. These Late Marcala cobs are relatively slender (14 mm in thickness), exhibit a great deal of morphological variability, and have between 12 and 14 rows.
The earliest cobs are morphometrically comparable to the later assemblage dating between 2,350 and 1,180 cal B.P. (Fig. 3). However, some of the cobs in the later assemblage have larger numbers of kernel rows and higher grain yields. Principal component analysis (PCA) was used to examine morphological changes in the maize cob assemblage through time. We grouped the morphometric variables into subsets based on relatedness and combined them into three primary components that account for ∼77% of all variation within the El Gigante assemblage (SI Appendix, Table S4C). Increasing cob size is a persistent trend in the local development or the introduction of new varieties to the El Gigante region. Cobs in the later assemblages dating to between 2,350 and 1,180 cal B.P. average ∼16 mm in diameter, and some exceed 20 mm in diameter. Later assemblages are more strongly associated with increased cob diameter, rachis diameter, and kernel row number than the earliest assemblage dating to the Late Marcala phase. These three characteristics are strongly associated with overall yield, because measurements of rachis size attest to varying amounts of productive surface area of the cob and kernel row number corresponds to the number of kernels present on a cob. ANOVA values reveal that all three of these critical variables increase significantly through time: cob diameter (F = 2.872, df = 4, P = 0.050), rachis diameter (F = 7.076, df = 4, P = 0.001), and row number (F = 9.729, df = 4, P = 0.000). This observation is consistent with (i) local selection for increasing grain yield in the absence of persistent teosinte introgression or (ii) the introduction of more productive nonlocal varieties lacking teosinte introgression.
Fig. 3.
Photographs showcasing the morphological variability of maize cobs during the Late Marcala (Left) and Estanzuela (Right) phases at El Gigante.
Maize cobs with 16 kernel rows first appear in the El Gigante assemblage at 2,300–2,060 cal B.P., during the early part of the Estanzuela Late Formative occupation. A specimen with 22 rows appears even later during a possible Terminal Formative/Early Classic period occupation (1,730–1,620 cal B.P.). Significant increase in kernel rows follows local selection for larger cob diameter. Considered altogether, increasing kernel row number and cob size drive variation among some later cobs in the assemblage dating after 2,310 cal B.P. None show obvious morphological evidence for teosinte introgression.
The significant increase in kernel row number and grain yield, evident in the latest cobs from El Gigante, is preceded by changes in cupule architecture. Changes in rachis segment length and cupule morphology also appear to be primary components of maize variation at El Gigante between the Late Marcala and Estanzuela phases (SI Appendix, Fig. S5). Cupule width increases significantly through time (F = 3.186, df = 4, P = 0.034), and rachis segment length decreases through time after it initially increased in the Late Marcala until the middle of the Estanzuela phase (after 2,000 cal B.P.). Individual specimens reflect a mosaic of cupule characteristics, consistent with local diversification, but a general trend toward increased kernel size (wider cupules with wider wing widths) and accessibility (shallower and shorter cupules) indicates early selection for these characteristics. In total, this suite of characters indicates increasing selection for higher grain yields, resulting in more productive staple grain varieties.
Discussion
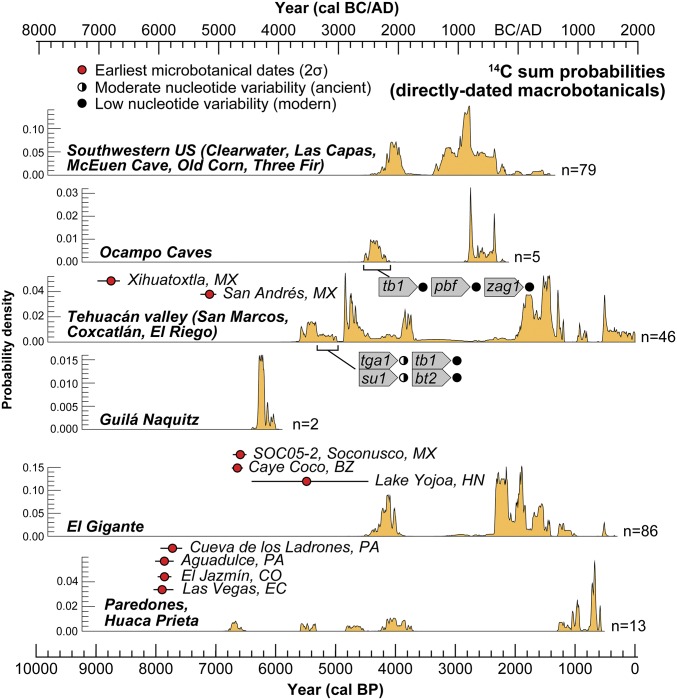
El Gigante is one of six dry rockshelters known with well-preserved macrobotanical assemblages in Mesoamerica. These rockshelters were all occupied episodically, and each captures a different and geographically distinct view of the transition from foraging to farming in this region (Fig. 4). Of these sites, El Gigante is the only known dry rockshelter south of the Mexican highlands that falls well outside the contemporary range of the direct progenitor of maize [parviglumis (7, 32, 33)]. At an elevation of 1,300 masl, El Gigante also falls outside the topographic range of mexicana and the limited distribution of Central American teosintes (nicaraguensis, luxurians, and huehuetenagensis). Bayesian chronological modeling of 89 14C dates from El Gigante show eight phases of rockshelter use between 11,010 and 980 cal B.P., with an extended hiatus in the record between 7,430 and 4,340 cal B.P. Maize is absent in deposits dating between 11,010 and 7,430 cal B.P., but it appears in the record starting at 4,340 cal B.P. The earliest preceramic cobs fall morphometrically within the range of cobs dating later in the sequence (2,350–1,180 cal B.P.).
Fig. 4.
Sum probability distributions of directly dated maize from sites and regions of interest alongside the 2σ-calibrated ranges of the earliest regional microbotanical dates (55). Nucleotide variability data are provided for the earliest cobs from the Tehuacan Valley (tga1, tb1, su1, and bt2) (24, 25) and Ocampo caves (tb1, pdf, and su1) (30).
Macrobotanical maize remains in preceramic deposits older than 4,000 cal B.P. are exceedingly rare in the Americas (Fig. 4). In Mesoamerica, AMS 14C-dated maize cobs with morphometric data come from Guila Naquitz (Oaxaca, two cobs) (22), San Marcos (Tehuacan, one cob) (23), Coxcatlan (Tehuacan, one cob) (23, 37), and El Riego (Tehuacan, one cob) (28). Additional AMS 14C-dated maize cobs earlier than 4,000 cal B.P. without detailed morphometric data come from San Marcos Cave in Tehuacan (seven cobs) (25) and from Romero and Valenzuela caves in the Ocampo region of Tamaulipas, Mexico (two cobs) (30). The earliest distichous cobs from Guila Naquitz (∼6,200 cal B.P.) (21) have two rows and nondisarticulating rachis size averaging 2 mm in diameter and 3 mm in length (22). Cupule width averages 3 mm. The early four-row cobs from Oaxaca are comparable to the earliest cobs from Tehuacan (5,300–4,950 cal B.P.) (38, 39) (Fig. 5) and indicate that kernels did not disarticulate naturally, meaning that humans were controlling the reproduction of these plants (22). Genomic evidence from maize macrofossils from San Marcos cave (Tehuacan) dating between 5,300 and 4,950 cal B.P. also indicates more maize-like alleles for inflorescence and seed architecture (td1 = tassel dwarf1; tb1 = teosinte branched1; ba1 = barren stalk1), glycogen biosynthesis (bt2 = brittle endosperm2), and circadian clock and flowering time (zmg1) (24, 25). However, more ancestral, teosinte-like alleles were documented in the same specimens that controlled ear shattering (zagl1 = MADS-box gene) and the starch production and biosynthesis in kernels (su1 = sugary 1 and wx1 = waxy 1), suggesting that initial selection pressures associated with domestication were still underway at ∼5,000 cal B.P. (24, 25).
Fig. 5.
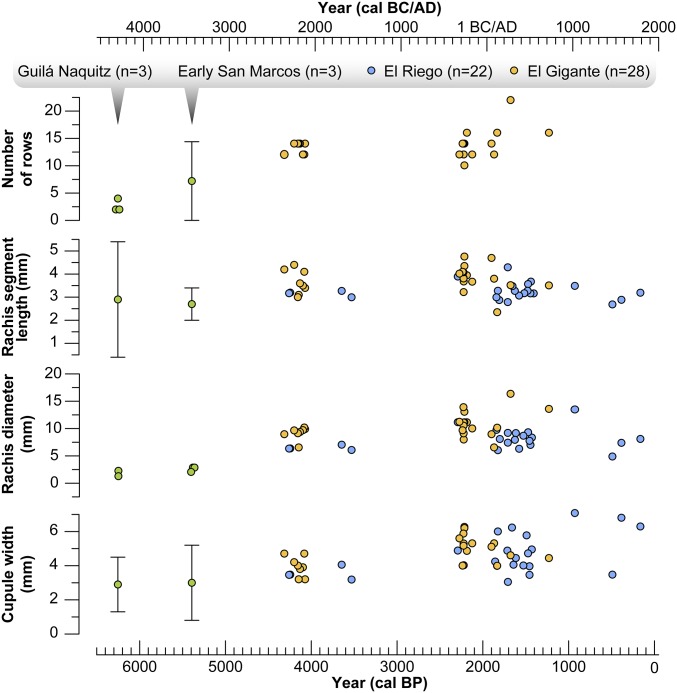
Comparison of measurements obtained from El Gigante maize versus the available morphological data from Tehuacan (San Marcos and El Reigo) and Oaxaca (Guila Naquitz) (22, 28).
The 10 earliest El Gigante maize cobs (4,300–4,000 cal B.P.) are three- to fourfold larger (12–14 rows) than the earliest Oaxacan and Tehuacan cobs (Fig. 5). The average cupule width of the El Gigante cobs (4 mm) is substantially larger than the average cupule width of the early Mexican cobs, indicating local adaptation and selection for larger seed size. Rachis diameter and segment length of the earliest El Gigante cobs are also comparable to later assemblages in Mesoamerica (28). All of these data indicate selection for increased maize productivity outside the range of parviglumis and mexicana, and suggest the influence of reproductive isolation in facilitating the fixation of robust domesticated traits (40). This observation is consistent with morphometric data (kernel row number, cob diameter, and cupule shape), indicating early increases in the size and productivity of maize in South America (Huaca Prieta and Paredones, Peru) between ∼6,700 and 4,000 cal B.P. after initial dispersal well outside the range of parviglumis and mexicana (5).
The morphometric data from the early El Gigante cobs compliment ancient DNA (aDNA) work on comparably aged cobs from Tamaulipas, Mexico, which also fall outside of the natural ranges of parviglumis/mexicana (Fig. 1). The cobs from Tamaulipas date to ∼4,200 cal B.P. and indicate the presence of alleles for controlling reproduction (zag1), protein storage (pbf), plant architecture (tb1), and starch production (pdf) comparable to modern maize (31, 41). This finding is consistent with morphological changes in the El Gigante cobs, suggesting stem and cob architecture were similar to modern maize varieties. Selection is also evident in rachis diameter (twofold thicker than the earliest Oaxacan and Tehuacan cobs), a classic marker for controlling seed dispersal. This structural change indicates selection to reduce shattering by early farmers and is consistent with previously documented early selection on the zag1 gene controlling shattering (31). Increased seed size in the El Gigante cobs parallels selection for alleles promoting protein storage (pbf) and sugar content in the Tamaulipas cobs (31). The morphological data from El Gigante, when combined with the body of regional genomic evidence (24, 25, 31, 41), suggest strong selection for a staple food crop outside the range of parviglumis/mexicana by 4,000 cal B.P.
The major changes in cob architecture evident in El Gigante by 4,300–4,000 cal B.P. are coincident with widespread adoption of this domesticate in Mesoamerica and the evolution of drought-resistant varieties during diffusion into the southwestern United States (∼4,100 cal B.P.) (4) (Fig. 4). Pollen, phytolith, and charcoal records from multiple lowland neotropical wetland environments indicate more persistent land clearance and burning after this time (20, 42–46). The Tehuacan Valley assemblages (San Marcos, Coxcatlan, and El Riego) show relatively gradual changes in rachis architecture and seed size after this time (28). In contrast, rachis segment length and diameter in the El Gigante assemblage are consistently larger than comparably aged material from Tehuacan and within the range of cobs dating later in time in both regions (SI Appendix, Table S5). This pattern suggests that earlier and stronger selection for increased maize productivity outside the natural distribution of parviglumis and mexicana was occurring by 4,300 cal B.P. in Central America. The lack of late morphological evidence for teosinte introgression also suggests that the Central American teosintes (nicaraguensis, luxurians, and huehuetenagensis) were as rare and sparsely distributed as they are today. We hypothesize that more productive varieties of maize were favored with reduced teosinte introgression in this region.
El Gigante is significant because it provides one of a limited number of well-preserved macrobotanical assemblages that reveal how early farmers selected specific morphological traits that led to landraces productive enough to serve as a staple grain. Our data indicate substantial increases in maize productivity in a region outside of the natural distribution of parviglumis/mexicana before the emergence of sedentary agricultural villages and hereditary social inequality that occurred in Mesoamerica after 4,000 cal B.P. (20, 46, 47). The maize macrofossils preserved in El Gigante also provide an important source of morphological and genetic information, given predictions of greater environmental instability and current concerns of decreasing maize diversity in the context of globalization, local loss of traditional farming techniques, the introduction of GM and improved varieties, and the agricultural basis of nation-states in this region.
Materials and Methods
Analysis of Maize Macrofossils.
Twenty-seven desiccated and seven charred cobs/cob fragments were studied under low-power magnification using a 7–46× stereoscopic microscope. This analysis considers eight diagnostic measurements appropriate for racial classification (48, 49) and productivity (yield) assessment (50) of cobs and cob fragments: rank/row number, rachis diameter, rachis segment length, cupule width, cupule length, cupule depth, cupule wing width, and cob diameter. Additional qualitative assessment included glume characteristics, presence of cupule hairs, and estimated cob shape.
Examination of these morphological characteristics followed established procedures (48, 51). Most measurements were recorded at midcob (if possible) to the nearest 0.01 mm using digital capture and measurement software calibrated to 0.1 μm. Cupule depth was evaluated using a dental probe with estimates rounded to the nearest 0.25 mm. For each cob/cob fragment, the reported values for rachis segment length, cupule width, cupule length, cupule depth, and cupule wing width represent the average of measurements from at least three separate ranks. Measurements were not adjusted to account for expansion or shrinkage due to charring.
PCA facilitated exploration of relationships between the eight directly measured morphometric variables; individual samples and their temporal associations are reported in SI Appendix, Tables S1 and S6. In this analysis, charred cobs/cob fragments were excluded from quantitative analysis because it is well established that measurements from carbonized assemblages compare poorly due to physical distortion (52, 53). Using IBM SPSS 24, the PCA correlation matrix standardized all of the variables and treated each with equal weight. Initial assessment of the appropriateness of this statistical evaluation revealed that all of the eight morphometric characteristics were correlated (r ≥ 0.3) with at least one other variable, providing a good indication that PCA would produce a meaningful result. The Kaiser–Meyer–Olkin measure of sampling adequacy was 0.625, above the recommended value of 0.6, and Bartlett’s test of sphericity was significant [χ2 (28) = 79.012, P < 0.000]. The commonalities were all above 0.3 (SI Appendix, Table S4C), further confirming that each item shared some common variance with other items and lending support to the inclusion of each variable in the analysis.
The initial eigenvalues (SI Appendix, Table S4B) showed that the first component explained 37.28% of the variance, the second component explained 25.67% of the variance, and the third component explained 14.14% of the variance. Evidence of substantive variable groupings and temporal associations based on the PCA were further evaluated for statistical significance. One-way ANOVA and post hoc Tukey honest significant difference tests assessed variation in key morphometric differences through time.
High-Precision AMS 14C.
Identified macrobotanical remains were prepared for AMS 14C dating at the The Pennsylvania State University. After removing adhering sediment, samples were subjected to standard acid/base/acid pretreatment consisting of repeated baths in 1 M HCl and NaOH at 70 °C for 30 min on a heater block. A final acid wash removed secondary carbonates formed during the base treatment. Samples were then returned to neutral pH with two 15-min baths in deionized water water at 70 °C to remove chlorides, and dried on a heater block. Sample CO2 was produced by combustion at 900 °C for 3 h in evacuated sealed quartz tubes using a CuO oxygen source and Ag wire to remove chloride compounds. Primary (OX-1) and secondary (FIRI-D/F, FIRI-H) standards and a Queets Wood background were selected to match the sample age and underwent the same chemical steps for quality assurance. Graphitization and high-precision AMS 14C measurements were made at the Keck Carbon Cycle Accelerator Mass Spectrometer facility (using a modified NEC 1.5SDH-1 instrument; National Electrostatics Corporation). All 14C ages were δ13C-corrected for mass-dependent fractionation with measured 13C/12C values (54).
Supplementary Material
Acknowledgments
We thank the Instituto Hondureño de Antropología e Historia for granting us permission to conduct this research. Financial support for this research came from the National Science Foundation (Archaeology Program, Grant BCS-100343 to K.H. and D.L.W. and Archaeometry Program, Grant BCS-1460367 to D.J.K. and B.J.C.) and The Pennsylvania State University.
Footnotes
Conflict of interest statement: The editor, D.R.P., and author L.K. are both affiliated with the Department of Anthropology, Smithsonian National Museum of Natural History.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1705052114/-/DCSupplemental.
References
- 1.Piperno DR, Ranere AJ, Holst I, Iriarte J, Dickau R. Starch grain and phytolith evidence for early ninth millennium B.P. maize from the central Balsas river valley, Mexico. Proc Natl Acad Sci USA. 2009;106:5019–5024. doi: 10.1073/pnas.0812525106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ranere AJ, Piperno DR, Holst I, Dickau R, Iriarte J. The cultural and chronological context of early Holocene maize and squash domestication in the central Balsas river valley, Mexico. Proc Natl Acad Sci USA. 2009;106:5014–5018. doi: 10.1073/pnas.0812590106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corral JAR, et al. Climatic adaptation and ecological descriptors of 42 Mexican maize races. Crop Sci. 2008;48:1502–1512. [Google Scholar]
- 4.da Fonseca RR, et al. The origin and evolution of maize in the southwestern United States. Nat Plants. 2015;1:14003. doi: 10.1038/nplants.2014.3. [DOI] [PubMed] [Google Scholar]
- 5.Grobman A, et al. Preceramic maize from Paredones and Huaca Prieta, Peru. Proc Natl Acad Sci USA. 2012;109:1755–1759. doi: 10.1073/pnas.1120270109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Heerwaarden J, et al. Genetic signals of origin, spread, and introgression in a large sample of maize landraces. Proc Natl Acad Sci USA. 2011;108:1088–1092. doi: 10.1073/pnas.1013011108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hufford MB, Martínez-Meyer E, Gaut BS, Eguiarte LE, Tenaillon MI. Inferences from the historical distribution of wild and domesticated maize provide ecological and evolutionary insight. PLoS One. 2012;7:e47659. doi: 10.1371/journal.pone.0047659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hufford MB, et al. Comparative population genomics of maize domestication and improvement. Nat Genet. 2012;44:808–811. doi: 10.1038/ng.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuoka Y, et al. A single domestication for maize shown by multilocus microsatellite genotyping. Proc Natl Acad Sci USA. 2002;99:6080–6084. doi: 10.1073/pnas.052125199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mercer K, Martínez-Vásquez Á, Perales HR. Asymmetrical local adaptation of maize landraces along an altitudinal gradient. Evol Appl. 2008;1:489–500. doi: 10.1111/j.1752-4571.2008.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vielle-Calzada J-P, Padilla J. The Mexican landraces: Description, classification and diversity. In: Bennetzen JL, Hake SC, editors. Handbook of Maize: Its Biology. Springer; New York: 2009. pp. 543–561. [Google Scholar]
- 12.Merrill WL, et al. The diffusion of maize to the southwestern United States and its impact. Proc Natl Acad Sci USA. 2009;106:21019–21026. doi: 10.1073/pnas.0906075106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbo S, Rubin B. Transgenic crops: A cautionary tale. Science. 2000;287:1927–1928. [PubMed] [Google Scholar]
- 14.Perales HR, Benz BF, Brush SB. Maize diversity and ethnolinguistic diversity in Chiapas, Mexico. Proc Natl Acad Sci USA. 2005;102:949–954. doi: 10.1073/pnas.0408701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellon MR, Hodson D, Hellin J. Assessing the vulnerability of traditional maize seed systems in Mexico to climate change. Proc Natl Acad Sci USA. 2011;108:13432–13437. doi: 10.1073/pnas.1103373108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doebley J. The genetics of maize evolution. Annu Rev Genet. 2004;38:37–59. doi: 10.1146/annurev.genet.38.072902.092425. [DOI] [PubMed] [Google Scholar]
- 17.Piperno DR. The origins of plant cultivation and domestication in the new world tropics: Patterns, process, and new developments. Curr Anthropol. 2011;52:S453–S470. [Google Scholar]
- 18.Pope KO, et al. Origin and environmental setting of ancient agriculture in the lowlands of Mesoamerica. Science. 2001;292:1370–1373. doi: 10.1126/science.292.5520.1370. [DOI] [PubMed] [Google Scholar]
- 19.Pohl MED, Piperno DR, Pope KO, Jones JG. Microfossil evidence for pre-Columbian maize dispersals in the neotropics from San Andres, Tabasco, Mexico. Proc Natl Acad Sci USA. 2007;104:6870–6875. doi: 10.1073/pnas.0701425104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennett DJ, et al. Pre-pottery farmers on the Pacific coast of southern Mexico. J Archaeol Sci. 2010;37:3401–3411. [Google Scholar]
- 21.Piperno DR, Flannery KV. The earliest archaeological maize (Zea mays L.) from highland Mexico: New accelerator mass spectrometry dates and their implications. Proc Natl Acad Sci USA. 2001;98:2101–2103. doi: 10.1073/pnas.98.4.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benz BF. Archaeological evidence of teosinte domestication from Guilá Naquitz, Oaxaca. Proc Natl Acad Sci USA. 2001;98:2104–2106. doi: 10.1073/pnas.98.4.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benz BF, Long A. Prehistoric maize evolution in the Tehuacan valley. Curr Anthropol. 2000;41:459–465. [PubMed] [Google Scholar]
- 24.Ramos-Madrigal J, et al. Genome sequence of a 5,310-year-old maize cob provides insights into the early stages of maize domestication. Curr Biol. 2016;26:3195–3201. doi: 10.1016/j.cub.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 25.Vallebueno-Estrada M, et al. The earliest maize from San Marcos Tehuacán is a partial domesticate with genomic evidence of inbreeding. Proc Natl Acad Sci USA. 2016;113:14151–14156. doi: 10.1073/pnas.1609701113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webster DL. Backward bottlenecks: Ancient teosinte/maize selection. Curr Anthropol. 2011;52:77–104. [Google Scholar]
- 27.Smalley J, Blake M. Sweet beginnings: Stalk sugar and the domestication of maize. Curr Anthropol. 2003;44:675–703. [Google Scholar]
- 28.Benz BF, Cheng L, Leavitt SW, Eastoe C. El riego and early maize agricultural evolution. In: Staller J, Tykot R, Benz B, editors. Histories of Maize. Elsevier; San Diego: 2006. pp. 73–80. [Google Scholar]
- 29.MacNeish R. A summary of the subsistence. In: Byers D, editor. The Prehistory of the Tehuacan Valley: Environment and Subsistence. Vol 1. Univ of Texas Press; Austin, TX: 1967. pp. 290–309. [Google Scholar]
- 30.Smith BD. Reconsidering the Ocampo caves and the era of incipient cultivation in Mesoamerica. Lat Am Antiq. 1997;8:342–383. [Google Scholar]
- 31.Jaenicke-Després V, et al. Early allelic selection in maize as revealed by ancient DNA. Science. 2003;302:1206–1208. doi: 10.1126/science.1089056. [DOI] [PubMed] [Google Scholar]
- 32.Doebley J. Molecular evidence and the evolution of maize. Econ Bot. 1990;44(Suppl 3):6–27. [Google Scholar]
- 33.Hufford MB, Bilinski P, Pyhäjärvi T, Ross-Ibarra J. Teosinte as a model system for population and ecological genomics. Trends Genet. 2012;28:606–615. doi: 10.1016/j.tig.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Hufford MB, et al. The genomic signature of crop-wild introgression in maize. PLoS Genet. 2013;9:e1003477. doi: 10.1371/journal.pgen.1003477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheffler TE, Hirth KG, Hasemann G. The El Gigante rockshelter: Preliminary observations on an early to late Holocene occupation in southern Honduras. Lat Am Antiq. 2012;23:597–610. [Google Scholar]
- 36.Bronk Ramsey C. 2013 OxCal 4.23 Manual. Available at https://c14.arch.ox.ac.uk/oxcalhelp/hlp_contents.html. Accessed November 17, 2016.
- 37.Reimer PJ, et al. IntCal13 and Marine13 radiocarbon age calibration curves 0–50,000 years cal BP. Radiocarbon. 2013;55:1869–1887. [Google Scholar]
- 38.Long A, Benz BF, Donahue DJ, Jull AJT, Toolin LJ. First direct AMS dates on early maize from Tehuacán, Mexico. Radiocarbon. 1989;31:1035–1040. [Google Scholar]
- 39.Benz B, Iltis H. Studies in archeological maize I: The “wild” maize from San Marcos cave reexamined. Am Antiq. 1990;55:500–511. [Google Scholar]
- 40.Civáň P, Ivaničová Z, Brown TA. Reticulated origin of domesticated emmer wheat supports a dynamic model for the emergence of agriculture in the fertile crescent. PLoS One. 2013;8:e81955. doi: 10.1371/journal.pone.0081955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaenicke-Després VR, Smith BD. Ancient DNA and the integration of archaeological and genetic approaches to the study of maize domestication. In: Staller JE, editor. Histories of Maize. Elsevier; Amsterdam: 2006. pp. 83–95. [Google Scholar]
- 42.Kennett DJ, Beach TP. Archaeological and environmental lessons for the Anthropocene from the Classic Maya collapse. Anthropocene. 2014;4:88–100. [Google Scholar]
- 43.Neff H, et al. Early Maya adaptive patterns: Mid-late Holocene paleoenvironmental evidence from Pacific Guatemala. Lat Am Antiq. 2006;17:287–315. [Google Scholar]
- 44.Pohl MD, et al. Early agriculture in the Maya lowlands. Lat Am Antiq. 1996;7:355–372. [Google Scholar]
- 45.Rosenswig RM, Pearsall DM, Masson MA, Culleton BJ, Kennett DJ. Archaic period settlement and subsistence in the Maya lowlands: New starch grain and lithic data from Freshwater Creek, Belize. J Archaeol Sci. 2014;41:308–321. [Google Scholar]
- 46.Flannery KV. The Early Mesoamerican Village. Academic; New York: 1976. [Google Scholar]
- 47.Rosenswig RM. A mosaic of adaptation: The archaeological record for Mesoamerica’s archaic period. J Archaeol Res. 2015;23:115–162. [Google Scholar]
- 48.Bird RM. Manual for the measurement of maize cobs. In: Johannesen S, Hastorf CA, editors. Corn and Culture in the Prehistoric New World. Westview; Boulder, CO: 1994. pp. 5–22. [Google Scholar]
- 49.Nickerson NH. Variation in cob morphology among certain archaeological and ethnological races of maize. Ann Mo Bot Gard. 1953;40:79–111. [Google Scholar]
- 50.Diehl MW. Morphological observations on recently recovered early agricultural period maize cob fragments from southern Arizona. Am Antiq. 2005;70:361–375. [Google Scholar]
- 51.Smith BD. Documenting domesticated plants in the archaeological record. In: Zeder MA, Bradley DG, Emshwiller E, Smith BD, editors. Documenting Domestication: New Genetic and Archaeological Paradigms. Univ of California Press; Los Angeles: 2006. pp. 15–24. [Google Scholar]
- 52.Goette S, Williams M, Johannessen S, Hastorf CA. Toward reconstructing ancient maize: Experiments in processing and charring. J Ethnobiol. 1994;14:1–21. [Google Scholar]
- 53.King FB. Variability in cob and kernel characteristics of North American maize cultivars. In: Johannessen S, Hastorf CA, editors. Corn and Culture in the Prehistoric New World. Westview; Boulder, CO: 1994. pp. 35–54. [Google Scholar]
- 54.Stuiver M, Polach HA. Discussion reporting of C-14 data. Radiocarbon. 1977;19:355–363. [Google Scholar]
- 55.Blake M, et al. 2017 Ancient Maize Map, version 2.1. Available at en.ancientmaize.com/. Accessed March 29, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.