Significance
Aberrant inflammation is the root cause of numerous human diseases, from autoimmunity to cancer. microRNA-146a (miR-146a), a member of a large class of small regulatory RNAs, functions as a critical molecular brake on inflammation and malignant transformation. However, the molecular mechanism through which miR-146a exerts its regulatory activity in immune cells is unclear. Using mouse genetics, we have examined the role of the miR-146a–Traf6 signaling axis and found that although this miRNA–target interaction is responsible for regulating normal myeloid cell development and autoimmunity, it is dispensable for hematopoietic stem cell homeostasis and tumor suppression.
Keywords: microRNA, inflammation, miR-146a, Traf6, IFN-γ
Abstract
microRNA-146a (miR-146a) has been previously implicated as an essential molecular brake, preventing immune overreaction and malignant transformation by attenuating NF-κB signaling, putatively via repression of the Traf6 and Irak1 genes. The exact contribution of miR-146a–mediated silencing of these genes to the control of immune activation is currently unknown. Therefore, we defined the role of the miR-146a–Traf6 signaling axis in the regulation of immune homeostasis using a genetic epistasis analysis in miR-146a−/− mice. We have uncovered a surprising separation of functions at the level of miR-146a targets. Lowering the Traf6 gene dose and consequent attenuation of NF-κB activation rescued several significant miR-146a−/− phenotypes, such as splenomegaly, aberrant myeloproliferation, and excessive inflammatory responses. In contrast, decreasing Traf6 expression had no effect on the development of the progressive bone marrow failure phenotype, as well as lymphomagenesis in miR-146a−/− mice, indicating that miR-146a controls these biological processes through different molecular mechanisms.
Aberrant activation of immune cells is an underlying cause for numerous human pathologies, from systemic autoimmunity to cancer. microRNA-146a (miR-146a), a member of a large class of small-noncoding RNAs, has recently emerged as a critical immune regulator that helps prevent immune overreaction and malignant transformation (1). miR-146a is expressed primarily in cells of hematopoietic origin and is sharply up-regulated in response to microbial infection (2, 3). It functions as a negative feedback regulator of NF-κB signaling and, thus, mice that lack expression of this miRNA display exaggerated inflammatory responses to bacterial challenges and develop a systemic autoimmune disease over time (2, 4). Furthermore, dysregulation of miR-146a expression was implicated in the development of multiple autoimmune disorders in humans, including rheumatoid arthritis (5), systemic lupus erythematosus (6), and Sjogren’s syndrome (7).
miR-146a plays an important role in the regulation of hematopoietic stem cell (HSC) homeostasis and myelopoiesis (8). Abrogation of miR-146a expression in mice results in splenomegaly due to massive myeloproliferation (2). Furthermore, miR-146a is recurrently down-regulated in the bone marrow (BM) of patients with myelodysplastic syndrome (MDS) (9, 10), while miR-146a–deficient mice (miR-146a−/−) display an age-dependent, MDS-like HSC exhaustion and develop BM failure as they age (2).
Finally, miR-146a has a tumor-suppressor role and controls malignant transformation (2, 4, 11). Aged miR-146a−/− mice spontaneously develop tumors of myeloid and lymphoid origin. Moreover, accumulating evidence suggests that several types of human hematological and solid tumors display impaired miR-146a activity (12).
Thus, on balance, miR-146a is an essential regulator of immune cell activation, hematopoiesis, and cancer. Nevertheless, our understanding of the molecular mechanisms through which this miRNA elicits a wide range of biological activities remains poor. Studies performed in several laboratories have identified a gamut of miR-146a target genes in immune cells, including Cxcr4, Irak1, Traf6, Card10, Fadd, Relb, and Stat1 (13–15). The two most validated miR-146a targets from this list are Traf6 and Irak1, which encode adaptor proteins that function in a signaling hub that connects various immune receptors to the downstream signaling cascades, such as Jun amino-terminal kinase, p38 mitogen activated protein kinases (MAPK), extracellular signal-regulated kinase, and NF-κB activation pathways. The current molecular model suggests that miR-146a mediates its functions by attenuating Traf6 and Irak1 expression and subsequent dampening of NF-κB signal. However, this model has never been validated in vivo and, therefore, the physiological relevance of Traf6 or Irak1 gene silencing in the context of miR-146a–mediated regulation of immune functions is presently unknown.
To fill this knowledge gap, we performed a genetic epistasis analysis of the miR-146a–Traf6 signaling axis in mice. Our findings suggest that tight control of Traf6 expression is absolutely required for the miR-146a–mediated regulation of immune cell activation and myelopoiesis. Lowering the Traf6 gene dose in miR-146a−/− mice rescued aberrant myeloproliferation and autoimmunity phenotypes. In contrast, we found that the miR-146a–Traf6 regulatory axis is completely dispensable for the tumor-suppressive function of this miRNA and has little role in the maintenance of HSC homeostasis. Taken together, our results further elucidate the role that miR-146a plays in immune regulation and uncover the unexpected separation of function at the level of miR-146a targets that might explain the ability of this small regulatory RNA to control diverse immune functions.
Results
Deletion of One Traf6 Allele in miR-146a–Deficient Mice Reduced TRAF6 Protein Expression to Near Wild-Type Levels.
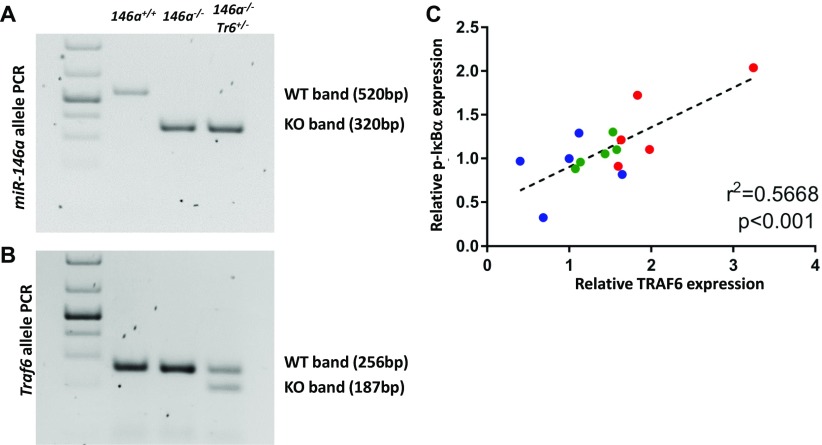
To define the role of Traf6 in mediating physiological functions of miR-146a, we performed a genetic epistasis analysis of the miR-146a–Traf6 signaling axis in mice. Because miR-146a deletion results in a modest Traf6 derepression (2, 16), we reasoned that lowering the Traf6 gene dose in miR-146a–deficient mice should reduce TRAF6 expression close to wild-type levels. To that end, we crossed miR-146a−/− (2) with mice carrying a targeted Traf6 deletion allele (Traf6+/−) (17). The genetic composition of the resulting miR-146a−/−Traf6+/− compound mutant mice was validated by genomic PCR analysis (Fig. S1 A and B).
Fig. S1.
Lowering the Traf6 gene dose attenuates TRAF6 expression and the downstream NF-κB signaling in miR-146a−/− mice. (A and B) Representative PCR confirming genotype of (A) miR-146a and (B) Traf6 alleles in genomic DNA extracted from miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6+/− mice. (C) Correlation of TRAF6 and phosphorylated IκBα protein levels from miR-146a+/+ (blue), miR-146a−/− (red), and miR-146a−/−Traf6+/− (green) splenocytes, (n = 5). Linear regression analysis and goodness of fit was determined using Prism 7 software, R2 = 0.5668.
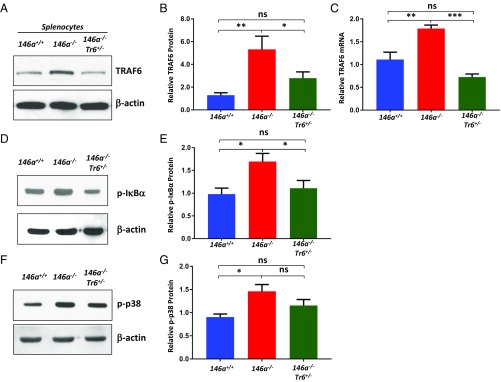
To examine how this genetic manipulation affected TRAF6 abundance, we compared the levels of TRAF6 protein in miR-146a+/+, miR-146a−/−, and miR-146a−/−Traf6+/− splenocytes by Western blot analysis. In agreement with the previously published results (2, 16), we found a significant increase (∼fivefold) in TRAF6 abundance in miR-146a−/− cells. In contrast, miR-146a−/−Traf6+/− splenocytes expressed TRAF6 at near wild-type level (Fig. 1 A and B). Analysis of Traf6 mRNA in miR-146a+/+, miR-146a−/−, and miR-146a−/−Traf6+/− splenocytes revealed a similar pattern (Fig. 1C). Furthermore, we determined how lowering the Traf6 gene dose impacts the activation of downstream signaling in miR-146a−/− cells. In agreement with previous reports (4, 16), we observed that abrogation of miR-146a activity results in the overactivation of the canonical NF-κB signaling cascade (Fig. 1 D and E). However, deletion of one Traf6 allele significantly attenuated the aberrant NF-κB signal in miR-146a−/− cells (Fig. 1 D and E and Fig. S1C). Western blot analysis of p38 MAPK activation in miR-146a−/−Traf6+/− splenocytes, another key signaling pathway that is triggered by TRAF6 (18), detected a trend for lower activity in comparison with miR-146a−/− cells (Fig. 1 F and G). Thus, collectively, our results strongly indicate that Traf6 haploinsufficiency may effectively restore the normal function of the miR-146a–Traf6 regulatory axis in the context of the miR-146a–null phenotype.
Fig. 1.
Lowering the Traf6 gene dose attenuates TRAF6 expression and the downstream NF-κB signaling in miR-146a−/− mice. (A) Western blot analysis of TRAF6 expression in miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6+/− splenocytes. (B) Quantification of TRAF6 protein expression in miR-146+/+ (n = 11), miR-146a−/− (n = 14), and miR-146a−/−Traf6+/− (n = 14) splenocytes. (C) Quantitative RT-PCR analysis of Traf6 mRNA levels in miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6+/− splenocytes (n = 5 per group). (D) Western blot analysis of phosphorylated IκBα in miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6+/− splenocytes. (E) Quantification of phosphorylated IκBα in miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6+/− splenocytes (n = 6). (F) Western blot analysis of phosphorylated p38 MAPK in miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6+/− splenocytes. (G) Quantification of phosphorylated p38 MAPK in miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6+/− splenocytes (n = 6 per group). TRAF6, phospho-IκBα, and phospho-p38 values were obtained from scanned X-ray films using ImageJ software. Each sample was first normalized to β-actin that was used as loading control. Data are shown as mean ± SEM. P values were calculated using a standard one-way ANOVA test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ns, not significant.
Lowering the Traf6 Gene Dose Rescued Aberrant Myeloproliferation in miR-146a−/− Mice.
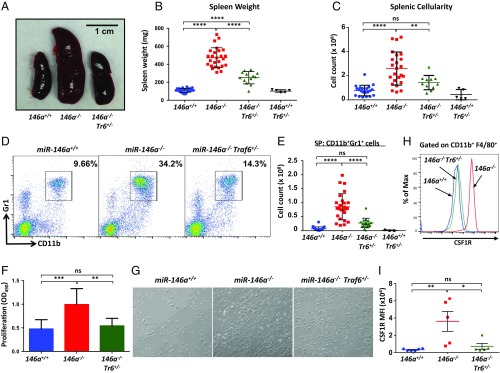
One of the most profound phenotypic changes in miR-146a−/− mice is a myeloproliferative disorder that is grossly manifested by a progressive splenomegaly. To investigate how lowering the Traf6 gene dose impacts aberrant myeloproliferation in miR-146a−/− mice, we performed a morphological analysis of aged (8- to 10-mo-old) cohorts of female miR-146a+/+, miR-146a−/−, and miR-146a−/−Traf6+/− mice. As expected, we observed a significant splenomegaly (∼fourfold increase in spleen weight) in miR-146a−/− animals (Fig. 2 A and B), while miR-146a−/−Traf6+/− mice displayed a partial rescue of this defect (∼twofold spleen weight increase in comparison with the miR-146a+/+ group) (Fig. 2 A and B). Furthermore, we also found a marked decrease in the total number of cells in miR-146a−/−Traf6+/− spleen in comparison with miR-146a+/+ organs (Fig. 2C). Of note, the spleen weight and cellularity in the miR-146a+/+Traf6+/− cohort were indistinguishable from miR-146a+/+ animals (Fig. 2 A–C).
Fig. 2.
miR-146a–dependent attenuation of Traf6 is required for control of myeloproliferation. (A–C) Rescue of the splenomegaly defect in miR-146a−/−Traf6+/− mice. (A) Photograph of spleens from miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6+/− mice. Spleen weight (B) and cellularity (C) in 9- to 10-mo-old female miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6+/− mice (n ≥ 5 per group). (D and E) Rescue of the myeloproliferation phenotype in miR-146a−/−Traf6+/− mice. (D) Flow cytometry analysis of splenocytes from miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6+/− mice using antibodies against CD11b and Gr1. Immature myeloid cells (CD11b+Gr1+) are gated, and numbers indicate the percentage of cells in the gate. (E) Total number of CD11b+Gr1+ cells in spleens of 9- to 10-mo-old female miR-146+/+, miR-146a−/−, miR-146a−/−Traf6+/−, and miR-146+/+Traf6+/− mice (n ≥ 16 per group). (F and G) Deletion of one Traf6 allele attenuates proliferation capacity of miR-146a−/− macrophages. (F) Quantification of BMDM proliferation. Macrophages were derived from miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6+/− BM in the presence of 50 ng/mL M-CSF for 3 d before their proliferation ability was measured using the MTS assay. Data were combined from three independent experiments (n ≥ 7 per group). (G) Representative pictures of miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6+/− BMDM cultures. (Magnification: 40×.) (H) Flow cytometry analysis of CSF1R expression on miR-146+/+ (blue line), miR-146a−/− (red line), and miR-146a−/−Traf6+/− (green line) macrophages. BMDMs were derived as described in F. Cells were pregated for the expression of CD11b and F4/80 markers first. (I) Quantification of cell surface CSF1R expression on miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6+/− BMDMs (n ≥ 5 per group). MFI, mean fluorescent intensity. Data are shown as mean ± SD. P values were calculated using a standard one-way ANOVA test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001; ns, not significant; SP, spleen.
Using flow cytometry, we quantified the accumulation of immature myeloid cells in the spleens of aged mice and determined that lowering the Traf6 gene dose reduces the frequency and absolute number of CD11b+Gr1+ myeloid cells to near wild-type levels, suggesting a clear rescue of this miR-146a−/− phenotype (Fig. 2 D and E).
To further characterize the role of the miR-146a–Traf6 signaling axis in the regulation of myeloid development, we generated BM-derived macrophages (BMDMs) from the three strains of mice and assessed their proliferative potential in vitro. Consistent with our previous findings (2), miR-146a−/− BMDMs displayed a clear proliferative advantage over wild-type BMDMs after 3 d in culture (Fig. 2 F and G). On the other hand, miR-146a−/−Traf6+/− macrophages divided much slower and resembled wild-type cells in their proliferation capacity (Fig. 2 F and G). We have previously linked enhanced proliferation of miR-146a−/− BMDMs to an increase in colony stimulating factor receptor 1 (CSF1R) expression (2). In agreement with this notion, we found that the cell surface levels of CSF1R on miR-146a−/−Traf6+/− macrophages were significantly down-regulated in comparison with miR-146a−/− cells (Fig. 2 H and I).
miR-146a–Mediated Control of Traf6 Expression Is Required for the Attenuation of Proinflammatory Cytokine Responses, Control of T Cell Tolerance, and Prevention of Autoimmunity.
Previous studies have firmly established miR-146a as a negative regulator of inflammation and autoimmunity (2, 13, 16). Because of its critical role in the activation of NF-κB signaling, Traf6 has long been proposed as a key molecular target through which miR-146a controls immune responses. However, formal in vivo evidence supporting this notion is missing. To determine the role of the miR-146a–Traf6 axis in the regulation of immune responses, we characterized several aspects of innate and adaptive immunity in miR-146a−/−Traf6+/− mice.
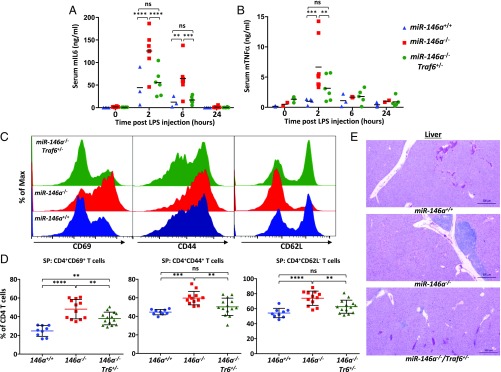
First, we examined how the compound mutant mice respond to a systemic bacterial endotoxin challenge. Age- and gender-matched cohorts of miR-146a+/+, miR-146a−/−, and miR-146a−/−Traf6+/− mice were challenged with a sublethal dose of bacterial lipopolysaccharides (LPS) and levels of proinflammatory cytokines in the serum were measured by ELISA. In agreement with previously published results (2), miR-146a−/− mice mounted an exaggerated proinflammatory cytokine response following LPS injection, manifested by a significantly elevated production of IL-6 and TNF-α proteins in comparison with wild-type animals (Fig. 3 A and B). On the other hand, miR-146a−/−Traf6+/− mice displayed a marked reduction in the production of these cytokines to near wild-type levels (Fig. 3 A and B), suggesting that miR-146a indeed controls Toll-like receptor (TLR)-mediated inflammatory responses by attenuating TRAF6 protein levels.
Fig. 3.
Lowering the Traf6 gene dose rescues aberrant inflammation phenotype in miR-146a–null mice. (A and B) Normal cytokine response to bacterial endotoxin challenge in miR-146a−/−Traf6+/− mice. Serum levels of IL-6 (A) and TNF-α (B) cytokines in miR-146+/+ (n ≥ 3), miR-146a−/− (n = 6), and miR-146a−/−Traf6+/− (n = 6) mice challenged intraperitoneally with a sublethal LPS (1 mg/kg) dose. Peripheral blood was collected at the indicated time points and cytokine concentrations were analyzed in the serum by sandwich ELISA. All mice were female and aged 3–4 mo. (C and D) Traf6 haploinsufficiency reverts the hyperactivated state of peripheral miR-146a−/− T cells. (C) FACS analysis of CD69, CD44, and CD62L activation markers on CD4+ T cells isolated from miR-146+/+ (blue plots), miR-146a−/− (red plots), and miR-146a−/−Traf6+/− (green plots) spleens. (D) Quantification of CD69, CD44, and CD62L expression levels on CD4+ T cells isolated from miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6+/− spleens (n > 9 animals for all genotypes, female mice aged 9–10 mo). Values are reported as percent of CD4+ T cells. (E) Lowering the Traf6 gene dose in miR-146a−/− mice decreases lymphohistiocytic infiltrates in liver. Representative H&E-stained sections from 9- to 10-mo-old miR-146+/+, miR-146a−/−, miR-146a−/−Traf6+/− mice. Data are shown as mean ± SD. P values were calculated using either standard one-way or two-way ANOVA tests. **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001; ns, not significant; SP, spleen. (Magnification: 20×.)
Abrogation of miR-146a expression was reported to result in the loss of peripheral T cell tolerance and subsequent development of the autoimmune disease (2). Analysis of the peripheral T cells in miR-146a−/− mice by flow cytometry revealed that the majority of splenic CD4+ T cells displayed an activated effector status as characterized by the loss of L-Selectin (CD62L), and a gain of PGP-1 (CD44) and CD69 expression (Fig. 3 C and D). In contrast, examination of the T cell populations in miR-146a−/−Traf6+/− mice found that lowering the Traf6 gene dose rescues this defect and leads to a significant reduction in the amount of the activated effector T cells (Fig. 3 C and D). Furthermore, histological analysis of liver sections from miR-146a−/−Traf6+/− mice revealed reduced lymphohistiocytic infiltrates compared with miR-146a−/− mice (Fig. 3E). Collectively, our results suggest that the miR-146a–Traf6 signaling axis plays a crucial role in maintaining the homeostasis of the immune system and preventing development of autoimmunity.
The miR-146a–Traf6 Regulatory Axis Is Dispensable for Tumor Suppression.
miR-146a was previously implicated in the regulation of tumorigenesis (2, 4); however, our molecular understanding of its tumor-suppressor activity is incomplete. Since chronic inflammation often promotes malignant transformation and because our findings suggest that the miR-146a–Traf6 signaling axis is essential for the regulation of inflammatory responses, we investigated the role of Traf6 in mediating the tumor-suppressor activity of miR-146a. Aged miR-146a−/− mice develop spontaneous tumors of both lymphoid and myeloid origin, however with relatively low penetrance and long latency (2, 4). Thus, to make our analysis of tumorigenesis in miR-146a−/− mice more robust, we chose to take advantage of the Eμ-myc transgenic mouse model. Eμ-myc transgenic mice carry a c-myc oncogene driven by the Ig heavy-chain enhancer and invariably develop a mix of pre-B and mature B cell lymphomas with a latency of about 24 wk (19). The protracted latent period before the onset of frank disease in this model likely reflects the inability of c-myc overexpression to initiate malignant transformation on its own, requiring cooperation from additional genetic lesions (20). Many aspects of the lymphomas that Eμ-myc mice develop resemble known features of human disease, including progression through a distinct premalignant phase (21).
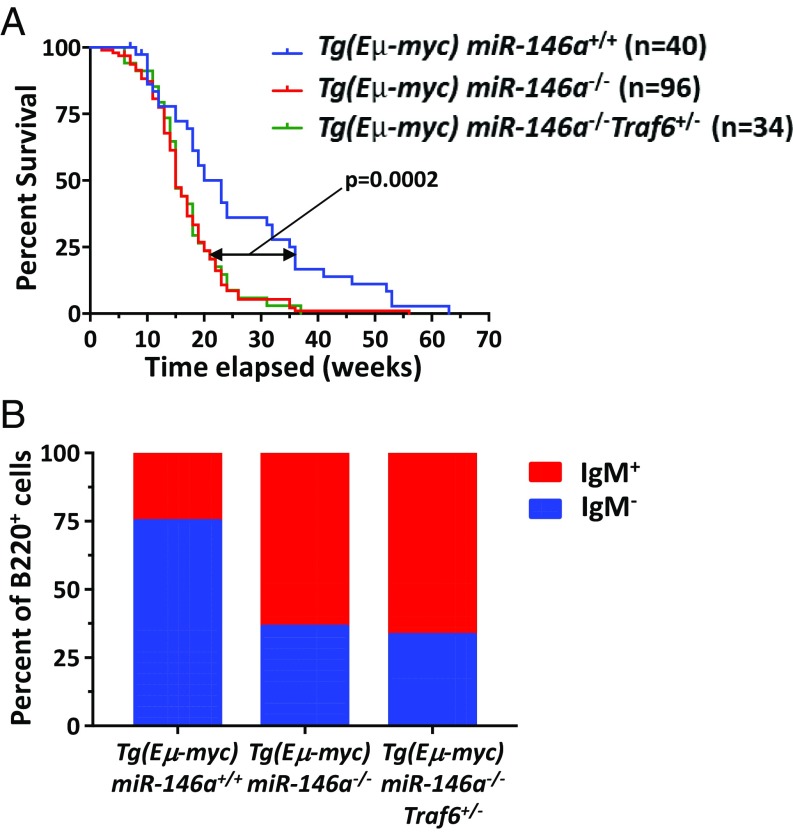
We bred Eμ-myc transgenic mice onto both the miR-146a−/− and miR-146a−/−Traf6+/− backgrounds and generated Tg(Eμ-myc);miR-146a−/− and Tg(Eμ-myc);miR-146a−/−Traf6+/−compound mutant mice. To determine whether miR-146a abrogation affects disease onset and mortality, we aged large cohorts of the compound mutant mice and monitored them for the appearance of lymph node tumors as well as signs of systemic illness. We found that Tg(Eμ-myc);miR-146a−/− mice displayed a significant increase in mortality in comparison with control Eμ-myc transgenic animals (the median time of survival dropped from 24 wk down to 15 wk) (Fig. 4A). Moreover, immunophenotyping of miR-146a–deficient and miR-146a–proficient lymphomas revealed a change in the maturation status of malignant B cells: Tg(Eμ-myc);miR-146a−/− tumors were derived predominantly from immature B cells (B220+IgM+), while the majority of tumors in Tg(Eμ-myc) mice originated from the early B cell precursors (B220+IgM−) (Fig. 4B). These observations are in good agreement with the recent findings by Contreras et al. (11), indicating that miR-146a loss accelerates MYC-driven lymphoma development.
Fig. 4.
miR-146a does not require Traf6 to exert its tumor-suppressive function. (A) Survival of female Tg(Eμ-Myc);miR-146a+/+ (n = 40), Tg(Eμ-Myc);miR-146a−/− (n = 96), and Tg(Eμ-Myc);miR-146a−/−Traf6+/− (n = 34) mice. Animals were monitored daily and euthanized once the tumor became palpable or the animals appeared to be in distress as per standard IACUC guidelines for care of tumor-bearing mice. The survival curve comparison was calculated using a Mantel–Cox (log rank) test, P = 0.0002. (B) FACS analysis of tumors from female Tg(Eμ-Myc);miR-146a+/+ (n = 9), Tg(Eμ-Myc);miR-146a−/− (n = 14), and Tg(Eμ-Myc);miR-146a−/−Traf6+/− (n = 11) mice using anti-B220 and anti-IgM antibodies. Cells were pregated for the expression of CD19 first. The IgM marker defines B cell development stage: early B cell precursors were identified as CD19+B220+IgM−, and immature B cells were identified as CD19+B220+IgM+.
Interestingly, lowering the Traf6 gene dose in Tg(Eμ-myc);miR-146a−/− mice did not rescue this phenotype, because Tg(Eμ-myc);miR-146a−/−Traf6+/− and Tg(Eμ-myc);miR-146a−/− mice were found to display identical mortality rates, as well as similar makeup of B cell lymphomas (Fig. 4). Together, our findings suggest that miR-146a–mediated control of Traf6 expression and downstream inflammatory responses is dispensable for the tumor-suppressor activity of this miRNA, indicating that miR-146a must engage other, perhaps not yet identified, molecular targets to exert its regulatory effect on malignant transformation.
Lowering the Traf6 Gene Dose Does Not Prevent HSC Depletion and Development of BM Failure in miR-146a−/− Mice.
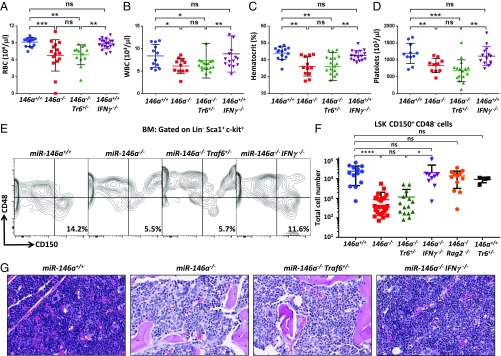
One of the more profound defects found in mice lacking a functional miR-146a gene is the progressive impairment of hematopoiesis. By 8 mo of age, miR-146a−/− mice display severe anemia, leukopenia, and thrombocytopenia (Fig. 5 A–D) that are consistent with the BM failure typically found in MDS or aplastic anemia (AA) (2, 10). This progressive pancytopenia is a consequence of the destruction of the normal HSC niche (10). Using the Signaling Lymphocyte Activation Molecule (SLAM) system of defining undifferentiated stem and progenitor cells, we confirmed the findings by Zhao et al. (10) that aged miR-146a−/− mice have a drastically reduced population of long-term HSCs (LT-HSCs) in the BM (Fig. 5 E and F). Furthermore, we also observed that BM architecture in aged miR-146a–deficient mice undergoes significant remodeling characterized by marked hypocellularity and morphologic features consistent with myelofibrosis (Fig. 5G). Surprisingly, in contrast to the above-described analyses of the myeloproliferation and autoimmunity defects, we found that Traf6 haploinsufficiency in miR-146a−/− mice had no impact on the development of the BM failure phenotype. Aged miR-146a−/−Traf6+/− mice have significantly diminished numbers of LT-HSCs in the BM (Fig. 5 E and F) and, consequently, display no rescue of the pancytopenia that is characteristic for miR-146a–deficient mice (Fig. 5 A–D). Thus, our findings argue that miR-146a most likely uses a Traf6-independent molecular mechanism for the regulation of HSC homeostasis.
Fig. 5.
BM failure in miR-146a−/− mice is rescued by IFN-γ ablation, but not by lowering the Traf6 gene dose. (A–D) Complete blood count tests of terminal blood collected from 9- to 10-mo-old female miR-146+/+, miR-146a−/−, miR-146a−/−Traf6+/−, and miR-146a−/−IFN-γ−/− mice (n ≥ 10 for each group). (A) Absolute red blood counts. (B) Absolute white blood counts (WBC). (C) Blood hematocrit. (D) Absolute platelet counts. (E and F) Analysis of LT-HSC cells from the BM of 9- to 10-mo-old female miR-146+/+, miR-146a−/−, miR-146a−/−Traf6+/−, and miR-146a−/−IFNγ−/− mice. Lineage depleted cells (Lin−) from BM were analyzed by FACS using anti–c-kit, -Sca1, -CD150, -CD48 antibodies. (E) Representative FACS analysis using the SLAM markers to identify the LT-HSC population as Lin−c-kit+Sca1+CD150+CD48−. Numbers indicate frequency of LT-HSCs. (F) Quantification of LT-HSCs using the SLAM marker system in 9- to 10-mo-old miR-146+/+, miR-146a−/−, miR-146a−/−Traf6+/−, miR-146a−/−IFNγ−/−, miR-146a−/−Rag2−/−, and miR-146a+/+Traf6+/− BM (n ≥ 13 for all groups except miR-146a+/+Traf6+/− n = 4). (G) Representative H&E-stained sections of tibias from 9- to 10-mo-old female miR-146+/+, miR-146a−/−, miR-146a−/−Traf6+/−, and miR-146a−/−IFN-γ−/− mice. Note that BM in miR-146a−/− and miR-146a−/−Traf6+/− mice is markedly hypocellular, whereas miR-146a−/−IFN-γ−/− BM appears closer to normal and is only minimally hypocellular. Data are shown as mean ± SD. P values were calculated using standard one-way ANOVA tests. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001; ns, not significant. (Magnification: 40×.)
Excessive IFN-γ Production by BM-Resident T Cells Destroys HSC Niche in miR-146a−/− Mice.
IFN-γ has a well-documented role in suppressing HSC maintenance and self-renewal (22, 23). Moreover, AA patients (who develop BM failure because of HSC exhaustion) frequently display elevated levels of IFN-γ in the peripheral blood, as well as impaired colony-forming potential of BM cells, which can be restored by treatment with IFN-γ neutralizing antibodies (24). Interestingly, miR-146a–deficient T cells are known to produce aberrantly high levels of IFN-γ that were shown to contribute to the loss of immunological tolerance (13, 16). These findings prompted us to hypothesize that dysregulation of IFN-γ production mediates HSC destruction in miR-146a−/− mice. To probe this notion genetically, we cross-bred IFN-γ−/− mice (25) with miR-146a−/− mice and generated miR-146a−/−IFN-γ−/− double-knockout mice. Our analysis of aged miR-146a−/−IFN-γ−/− mice revealed normal peripheral blood cell counts (Fig. 5 A–D), suggesting that IFN-γ ablation can rescue the BM failure defect caused by miR-146a deficiency. In agreement with this conclusion, we also found that the number of LT-HSCs in miR-146a−/−IFN-γ−/− BM rebounded to near wild-type level (Fig. 5 E and F), while histological analysis of the double-knockout BM revealed partial rescue of the hypocellulartity and apparent fibrosis (Fig. 5G). Depletion of B and T lymphocytes via Rag2 deletion also prevented LT-HSC destruction in miR-146a−/− BM (Fig. 5F), pointing to an HSC-extrinsic nature of this defect.
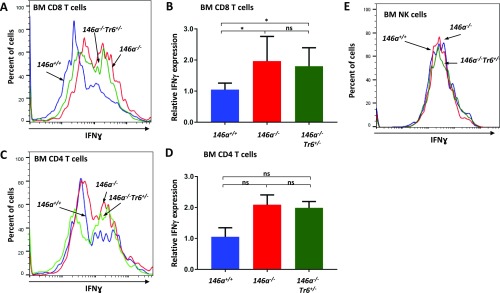
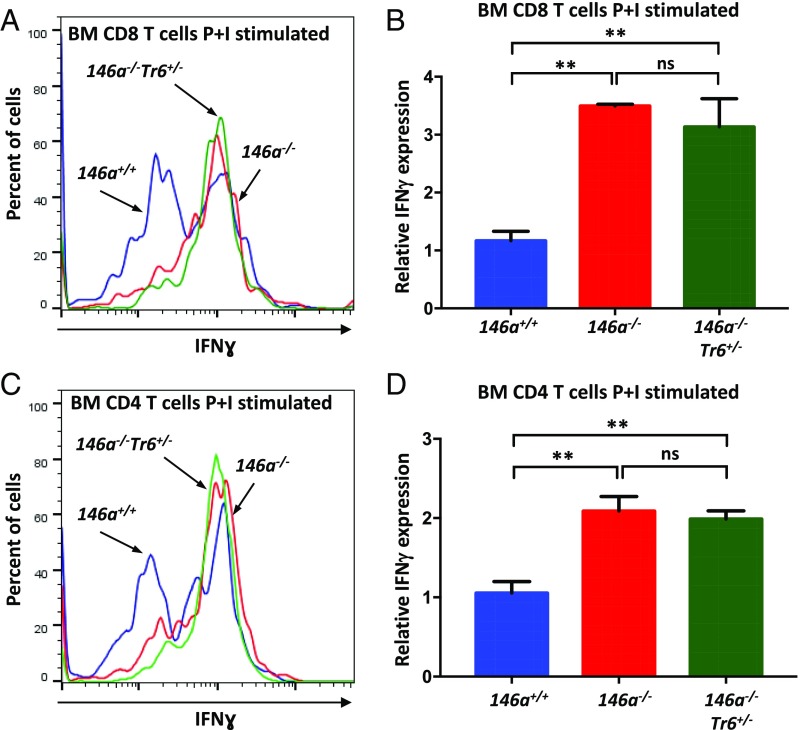
Since activated T cells are considered the main source of IFN-γ in the body, we examined production of this proinflammatory cytokine by BM-resident miR-146a−/− T cells. Of note, CD3+ T cells were previously reported to constitute 1–5% of all mononuclear cells in the mouse BM (26). Using a flow cytometry protocol for intracellular staining, we detected slightly elevated basal level of IFN-γ production in CD8+ T cells isolated from miR-146a−/− BM (Fig. S2 A and B). IFN-γ expression in BM-resident miR-146a−/− CD4+ T cells followed the same trend, but the difference was not statistically significant (Fig. S2 C and D). However, stimulation with phorbol 12-myristate 13-acetate and ionomycin confirmed that both CD8+ and CD4+ T cells from miR-146a−/− BM have a much greater IFN-γ productive potential than wild-type cells (Fig. 6). Interestingly, we found that IFN-γ overproduction by BM-resident miR-146a−/− T cells was largely refractory to the lowering of the Traf6 gene dose (Fig. 6 and Fig. S2 A–D). The exaggerated production of IFN-γ in response to stimulation was also observed in miR-146–deficient T cells isolated from the spleen, although the defect was slightly less pronounced than in BM-resident T cells (Fig. S3). Natural killer (NK) cells are another known source of IFN-γ in the body; however, our analysis revealed that IFN-γ secretion by NK cells from miR-146a−/− BM is comparable to wild-type cells (Fig. S2E). Thus, our experiments suggest that Traf6-independent dysregulation of IFN-γ expression in BM-resident T cells is responsible for the destruction of the HSC niche in miR-146a−/− mice.
Fig. S2.
IFN-γ expression in BM-resident lymphocytes. FACS analysis of IFN-γ production by CD8+ T (A and B), CD4+ T (C and D), and NK (E) cells isolated from miR-146+/+, miR-146a−/−, miR-146a−/−Traf6+/− BM. BM cells harvested from 8-wk-old female mice were analyzed by flow cytometry for intracellular IFN-γ expression. Representative FACS plots showing IFN-γ expression in CD8+ T (A), CD4+ T (C), and NK (E) cells from miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6−/+ BM. Quantification of intracellular IFN-γ expression in stimulated CD8+ (B) and CD4+ (D) T cells from miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6+/− BM. IFN-γ MFI values were calculated using the FlowJo X software and then were normalized to miR-146+/+ samples. Data are representative of at least three different experiments with n ≥ 3 for each genotype. Data are shown as mean ± SD. P values were calculated using standard one-way ANOVA tests. *P ≤ 0.05; ns, not significant.
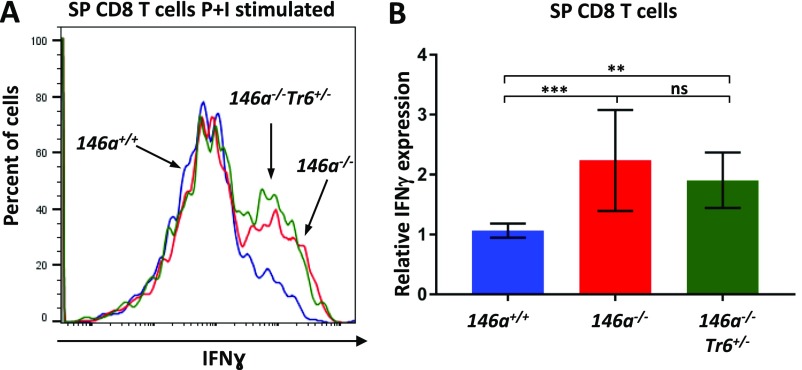
Fig. 6.
BM-resident T cells are the main producers of aberrant IFN-γ driving the HSC destruction in miR-146a−/− mice. FACS analysis of IFN-γ production by CD8+ (A and B) and CD4+ (C and D) T cells isolated from miR-146+/+, miR-146a−/−, miR-146a−/−Traf6+/− BM. BM cells harvested from 8-wk-old female mice were stimulated with phorbol 12-myristate 13-acetate and ionomycin (P+I) for 4 h and analyzed by flow cytometry for intracellular IFN-γ expression. Representative FACS plots showing IFN-γ expression in stimulated CD8+ (A) and CD4+ (C) T cells from miR-146+/+ (blue line), miR-146a−/− (red line), and miR-146a−/−Traf6+/− (green line) BM. Quantification of intracellular IFN-γ expression in stimulated CD8+ (B) and CD4+ (D) T cells from miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6+/− BM. IFN-γ MFI values were calculated using the FlowJo X software and then were normalized to miR-146+/+ samples. Data are representative of at least three different experiments with n ≥ 3 for each genotype. Data are shown as mean ± SD. P values were calculated using standard one-way ANOVA tests. **P ≤ 0.01; ns, not significant.
Fig. S3.
IFN-γ expression in stimulated splenic T lymphocytes. FACS analysis of IFN-γ production by CD8+ T cells isolated from miR-146+/+, miR-146a−/−, miR-146a−/−Traf6+/− spleens. Splenocytes harvested from 12- to 14-wk-old female mice were stimulated with phorbol 12-myristate 13-acetate and ionomycin for 4 h and analyzed by flow cytometry for intracellular IFN-γ expression. Representative FACS plots showing IFN-γ expression in CD8+ T (A) cells from miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6−/+ spleens. (B) Quantification of intracellular IFN-γ expression in stimulated CD8+ T cells from miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6+/− spleens. IFN-γ MFI values were calculated using the FlowJo X software and then were normalized to miR-146+/+ samples. Data are representative of at least two different experiments with n ≥ 3 for each genotype. Data are shown as mean ± SD. P values were calculated using standard one-way ANOVA tests. **P ≤ 0.01; ***P ≤ 0.001; ns, not significant; P+I, phorbol 12-myristate 13-acetate and ionomycin.
Discussion
Previous work by us and other investigators has established miR-146a as an essential immune regulator that controls activation and malignant transformation of immune cells (2, 13, 16). However, the exact molecular mechanism through which miR-146a exerts its numerous regulatory activities is unclear. In the present study using genetic epistasis analysis, we have examined the role of Traf6, one of the most validated miR-146a targets, in mediating some of the known physiological functions of this miRNA. Our findings uncovered an unexpected separation of function at the level of miR-146a target genes. On the one hand, lowering of the Traf6 gene dose effectively rescued the myeloproliferative and autoimmune phenotypes in miR-146a–deficient mice. In contrast, the Traf6 halploinsufficiency had little effect on the progressive HSC destruction and subsequent development of BM failure in miR-146a–null mice. Furthermore, we found that attenuation of Traf6 expression by miR-146a is largely dispensable for the tumor-suppressive activity of this miRNA in B lymphocytes.
TRAF6 is a versatile adapter protein with a K63-linked ubiquitin ligase activity that triggers NF-κB and MAPK signaling downstream of a multitude of immune receptors (27). As evidenced by the overexpression studies, TRAF6 abundance has to be tightly regulated to prevent aberrant NF-κB signaling (28, 29). Hence, miR-146a, which binds to three evolutionary conserved 8-mer “seed” sequences in the Traf6 3′UTR (3), has evolved as a crucial molecular brake on Traf6 expression and subsequent NF-κB activation. Because NF-κB signaling plays an essential role in the initiation and execution of immune responses, Traf6 is often viewed as a key miR-146a target, through which miR-146a mediates most, if not all, of its immunomodulatory activities. However, our epistasis analysis in miR-146a–deficient mice challenges this notion and suggests that miR-146a engages additional molecular targets to mediate its biological functions.
Confirming the importance of the miR-146a–Traf6 regulatory axis, our findings implicate Traf6 derepression and subsequent NF-κB overactivation as the main drivers of aberrant myeloproliferation and autoimmunity in miR-146a−/− mice. This conclusion is in good agreement with the known role of the NF-κB signaling pathway in myeloid cell differentiation and proinflammatory signaling (30). For example, ablation of IκBα, a critical NF-κB pathway inhibitor, was previously shown to result in aberrant granulocytopoiesis because of constitutive NF-κB activation (30–32). Additionally, lowering of the Nfkb1 gene dose in miR-146a–null mice was reported to partially rescue the splenomegaly and abnormal accumulation of CD11b+Gr1+ myeloid cells (10).
Constitutive NF-κB activation is a hallmark of many hematopoietic and solid tumors (33–37). Therefore, the significant susceptibility of aged miR-146a–deficient mice to cancer was often considered in the context of the TRAF6–NF-κB signaling axis dysregulation. However, our genetic dissection of the tumor-suppressor activity of miR-146a in B cells does not support this hypothesis. Lowering of the Traf6 gene dose had no effect on the accelerated tumor development in the Eμ-myc lymphoma mouse model, despite a noticeable effect of Traf6 haploinsufficiency on aberrant splenic myeloproliferation, proinflammatory cytokine production, and hepatic immune cell infiltrates in miR-146a−/− mice. Thus, it appears that miR-146a engages a different molecular target, and not Traf6, to control malignant transformation of B cells. This conclusion is supported by the findings of Contreras et al. (11), who also observed an accelerated B cell lymphomagenesis in the Eμ-myc transgenic mice upon miR-146a deletion, but found no major change in NF-κB–dependent signaling in miR-146a–deficient tumors. Instead, Contreras et al. proposed that miR-146a suppresses tumorigenesis by downregulating expression of EGR1, a transcription factor associated with cellular proliferation and survival. However, Egr1 is unlikely to function as a direct miR-146a molecular target in mouse cells, because the miR-146a seed sequence in the mouse Egr1 3′UTR is not conserved. Syk, a critical signaling mediator downstream of B cell antigen receptor, is another potential target through which miR-146a may impact B cell proliferation. Abrogation of miR-146a–mediated repression of SYK was recently implicated as a major driving factor in Hoxa9/Meis1-induced mouse model of myeloid leukemogenesis (38). Although this exact target interaction may not be applicable to human leukemias because of the lack of any miR-146a binding sites in the human Syk 3′UTR, it is illustrative of the potential role of miR-146a dysfunction in driving a variety of human cancers. The list of bioinformatically defined miR-146a targets is relatively rich in genes that are known to contribute to the regulation of cellular growth and, thus, future genetic experiments will be required to identify the key molecular targets through which miR-146a exerts its tumor-suppressor activity in different immune cells.
As in the case of tumor suppression, the miR-146a–mediated regulation of HSC homeostasis is apparently not dependent on its ability to downmodulate Traf6 expression. We found no rescue of the progressive BM failure phenotype in miR-146a−/− mice with Traf6 haploinsufficiency. In contrast, our genetic analysis implicated IFN-γ as a critical driver of the HSC destruction and resulting pancytopenia in miR-146a−/− mice. The strong negative effect of chronic IFN-γ production on HSC maintenance is a well-established notion (22, 23, 39). Our data reveal that resident T cells are the most likely source of the aberrant IFN-γ in miR-146a−/− BM. This finding is in line with our previous observations that CD4+ T cells in miR-146a−/− mice are skewed toward Th1 phenotype and secrete excessive amounts of IFN-γ upon activation (13). Furthermore, the role of miR-146a–deficient T cells in the progressive BM failure phenotype is supported by the partial rescue of this defect in miR-146a−/− mice with a severe depletion of mature lymphocytes because of either Rag2 or Rag1 deletion [Fig. 5F and data from Zhao et al. (10), respectively].
Presumably, IFN-γ is not the only mediator of inflammation that contributes to the destruction of the HSC niche in miR-146a−/− BM. Ablation of Il-6, a proinflammatory cytokine also found to be overproduced in miR-146a–deficient BM, was shown to rescue the progressive BM failure phenotype in miR-146a−/− mice (10). The primary cellular targets and the hierarchical framework between these two cytokines in HSC homeostasis are not yet clear. Interestingly however, IFN-γ−mediated stimulation of the HSC niche in response to an acute viral infection was dependent on IL-6 production by mesenchymal stromal cells in the BM (40). Cytotoxic T cells exposed to a viral antigen apparently secrete IFN-γ to promote IL-6 production by mesenchymal stromal cells that in its turn stimulates multipotent hematopoietic progenitors in the BM and triggers emergency myelopoiesis. Collectively, our observations strongly suggest that the progressive BM failure in miR-146a−/− mice is an HSC-extrinsic defect closely resembling the pathophysiological changes during AA. Similar to the situation with miR-146a−/− mice, AA is associated with an expansion of autoreactive T cells and, hence, is responsive to immunosuppressive therapies. AA patients exhibit increased levels of circulating IFN-γ, while blockade of this cytokine significantly improves hematopoietic colony-forming activity of the BM cells from afflicted subjects (24). Moreover, a genetic polymorphism that enhances IFN-γ production is strongly associated with the risk of developing AA (41).
Our inference about the nonessential role of Traf6 as a miR-146a target in the progressive BM failure and lymphoma phenotypes has one potential caveat. It is certainly conceivable that lowering the Traf6 gene dose did not sufficiently reduce expression of this adapter protein in all relevant miR-146a−/− cell types, thus explaining the lack of rescue of some defects in miR-146a−/−Traf6+/− mice. However, in the instance of progressive BM failure, this argument is not very compelling, because our data indicate that HSC destruction in miR-146a−/− mice is driven by aberrantly activated T cells; therefore, it is not obvious why Traf6 haploinsufficiency, while having a profound effect on other T cell-dependent defects in miR-146a−/− mice, would have little impact on BM failure. Of note, our conclusion on the role of Traf6 runs contrary to some observations that implicated this adapter molecule in the regulation of HSC homeostasis and the pathophysiology of MDS (10, 41, 42).
The identity of the molecular target that miR-146a engages in T cells to regulate IFN-γ production is currently unknown. One putative candidate is Irak1, because of its ability to trigger activation of the inflammasome and subsequent IL-18 production (43, 44). IL-18 is known to stimulate IFN-γ secretion and Irak1-deficient memory CD8+ T cells are severely compromised in IFN-γ production. Alternatively, miR-146a could target Stat1 to control IFN-γ expression. As was previously reported, effector CD4+ T cells from miR-146a−/−Stat1+/− mice display significantly attenuated levels of IFN-γ production (13).
Collectively, our genetic analysis suggests that separation of function at the level of miR-146a can explain its capacity to regulate diverse immunological processes (Fig. 7). This mode of regulation is not unique to miR-146a and is apparently used by other immunological miRNAs with pleotropic functions. For example, miR-155 can interfere with Th2 differentiation by modulating levels of c-Maf (45), regulate plasma cell differentiation through PU.1 (46), and control generation and function of follicular helper T cells by targeting Peli1 (47). In addition, the miR-142–BAFF-R signaling axis was implicated in the control of peripheral B cell proliferation, but was found to be dispensable for the regulation of B cell effector responses (48). As the understanding and awareness of specialized immune cell subsets increases, it is crucial to precisely explicate what mechanisms control and engage their unique gene-expression programs. Opportunities for more precise targeted interventions will arise if there are miRNA-target gene pairings that can be identified as interacting only in a particular cell type or under a certain gene expression program. Once better understood, this cell- and context-specific regulation of cognate targets by miRNAs can be effectively exploited to generate novel therapeutic approaches to treat various immune-related diseases and cancer.
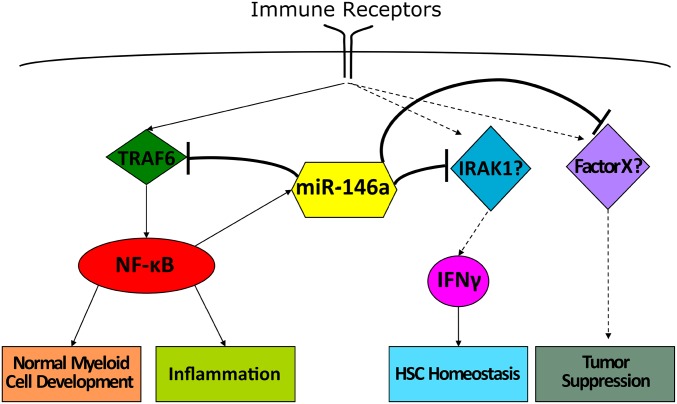
Fig. 7.
Separation of function at the level of molecular targets allows miR-146a to control multiple immunological functions. Graphical model describing molecular mechanisms by which miR-146a regulates inflammation, myeloproliferation, HSC homeostasis, and cancer.
Materials and Methods
Mice.
Mice with a homozygous deletion of the miR-146 gene were generated as previously described (2). Wild-type littermates generated during the initial creation of the miR-146a knockout mice were used as wild-type controls (miR-146a+/+). miR-146a−/− mice were mated to mice bearing a heterozygous deletion of Traf6 (17) to create mice with the miR-146a−/−Traf6+/− genotype. To create the miR-146a−/−IFN-γ−/− double-knockout mouse, miR-146a−/− mice were mated to mice with a homozygous deletion of IFN-γ (obtained from the Jackson Laboratories) (25).
Male Tg(Eμ-myc) mice (19) were purchased from the Jackson Laboratories and bred to female miR-146a−/− mice to produce the Tg(Eμ-myc);miR-146a−/− strain. Male Tg(Eμ-myc);miR-146a−/− mice were mated with female miR-146a−/−Traf6+/− mice to produce the Tg(Eμ-myc);miR-146a−/−Traf6+/− line.
Mice were housed at the Beckman Research Institute of the City of Hope in accordance with stated Institutional Review Board and Institutional Animal Care and Use Committee (IACUC) protocols (49).
Genomic PCR.
DNA was extracted from clipped tails using the KAPA Express Extract buffer (KAPA Biosystems). PCR to detect Traf6 and miR-146a alleles was performed using the KAPA2G Fast Genotyping Mix (KAPA Biosystems) and using primers that were previously reported (2, 17).
Western Blots.
Mouse spleens were dissociated through a 45-μm mesh and treated with red blood counts (RBC) lysis buffer (BioLegend). Following RBC lysis and washing, single-cell suspensions of the remaining cells were made in complete RPMI medium containing 10% (vol/vol) FBS along with penicillin and streptomycin. Portions of this single-cell splenocyte suspension were pelleted, washed twice with PBS to remove excess medium, and lysed in cOmplete EDTA-free lysis buffer supplemented with cOmplete EDTA-free protease inhibitor mixture and Phos-Stop phosphatase inhibitor mixture (Roche). Total protein extract concentration was determined using the Bradford dye-binding reagent (Bio-Rad). Samples were run on an SDS-PAGE gel using 20 μg of protein per sample and transferred to nitrocellulose membranes. Anti-TRAF6 antibody (Cat# 597; MBL) was used to detect mouse TRAF6 protein, anti–phospho-IκBα antibody (Cat# 2859; Cell Signaling Technologies) was used to detect phosphorylated IκBα, anti–phospho-p38 MAPK antibody (Cat# 9215; Cell Signaling Technologies) was used to detect phosphorylated p38 MAPK. ImageJ software was used to quantify the relative amounts of TRAF6, phospho-IκBα, and phospho-p38 MAPK normalized to β-actin.
Flow Cytometry.
Single-cell suspensions from mouse spleens were prepared as described for BM cells in Western blot sections. One-million cells per sample of a single-cell suspension were stained with appropriate cell-surface markers and analyzed on a BD Accuri C6 flow cytometer. Fluorochrome conjugated antibodies against CD11b, GR1, Ter119, CD4, CD8, CD44, CD6L, CD69, CD150, CD48, CD117, Sca1, CSF1R, F480, were purchased from Biolegend or eBioscience and used according to the manufacturer’s instructions.
BMDM Proliferation.
BM cells from 10-wk-old female miR-146+/+, miR-146a−/−, and miR-146a−/−Traf6+/− mice were plated in triplicate on a 96-well plate at a density of 2 × 104 cells per well. Cells were cultured in complete DMEM supplemented with 50 ng/mL M-CSF. Proliferation of BMDMs was measured using the MTS assay (CellTiter 96; Promega) on day 3 postplating. For quantification of CSF1R, BM cells were plated in six-well dishes at a density of 6 × 105 cells per well. Cells were cultured in complete DMEM supplemented with 50 ng/mL M-CSF with a complete wash and change of medium on day 4.
Sublethal LPS Challenge.
Mice were injected intraperitoneally with LPS at a dose of 1 mg/kg of body weight. Peripheral blood was collected by retro-orbital bleeding before LPS injection as well as at 2- and 6-h post-LPS injection. At 24-h postinjection the mice were euthanized and blood was collected via cardiac puncture. Serum separator microtainer tubes (BD) were used to separate serum from whole blood.
Cytokine ELISA.
Sandwich ELISAs were performed using 96-well Maxisorp flat bottom plates (NUNC). Cytokine levels in mouse serum were detected using specific anti–IL-6 or anti–TNF-α capture and detection antibodies (eBioscience). Absolute cytokine levels were quantified by comparison with a standard curve produced using either recombinant mouse IL-6 or recombinant mouse TNF-α (eBioscience).
IFN-γ Detection in Stimulated Cells.
BM was collected in complete RPMI medium by using a 27-gauge needle to flush out mouse femurs and tibias. Following RBC lysis, the marrow was suspended in complete RPMI medium and plated in a six-well plate at a density of 2 × 106 cells per well. Cells were stimulated with 25 ng of phorbol 12-myristate 13-acetate (Abcam) and 500 ng of Ionomycin (Sigma) for 4 h in the presence of Monensin (BioLegend) to block cytokine secretion. Cells were stained with antibodies against surface markers, then fixed and permeabilized overnight using a fixation and intracellular permeabilization buffer kit (BioLegend). Following permeabilization, cells were stained with anti–IFN-γ antibody and analyzed on an Accuri C6 flow cytometer. Anti-rat IgG1-APC was used as an isotype control.
Histopathology.
Freshly harvested tissues were fixed in 10% formalin for 48 h and then washed in 70% ethanol followed by embedding in paraffin blocks, sectioning, and H&E staining. Slides were reviewed by a trained pathologist.
Complete Blood Counts.
Whole blood was obtained from freshly euthanized mice via cardiac puncture and analyzed on a Hemavet 950FS (Drew Scientific) within 1 h of collection.
Statistical Analyses.
All statistical analysis was performed using Prism 7 (Graphpad Software). P values were calculated using a standard ANOVA using Tukey’s multiple comparison tests with a single pooled variance. Survival curve comparisons were calculated using the Mantel–Cox log-rank test.
Acknowledgments
We thank the City of Hope Animal Research Center for their help and care in breeding and maintaining our mouse colonies. Research reported in this publication includes work performed in the Analytical Cytometry Core supported by the National Cancer Institute of the NIH under Award P30CA033572. This work was funded in part by the Nesvig Lymphoma Research Fund at the City of Hope and the Research Career Development award (to M.P.B.) by the STOP CANCER Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706833114/-/DCSupplemental.
References
- 1.Boldin MP, Baltimore D. MicroRNAs, new effectors and regulators of NF-κB. Immunol Rev. 2012;246:205–220. doi: 10.1111/j.1600-065X.2011.01089.x. [DOI] [PubMed] [Google Scholar]
- 2.Boldin MP, et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med. 2011;208:1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao JL, et al. NF-kappaB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. Proc Natl Acad Sci USA. 2011;108:9184–9189. doi: 10.1073/pnas.1105398108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakasa T, et al. Expression of microRNA-146 in rheumatoid arthritis synovial tissue. Arthritis Rheum. 2008;58:1284–1292. doi: 10.1002/art.23429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang Y, et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009;60:1065–1075. doi: 10.1002/art.24436. [DOI] [PubMed] [Google Scholar]
- 7.Pauley KM, et al. Altered miR-146a expression in Sjögren’s syndrome and its functional role in innate immunity. Eur J Immunol. 2011;41:2029–2039. doi: 10.1002/eji.201040757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao JL, Starczynowski DT. Role of microRNA-146a in normal and malignant hematopoietic stem cell function. Front Genet. 2014;5:219. doi: 10.3389/fgene.2014.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starczynowski DT, et al. Identification of miR-145 and miR-146a as mediators of the 5q- syndrome phenotype. Nat Med. 2010;16:49–58. doi: 10.1038/nm.2054. [DOI] [PubMed] [Google Scholar]
- 10.Zhao JL, Rao DS, O’Connell RM, Garcia-Flores Y, Baltimore D. MicroRNA-146a acts as a guardian of the quality and longevity of hematopoietic stem cells in mice. eLife. 2013;2:e00537. doi: 10.7554/eLife.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Contreras JR, et al. MicroRNA-146a modulates B-cell oncogenesis by regulating Egr1. Oncotarget. 2015;6:11023–11037. doi: 10.18632/oncotarget.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labbaye C, Testa U. The emerging role of MIR-146A in the control of hematopoiesis, immune function and cancer. J Hematol Oncol. 2012;5:13. doi: 10.1186/1756-8722-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu LF, et al. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010;142:914–929. doi: 10.1016/j.cell.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labbaye C, et al. A three-step pathway comprising PLZF/miR-146a/CXCR4 controls megakaryopoiesis. Nat Cell Biol. 2008;10:788–801. doi: 10.1038/ncb1741. [DOI] [PubMed] [Google Scholar]
- 15.Crone SG, et al. MicroRNA-146a inhibits G protein-coupled receptor-mediated activation of NF-κB by targeting CARD10 and COPS8 in gastric cancer. Mol Cancer. 2012;11:71. doi: 10.1186/1476-4598-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, et al. miR-146a controls the resolution of T cell responses in mice. J Exp Med. 2012;209:1655–1670. doi: 10.1084/jem.20112218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naito A, et al. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells. 1999;4:353–362. doi: 10.1046/j.1365-2443.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi N, et al. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001;20:1271–1280. doi: 10.1093/emboj/20.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris AW, et al. The E mu-myc transgenic mouse. A model for high-incidence spontaneous lymphoma and leukemia of early B cells. J Exp Med. 1988;167:353–371. doi: 10.1084/jem.167.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–2669. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langdon WY, Harris AW, Cory S, Adams JM. The c-myc oncogene perturbs B lymphocyte development in E-mu-myc transgenic mice. Cell. 1986;47:11–18. doi: 10.1016/0092-8674(86)90361-2. [DOI] [PubMed] [Google Scholar]
- 22.de Bruin AM, Voermans C, Nolte MA. Impact of interferon-γ on hematopoiesis. Blood. 2014;124:2479–2486. doi: 10.1182/blood-2014-04-568451. [DOI] [PubMed] [Google Scholar]
- 23.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zoumbos NC, Gascon P, Djeu JY, Young NS. Interferon is a mediator of hematopoietic suppression in aplastic anemia in vitro and possibly in vivo. Proc Natl Acad Sci USA. 1985;82:188–192. doi: 10.1073/pnas.82.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalton DK, et al. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 26.Zhao E, et al. Bone marrow and the control of immunity. Cell Mol Immunol. 2012;9:11–19. doi: 10.1038/cmi.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh MC, Lee J, Choi Y. Tumor necrosis factor receptor- associated factor 6 (TRAF6) regulation of development, function, and homeostasis of the immune system. Immunol Rev. 2015;266:72–92. doi: 10.1111/imr.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 29.Ishida T, et al. Identification of TRAF6, a novel tumor necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J Biol Chem. 1996;271:28745–28748. doi: 10.1074/jbc.271.46.28745. [DOI] [PubMed] [Google Scholar]
- 30.Ward C, et al. NF-kappaB activation is a critical regulator of human granulocyte apoptosis in vitro. J Biol Chem. 1999;274:4309–4318. doi: 10.1074/jbc.274.7.4309. [DOI] [PubMed] [Google Scholar]
- 31.Huxford T, Malek S, Ghosh G. Structure and mechanism in NF-kappa B/I kappa B signaling. Cold Spring Harb Symp Quant Biol. 1999;64:533–540. doi: 10.1101/sqb.1999.64.533. [DOI] [PubMed] [Google Scholar]
- 32.May MJ, Ghosh S. IkappaB kinases: Kinsmen with different crafts. Science. 1999;284:271–273. doi: 10.1126/science.284.5412.271. [DOI] [PubMed] [Google Scholar]
- 33.Shepard LW, et al. Constitutive activation of NF-kappa B and secretion of interleukin-8 induced by the G protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus involve G alpha(13) and RhoA. J Biol Chem. 2001;276:45979–45987. doi: 10.1074/jbc.M104783200. [DOI] [PubMed] [Google Scholar]
- 34.Bosman MC, Schuringa JJ, Vellenga E. Constitutive NF-κB activation in AML: Causes and treatment strategies. Crit Rev Oncol Hematol. 2016;98:35–44. doi: 10.1016/j.critrevonc.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Pham LV, Tamayo AT, Yoshimura LC, Lin-Lee YC, Ford RJ. Constitutive NF-kappaB and NFAT activation in aggressive B-cell lymphomas synergistically activates the CD154 gene and maintains lymphoma cell survival. Blood. 2005;106:3940–3947. doi: 10.1182/blood-2005-03-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: From innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 37.Kim DW, et al. Activation of NF-kappaB/Rel occurs early during neoplastic transformation of mammary cells. Carcinogenesis. 2000;21:871–879. doi: 10.1093/carcin/21.5.871. [DOI] [PubMed] [Google Scholar]
- 38.Mohr S, et al. Hoxa9 and Meis1 cooperatively induce addiction to Syk signaling by suppressing miR-146a in acute myeloid leukemia. Cancer cell. 2017;31:549–562 e511. doi: 10.1016/j.ccell.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J, Feng X, Desierto MJ, Keyvanfar K, Young NS. IFN-γ-mediated hematopoietic cell destruction in murine models of immune-mediated bone marrow failure. Blood. 2015;126:2621–2631. doi: 10.1182/blood-2015-06-652453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schürch CM, Riether C, Ochsenbein AF. Cytotoxic CD8+ T cells stimulate hematopoietic progenitors by promoting cytokine release from bone marrow mesenchymal stromal cells. Cell Stem Cell. 2014;14:460–472. doi: 10.1016/j.stem.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Dufour C, et al. Associazione Italiana di Emato-Oncologia Pediatrica (AIEOP); Department of Hematology, Ospedale S. Martino, Genoa, Italy Homozygosis for (12) CA repeats in the first intron of the human IFN-gamma gene is significantly associated with the risk of aplastic anaemia in Caucasian population. Br J Haematol. 2004;126:682–685. doi: 10.1111/j.1365-2141.2004.05102.x. [DOI] [PubMed] [Google Scholar]
- 42.Varney ME, et al. Loss of Tifab, a del(5q) MDS gene, alters hematopoiesis through derepression of Toll-like receptor-TRAF6 signaling. J Exp Med. 2015;212:1967–1985. doi: 10.1084/jem.20141898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernandes-Alnemri T, et al. Cutting edge: TLR signaling licenses IRAK1 for rapid activation of the NLRP3 inflammasome. J Immunol. 2013;191:3995–3999. doi: 10.4049/jimmunol.1301681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin KM, et al. IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc Natl Acad Sci USA. 2014;111:775–780. doi: 10.1073/pnas.1320294111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su W, et al. The p53 transcription factor modulates microglia behavior through microRNA-dependent regulation of c-Maf. J Immunol. 2014;192:358–366. doi: 10.4049/jimmunol.1301397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu D, et al. The miR-155-PU.1 axis acts on Pax5 to enable efficient terminal B cell differentiation. J Exp Med. 2014;211:2183–2198. doi: 10.1084/jem.20140338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu WH, et al. A miR-155-Peli1-c-Rel pathway controls the generation and function of T follicular helper cells. J Exp Med. 2016;213:1901–1919. doi: 10.1084/jem.20160204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kramer NJ, et al. Altered lymphopoiesis and immunodeficiency in miR-142 null mice. Blood. 2015;125:3720–3730. doi: 10.1182/blood-2014-10-603951. [DOI] [PubMed] [Google Scholar]
- 49.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed National Academies Press; Washington, DC: 2011. [Google Scholar]