Abstract
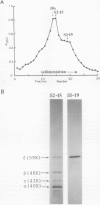
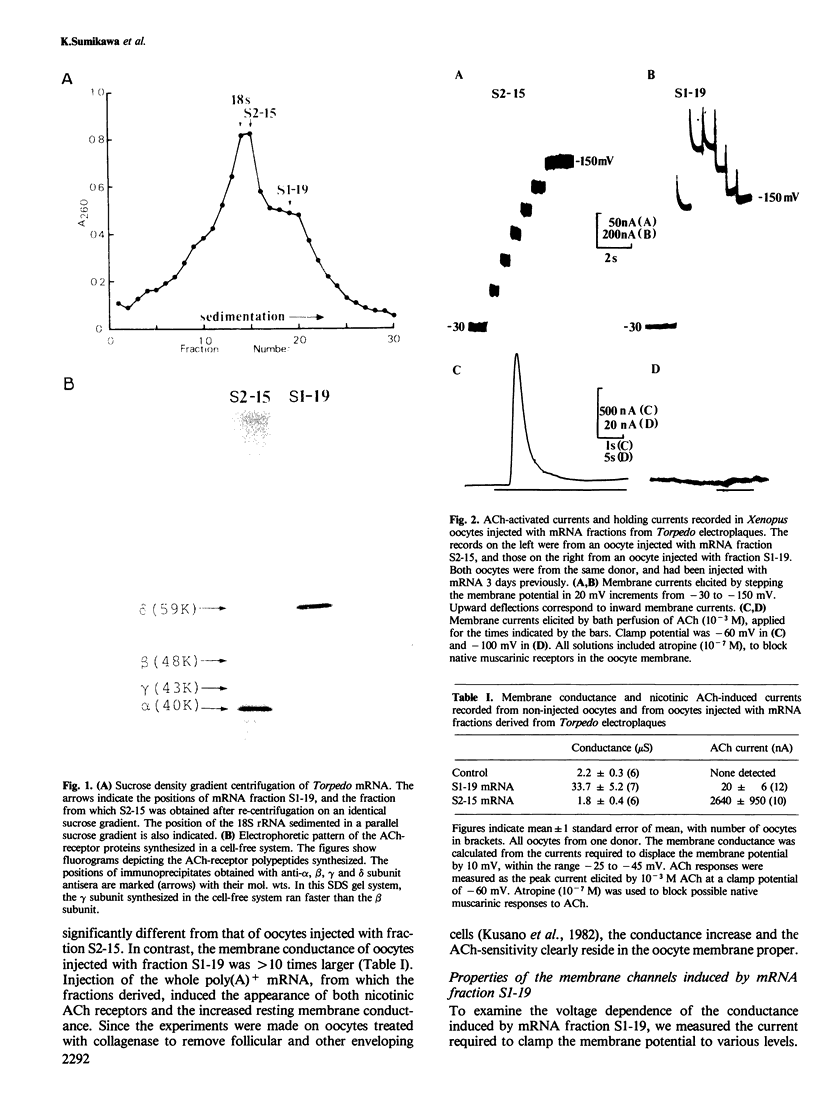
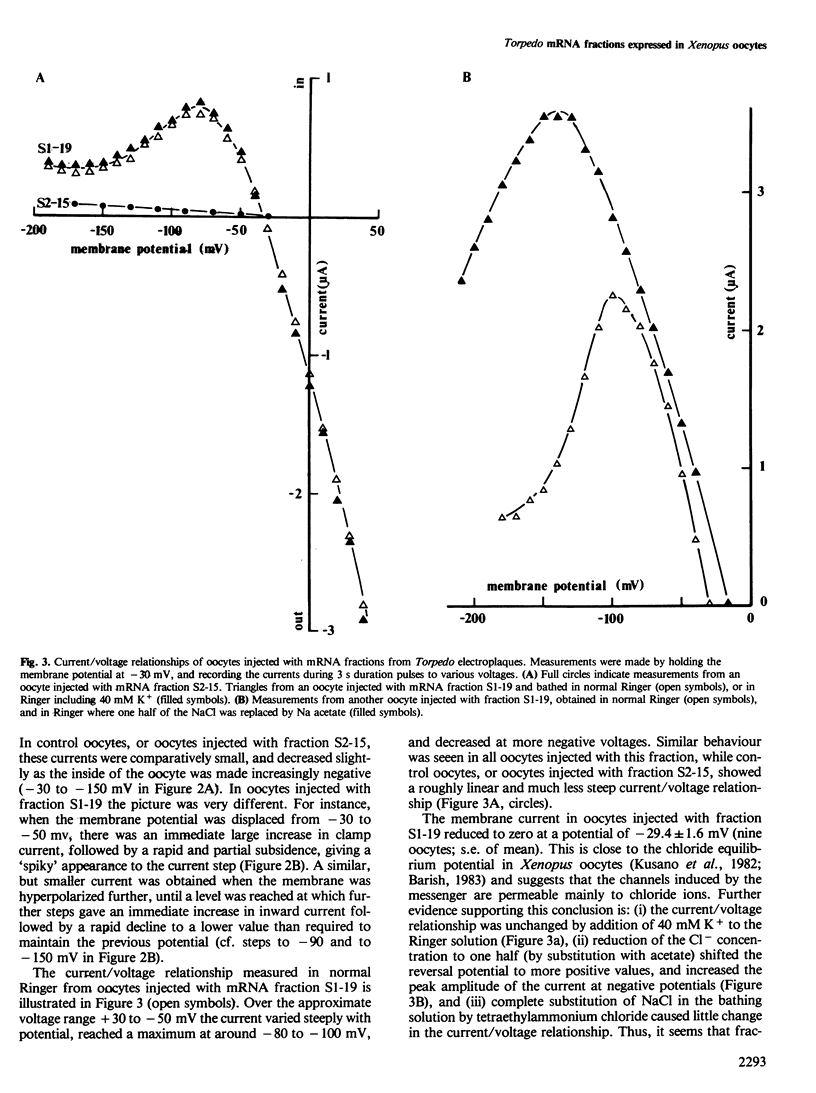
Poly(A)+ mRNA extracted from the electric organ of Torpedo was fractionated by sucrose density gradient centrifugation. After injection into Xenopus oocytes one mRNA fraction induced the appearance of chloride channels in the oocyte membrane. Many of these channels were normally open, and the ensuing chloride current kept the resting potential of injected oocytes close to the chloride equilibrium potential. When the membrane was hyperpolarized, the chloride current was reduced. A separate fraction of mRNA induced the incorporation of acetylcholine receptors into the oocyte membrane. When translated in a cell-free system this fraction directed the synthesis of the alpha, beta, gamma, and delta subunits of the acetylcholine receptor. In contrast, the mRNA fraction that induced the chloride channels caused the synthesis of the delta subunit, a very small amount of alpha, and no detectable beta or gamma subunits. This suggests that the size of the mRNA coding for the chloride channel is similar to the preponderant species of mRNA coding for the delta subunit of the acetylcholine receptor.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. In vitro synthesis, glycosylation, and membrane insertion of the four subunits of Torpedo acetylcholine receptor. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5598–5602. doi: 10.1073/pnas.78.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish M. E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983 Sep;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard E. A., Miledi R., Sumikawa K. Translation of exogenous messenger RNA coding for nicotinic acetylcholine receptors produces functional receptors in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1982 May 22;215(1199):241–246. doi: 10.1098/rspb.1982.0040. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982 Jul 22;215(1201):491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Sumikawa K. Properties of acetylcholine receptors translated by cat muscle mRNA in Xenopus oocytes. EMBO J. 1982;1(11):1307–1312. doi: 10.1002/j.1460-2075.1982.tb01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. Integral membrane channels: studies in model membranes. Physiol Rev. 1983 Oct;63(4):1209–1242. doi: 10.1152/physrev.1983.63.4.1209. [DOI] [PubMed] [Google Scholar]
- Mishina M., Kurosaki T., Tobimatsu T., Morimoto Y., Noda M., Yamamoto T., Terao M., Lindstrom J., Takahashi T., Kuno M. Expression of functional acetylcholine receptor from cloned cDNAs. Nature. 1984 Feb 16;307(5952):604–608. doi: 10.1038/307604a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Hirose T., Asai M., Takashima H., Inayama S., Miyata T. Primary structures of beta- and delta-subunit precursors of Torpedo californica acetylcholine receptor deduced from cDNA sequences. Nature. 1983 Jan 20;301(5897):251–255. doi: 10.1038/301251a0. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Houghton M., Emtage J. S., Richards B. M., Barnard E. A. Active multi-subunit ACh receptor assembled by translation of heterologous mRNA in Xenopus oocytes. Nature. 1981 Aug 27;292(5826):862–864. doi: 10.1038/292862a0. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Houghton M., Smith J. C., Bell L., Richards B. M., Barnard E. A. The molecular cloning and characterisation of cDNA coding for the alpha subunit of the acetylcholine receptor. Nucleic Acids Res. 1982 Oct 11;10(19):5809–5822. doi: 10.1093/nar/10.19.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. M., Miller C. A voltage-gated anion channel from the electric organ of Torpedo californica. J Biol Chem. 1979 Oct 25;254(20):10161–10166. [PubMed] [Google Scholar]