Significance
Glycan binding proteins (GBPs) play an important and ever-emerging role in decoding the structural diversity of cell surface glycans into function. New GBPs provide useful tools to probe and manipulate biological processes. Here we describe the characterization of the Y3 protein from the mushroom Coprinus comatus as a unique GBP that shows selective cytotoxicity toward human T-cell leukemia Jurkat cells through caspase-associated apoptosis. Structural analysis along with glycan array screening of Y3 reveals a unique tertiary structure and a specific interaction with GalNAcβ1-4(Fucα1-3)GlcNAc, a glycan abundant in invertebrates but uncommon in humans. This work expands on promising novel GBPs available in less-explored sources for biomedical and research applications.
Keywords: glycan binding protein, Coprinus comatus, cytotoxicity, LDNF, crystal structure
Abstract
Glycans possess significant chemical diversity; glycan binding proteins (GBPs) recognize specific glycans to translate their structures to functions in various physiological and pathological processes. Therefore, the discovery and characterization of novel GBPs and characterization of glycan–GBP interactions are significant to provide potential targets for therapeutic intervention of many diseases. Here, we report the biochemical, functional, and structural characterization of a 130-amino-acid protein, Y3, from the mushroom Coprinus comatus. Biochemical studies of recombinant Y3 from a yeast expression system demonstrated the protein is a unique GBP. Additionally, we show that Y3 exhibits selective and potent cytotoxicity toward human T-cell leukemia Jurkat cells compared with a panel of cancer cell lines via inducing caspase-dependent apoptosis. Screening of a glycan array demonstrated GalNAcβ1–4(Fucα1–3)GlcNAc (LDNF) as a specific Y3-binding ligand. To provide a structural basis for function, the crystal structure was solved to a resolution of 1.2 Å, revealing a single-domain αβα-sandwich motif. Two monomers were dimerized to form a large 10-stranded, antiparallel β-sheet flanked by α-helices on each side, representing a unique oligomerization mode among GBPs. A large glycan binding pocket extends into the dimeric interface, and docking of LDNF identified key residues for glycan interactions. Disruption of residues predicted to be involved in LDNF/Y3 interactions resulted in the significant loss of binding to Jurkat T-cells and severely impaired their cytotoxicity. Collectively, these results demonstrate Y3 to be a GBP with selective cytotoxicity toward human T-cell leukemia cells and indicate its potential use in cancer diagnosis and treatment.
Polysaccharides (glycans) are one fundamental building block of life, ubiquitously expressed in all organisms and essential to numerous biological processes including adhesion and growth, signaling, infection, and tumor pathogenesis (1–3). In addition, aberrant glycosylation is directly linked with many human diseases (4). Of note, glycans serve as useful biomarkers of various cancers and targets of therapeutic intervention (4–7). Glycan binding proteins (GBPs) read the diversity and complexity of glycans in a relatively specific manner and execute the physiological or pathological information encoded by the polymers (1, 8). The specificity of GBP–glycan interactions is determined by multiple factors, including composition, site-specific modifications, and tertiary structure of the glycans. Lectins form one major group of GBPs and are widely distributed among organisms (e.g., viruses, bacteria, fungi, insects, plants, and animals) (9, 10). Lectins adopt at least 14 different folds and common examples include the ricin-like β-trefoil, galectin-like fold, actinoporin-like fold, and β-propeller (11, 12). However, fold can show low sequence identities among family members (13). Indeed, significant diversity in sequence and folds highlights the divergent and convergent evolution of protein functions and poses challenges to accurately and reliably annotate new members (14, 15).
A number of GBPs are small proteins, less than 150 amino acids, and over the past decades the functions of many small proteins have been discovered to be critical to various cellular processes, primarily through serendipitous studies (16). For example, galectin-1 with an affinity for β-galactose (Gal) was shown to be essential to neuronal cell differentiation and be associated with malignant tumor progression in human (17, 18). Small proteins also regulate essential cellular processes of bacteria (e.g., the 43-aa SgrS) (19), yeast (20), and animals (16). In recent years, systems biology studies have demonstrated that organisms commonly express hundreds of small proteins that often have no characterized homologs (16, 21–23). Accurately assigning a biochemical, cellular, or physiological function to these proteins is a rapidly evolving research area whose advances require the integration of multiple disciplines (16, 24).
Mushrooms have been used for food, medicine, or other purposes for thousands of years (25) but 90% of mushroom species in nature remain unexplored (26, 27). The potential of this untapped source in the discovery of useful substances is exemplified by the semisynthetic analog of pleuromutilin (retapamulin) as clinically used antibiotic (28), illudins as anticancer drugs (29), and peptidic omphalotins as nematicidal agents (30). Addition to low molecular weight (MW) secondary metabolites, mushrooms produce a variety of proteins, such as lectins, with antitumor, antiviral, antimicrobial, antioxidative, and/or immunomodulatory activities (31). Recent genomic and computational studies have further identified an enormous number of genes encoding small proteins from fungal genomes (32). These small proteins awaiting assigned functions can become useful biotechnological and biomedical agents (31).
The mushroom Coprinus comatus has an annual production of about 0.4 million tons globally and shows a range of bioactivities (e.g., immunomodulation and anticancer) (33, 34). Y3 is a 130-aa protein isolated from C. comatus and initial characterization showed it inhibits the infection and multiplication of the tobamovirus, Tobacco mosaic virus (TMV) (35). Our search of publicly available genome database yielded a limited number of Y3 homologs with only low sequence similarity, and none has been characterized (Fig. 1A). Herein, we disclose the detailed biochemical, functional, and structural characterization of this fungal small protein. Our biochemical studies of recombinant mature Y3 produced in a yeast expression system validated its anti-tobamovirus activity and revealed its glycan binding capability. Given the significance of glycans to cancer development and apoptosis (4–6), we further demonstrated that Y3 selectively and potently induced caspase-dependent apoptosis of human T-cell leukemia Jurkat cells. Glycan array screening identified a glycan antigen that Y3 showed strong specific interactions with. Finally, we determined a high-resolution crystal structure of Y3, and characterized its glycan binding pocket. These results lay the foundation to identify and characterize Y3 homologs in other fungal species and to develop this GBP scaffold for biomedical and research applications. This work also suggests the exploration of untapped potential of increasingly available small proteins in drug research using a multiple disciplinary approach.
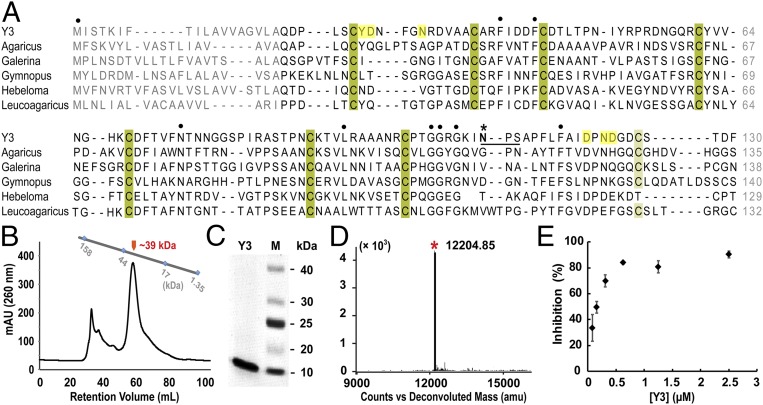
Fig. 1.
Biochemical characterization of Y3. (A) Sequence alignment of Y3 and its homologs from fungal species. Conserved cysteine residues that potentially form disulfide bridges are shaded in dark- and light green and all other conserved residues are indicated with dots (·). Potential N-glycosylation site (Asn110-Ser112) in Y3 is underlined with a dark line, and Asn110 is labeled with an asterisk (*). Residues potentially involved in ligand binding are shaded in yellow. Y3: C. comatus, GenBank: ADK35888.1; Agaricus: Agaricus bisporus var. burnettii JB137-S8, GenBank: XP_007333380.1; Galerina: Galerina marginata CBS 339.88, GenBank: KDR84548.1; Gymnopus: Gymnopus luxurians FD-317 M1, GenBank: KIK60251.1; Hebeloma: Hebeloma cylindrosporum h7, GenBank: KIM42673.1; Leucoagaricus: Leucoagaricus sp. SymC.cos, GenBank: KXN92904.1. (B) Recombinant Y3 was a putative dimer as shown in SEC analysis. Retention volumes of molecular standards of 1.35, 17, 44, and 158 kDa are indicated. (C) SDS/PAGE analysis showed the high purity of recombinant Y3. (D) The m/z value of purified Y3 at 12,204.85 in MALDI-MS analysis matched with calculated molecular weight of mature Y3 monomer (12,229.54 Da). (E) The inhibitory curve of recombinant Y3 on TMGMV infection. Data are presented as mean ± SD (n = 6).
Results and Discussion
Biochemical Characterization of Recombinant Y3.
In an initial report, Y3 was isolated from the fungal fruiting body and showed anti-TMV activity which was retained after incubating the protein at 90 °C for 10 min (35). A bioinformatic analysis of Y3 predicted an 18-aa signal peptide (SP) at its N-terminus and a potential N-glycosylation site (Asn110) (Fig. 1A). Indeed, native Y3 was reported to be a glycoprotein (35). Considering these putative postmodifications, we expressed both full-length and SP-free genes in Pichia pastoris (SI Appendix, Fig. S1). To preserve the original structure and activity, no purification tag was included. Recombinant Y3 was produced only from the SP-free gene (Fig. 1 B and C). The yield of recombinant Y3 was about 20 mg/L after filtration, concentration, and dialysis of the P. pastoris culture supernatant. The size-exclusion chromatography (SEC) analysis of purified Y3 led to a single peak with an estimated MW of ∼39 kDa (Fig. 1B). Denaturing SDS/PAGE analysis revealed a single band of ∼12 kDa, similar to that of native Y3 directly isolated from C. comatus. Treatment with heat (90 °C for 20 min) or a range of acid/base (pH 3.0–11.5) had no effect on the SDS/PAGE profile of recombinant Y3. The impressive stability of Y3 may be ascribed to the presence of multiple disulfide bridges from its eight cysteine residues (Fig. 1A). Indeed, Ellman's analysis excluded the presence of any free thiol in the recombinant protein (36) (SI Appendix, Fig. S2).
We next determined the MW of recombinant Y3 using MALDI-TOF mass spectrometry (MS). The observed MW of 12.20 kDa agreed well with the calculated MW of SP-free Y3 (12.22 kDa) (Fig. 1D). Treating the recombinant Y3 with PNGase F, which removes potential N-glycan of glycoproteins, led to no MW change in the MS analysis, further suggesting that Y3 is not a glycoprotein. Strikingly, we identified a 38% of total carbohydrates in the Y3 sample (wt/wt) in phenol sulfuric acid analysis (37, 38) (SI Appendix, Fig. S3A), and dialysis of Y3 against PBS buffer (vol/vol, 1:500) gradually reduced the carbohydrate content to about 27% over the period of 6 d, suggesting a noncovalent interaction. In light of the above result, we reexamined a broad peak in the electrospray ionization mass spectrometry (ESI-MS) profile that did not possess proteinous UV-vis absorbance at 260 nm (SI Appendix, Fig. S4 A–C). The peak consisted of a wide range of MWs and likely represented a mixture of potential carbohydrate fragments bound to Y3, which possibly contribute to the observed MW of Y3 in the SEC analysis (Fig. 1B). Copurification of endogenous carbohydrates with recombinant proteins has been uncommonly encountered in previous studies (39) because of generally relatively low binding affinity (∼millimolar) between noncognate glycans and GBPs (40). Nonetheless, our studies strongly suggest Y3 to be a GBP.
We further evaluated the anti-TMV activity of recombinant Y3 and showed it reduced the infectivity of purified Tobacco mild green mosaic virus (TMGMV) (26.5 µg/mL) by 50% at 0.12 μM (Fig. 1E), more effective than that reported for native Y3 and TMV (0.17 μM) (35). When mixed with TMGMV at room temperature and on ice, recombinant Y3 (0.078 μM) rapidly reduced the infectivity of TMGMV by 40 and 10.7%, respectively. A 20-min incubation period substantially increased the corresponding inhibition to 52.1 and 39.2%, suggesting the effects of both temperature and incubation time. Collectively, these data demonstrated that recombinant Y3 from the yeast system retained the proven antiviral activity.
Y3-Induced Caspase-Dependent Apoptosis of Human T-Cell Leukemia Jurkat Cells.
Given the diverse and important roles glycans play in cancer biology, we assessed potential cytotoxicity of Y3 against a panel of human cancer cell lines (SI Appendix, Table S1). At 10 µM concentration, Y3 showed only modest to weak growth inhibition of cervical cancer HeLa cells, liver carcinoma HepG2 cells, pancreas carcinoma Dan-G cells, and prostatic cancer DU-145 cells (SI Appendix, Fig. S5A). By contrast, however, Y3 exhibited potent activity toward human T-cell leukemia Jurkat cells at the nanomolar level (SI Appendix, Fig. S5B). The high selectivity was further indicated with no observed cytotoxic effect on pancreas carcinoma MIA-PaCa-2, kidney HEK293, and head and neck squamous carcinoma UM-SCC-1 cells. To further probe the effects of Y3 on Jurkat cells, we examined the modes of cell death by using 7-aminoactinomycin D (7-AAD) and Annexin V double staining (41). This analysis revealed that Y3 induced both early and late apoptosis of Jurkat cells in a dose-dependent manner (Fig. 2A). Treatment at 0.1 μM for 4h induced 45 ± 2.5% of Jurkat cells to enter early stage apoptosis as indicated by Annexin V staining, while 23 ± 1.3% were stained by both 7-AAD and Annexin V, suggesting late-phase apoptosis (Fig. 2A and SI Appendix, Fig. S6). These percentage values of early- and late-phase apoptosis were shifted to 17 ± 3.0% and 73 ± 4.8%, respectively, at 20 h, giving apoptotic cells as 90% of total cells. These findings indicate a potent and rapid cytotoxicity of Y3 against leukemia cells. Of note, Y3 samples dialyzed to remove bound carbohydrate exhibited the same extent of anti-Jurkat activity (SI Appendix, Fig. S3B), indicating a minimal effect of copurified carbohydrates on the observed bioactivity.
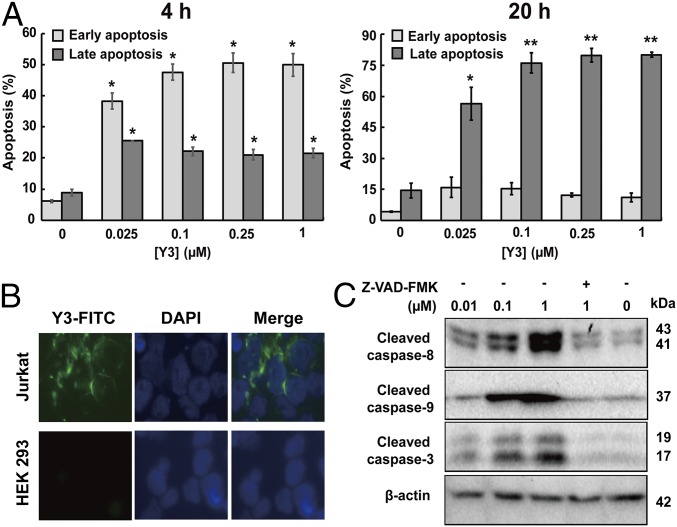
Fig. 2.
Y3 induced the apoptosis of Jurkat cells. (A) Annexin V and 7-AAD staining quantitated cell populations in early and late apoptosis after treatment with serial concentrations of Y3 for 4 and 20 h. Data are shown as the means ± SD (n = 3). Significant differences between control (0 µM) and treatments are shown (*P < 0.05; **P < 0.01). (B) Y3-FITC showed strong binding to the cell surface of Jurkat cells while minimal-to-no binding to HEK 293 in fluorescence microscopy images. (C) Caspases-3, 8, and 9 in Jurkat cells were activated by Y3 as indicated by Western blotting analysis using specific antibodies. Jurkat cells were treated with different concentrations of Y3 and pan-caspase inhibitor Z-VAD-FMK (20 µM) for 20 h.
To further investigate this activity, we labeled recombinant Y3 with a fluorescein-5-isothiocyanate (FITC) fluorescent probe. FITC-Y3 retained a similar level of anti-Jurkat activity as observed with Y3 (SI Appendix, Fig. S7A), and demonstrated to bind to the cell surface of Jurkat cells in a dose-dependent manner (Fig. 2B and SI Appendix, Fig. S7B). We did not observe any transport of FITC-Y3 into the cells. By contrast, FITC-Y3 showed minimal binding to the control HEK293 cells (Fig. 2B). These results suggest that Y3 triggered the apoptosis pathways through the binding to the cell surface of Jurkat cells, and highlight potential applications in the management of acute T-cell leukemia.
We next examined the potential mechanism of the cytotoxicity of Y3. As the activation of caspases is essential in both intrinsic and extrinsic apoptotic pathways (42), we measured the levels of cleaved caspases in Jurkat cells after the treatment of Y3 at 0.01–1 µM for 20 h (Fig. 2C). Western blotting analysis clearly demonstrated that Y3 induced the activation of caspases 3, 8, and 9 in a dose-dependent manner, while cotreatment with the pan-caspase inhibitor z-VAD-FMK (20 µM) significantly blocked the activation of all three caspases, confirming a caspase-dependent mechanism of Y3′s action.
Glycan Binding Profile of Y3.
As discussed, glycans are critical to various essential molecular and cellular processes related to diseases and health, for example as important biomarkers and potential therapeutic and diagnostic targets of cancers (4–7). To assess the glycan binding profile of Y3, we prepared biotinylated Y3 to screen against a mammalian glycan array consisting of 600 glycans in replicates of 6 at the Consortium for Functional Glycomics (CFG). Detailed analysis of the concentration-dependent binding of Y3 showed the strongest binding affinity to GalNAcβ1–4(Fucα1–3)GlcNAc (LDNF) (Fig. 3A). LDNF is a member of the LacdiNAc (GalNAcβ1–4GlcNAc, LDN) family of glycans, which are abundant in invertebrates such as parasitic helminths and insects (43). In humans, the LDN-type glycans are less common and may be associated with the development of some cancers (44, 45). The binding affinity of the LDNF glycan epitope and Y3 was 10–102 × higher than closely related LDN or sulfated analogs that were included in the array (Fig. 3A). Additionally, 6′-sulfation of LDNF resulted in a weaker binding (∼8× lower) while a 3-sulfation group completely blocked the glycan–Y3 interaction, illustrating a specific Y3–glycan interaction.
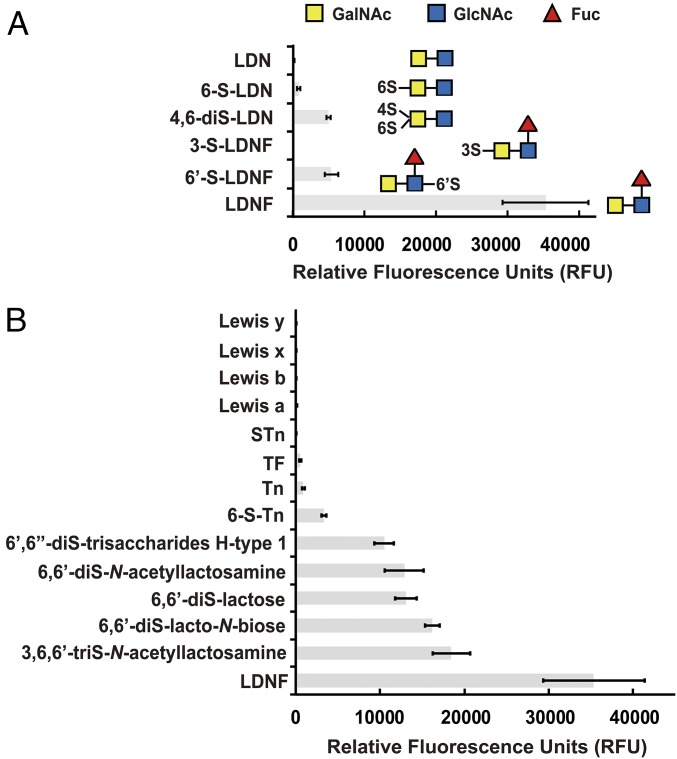
Fig. 3.
Glycan array screening identified LDNF as a specific ligand of Y3. (A) Y3 showed varying binding to glycans of the LDL family with LDNF as the best ligand. Blue square: GlcNAc; yellow square: GalNAc; red triangle: Fuc; S: sulfo. (B) Fluorescence signals of other top glycans and common human antigens in binding to Y3. Figures were generated from data with Y3 at 50 µg/mL in the screening. Data are presented as mean ± SD (n = 6).
In general, there was a distinct trend of sulfation in the top ligands of Y3, such as disaccharides 3,6,6′-trisulfo-N-acetyllactosamine, 6,6′-disulfo-lacto-N-biose, 6,6′-disulfo-lactose, 6,6′-disulfo-N-acetyllactosamine, and trisaccharide Fucα1–2(6S)Galβ1–3(6S)GlcNAc (6′,6′′-disulfo-trisaccharides H-type 1) (Fig. 3B). In fact, sulfo modification occurred on the majority of top 20 glycans, suggesting that this group could mediate specific interactions with Y3 (SI Appendix, Table S2). GlcNAc, Glc, or their 6-sulfated analogs appeared at the reducing end of all top 20 glycans of Y3. In fact, β-6-sulfo-GlcNAc was the best monosaccharide glycan in the Y3 glycan screening, whose signal was 11× lower than LDNF (Fig. 3B). Also, Y3 strongly favored terminal, nonreducing GalNAc or Gal that is modified by mono- or disulfo groups at 6, 3, or 4 position (SI Appendix, Table S2). However, Y3 showed only weak binding to GalNAc itself, also known as Tn antigen that is commonly found on cancer cells (46). Other cancer-relevant Tn antigen derivatives including the Thomsen–Friedenreich antigen (Galβ1–3GalNAc) and sialyl Tn antigen (Neu5Acα2–6GalNAc) also showed minimal binding to Y3 (Fig. 3B). Glycan profiling of Y3 further suggested that a β1,3-glycosidic bond was favored slightly more than a β1,4-linkage (e.g., 6,6′-disulfo-lacto-N-biose vs. 6,6′-disulfo-N-acetyllactosamine, Fig. 3B). In this regard, Y3 demonstrated minimal binding to neither β1–3 bond-containing histo-blood group antigens Lewis a [Galβ1–3(Fucα1–4)GlcNAc] and b [Fucα1–2Galβ1–3(Fucα1–4)GlcNAc] nor β1–4 bond-related cancer antigens Lewis x [Galβ1–4(Fucα1–3)GlcNAc] and y [Fucα1–2Galβ1–4(Fucα1–3)GlcNAc] (Fig. 3B). Strikingly, the only structural difference between LDNF and Lewis x is the terminal GalNAc and Gal, which varies their binding affinities toward Y3 at 50 µg/mL by over 17,000× (Fig. 3B). Overall, the glycan screening suggested that Y3 exclusively recognized the LDNF moiety and modestly interacted with sulfated di- or trisaccharides primarily consisting of GalNAc, Gal, GlcNAc, Glc, Fuc, and their sulfated analogs. Two Ca2+-dependent lectins macrophage galactose-type lectin (MGL) and dendritic cell-specific C-type lectin DC-SIGN are other characterized GBPs that show a relatively tight interaction with LDNF (47, 48). However, MGL binds to the LDN antigen 1.3× more tightly than LDNF, while DC-SIGN shows significantly stronger interaction with Lewis x (49), marking Y3 as the only known LDNF-specific GBP.
High-Resolution Crystal Structure of Y3.
To gain structural insights into Y3 as a specific LDNF-binding GBP, we successfully determined its crystal structure by single-wavelength anomalous diffraction analysis (SAD). Native Y3 crystal diffracted to 1.2-Å resolution bearing translational noncrystallographic symmetry (SI Appendix, Table S3). Heavy atom soaking of crystals resulted in an orphan dataset (Y3-Pt) in an alternative lattice used for experimental phasing, in the absence of any reference structure suitable for molecular replacement (SI Appendix, Table S3). Depending on the lattices, Y3 consists of one or two dimers per asymmetric unit.
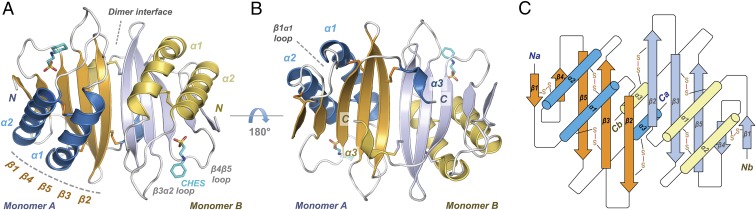
Y3 monomer forms a compact single-domain αβα-sandwich consisting of three α-helices and a five-stranded β-sheet (Fig. 4). The β-sheet was linked as β1-β4-β5-β3-β2 from edge to center in an antiparallel orientation. Two long α-helices (α1 and α2) pack against one side of the β-sheet, while a short C-terminal α3 is located on the opposite side. The monomer contains four-intramolecular disulfide bridges, Cys24-Cys101, Cys36-Cys69, Cys61-Cys126, and Cys44-Cys90 (Fig. 4 and SI Appendix, Fig. S8A), agreeing with the results of Ellman’s (SI Appendix, Fig. S2) and MS analysis (Fig. 1D). The majority of these disulfide bridges are among conserved cysteines through Y3 homologs from other fungal species (Fig. 1A). Another modification on Y3 was the formation of pyroglutamic acid from Gln19 presumably by a glutamine cyclase after translation (50) (SI Appendix, Fig. S8B); this is not uncommonly observed on +1 residue that follows the signal peptide. Also, one molecule of N-cyclohexyl-2-aminoethanesulfonic acid from the crystallization buffer was clearly resolved between the β3α2 and β4β5 loops (SI Appendix, Fig. S8C). Its anionic sulfate group interacts with NPhe115, NSer86, and the Asn89 sidechain, while the hydrophobic cyclohexane extends into the bulk solvent. In the crystallographic asymmetric unit, the four Y3 monomers have an rmsd range of 0.14–0.36 Å (among monomers) and differ primarily in the β1α1-loop regions (residues 26–31), with a Cα (backbone carbon) movement of up to 4.5 Å in this region (SI Appendix, Fig. S8D). The five-stranded, antiparallel β-sheets from two Y3 monomers assemble to a large, intermolecular 10-stranded, antiparallel β-sheet with all α1 and α2 helices on one side and α3 on the opposite side (Fig. 4 A and B). Additional dimeric interactions include intermolecular hydrogen bonds between Arg′53 of the α1β2 loop and the neighboring Val63, and water-mediated H bonds between Gln59/Gln′59 (SI Appendix, Fig. S8E).
Fig. 4.
The X-ray crystal structure of Y3. (A–B) Y3 dimer forms a 10-stranded β-sheet with helices α1 and α2 on one side of the dimeric interface while two α3 on the other side. (C) Structure topology illustration of Y3 dimer with the indication of disulfide bridges.
Structural homology searches (DALI server) (51) revealed that Y3 shares limited structural similarity with known structures. The most relevant in weak overall structural homology is an α-Gal binding lectin LDL from the mushroom Lyophyllum decastes [Protein Data Bank (PDB) ID code 4NDV, Z score 8.7, rmsd 2.2 Å)] (52), which shares 12% sequence identity with Y3 (SI Appendix, Fig. S9A). The LDL structure contains a four-stranded, antiparallel β-sheet and two α-helices being packed against one side of the β-sheet. However, the structure of Y3 shows significant differences from LDL (SI Appendix, Fig. S9B), including the number and locations of disulfide bridges, the number of β-sheets, and the α3 helix present in Y3. Importantly, LDL forms an alternate dimer by the stacking of the exposed sides of the β-strands of the two subunits as an αβ2α-sandwich (53) (SI Appendix, Fig. S9C). By contrast, the Y3 monomer is featured with the presence of a third α-helix (α3) and is incompatible of forming such β-stack-β conformation (SI Appendix, Fig. S9D). Finally, up to 1 mM of recombinant Y3 did not agglutinate human and rabbit erythrocytes (SI Appendix, Fig. S10), strikingly different from the characteristic feature of other reported lectins. Indeed, Y3 exhibited insignificant interactions with glycans (e.g., blood group glycans) other than LDNF in the microarray screening (Fig. 3). In this regard, the reported hemagglutination activity of native Y3 purified from the mushroom, in an early report (35), might be related to impurities from the partially purified sample or possibly suggest a different activity spectrum of Y3 from the native source. All of these structural and functional findings indicate that Y3 adopts a unique glycan-binding mode.
Glycan Binding Site of Y3.
The Y3 dimer forms a Janus conformation with the majority of hydrophilic residues assembled on one side. The other surface forms a hydrophilic pocket that sits on the 10-stranded, antiparallel β-sheet and is surrounded by the two α3 helices, one from each monomer (Fig. 5A). The large pocket is a unique feature compared with other reported GBPs and suggests that Y3 most likely interacts with complex glycan chains rather than mono- or disaccharides. Indeed, GalNAc, 6-sulfo-GlcNAc, d-Man, d-Glu, d-Gal, d-Fuc, or d-Lac did not show an interaction with Y3 using isothermal titration calorimetry or biolayer interferometry analysis. Additionally, soaking of Y3 crystals with any of these sugars did not allow clear interpretable ligand electron difference maps. These results are consistent with the glycan binding array data suggesting LDNF as a favored ligand.
Fig. 5.
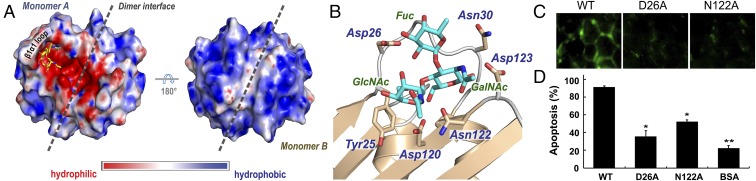
Y3 contained a large pocket for interacting with LDNF primarily through H-bonding network. (A) The putative glycan binding pocket of Y3. Left, an electrostatic surface representation of the Y3 dimer. The LDNF motif was modeled into the Y3 dimer and aligned along with the β1α1 loop. Hydrophilic and hydrophobic regions are shown with red and blue, respectively. Right, the dimer rotated by 180° to show the hydrophobic face. (B) A close-up view of the binding site with the docked LDNF. Key residues involved in H-bond interactions are labeled. (C) Fluorescence microscopy images of Jurkat cells after treatment with FITC-labeled Y3, Y3D26A, and Y3N122A. (D) Annexin V/7-AAD apoptotic assays revealed significantly decreased anti-Jurkat activity of Y3D26A and N122A mutants (1 µM). Data are presented as means ± SD (n = 3). Significant differences between WT and mutants are shown (*P < 0.05; **P < 0.01).
To provide additional insights into the Y3/glycan recognition, the LDNF structure was modeled into the Y3 dimer (Fig. 5B). LDNF was aligned along the β1α1 loop in the top docking result. Key hydrogen bond interactions between the protein and sugar include Asp26, Asn30, Asp120, Asn122, and Asp123. The GalNAc moiety sits in the cavity between the β3 and the β1α1 loop, and the terminal GlcNAc is near Asp120/Asn122, forming an interaction with Tyr25 (Fig. 5B). The similar interaction mode with GlcNAc is present in the structures of Wisteria floribunda lectin (PDB ID code 5KXC) and Clitocybe nebularis ricin B-like lectin (PDB IC code 3NBE) (54) (SI Appendix, Fig. S11). In addition, the LDNF motif could further extend to the dimeric interface of Y3 leading to improved glycan binding affinity. Of note, we did observe unassigned electron density near the GlcNAc interacting region in our high-resolution structure (Fig. 5A and SI Appendix, Fig. S12). The density most likely corresponds to a polysaccharide fragment of bound endogenous glycan (likely mannose), indirectly supporting our docking model and agreeing with the results of phenol sulfuric acid and ESI-MS analysis (SI Appendix, Figs. S3 and S4). To provide a structure/function relationship of key residues in ligand binding, we created Y3 D26A and N122A mutants (SI Appendix, Fig. S13), whose carbohydrate contents were approximately 33 and 28%, respectively (SI Appendix, Fig. S3C). Both mutants nearly lost their ability to bind Jurkat cells (Fig. 5C). Furthermore, the cytotoxicity of both mutants was significantly decreased compared with the level of a negative control BSA (Fig. 5D). These results provided strong supportive evidence for our docking model, and offered insights into the specific interactions of Y3-LDNF. After binding to the glycan, Y3 may initiate apoptosis pathways in Jurkat cells.
Conclusion
Our studies describe and characterize Y3 from the edible mushroom C. comatus as a GBP with a unique topology and tertiary structure. Its cytotoxicity toward Jurkat cells is mediated by the activation of caspase cascade that is presumably induced by specific LDNF/Y3 interactions. T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive hematological malignancy, and the long-term survival of T-ALL patients remains extremely poor due to the remission and recurrence (55, 56). The critical need of an improved treatment regimen of aggressive acute T-cell leukemia lends obvious significance to Y3 for developing potential novel treatment and diagnosis options. Future work includes the functional characterization of Y3 homologs from other fungal species, which will show considerable variations of key residues in the glycan binding pocket (Fig. 1A), the detailed investigation of Y3 signaling in cancer cells (e.g., its receptor), and the development of Y3 and its homologs for biomedical applications. We envision that such work will discover GBPs as potential therapeutic agents toward other diseases and provide new insight into the structure–function-relationship of this family of GBPs.
Methods
Screens for Anticancer Activity.
The cells shown in SI Appendix, Table S1 were cultured in DMEM containing 10% FBS, 100 U·mL−1 penicillin and streptomycin, and maintained at 37 °C in a humidified incubator under 5% CO2. The cells (104 cells in 100 μL), seeded in 96-well plate, were treated with Y3 (10 μM). After incubation at 37 °C for 48 h, a thiazolyl blue tetrazolium bromide protocol was followed to determine cell viability using a UV/vis microplate spectrophotometer (BioTek). Six replications were performed per treatment and the percent inhibition was calculated as (1 − test OD570/nontreated OD570) × 100%. Serial concentrations of Y3 (0, 0.016, 0.031, 0.063, 0.13, 0.25, 0.5, and 1 μM) were used to treat Jurkat cells.
Glycan Microarray Analysis of Y3′s Glycan Binding Profile.
Glycan microarray analysis was performed by the Consortium for Functional Glycomics (Core H). The Mammalian Printed Array, version 5.3, consists of 600 glycans in replicates of 6 and was used in this work. For screening the array, Y3 was biotinylated using EZ-Link NHS-PEG4-Biotinylation Kit (Thermo Scientific) according to manufacturer’s instructions. Biotin-labeled Y3 at 5 and 50 μg/mL was analyzed as previously described (57). Streptavidin-488 was used to detect biotinylated Y3 that bound to the glycans on the array. The average binding for each glycan target as well as SD was calculated after the highest and lowest point from each set of six replicates were removed, and glycans were then ranked and sorted. The scanner response was linear to a maximal relative fluorescence unit value of about 50,000.
Structure Determination of Y3.
Briefly, Y3 crystallization was performed in a hanging drop format. Heavy atoms derivative crystals for SAD were obtained through cocrystallization or soaking. Y3 native and derivative crystal X-ray diffraction data sets were collected on beamlines 21-ID-G and 21-ID-F of the Life Sciences Collaborative Access Team (LS-CAT) facility at the Advanced Photon Source, Argonne National Laboratory (ANL) with a wavelength of 0.9786 Å at 100 K. The structure of Y3 was determined with Pt-SAD with details shown in SI Appendix. Statistics on data collection and atomic structure refinement are given in SI Appendix, Table S3. The refined coordinates have been deposited in the PDB (ID codes 5V6I and 5V6J).
Supporting Information.
Materials and methods, including heterologous expression, Ellman's test for free thiol determination, quantitation of carbohydrate content in protein samples, anti-tobamovirus assay, cell apoptosis quantitation, cell imaging, Western blotting, hemagglutination assays, determination of the carbohydrate content, the carbohydrate binding to Y3, Y3 crystallization, diffraction data collection and processing, atomic structure determination and refinement, docking, MS analysis, and statistical analysis are described in detail in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Prof. Hui Feng (Boston University) for important experiment materials. We also thank Dr. Chen Liu and Dr. Qunfeng Wu (Rutgers University) for informative discussions and Mr. Yi Zhang and Mr. Julian Rashid for technical support. We thank the staff of LS-CAT, ANL for help with data collection and discussion. Octet RED384 is available at the Center for Translational Research in Neurodegenerative Disease at University of Florida (UF). We acknowledge the participation of the Protein-Glycan Interaction Resource of the CFG (NIH Grant R24 GM098791) and the National Center for Functional Glycomics at Beth Israel Deaconess Medical Center, Harvard Medical School (NIH Grant P41 GM103694). Part of this work was supported by College of Pharmacy and UF. Y.D. is an Air Force Office of Scientific Research Young Investigator.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 5V6I and 5V6J).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706894114/-/DCSupplemental.
References
- 1.Varki A. Biological roles of glycans. Glycobiology. 2017;27:3–49. doi: 10.1093/glycob/cww086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13:448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hart GW. Thematic minireview series on glycobiology and extracellular matrices: Glycan functions pervade biology at all levels. J Biol Chem. 2013;288:6903. doi: 10.1074/jbc.R113.453977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinho SS, Reis CA. Glycosylation in cancer: Mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 5.Stowell SR, Ju T, Cummings RD. Protein glycosylation in cancer. Annu Rev Pathol. 2015;10:473–510. doi: 10.1146/annurev-pathol-012414-040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dube DH, Bertozzi CR. Glycans in cancer and inflammation–potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 7.Hung TC, Lin CW, Hsu TL, Wu CY, Wong CH. Investigation of SSEA-4 binding protein in breast cancer cells. J Am Chem Soc. 2013;135:5934–5937. doi: 10.1021/ja312210c. [DOI] [PubMed] [Google Scholar]
- 8.Potapenko IO, et al. Glycan gene expression signatures in normal and malignant breast tissue; possible role in diagnosis and progression. Mol Oncol. 2010;4:98–118. doi: 10.1016/j.molonc.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharon N. Lectins: Carbohydrate-specific reagents and biological recognition molecules. J Biol Chem. 2007;282:2753–2764. doi: 10.1074/jbc.X600004200. [DOI] [PubMed] [Google Scholar]
- 10.Sharon N, Lis H. History of lectins: From hemagglutinins to biological recognition molecules. Glycobiology. 2004;14:53R–62R. doi: 10.1093/glycob/cwh122. [DOI] [PubMed] [Google Scholar]
- 11.Gabius HJ, André S, Jiménez-Barbero J, Romero A, Solís D. From lectin structure to functional glycomics: Principles of the sugar code. Trends Biochem Sci. 2011;36:298–313. doi: 10.1016/j.tibs.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Hassan MA, Rouf R, Tiralongo E, May TW, Tiralongo J. Mushroom lectins: Specificity, structure and bioactivity relevant to human disease. Int J Mol Sci. 2015;16:7802–7838. doi: 10.3390/ijms16047802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Coq J, Ghosh P. Conservation of the C-type lectin fold for massive sequence variation in a Treponema diversity-generating retroelement. Proc Natl Acad Sci USA. 2011;108:14649–14653. doi: 10.1073/pnas.1105613108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant OC, Woods RJ. Recent advances in employing molecular modelling to determine the specificity of glycan-binding proteins. Curr Opin Struct Biol. 2014;28:47–55. doi: 10.1016/j.sbi.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor ME, Drickamer K. Convergent and divergent mechanisms of sugar recognition across kingdoms. Curr Opin Struct Biol. 2014;28:14–22. doi: 10.1016/j.sbi.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saghatelian A, Couso JP. Discovery and characterization of smORF-encoded bioactive polypeptides. Nat Chem Biol. 2015;11:909–916. doi: 10.1038/nchembio.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: A small protein with major functions. Glycobiology. 2006;16:137R–157R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- 18.Barondes SH, et al. Galectins: A family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 19.Wadler CS, Vanderpool CK. A dual function for a bacterial small RNA: SgrS performs base pairing-dependent regulation and encodes a functional polypeptide. Proc Natl Acad Sci USA. 2007;104:20454–20459. doi: 10.1073/pnas.0708102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kastenmayer JP, et al. Functional genomics of genes with small open reading frames (sORFs) in S. cerevisiae. Genome Res. 2006;16:365–373. doi: 10.1101/gr.4355406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramamurthi KS, Storz G. The small protein floodgates are opening; now the functional analysis begins. BMC Biol. 2014;12:96. doi: 10.1186/s12915-014-0096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz-Orera J, Messeguer X, Subirana JA, Alba MM. Long non-coding RNAs as a source of new peptides. eLife. 2014;3:e03523. doi: 10.7554/eLife.03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma J, et al. Improved identification and analysis of small open reading frame encoded polypeptides. Anal Chem. 2016;88:3967–3975. doi: 10.1021/acs.analchem.6b00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang H, et al. A general strategy for the discovery of metabolic pathways: D-threitol, L-threitol, and erythritol utilization in Mycobacterium smegmatis. J Am Chem Soc. 2015;137:14570–14573. doi: 10.1021/jacs.5b08968. [DOI] [PubMed] [Google Scholar]
- 25.Erjavec J, Kos J, Ravnikar M, Dreo T, Sabotič J. Proteins of higher fungi–from forest to application. Trends Biotechnol. 2012;30:259–273. doi: 10.1016/j.tibtech.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Hibbett DS, et al. A higher-level phylogenetic classification of the Fungi. Mycol Res. 2007;111:509–547. doi: 10.1016/j.mycres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Lindequist U, Niedermeyer TH, Jülich WD. The pharmacological potential of mushrooms. Evid Based Complement Alternat Med. 2005;2:285–299. doi: 10.1093/ecam/neh107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirst HA. Developing new antibacterials through natural product research. Expert Opin Drug Discov. 2013;8:479–493. doi: 10.1517/17460441.2013.779666. [DOI] [PubMed] [Google Scholar]
- 29.Tanasova M, Sturla SJ. Chemistry and biology of acylfulvenes: Sesquiterpene-derived antitumor agents. Chem Rev. 2012;112:3578–3610. doi: 10.1021/cr2001367. [DOI] [PubMed] [Google Scholar]
- 30.Buchel E, Mayer A, Martini U, Anke H, Sterner O. Structure elucidation of omphalotin, a cyclic dodecapeptide with potent nematicidal activity isolated from Omphalotus olearius. Pestic Sci. 1998;54:309–311. [Google Scholar]
- 31.Xu X, Yan H, Chen J, Zhang X. Bioactive proteins from mushrooms. Biotechnol Adv. 2011;29:667–674. doi: 10.1016/j.biotechadv.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Kumar A, et al. An integrated approach for finding overlooked genes in yeast. Nat Biotechnol. 2002;20:58–63. doi: 10.1038/nbt0102-58. [DOI] [PubMed] [Google Scholar]
- 33.Li B, Lu F, Suo X, Nan H, Li B. Antioxidant properties of cap and stipe from Coprinus comatus. Molecules. 2010;15:1473–1486. doi: 10.3390/molecules15031473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan J, et al. Structural elucidation of a neutral fucogalactan from the mycelium of Coprinus comatus. Carbohydr Res. 2006;341:1130–1134. doi: 10.1016/j.carres.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 35.Wu L, Wu Z, Lin Q, Xie L. [Purification and activities of an alkaline protein from mushroom Coprinus comatus] Wei Sheng Wu Xue Bao. 2003;43:793–798. Chinese. [PubMed] [Google Scholar]
- 36.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 37.Masuko T, et al. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal Biochem. 2005;339:69–72. doi: 10.1016/j.ab.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 39.Tateno H, Winter HC, Petryniak J, Goldstein IJ. Purification, characterization, molecular cloning, and expression of novel members of jacalin-related lectins from rhizomes of the true fern Phlebodium aureum (L) J. Smith (Polypodiaceae) J Biol Chem. 2003;278:10891–10899. doi: 10.1074/jbc.M211840200. [DOI] [PubMed] [Google Scholar]
- 40.Liao JH, et al. A multivalent marine lectin from Crenomytilus grayanus possesses anti-cancer activity through recognizing globotriose Gb3. J Am Chem Soc. 2016;138:4787–4795. doi: 10.1021/jacs.6b00111. [DOI] [PubMed] [Google Scholar]
- 41.George TC, et al. Distinguishing modes of cell death using the ImageStream multispectral imaging flow cytometer. Cytometry A. 2004;59:237–245. doi: 10.1002/cyto.a.20048. [DOI] [PubMed] [Google Scholar]
- 42.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nyame AK, Leppänen AM, Bogitsh BJ, Cummings RD. Antibody responses to the fucosylated LacdiNAc glycan antigen in Schistosoma mansoni-infected mice and expression of the glycan among schistosomes. Exp Parasitol. 2000;96:202–212. doi: 10.1006/expr.2000.4573. [DOI] [PubMed] [Google Scholar]
- 44.Machado E, et al. N-Glycosylation of total cellular glycoproteins from the human ovarian carcinoma SKOV3 cell line and of recombinantly expressed human erythropoietin. Glycobiology. 2011;21:376–386. doi: 10.1093/glycob/cwq170. [DOI] [PubMed] [Google Scholar]
- 45.Hirano K, Matsuda A, Shirai T, Furukawa K. Expression of LacdiNAc groups on N-glycans among human tumors is complex. BioMed Res Int. 2014;2014:981627. doi: 10.1155/2014/981627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ju T, Aryal RP, Kudelka MR, Wang Y, Cummings RD. The Cosmc connection to the Tn antigen in cancer. Cancer Biomark. 2014;14:63–81. doi: 10.3233/CBM-130375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Vliet SJ, et al. Carbohydrate profiling reveals a distinctive role for the C-type lectin MGL in the recognition of helminth parasites and tumor antigens by dendritic cells. Int Immunol. 2005;17:661–669. doi: 10.1093/intimm/dxh246. [DOI] [PubMed] [Google Scholar]
- 48.van Die I, et al. The dendritic cell-specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis x. Glycobiology. 2003;13:471–478. doi: 10.1093/glycob/cwg052. [DOI] [PubMed] [Google Scholar]
- 49.van Liempt E, et al. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett. 2006;580:6123–6131. doi: 10.1016/j.febslet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Huang KF, Liu YL, Cheng WJ, Ko TP, Wang AH. Crystal structures of human glutaminyl cyclase, an enzyme responsible for protein N-terminal pyroglutamate formation. Proc Natl Acad Sci USA. 2005;102:13117–13122. doi: 10.1073/pnas.0504184102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holm L, Rosenström P. Dali server: Conservation mapping in 3D. Nucleic Acids Res. 2010;38:W545-9. doi: 10.1093/nar/gkq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Eerde A, Grahn EM, Winter HC, Goldstein IJ, Krengel U. Atomic-resolution structure of the α-galactosyl binding Lyophyllum decastes lectin reveals a new protein family found in both fungi and plants. Glycobiology. 2015;25:492–501. doi: 10.1093/glycob/cwu136. [DOI] [PubMed] [Google Scholar]
- 53.Goldstein IJ, et al. A new alpha-galactosyl-binding protein from the mushroom Lyophyllum decastes. Arch Biochem Biophys. 2007;467:268–274. doi: 10.1016/j.abb.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 54.Pohleven J, et al. Bivalent carbohydrate binding is required for biological activity of Clitocybe nebularis lectin (CNL), the N,N′-diacetyllactosediamine (GalNAcβ1-4GlcNAc, LacdiNAc)-specific lectin from basidiomycete C. nebularis. J Biol Chem. 2012;287:10602–10612. doi: 10.1074/jbc.M111.317263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maude SL, Teachey DT, Porter DL, Grupp SA. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125:4017–4023. doi: 10.1182/blood-2014-12-580068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belver L, Ferrando A. The genetics and mechanisms of T cell acute lymphoblastic leukaemia. Nat Rev Cancer. 2016;16:494–507. doi: 10.1038/nrc.2016.63. [DOI] [PubMed] [Google Scholar]
- 57.Smith DF, Song X, Cummings RD. Use of glycan microarrays to explore specificity of glycan-binding proteins. Methods Enzymol. 2010;480:417–444. doi: 10.1016/S0076-6879(10)80033-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.