Significance
Calcium influx through the cardiac voltage-dependent L-type calcium channel (CaV1.2) increases during “fight or flight” through activation of the β-adrenergic and protein kinase A (PKA) signaling pathway. None of the previously identified sites in the α1C subunit, each painstakingly and singly investigated, were shown to be required for adrenergic modulation of CaV1.2. Our approach allowed an unprecedented and massive increase in throughput, because we mutated many potential PKA phosphorylation sites throughout α1C. By creating transgenic mice expressing either α1C with alanines substituted for all conserved consensus PKA phosphorylation sites or, separately, α1C without C-terminal proteolytic cleavage, our paradigm-shifting results show that acute β-adrenergic regulation of CaV1.2 does not require phosphorylation of any conserved Ser/Thr of α1C or the proteolytic cleavage of the C terminus of α1C.
Keywords: calcium channels, adrenergic, phosphorylation, heart, transgenic mice
Abstract
Calcium influx through the voltage-dependent L-type calcium channel (CaV1.2) rapidly increases in the heart during “fight or flight” through activation of the β-adrenergic and protein kinase A (PKA) signaling pathway. The precise molecular mechanisms of β-adrenergic activation of cardiac CaV1.2, however, are incompletely known, but are presumed to require phosphorylation of residues in α1C and C-terminal proteolytic cleavage of the α1C subunit. We generated transgenic mice expressing an α1C with alanine substitutions of all conserved serine or threonine, which is predicted to be a potential PKA phosphorylation site by at least one prediction tool, while sparing the residues previously shown to be phosphorylated but shown individually not to be required for β-adrenergic regulation of CaV1.2 current (17-mutant). A second line included these 17 putative sites plus the five previously identified phosphoregulatory sites (22-mutant), thus allowing us to query whether regulation requires their contribution in combination. We determined that acute β-adrenergic regulation does not require any combination of potential PKA phosphorylation sites conserved in human, guinea pig, rabbit, rat, and mouse α1C subunits. We separately generated transgenic mice with inducible expression of proteolytic-resistant α1C. Prevention of C-terminal cleavage did not alter β-adrenergic stimulation of CaV1.2 in the heart. These studies definitively rule out a role for all conserved consensus PKA phosphorylation sites in α1C in β-adrenergic stimulation of CaV1.2, and show that phosphoregulatory sites on α1C are not redundant and do not each fractionally contribute to the net stimulatory effect of β-adrenergic stimulation. Further, proteolytic cleavage of α1C is not required for β-adrenergic stimulation of CaV1.2.
Ca2+ influx through the cardiac voltage-dependent L-type calcium channel (Cav1.2) initiates excitation–contraction coupling. As part of the “fight-or-flight” response, β-adrenergic agonists signaling through the protein kinase A (PKA) pathway rapidly enhance Ca2+ current by increasing the mean open time (mode 2 gating) and open probability of CaV1.2 channels (1). The molecular mechanisms of this fundamental regulatory process remain unknown despite decades of investigation, although it is well-established that cAMP-PKA–mediated phosphorylation is a fundamental event (2–4). PKA phosphorylation of Ser1928 in the cardiac α1C subunit was initially proposed to be required for adrenergic modulation (5), but this was ruled out by expression, using adenovirus or knock-in strategies, of an α1C subunit with substitution of Ser1928 by Ala (6, 7). Ser1512 and Ser1570 in α1C, which are essential for modulation of CaV1.2 by Ca2+/calmodulin-dependent protein kinase II (CaMKII), are also not required for adrenergic stimulation (8). Ser1700 was then proposed as the primary and essential phosphorylation site responsible for PKA modulation of CaV1.2, based on experiments in tsA-201 cells (9). Phosphorylation of Thr1704 by casein kinase II was also proposed to modulate basal CaV1.2 function. We recently showed, however, that Ser1700 and Thr1704 are not required for β-adrenergic stimulation of CaV1.2 in cardiomyocytes (10). PKA targets in the CaV1.2 auxiliary β2 subunits Ser459, Ser478, and Ser479 (11) are also nonessential (6, 8, 12). Thus, CaV1.2 residues previously identified as PKA targets by standard biochemical methodologies, including mass spectrometry and site-directed mutagenesis, are not essential for the rapid β-adrenergic stimulation of CaV1.2 in the heart.
The failure to identify a single site as essential for the acute β-adrenergic modulation of CaV1.2 led us to propose several possible explanations for these findings: (i) that the phosphorylation of any one of several α1C residues can induce the adrenergic stimulation of CaV1.2 current (redundancy), (ii) that each phosphorylated residue contributes a small fraction of the total effect, and/or (iii) that the critical PKA phosphorylation sites in α1C have not yet been identified. To test these hypotheses, we replaced with Ala all consensus intracellular PKA phosphorylation sites in α1C that are conserved in the rabbit, human, guinea pig, mouse, and rat and determined whether elimination of PKA phosphorylation sites in α1C blocked CaV1.2’s responsiveness to adrenergic modulation in cardiomyocytes.
In addition to phosphorylation by PKA, proteolytic cleavage of the α1C C terminus, occurring in greater than 80% of cardiac CaV1.2 channels, has been posited to play an essential role in adrenergic regulation of CaV1.2. Specifically, cleavage is proposed to set the basal CaV1.2 activity, which is then augmented by adrenergic stimulation (5, 9, 13–18). The functional relevance of proteolytic cleavage of α1C, however, has not been demonstrated in cardiomyocytes. Indirect evidence, consisting of mass spectrometric analysis of skeletal muscle α1S proteolytic peptides and sequence alignments of α1S and α1C, was the basis for initially proposing Ala1800 in the context of a 1798NNAN motif as the α1C proteolytic site (13). Although deletion of the 1798NNAN motif did not alter the proteolytic cleavage of α1C (10), cleavage likely occurs in this general region based upon the observed molecular weight of the truncated α1C (Fig. 1A). Moreover, the persistence of α1C cleavage after deletion of the 1798NNAN motif could result from the presence of a similar motif, 1794NANI1797, which is still present when NNAN is deleted. Although the protease responsible for cleavage of α1C is not known, it was speculated to be calpain-like (13, 15, 17, 19), and a conserved motif rich in Pro, Glu, Ser, and Thr (PEST), which can serve as substrate recognition sites for calpains (20), is just N-terminal to Ala1800. We hypothesized that deletion of these motifs may abrogate C-terminal proteolytic cleavage and enable us to explore the role of proteolytic cleavage in fostering β-adrenergic regulation of CaV1.2. Thus, we created two transgenic mouse lines with deletion of either the PEST sequence 1769DTESP alone (ΔPEST) or the PEST sequence and 1794NANINNANN1802 (ΔPEST/PRO) to test the role of proteolytic cleavage of α1C in regulating CaV1.2 function in cardiomyocytes.
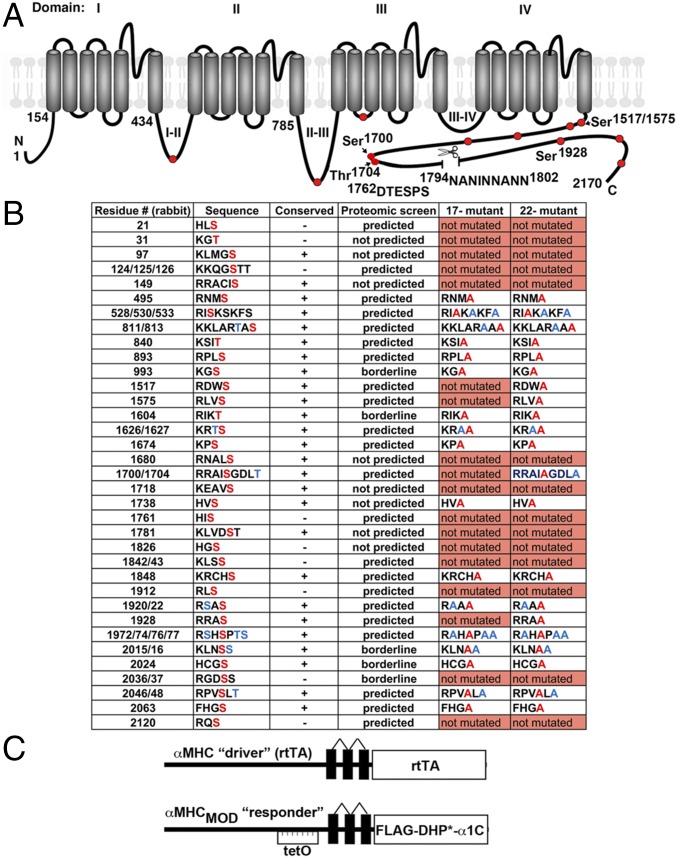
Fig. 1.
Putative PKA phosphorylation sites and proteolytic cleavage site in CaV1.2 α1C. (A) Schematic of rabbit cardiac α1C subunit topology. The putative proteolytic cleavage region is identified; the residues of deleted 1794NANINNANN are shown. The PEST sequence, 1762DTESPS, is also shown. Solid red circles represent some of the putative PKA phosphorylation sites. (B) Putative PKA phosphorylation sites in rabbit α1C. Residues in red are predicted phosphorylation sites and were mutated to Ala in the 17-mutant or 22-mutant transgenic mice. Ser and Thr, shown in blue, although not predicted to be phosphorylated residues, were mutated to Ala. Conserved indicates conserved in the human, guinea pig, rabbit, rat, and mouse. Predicted indicates predicted by at least one of the prediction tools, protein kinase A phosphorylation sites using the simplified kinase binding model (pKaPS), Disorder-Enhanced Phosphorylation Sites Predictor (DISPHOS), GPS, NetPhos, and Scansite. The borderline indicates within the “twilight” zone of pKaPS (31). (C) Schematic representation of the binary transgene system. The αMHC-rtTA is the standard cardiac-specific reverse tetracycline-controlled transactivator system. The αMHCMOD construct is a modified αMHC promoter containing the tet operon (tetO) for regulated expression of FLAG-tagged DHP-resistant (DHP*) α1C.
Materials and Methods
Reagents.
Nisoldipine and Rp-8-Br-cAMPS were purchased from Santa Cruz Biotechnology. All other chemicals were acquired from Sigma.
Animals.
The α1C transgenic constructs were generated by fusing rabbit Cacna1c cDNA (accession no. X15539) to the modified murine α-myosin heavy chain (MHC), tetracycline-inducible promoter (“responder” line) vector (a gift of Jeffrey Robbins and Jeffrey Molkentin, University of Cincinnati, Cincinnati) (21, 22). The α1C subunit was engineered to be dihydropyridine (DHP)-insensitive with the substitutions T1066Y and Q1070M (23, 24). These mice were bred with cardiac-specific (αMHC) doxycycline-regulated, codon-optimized reverse transcriptional transactivator (rtTA) mice obtained via Mutant Mouse Resource and Research Centers (MMRRC) (25) to generate double-transgenic mice. To induce expression, animals received 0.2 g/kg of doxycycline-impregnated food (catalog no. S3888; Bio Serv) for 1 d. The Institutional Animal Care and Use Committee at Columbia University approved all animal experiments.
Immunoblots and Immunofluorescence.
Cardiac lysates from 8- to 12-wk-old doxycycline-fed transgenic mice were prepared as described (10). Proteins were size-fractionated, transferred to nitrocellulose membranes, and probed with anti-FLAG antibody (Sigma), anti-α1C (10), and antitubulin (Santa Cruz Biotechnology) antibodies. Detection was performed with a CCD camera (Carestream Imaging), and ImageQuant software was used for quantification. Isolated cardiomyocytes were fixed for 15 min in 4% paraformaldehyde, and indirect immunofluorescence performed using a 1:200 rabbit anti-FLAG antibody and 1:200 FITC-labeled goat–anti-rabbit antibody (Sigma). Images were acquired using a confocal microscope.
Cellular Electrophysiology.
Membrane currents were measured by the whole-cell patch-clamp method using a MultiClamp 700B amplifier and pCLAMP10 software (Molecular Devices) as described elsewhere (10). The pipette solution contained 40 mM CsCl, 90 mM Cs gluconate, 10 mM 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), 1 mM MgCl2, 4 mM Mg-ATP, 2 mM CaCl2, and 10 mm Hepes, adjusted to pH 7.2 with CsOH. After the isolated cardiomyocytes were adequately buffered with 10 mM BAPTA in the internal solution, the isolated cardiomyocytes (26) were superfused with 140 mM tetraethylammonium-Cl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM Hepes, adjusted to pH 7.4 with CsOH. Pipette series resistances were usually <1 MΩ after 60% compensation. Leak currents and capacitance transients were subtracted by a P/4 protocol. To measure Ca2+ peak currents, the cell membrane potential was held at −50 mV and stepped to +10 mV for 350 ms every 10 s. To evaluate the current–voltage (I-V) relationship for Ca2+ currents, the same protocol was repeated with steps between −40 mV and +60 mV in 10-mV increments. All experiments were performed at room temperature: 22 ± 1 °C. For I-V curves, we used a Boltzmann distribution: I(V) = Gmax * (V − Erev)/[1 + exp(Vmid − V)/k)], where Gmax is maximal conductance, Erev is reversal potential, Vmid is the midpoint, and k is the slope factor.
Fractional Shortening.
Freshly isolated myocytes were superfused with Tyrode’s solution containing 1.0 mM CaCl2 and 300 nM nisoldipine. Myocytes were field-stimulated at 1 Hz. Fractional shortening of sarcomere length was measured using the SarcLen module of Ionoptix.
Statistical Analysis.
Results are presented as mean ± SEM. For multiple group comparisons, one-way ANOVA, followed by Tukey’s post hoc test, was performed. For comparisons between two groups, an unpaired Student’s t test was used. Statistical analyses were performed using Prism 6 (Graphpad Software). Differences were considered statistically significant at values of P < 0.05.
Results
Generation of Inducible, Cardiac-Specific PKA Phosphorylation Site Mutant Mice.
The optimal PKA phosphorylation motif is a tetrapeptide with Arg at the second and third positions (termed -2 and -3) before the phosphorylated Ser or Thr and a large hydrophobic residue immediately thereafter (R-R-X-S/T-Φ) (27–29). The positions between -4 and -1 have a strong preference for Arg and, to a lesser extent, for His or Lys (28, 30). We identified all potential intracellular PKA phosphorylation sites (Fig. 1B) in rabbit α1C using both manual sequence analysis and several web-based PKA phosphorylation prediction tools, including prediction of protein kinase A phosphorylation sites using the simplified kinase binding model (pKaPS) (31), Disorder-Enhanced Phosphorylation Sites Predictor (DISPHOS) (32), GPS (33), NetPhos (34), and Scansite (35). Each phosphorylation site was mutated to Ala if the Ser or Thr in rabbit α1C was conserved in the rat, mouse, guinea pig, or human. For those conserved sites, we also mutated additional Ser and Thr within several amino acid residues C-terminal to the Arg or Lys to ensure that we fully tested each phosphoregulatory site (residues labeled blue in Fig. 1B). If the site was not conserved among all species, and there was no alternative putative consensus PKA phosphorylation site nearby, we excluded the site from consideration. We also excluded those sites predicted to be extracellular or within the plasma membrane.
Based on the list, we generated two transgenic mice with inducible cardiomyocyte-specific expression of an N-terminal 3× FLAG-epitope–tagged DHP-resistant α1C with Ala substitutions of all conserved Ser or Thr, either not including (“17-mutant”) or including (“22-mutant”) residues previously shown to be not required for β-adrenergic stimulation of CaV1.2 (rabbit Ser1517, Ser1575, Ser1700, Thr1704, and Ser1928) (Fig. 1 B and C). Several pseudo-wild-type (pWT), 17-mutant, and 22-mutant founder transgenic lines were originally created, and lines demonstrating doxycycline-induced α1C expression after crossing with the αMHC-rtTA mice were expanded and used for this study. Because the results were consistent within each mouse line, the data were pooled for gender and founders.
Functional Studies of PKA Phosphorylation Site Mutant Mice.
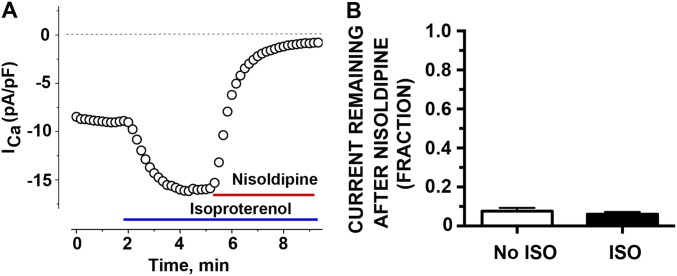
As in prior studies, we used a concentration of 300 nM nisoldipine, which blocked >98% of heterologously expressed WT CaV1.2 current in tsA-201 cells, but only blocked 34.6% of DHP-insensitive α1C (10). In cardiomyocytes isolated from nontransgenic mice, 300 nM nisoldipine inhibited 92.4 ± 1.6% of peak current in the absence of β-adrenergic stimulation (10) and 94.0 ± 1.2% of peak current after 200 nM isoproterenol (Fig. S1). Thus, when recording from cardiomyocytes expressing DHP-resistant transgenic α1C subunits (discussed below), these data demonstrate that we have effectively blocked the endogenous channels and isolated the transgenic currents, both in the absence and presence of isoproterenol.
Fig. S1.
Nisoldipine inhibits basal and β-adrenergic–stimulated endogenous CaV1.2 current equivalently. (A) Diary plot of Ca2+ current (ICa) amplitude at +10 mV in picoamperes per picofarad (pA/pF) before, after 200 nM isoproterenol, and after 300 nM nisoldipine in presence of 200 nM isoproterenol from nontransgenic mouse. Data are representative of seven similar experiments. (B) Bar graph of fraction current remaining after nisoldipine in cells without isoproterenol (ISO) superfusion (n = 12) and cells with isoproterenol superfusion (n = 7). Mean ± SEM (P = 0.75 by unpaired t test).
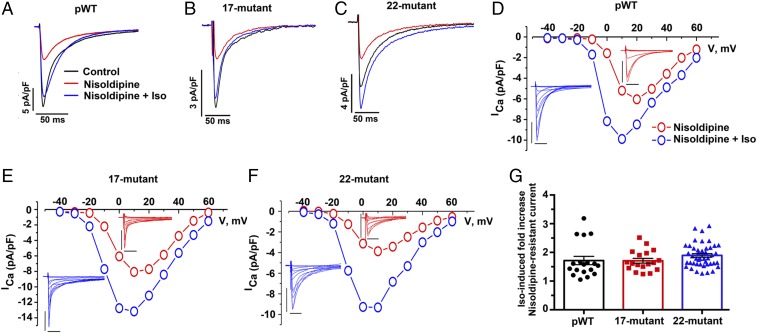
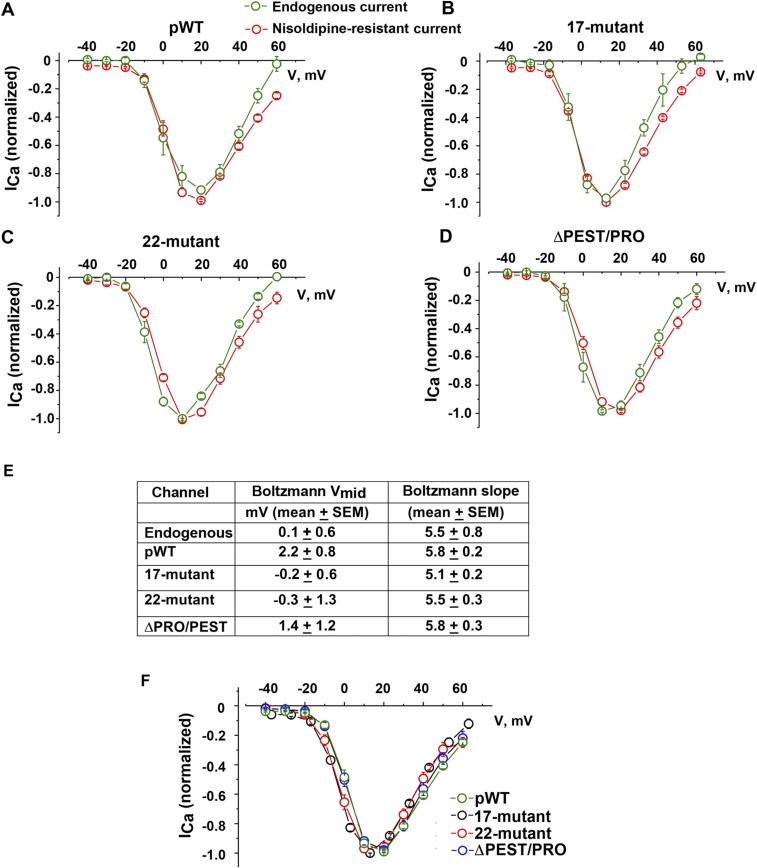
Transgenic mice were fed doxycycline-impregnated food overnight to induce the expression of the DHP-resistant transgenic α1C subunits. Nisoldipine (300 nM) inhibited 51.7 ± 5.8% of peak current in cardiomyocytes isolated from doxycycline-fed pWT α1C transgenic mice, 33.5 ± 3.9% of peak current in doxycycline-fed 17-mutant α1C transgenic mice, and 69.5 ± 1.6% of peak current in doxycycline-fed 22-mutant α1C mice (Fig. 2 A–C). The midpoint potentials, derived from the Boltzmann function, for steady-state activation of the transgenic, nisoldipine-resistant 17-mutant and 22-mutant channels demonstrated a nonsignificant small leftward shift compared with pWT channels, and the slope factors for 17-mutant and 22-mutant transgenic channels were not different from pWT channels (Fig. S2 E and F). Our interpretation of these results is that under basal conditions, the activation of the transgenic 17-mutant and 22-mutant CaV1.2 channels is similar to endogenous CaV1.2 channels.
Fig. 2.
β-Adrenergic stimulation of CaV1.2 current does not require conserved PKA phosphorylation sites in α1C. (A–C) Exemplar whole-cell Ca2+ currents (ICa) recorded from freshly dissociated cardiomyocytes of pWT, 17-mutant, and 22-mutant α1C transgenic mice. Pulses ranged from −70 mV to +10 mV before (black traces) and 3 min after (red traces) 300 nM nisoldipine and 3 min after 200 nM isoproterenol in the presence of nisoldipine (blue traces). (D–F) I-V relationships of pWT, 17-mutant, and 22-mutant α1C before and after 200 nM isoproterenol, in the presence of 300 nM nisoldipine. (Insets) Series of whole-cell CaV1.2 currents recorded from a series of pulses between −40 mV and +60 mV from a holding potential of −50 mV in the absence of 200 nM isoproterenol (red traces) and 3 min after isoproterenol (blue traces). The calibration was 5 picoamperes per picofarad (pA/pF) for 50 ms. Data are representative of pWT (n = 14), 17-mutant (n = 12), and 22-mutant (n = 10) I-V curves. The Boltzmann midpoint (ΔVmid) induced by isoproterenol: pWT (−2.4 ± 0.5, P < 0.001 by paired t test, n = 12) 17-mutant (−4.5 ± 0.5, P < 0.0001 by paired t test, n = 12), and 22-mutant (−2.8 ± 0.3, P < 0.0001 by paired t test). (G) Combined bar and column scatter plot depicting the fold increase in peak current caused by isoproterenol. Bar graphs are mean ± SEM (n = 18 cardiomyocytes from eight pWT α1C mice, n = 18 cardiomyocytes from four 17-mutant α1C mice, and n = 44 cardiomyocytes from eight 22-mutant α1C mice; P = 0.21 by one-way ANOVA).
Fig. S2.
I-V relationships for transgenic CaV1.2 channels and endogenous CaV1.2 channels are not different. Normalized I-V relationships of Ca2+ currents (ICa) recorded from pWT α1C (A), 17-mutant α1C (B), 22-mutant α1C (C), and ΔPEST/PRO α1C (D). Whole-cell CaV1.2 currents were recorded from a series of pulses between −40 mV and +60 mV from a holding potential of −70 mV before and 3 min after superfusion of 300 nM nisoldipine (red trace). I-V curve of endogenous CaV1.2 (green trace) was derived by subtracting nisoldipine-resistant current from the total CaV1.2 current. Mean ± SEM (pWT, n = 10; 17-mutant, n = 7; 22-mutant, n = 8; ΔPEST/PRO, n = 4). (E) Table of Boltzmann function, midpoint (Vmid), and slope for endogenous Ca2+ channels and nisoldipine-resistant mutant transgenic Ca2+ channels [P = not significant by ANOVA (P = 0.30); endogenous channels, n = 11; pWT, n = 8; 17-mutant, n = 12; 22-mutant, n = 10; ΔPRO/PEST, n = 11]. (F) Normalized I-V relationship of nisoldipine-resistant current of pWT, 17-mutant, 22-mutant, and ΔPEST/PRO. Mean ± SEM.
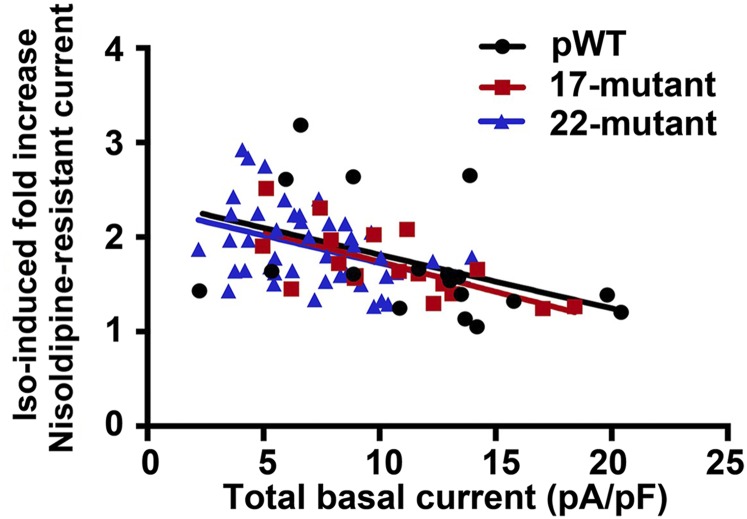
In the cardiomyocytes isolated from pWT α1C transgenic mice, isoproterenol increased the nisoldipine-insensitive peak current by a mean of 1.7 ± 0.1-fold (Fig. 2 D and G). In the cardiomyocytes isolated from the 17-mutant and 22-mutant transgenic mice, isoproterenol increased nisoldipine-resistant peak CaV1.2 current by a mean of 1.7 ± 0.1-fold and 1.9 ± 0.1-fold, respectively, which is nearly identical to the isoproterenol-induced augmentation of nisoldipine-resistant current in pWT α1C transgenic cardiomyocytes (Fig. 2 E–G). We observed a hyperpolarizing shift in the I-V curves for currents from both transgenic mutants, similar to the shift observed for pWT (Fig. 2 D–F). Also, similar to the pWT α1C transgenic mice, the magnitude of the isoproterenol-induced increase in the nisoldipine-resistant Ca2+ current was inversely correlated with the basal total CaV1.2 current (Fig. S3). The slopes and intercepts of the linear regression lines describing the relationship of total basal current density and response to isoproterenol were not statistically different. This inverse relationship has also been observed for endogenous Ca2+ channels in the guinea pig and rat (12).
Fig. S3.
Isoproterenol-induced fold increase in Ca2+ current stratified by total basal Ca2+ current in picoamperes per picofarad (pA/pF). Lines were fitted by linear regression.
Although we had eliminated the predicted PKA phosphorylation sites in α1C, the isoproterenol-induced increased current in the cardiomyocytes isolated from the 17-mutant and 22-mutant α1C transgenic mice was due to PKA, and not due to CaMKII, because inclusion of 10 mM BAPTA in the pipette solution should strongly and rapidly buffer [Ca2+], thereby inhibiting CaMKII activity. Moreover, in the 22-mutant mice, we ablated with Ala substitutions the known CaMKII phosphorylation sites in α1C (36). Taken together, these results demonstrate that the adrenergic regulation of CaV1.2 in the heart is dependent upon neither any combination of the five previously identified phosphoregulatory sites in α1C nor any of the additional 17 consensus PKA phosphorylation sites in α1C that are conserved among five species.
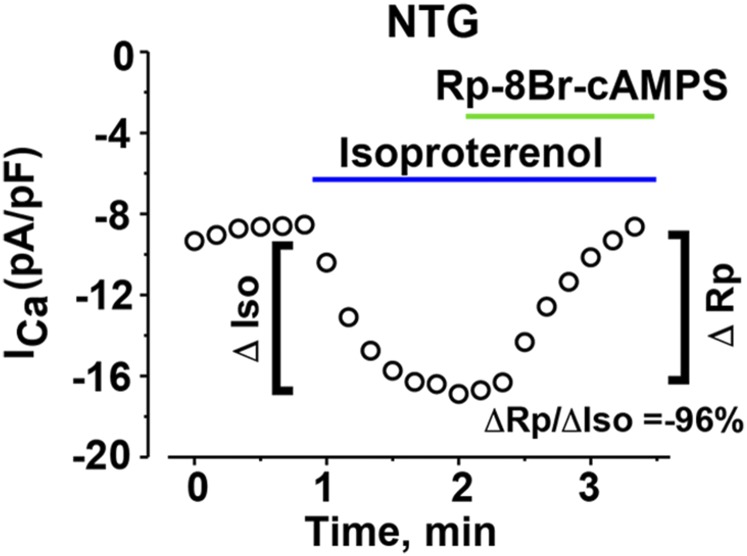
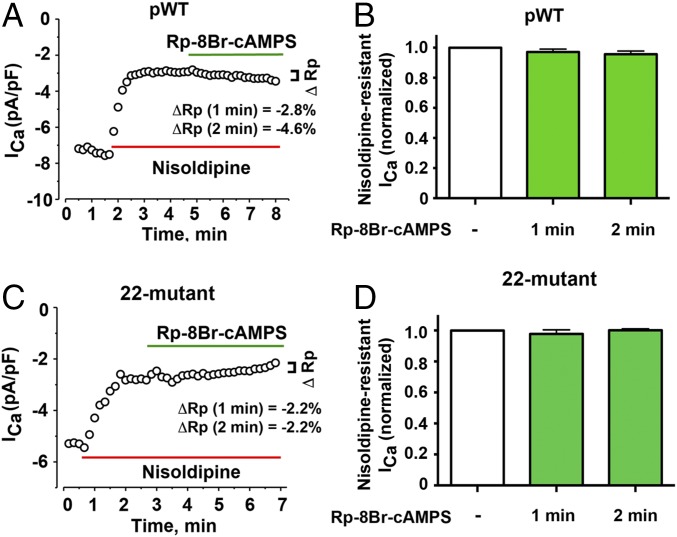
We considered the possibility that our results were confounded by basal phosphorylation even though the isolated cells are maintained in a physiological solution without catecholamines. To address whether the CaV1.2 channels have basal PKA phosphorylation and whether this phosphorylation leads to increased Ca2+ currents, we used a cell-permeable cAMP-PKA inhibitor (Rp-8-Br-cAMPS), which functions by occupying cAMP binding sites, thereby preventing dissociation and activation of the PKA holoenzyme (37). The inhibitor can rapidly reverse isoproterenol-mediated up-regulation of CaV1.2 of nontransgenic CaV1.2 channels by 96.5 ± 12.8% within 1 min (Fig. S4), implying that the cAMP-PKA inhibitor effectively reverses any PKA-mediated increase of basal CaV1.2 current. These results also provide additional evidence that the effect of isoproterenol is mediated via PKA, and not CaMKII. In cardiomyocytes isolated from both pWT and the 22-mutant α1C transgenic mice, the cAMP-PKA inhibitor had no significant inhibitory effect on nisoldipine-resistant basal CaV1.2 currents at 1 or 2 min (Fig. 3 A–D). These data indicate that neither endogenous nor transgenic CaV1.2 channels are basally activated by PKA in freshly isolated cardiomyocytes.
Fig. S4.
Diary plot of Ca2+ current (ICa) amplitude at +10 mV in picoamperes per picofarad (pA/pF) before isoproterenol, after 200 nM isoproterenol, and after 200 nM isoproterenol + 30 μM Rp-8Br-cAMPS in cardiomyocytes isolated from nontransgenic (NTG) mice. Data are representative of 10 similar experiments. The isoproterenol-induced increase in Ca2+ current was inhibited by 96% (ΔRp/ΔIso) within 1 min of exposure to Rp-8Br-cAMPS, in the continued presence of isoproterenol.
Fig. 3.
Basal Ca2+ current in isolated cardiomyocytes is not reduced by cAMP-PKA inhibitor. (A) Diary plot of Ca2+ current (ICa) amplitude at +10 mV picoamperes per picofarad (pA/pF) of cardiomyocytes isolated from pWT α1C transgenic mice. The current amplitude after inhibition of endogenous Ca2+ current by nisoldipine was set as the baseline. Data are representative of 14 cardiomyocytes for which the Ca2+ current was nominally inhibited by 2.8% at 1 min and 4.6% at 2 min after Rp-8Br-cAMPS exposure in the presence of nisoldipine. (B) Bar graph of normalized nisoldipine-resistant Ca2+ current before and after Rp-8Br-cAMPS. Mean ± SEM (n = 14 cardiomyocytes; P = 0.2 by repeated measures one-way ANOVA). (C) Diary plot of current amplitude at +10 mV (pA/pF) of cardiomyocytes isolated from 22-mutant α1C transgenic mice. The current amplitude after inhibition of endogenous Ca2+ current by nisoldipine was set as the baseline. Data are representative of eight cardiomyocytes, for which the Ca2+ current was nominally inhibited by 2.2% at 1 min and 2.2% at 2 min after Rp-8Br-cAMPS exposure in presence of nisoldipine. (D) Bar graph of the normalized nisoldipine-resistant current (pA/pF) before and after Rp-8Br-cAMPS. Mean ± SEM (n = 8 cardiomyocytes; P = 0.48 by repeated measures one-way ANOVA).
Proteolytic Processing of α1C.
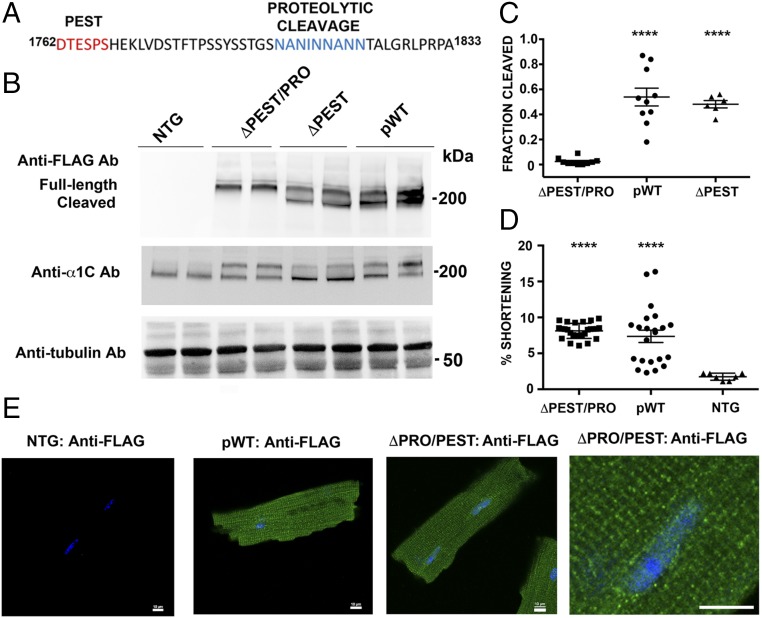
In cardiomyocytes isolated from nontransgenic mice, native α1C is detected as a full-length ∼240-kDa band and a cleaved ∼210-kDa band, using an anti-α1C antibody created against an internal epitope within the intracellular loop of domains II and III (10). To investigate the role of channel cleavage, we created two transgenic mice. In one, we deleted the PEST sequence (ΔPEST) alone. In the second, we deleted both the PEST sequence and 1794NANINNANN1802 (ΔPEST/PRO) (Fig. 4A). Hearts were resected, washed of blood, and immediately frozen. The ratio of cleaved to full-length ΔPEST transgenic α1C was 48.7 ± 3.0%, which is not significantly different from the 53.9 ± 7.1% cleavage of the pWT α1C (mean ± SEM, P = not significant by Tukey’s multiple comparison test; Fig. 4 B and C). In contrast, the ratio of cleaved to full-length ΔPEST/PRO was 2.4 ± 0.8% (mean ± SEM, P < 0.0001 by ANOVA/Tukey’s multiple comparison text compared with both pWT and ΔPEST; Fig. 4 B and C), indicating that proteolytic cleavage requires the presence of 1794NANINNANN1802.
Fig. 4.
Identification of C-terminal proteolytic cleavage of α1C. (A) Sequence of PEST and proteolytic cleavage site of rabbit α1C. (B) Anti-FLAG antibody (Ab; Upper), anti-α1C antibody (Middle), and antitubulin antibody (Lower) immunoblots showing FLAG epitope-tagged α1C expression in isolated cardiomyocytes from nontransgenic (NTG), pWT α1C, ΔPEST α1C, and ΔPEST/PRO α1C transgenic mice. (C) Bar graph of densitometries of cleaved α1C band divided by truncated + full-length α1C bands (n = 10 pWT α1C mice, n = 6 ΔPEST α1C mice, and n = 10 ΔPEST/PRO α1C mice; P < 0.0001 by ANOVA, ****P < 0.0001 by Tukey’s multiple comparison test). (D) Percent shortening of sarcomere length after incubation and superfusion of 300 nM nisoldipine-containing solution. Cardiomyocytes were field-stimulated at 1 Hz (P < 0.0001 by ANOVA and Tukey’s multiple comparison test). (E) Immunostaining of ΔPEST/PRO cardiomyocytes. Anti-FLAG antibody, FITC-conjugated secondary antibody, and nuclear labeling with Hoechst stain are shown. Images were obtained with a confocal microscope at 40× magnification. (Scale bar: 10 μm.)
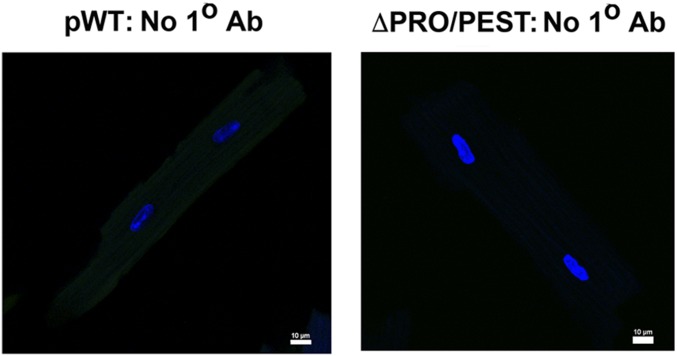
Having generated a mutant CaV1.2 in cardiomyocytes for which the C terminus was resistant to proteolytic cleavage by the endogenous proteases that act on the endogenous CaV1.2, we then asked whether proteolytic cleavage of the C terminus was required for either trafficking or function of cardiac CaV1.2. We used two complementary methods to assess appropriate trafficking to the t-tubules. First, we assessed whether the transgenic myocytes contracted normally in response to Ca2+ influx through the ΔPEST/PRO CaV1.2. Cardiomyocyte contraction requires Ca2+ influx via CaV1.2 in t-tubules, which triggers Ca2+-induced Ca2+ release from neighboring ryanodine receptor intracellular Ca2+ release channels and subsequent contraction. Superfusion of nisoldipine inhibited the contraction of nontransgenic cardiomyocytes to electric field stimulation at 1 Hz (Fig. 4D), confirming the necessity of CaV1.2. In cardiomyocytes isolated from both pWT and ΔPEST/PRO α1C mutant transgenic mice, field-stimulated contraction persisted in the presence of nisoldipine (Fig. 4D; P < 0.0001 for both pWT and ΔPEST/PRO compared with nontransgenic cardiomyocytes by ANOVA, Tukey’s multiple comparison test, P = not significant between pWT and ΔPEST/PRO). Thus, the nisoldipine-resistant ΔPEST/PRO channels can trigger contraction similar to the pWT channels. Second, we observed the location of ΔPEST/PRO CaV1.2 channels by immunocytochemistry. Anti-FLAG antibody immunofluorescence of fixed cardiomyocytes from pWT and ΔPEST/PRO mutant transgenic mice also showed a striated pattern consistent with surface membrane distribution of expressed α1C subunits and t-tubular localization (Fig. 4E). No anti-FLAG antibody immunofluorescent signal was detected in cardiomyocytes isolated from nontransgenic mice, and no immunofluorescence was detected in either pWT or ΔPEST/PRO transgenic cardiomyocytes when the anti-FLAG antibody was omitted (Fig. 4E and Fig. S5).
Fig. S5.
Immunostaining of pWT and ΔPEST/PRO cardiomyocytes. Incubation was performed with FITC-conjugated secondary antibody and nuclear labeling with Hoechst stain, but without anti-FLAG antibody. Images were obtained with a confocal microscope at a magnification of 40×. (Scale bar: 10 μm.)
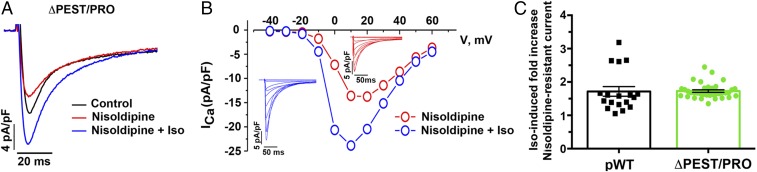
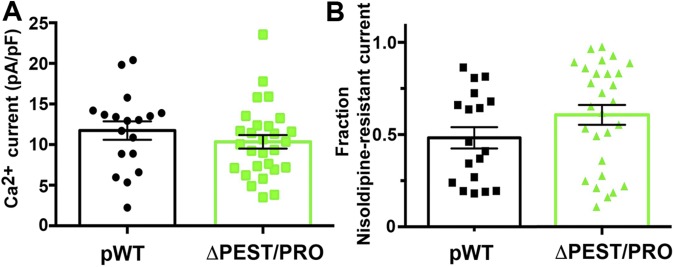
With this foundation, we then tested whether the absence of C-terminal cleavage affected the ability of the ΔPEST/PRO CaV1.2 channels to respond to isoproterenol. We measured CaV1.2 currents in adult cardiomyocytes from ΔPEST/PRO α1C mutant transgenic mice. Nisoldipine (300 nM) inhibited only 41.9 ± 5.3% of peak current, implying that the transgenic channels trafficked to the membrane and were functional (Fig. 5 and Figs. S2D and S6). The midpoint and slope of the ΔPEST/PRO α1C channels were not different from the midpoint and slope of pWT channels (Fig. S2 E and F). These findings imply that under basal conditions, the C-terminal proteolytic cleavage of α1C is not required for expression and basal function of the Ca2+ channel. In the cardiomyocytes isolated from ΔPEST/PRO α1C transgenic mice, isoproterenol increased the nisoldipine-insensitive peak current by a mean of 1.7 ± 0.04-fold (Fig. 5 A–C). Taken together, these results demonstrate that C-terminal proteolytic cleavage of α1C is not required for β-adrenergic regulation of CaV1.2 in the heart.
Fig. 5.
DHP-resistant currents in ΔPEST/PRO transgenic mice. (A) Exemplar whole-cell CaV1.2 currents recorded from pulses from −70 mV to +10 mV before (black trace), 3 min after 300 nM nisoldipine (red trace), and 3 min after 200 nM isoproterenol in the presence of nisoldipine (blue trace). (B) Representative I-V relationship of ΔPEST/PRO Ca2+ current (ICa) without and with 200 nM isoproterenol. (Insets) Series of whole-cell CaV1.2 currents recorded from a series of pulses between −40 mV and +60 mV from a holding potential of −70 mV in the absence of isoproterenol (red traces) and 3 min after 200 nM isoproterenol (blue traces). Data are representative of 14 similar I-V curves. The Boltzmann midpoint (ΔVmid) is induced by isoproterenol: ΔPEST/PRO: −2.4 ± 0.7 (P < 0.01 by paired t test; n = 12). (C) Combined bar and column scatter plot depicting the fold increase in peak current caused by 200 nM isoproterenol. Bar graphs are mean ± SEM. Presented pWT data are the same as in Fig. 2 (n = 18 cardiomyocytes from eight pWT α1C mice and n = 36 cardiomyocytes from six ΔPEST/PRO α1C mice; P = 0.92 by unpaired t test).
Fig. S6.
(A) Combined bar graph and column scatter plot for total peak current density in picoamperes per picofarad (pA/pF) of cardiomyocytes isolated from pWT and ΔPEST/PRO α1C transgenic mice. Mean ± SEM (P = 0.32 by unpaired t test). (B) Fraction of nisoldipine-resistant current in pWT and ΔPEST/PRO transgenic cardiomyocytes (P = 0.14 by unpaired t test).
Discussion
Prior studies used biochemical and mass spectrometry to identify single residues in α1C and β2 subunits phosphorylated by PKA as candidate targets for acute β-adrenergic regulation of CaV1.2 in heart. Our data show that none of those identified sites in α1C are required for the acute fight-or-flight modulation of CaV1.2 by β-adrenergic agonists in the heart. For both the 17-mutant and 22-mutant mouse lines, we found that acute β-adrenergic regulation of CaV1.2 was not diminished, implying that Ser or Thr residues in α1C that are common to the five species and within a consensus PKA phosphorylation site are not the functionally relevant PKA phosphorylation sites for acute modulation of CaV1.2 by β-adrenergic agonists. A role for the previously identified PKA phosphorylation sites within the CaV1.2 β2 subunit has been excluded by several studies (6, 8, 12). Our results suggest that the field may have been focusing on the wrong targets.
Most recently, Ser1700 has been proposed as the critical PKA phosphorylation site in α1C for mediating acute β-adrenergic regulation of CaV1.2 in the heart (9, 38). Our studies of Ser1700, in isolation (10) and now in combination with other putative PKA phosphorylation sites, do not support its role as was suggested by a knock-in mouse (38). Advantages of our approach are that expression of the mutant channels is conditional, short-term, and limited, thereby leading to fewer secondary compensatory effects, such as heart failure, which reduces normal β-adrenergic responsiveness. The potential confounders due to compensatory changes with a chronic, knock-in model are also illustrated by the decreased basal CaV1.2 current in cardiomyocytes isolated from an S1700A knock-in mouse, which was speculated to be secondary to reduced basal phosphorylation of Ser1700 by PKA (38, 39). Using a potent cAMP-PKA inhibitor that can fully reverse β-adrenergic stimulation of CaV1.2 when applied after isoproterenol, we demonstrate that CaV1.2 current is not up-regulated by cAMP-PKA–mediated pathways in unstimulated cardiomyocytes isolated from pWT α1C mice.
Our approach also allowed us to investigate the role of proteolytic cleavage of the α1C C terminus. Proteolytic cleavage and the subsequent assembly of the cleaved C-terminal fragment with the truncated α1C have been proposed to play important roles in basal and adrenergic regulation of CaV1.2 (9, 17, 18). Truncation of α1C, either at Gly1796 (immediately N-terminal to the identified protease cleavage site) or Asp1904 caused a marked reduction in the cell surface expression of α1C and in the CaV1.2 current in the heart, but not in vascular smooth muscle, leading to cardiac failure and perinatal death (18, 40). Because the distal C terminus, after cleavage from an endogenous channel, likely remains associated with the cardiac CaV1.2 complex (41), expression of α1C with a truncated distal C terminus does not mimic a physiological state. Here, we identified the proteolytic site of the cardiac Ca2+ channels and disrupted it. This strategy enabled us to explore adrenergic regulation with the C terminus of α1C still present but unable to be cleaved. We found that prevention of C-terminal proteolytic cleavage did not alter the expression, trafficking, basal function, or adrenergic regulation of the CaV1.2 in the heart. The functional effects of cleavage of α1C remain unclear, but our studies demonstrate that proteolytic cleavage of α1C in the heart is not required for β-adrenergic regulation of CaV1.2.
Taken together, our studies rule out a role for conserved PKA phosphorylation sites in α1C contributing to acute regulation of β-adrenergic stimulation of CaV1.2. Moreover, our data show that these key residues are not redundant and do not each contribute combinatorially to a net stimulatory effect. Whether redundancy could exist with the CaV1.2 β subunit is not clear at this stage.
Acknowledgments
This work was supported by NIH Grants R01 HL113136 and R01 HL121253. J.A. was supported by Grant F30 HL129628, and J.K. was supported by Grant T32 HL007343.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706054114/-/DCSupplemental.
References
- 1.Yue DT, Herzig S, Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci USA. 1990;87:753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartzell HC, Méry PF, Fischmeister R, Szabo G. Sympathetic regulation of cardiac calcium current is due exclusively to cAMP-dependent phosphorylation. Nature. 1991;351:573–576. doi: 10.1038/351573a0. [DOI] [PubMed] [Google Scholar]
- 3.Brum G, Flockerzi V, Hofmann F, Osterrieder W, Trautwein W. Injection of catalytic subunit of cAMP-dependent protein kinase into isolated cardiac myocytes. Pflugers Arch. 1983;398:147–154. doi: 10.1007/BF00581064. [DOI] [PubMed] [Google Scholar]
- 4.Osterrieder W, et al. Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature. 1982;298:576–578. doi: 10.1038/298576a0. [DOI] [PubMed] [Google Scholar]
- 5.De Jongh KS, et al. Specific phosphorylation of a site in the full-length form of the alpha 1 subunit of the cardiac L-type calcium channel by adenosine 3′,5′-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35:10392–10402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- 6.Ganesan AN, Maack C, Johns DC, Sidor A, O’Rourke B. Beta-adrenergic stimulation of L-type Ca2+ channels in cardiac myocytes requires the distal carboxyl terminus of alpha1C but not serine 1928. Circ Res. 2006;98:e11–e18. doi: 10.1161/01.RES.0000202692.23001.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemke T, et al. Unchanged beta-adrenergic stimulation of cardiac L-type calcium channels in Ca v 1.2 phosphorylation site S1928A mutant mice. J Biol Chem. 2008;283:34738–34744. doi: 10.1074/jbc.M804981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandmayr J, et al. Deletion of the C-terminal phosphorylation sites in the cardiac β-subunit does not affect the basic β-adrenergic response of the heart and the Ca(v)1.2 channel. J Biol Chem. 2012;287:22584–22592. doi: 10.1074/jbc.M112.366484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA. Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci Signal. 2010;3:ra70. doi: 10.1126/scisignal.2001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L, et al. β-Adrenergic regulation of the L-type Ca2+ channel does not require phosphorylation of α1C Ser1700. Circ Res. 2013;113:871–880. doi: 10.1161/CIRCRESAHA.113.301926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerhardstein BL, Puri TS, Chien AJ, Hosey MM. Identification of the sites phosphorylated by cyclic AMP-dependent protein kinase on the beta 2 subunit of L-type voltage-dependent calcium channels. Biochemistry. 1999;38:10361–10370. doi: 10.1021/bi990896o. [DOI] [PubMed] [Google Scholar]
- 12.Miriyala J, Nguyen T, Yue DT, Colecraft HM. Role of CaVbeta subunits, and lack of functional reserve, in protein kinase A modulation of cardiac CaV1.2 channels. Circ Res. 2008;102:e54–e64. doi: 10.1161/CIRCRESAHA.108.171736. [DOI] [PubMed] [Google Scholar]
- 13.Hulme JT, et al. Sites of proteolytic processing and noncovalent association of the distal C-terminal domain of CaV1.1 channels in skeletal muscle. Proc Natl Acad Sci USA. 2005;102:5274–5279. doi: 10.1073/pnas.0409885102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Jongh KS, Warner C, Colvin AA, Catterall WA. Characterization of the two size forms of the alpha 1 subunit of skeletal muscle L-type calcium channels. Proc Natl Acad Sci USA. 1991;88:10778–10782. doi: 10.1073/pnas.88.23.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Jongh KS, Merrick DK, Catterall WA. Subunits of purified calcium channels: A 212-kDa form of alpha 1 and partial amino acid sequence of a phosphorylation site of an independent beta subunit. Proc Natl Acad Sci USA. 1989;86:8585–8589. doi: 10.1073/pnas.86.21.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao T, et al. Identification and subcellular localization of the subunits of L-type calcium channels and adenylyl cyclase in cardiac myocytes. J Biol Chem. 1997;272:19401–19407. doi: 10.1074/jbc.272.31.19401. [DOI] [PubMed] [Google Scholar]
- 17.Hulme JT, Yarov-Yarovoy V, Lin TW, Scheuer T, Catterall WA. Autoinhibitory control of the CaV1.2 channel by its proteolytically processed distal C-terminal domain. J Physiol. 2006;576:87–102. doi: 10.1113/jphysiol.2006.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu Y, et al. Deletion of the distal C terminus of CaV1.2 channels leads to loss of beta-adrenergic regulation and heart failure in vivo. J Biol Chem. 2011;286:12617–12626. doi: 10.1074/jbc.M110.175307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Jongh KS, Colvin AA, Wang KK, Catterall WA. Differential proteolysis of the full-length form of the L-type calcium channel alpha 1 subunit by calpain. J Neurochem. 1994;63:1558–1564. doi: 10.1046/j.1471-4159.1994.63041558.x. [DOI] [PubMed] [Google Scholar]
- 20.Rechsteiner M. PEST sequences are signals for rapid intracellular proteolysis. Semin Cell Biol. 1990;1:433–440. [PubMed] [Google Scholar]
- 21.Sanbe A, et al. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ Res. 2003;92:609–616. doi: 10.1161/01.RES.0000065442.64694.9F. [DOI] [PubMed] [Google Scholar]
- 22.Hambleton M, et al. Inducible and myocyte-specific inhibition of PKCalpha enhances cardiac contractility and protects against infarction-induced heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H3768–H3771. doi: 10.1152/ajpheart.00486.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He M, Bodi I, Mikala G, Schwartz A. Motif III S5 of L-type calcium channels is involved in the dihydropyridine binding site. A combined radioligand binding and electrophysiological study. J Biol Chem. 1997;272:2629–2633. doi: 10.1074/jbc.272.5.2629. [DOI] [PubMed] [Google Scholar]
- 24.Hockerman GH, et al. Construction of a high-affinity receptor site for dihydropyridine agonists and antagonists by single amino acid substitutions in a non-L-type Ca2+ channel. Proc Natl Acad Sci USA. 1997;94:14906–14911. doi: 10.1073/pnas.94.26.14906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valencik ML, McDonald JA. Codon optimization markedly improves doxycycline regulated gene expression in the mouse heart. Transgenic Res. 2001;10:269–275. doi: 10.1023/a:1016601928465. [DOI] [PubMed] [Google Scholar]
- 26.O’Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 27.Bramson HN, Kaiser ET, Mildvan AS. Mechanistic studies of cAMP-dependent protein kinase action. CRC Crit Rev Biochem. 1984;15:93–124. doi: 10.3109/10409238409102298. [DOI] [PubMed] [Google Scholar]
- 28.Kemp BE, Graves DJ, Benjamini E, Krebs EG. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J Biol Chem. 1977;252:4888–4894. [PubMed] [Google Scholar]
- 29.Moore MJ, Adams JA, Taylor SS. Structural basis for peptide binding in protein kinase A. Role of glutamic acid 203 and tyrosine 204 in the peptide-positioning loop. J Biol Chem. 2003;278:10613–10618. doi: 10.1074/jbc.M210807200. [DOI] [PubMed] [Google Scholar]
- 30.Songyang Z, et al. Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr Biol. 1994;4:973–982. doi: 10.1016/s0960-9822(00)00221-9. [DOI] [PubMed] [Google Scholar]
- 31.Neuberger G, Schneider G, Eisenhaber F. pkaPS: Prediction of protein kinase A phosphorylation sites with the simplified kinase-substrate binding model. Biol Direct. 2007;2:1. doi: 10.1186/1745-6150-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iakoucheva LM, et al. The importance of intrinsic disorder for protein phosphorylation. Nucleic Acids Res. 2004;32:1037–1049. doi: 10.1093/nar/gkh253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou FF, Xue Y, Chen GL, Yao X. GPS: A novel group-based phosphorylation predicting and scoring method. Biochem Biophys Res Commun. 2004;325:1443–1448. doi: 10.1016/j.bbrc.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 35.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blaich A, et al. Facilitation of murine cardiac L-type Ca(v)1.2 channel is modulated by calmodulin kinase II-dependent phosphorylation of S1512 and S1570. Proc Natl Acad Sci USA. 2010;107:10285–10289. doi: 10.1073/pnas.0914287107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dostmann WR, et al. Probing the cyclic nucleotide binding sites of cAMP-dependent protein kinases I and II with analogs of adenosine 3′,5′-cyclic phosphorothioates. J Biol Chem. 1990;265:10484–10491. [PubMed] [Google Scholar]
- 38.Fu Y, Westenbroek RE, Scheuer T, Catterall WA. Phosphorylation sites required for regulation of cardiac calcium channels in the fight-or-flight response. Proc Natl Acad Sci USA. 2013;110:19621–19626. doi: 10.1073/pnas.1319421110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang L, et al. Loss of β-adrenergic-stimulated phosphorylation of CaV1.2 channels on Ser1700 leads to heart failure. Proc Natl Acad Sci USA. 2016;113:E7976–E7985. doi: 10.1073/pnas.1617116113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domes K, et al. Truncation of murine CaV1.2 at Asp-1904 results in heart failure after birth. J Biol Chem. 2011;286:33863–33871. doi: 10.1074/jbc.M111.252312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerhardstein BL, et al. Proteolytic processing of the C terminus of the alpha(1C) subunit of L-type calcium channels and the role of a proline-rich domain in membrane tethering of proteolytic fragments. J Biol Chem. 2000;275:8556–8563. doi: 10.1074/jbc.275.12.8556. [DOI] [PubMed] [Google Scholar]