Significance
A rich archaeological record of fish-bone remains testifies to the millennia-long human exploitation of the natural resources of the oceans. In Europe, historical evidence demonstrates that an extensive international industry developed during the Middle Ages that exported preserved cod from the Lofoten Archipelago, northern Norway, to expanding urban centers around the North and Baltic Sea regions. The early origins of this iconic exchange, however, have long been debated. We genetically trace the ancestry of Viking Age fish from mainland Europe to the North East Arctic cod population that supports the modern Lofoten fisheries. This application of genome-wide analyses from ancient fish bone reveals an early origin of what became an economically important trade, with implications for archaeology and environmental history.
Keywords: genomics, high-throughput sequencing, trade, chromosomal inversion, fish bone
Abstract
Knowledge of the range and chronology of historic trade and long-distance transport of natural resources is essential for determining the impacts of past human activities on marine environments. However, the specific biological sources of imported fauna are often difficult to identify, in particular if species have a wide spatial distribution and lack clear osteological or isotopic differentiation between populations. Here, we report that ancient fish-bone remains, despite being porous, brittle, and light, provide an excellent source of endogenous DNA (15–46%) of sufficient quality for whole-genome reconstruction. By comparing ancient sequence data to that of modern specimens, we determine the biological origin of 15 Viking Age (800–1066 CE) and subsequent medieval (1066–1280 CE) Atlantic cod (Gadus morhua) specimens from excavation sites in Germany, Norway, and the United Kingdom. Archaeological context indicates that one of these sites was a fishing settlement for the procurement of local catches, whereas the other localities were centers of trade. Fish from the trade sites show a mixed ancestry and are statistically differentiated from local fish populations. Moreover, Viking Age samples from Haithabu, Germany, are traced back to the North East Arctic Atlantic cod population that has supported the Lofoten fisheries of Norway for centuries. Our results resolve a long-standing controversial hypothesis and indicate that the marine resources of the North Atlantic Ocean were used to sustain an international demand for protein as far back as the Viking Age.
The global trade of animal products is driven by an increasingly international economy that geographically separates consumer demands from their ecological footprint elsewhere (1). However, long-range trade has an extensive history (2). For instance, in medieval and postmedieval Europe, the onset of urbanized market economies has been linked to the growth of long-range trade by historical and archaeological evidence (3, 4). The exploitation of increasingly distant fish populations has proven to be one of the clearest demonstrations of this ecological globalization (5–9). Fisheries around the coastal regions of the Lofoten Archipelago in Norway have a particularly long history; in this region, the regular arrival of seasonal spawning aggregations of Atlantic cod—migrating southwards from the Arctic Barents Sea (10, 11)—coincides with those climatic conditions ideal for the freeze drying and long-term preservation of cod without the use of expensive salt. These unique conditions allowed the development of an extensive long-distance trade and fishery that for centuries provided a high-quality yet affordable source of protein with a long shelf-life to urban centers around southern North and Baltic Sea regions (12). Identifying the emergence and growth of such extensive fisheries is a fundamental step in the study of past impacts of human exploitation (13–16). However, in the centuries before systematic historical records, this crucially depends on the ability to determine the biological source of archaeological samples.
Ancient DNA (aDNA) methods provide unique opportunities to elucidate diversification events, genetic admixture, or migration routes (e.g., see ref. 17) and have been used to assign historic individuals to their most likely geographic origin (18–20). In particular, the genome-wide analysis of 1,000s or more single nucleotide polymorphisms (SNPs) allows the determination of an individual’s geographic origin with accuracy and precision, even when limited genetic differentiation exists among regions (20–22). Since low levels of genetic differentiation are typical for marine species with high dispersal capabilities like Atlantic cod (23, 24), such genome-wide approaches are essential to increase the assignment power of modern (22) and ancient samples of these species (25). So far, however, aDNA studies using genome-wide approaches are lacking for marine fishes.
Here, we compare whole genome data of 15 Viking Age and medieval Atlantic cod from five archaeological sites to those of 168 modern specimens from six populations (Fig. 1). We aim to discover where the cod were caught by analyzing fish bones from these sites. For example, were the cod eaten in a Viking Age (800–1066 CE) town of the western Baltic (Haithabu) caught in local waters, in the nearby North Sea or in truly distant waters of Arctic Norway (12), the Northern Isles of Scotland (26), and/or Iceland (6)? All of these distant regions are known to have produced dried fish for export later in the Middle Ages (27).
Fig. 1.
Approximate sampling locations of Atlantic cod in the northern Atlantic region in Europe. (A) Atlantic cod (Gadus morhua) specimens (sample size is indicated between brackets) were obtained from modern populations (black) and archaeological excavations (red). The NEA sample was obtained in winter, when this population migrates southwards from the Barents Sea to the Lofoten Archipelago to spawn. The Lofoten population was sampled during summer when the NEA cod are absent from this region. The modern range of Atlantic cod is indicated by blue shading. (B) Archaeological Atlantic cod jaw-bone (premaxilla) from Orkney.
To improve our ability to identify the source of these ancient samples, we exploit our knowledge of patterns of genomic variation found among modern Atlantic cod populations. Distinct genomic regions with elevated population differentiation have been identified for this species (22, 28–30). Several of these regions are colocalized into four Mbp-scale, polymorphic inversions with high linkage disequilibrium (31–34). These polymorphisms segregate with a distinct geographical distribution and have been associated with temperature clines and ecotype-specific migratory behavior (31, 32, 34). Their divergence can be used to improve the traceability of individuals (22) and, given their large size (between ∼5 and ∼17 Mbp), the inversion state of ancient samples can be easily determined from low coverage sequencing data.
We specifically focus on fish bones from Haithabu (n = 5, dated 800–1066 CE) for several reasons. First, a unique ninth-century account records the voyage of a Viking chieftain and trader from Arctic Norway to Haithabu. While his cargo is not fully specified—except for walrus tusks to be gifted to Alfred the Great of England (35) —a mundane consignment of dried cod may not have merited historical record. Second, artifactual evidence has confirmed contact between Haithabu and Scandinavian settlements of the North Atlantic (36). Third, Haithabu (and neighboring Schleswig, which later replaced Haithabu) preceded Lübeck as the main focus of trade in the western Baltic region. Because Lübeck came to control much of Europe’s dried cod trade (37), it is possible that its predecessors were already engaged in this exchange—at dates for which the historical record is incomplete. Fourth, the cod finds from Haithabu occurred alongside species such as saithe (Pollachius virens), ling (Molva molva), and halibut (Hippoglossus hippoglossus), which is more consistent with fishing in the North Sea or North Atlantic than in the Kattegat or Baltic Sea (38–40). Finally, isotope analyses using bone collagen (8, 41) or bone carbonate (9) suggest that the Haithabu cod were not locally caught, although their origin remains ambiguous. This previous research pinpoints the fish bones from Haithabu as ideal material with which to test the hypothesis of Viking Age transport of cod from northern Norway by using genomic methods. Without definitive interpretation of the Haithabu cod, the earliest secure historical and archaeological evidence for dried cod imports to towns around the North and Baltic Seas dates to the 12th, 13th, and 14th centuries (7, 8, 37, 42, 43). Fishing in Arctic Norway occurred long before the Viking Age (44), but most arguments for pre-12th-century long-range exports have had to rely on historical sources such as Icelandic sagas that postdate the events that they nominally described (43, 45).
In addition to the specimens from Haithabu, we include two bones (dated 1100–1280 CE) from Schleswig, one bone (dated 700–950 CE) from the small inland trading settlement of Bjørkum in western Norway and two bones (dated 1025–1175 CE) from Oslo. Lastly, five cod bones (dated 1000–1200 CE) are from a fishing settlement in Orkney, northern Scotland, where a local catch can be confidently assumed (26).
Results
We obtained 1,013 million paired reads (average read length 40–78 bp) that contained 15–46% endogenous DNA and resulted in 1- to 3.4-fold nuclear coverage for 15 of 19 ancient cod samples (Table S1). Analyses of postmortem degradation patterns showed the typical fragmentation and elevated deamination rates as expected from authentic ancient DNA (Fig. S1). For the modern data (Fig. 1), an average of 47 million paired reads were obtained per specimen, resulting in ∼ninefold individual coverage of the nuclear genome (Table S2). After SNP calling and filtering, we obtained a dataset of 156,695 SNPs.
Table S1.
Specimen ID, location, estimated date, and bone type of ancient Atlantic cod samples
| Specimen | Country | Location | Date, CE | Bone type | Reads, millions | Clonality, % | Endogenous DNA, % | Average insert length, bp | Fold coverage |
| COD003 | Germany | Schleswig | c.1100–1200 | Vertebra | 52 | 14 | 33 | 69 | 1.9 |
| COD023 | Germany | Schleswig | c.1200–1280 | Articular | 67 | 6 | 37 | 51 | 2.0 |
| COD027 | United Kingdom | Orkney | c.1000–1200 | Premaxilla | 59 | 9 | 42 | 75 | 2.9 |
| COD028 | United Kingdom | Orkney | c.1000–1200 | Premaxilla | 84 | 4 | 15 | 72 | 1.4 |
| COD029 | United Kingdom | Orkney | c.1000–1200 | Premaxilla | 58 | 5 | 33 | 71 | 2.1 |
| COD030 | United Kingdom | Orkney | c.1000–1200 | Premaxilla | 55 | 8 | 25 | 72 | 1.6 |
| COD034 | United Kingdom | Orkney | c.1000–1200 | Premaxilla | 49 | 23 | 27 | 72 | 1.5 |
| COD053 | Germany | Haithabu | c.800–1066 | Cleithrum | 83 | 8 | 28 | 47 | 1.7 |
| COD054 | Germany | Haithabu | c.800–1066 | Cleithrum | 58 | 8 | 37 | 67 | 2.3 |
| COD061 | Germany | Haithabu | c.800–1066 | Vertebra | 72 | 11 | 24 | 40 | 1.1 |
| COD062 | Germany | Haithabu | c.800–1066 | Vertebra | 76 | 8 | 25 | 46 | 1.4 |
| COD063 | Germany | Haithabu | c.800–1066 | Articular | 95 | 6 | 32 | 46 | 2.2 |
| COD076 | Norway | Bjørkum | c.700–950 | Dentary | 74 | 27 | 18 | 48 | 1.0 |
| COD086 | Norway | Oslo | c.1025–1175 | Ceratohyal | 59 | 12 | 46 | 78 | 3.4 |
| COD092 | Norway | Oslo | c.1025–1175 | Cleithra | 72 | 16 | 21 | 47 | 1.2 |
All bones were morphologically identified as Atlantic cod (Gadus morhua). WGS shotgun libraries were paired-end sequenced, and we report the number of collapsed reads, their clonality, their endogenous DNA content (defined as the unique, nonrepetitive fraction of reads aligning toward the gadmor2 reference genome with a minimum MapQ value of 25), the average insert length, and the fold coverage obtained for the nuclear genome. Four other specimens (not shown) were extracted of which two did not yield libraries, and two had endogenous DNA content below 1%.
Fig. S1.
aDNA fragmentation and misincorporation patterns of sequencing read data from 15 Atlantic cod samples. Patterns were obtained by using MapDamage v. 2.0.6 after down-sampling BAM files to 1 million reads. For visualization purposes, we only show the typical increase in C > T misincorporations due to cytosine deamination at the 5′-end of DNA fragments and the corresponding increase of G > A misincorporations at the 3′-end.
Table S2.
Population, specimen ID and sample date of modern Atlantic cod specimens
| Population | Specimen_ID | Sample date | Spawning population | No. of reads, millions | Fold coverage |
| Eastern Baltic | ARK_4001 | 2012, May | Yes | 40 | 7.4 |
| Eastern Baltic | ARK_4002 | 2012, May | Yes | 20 | 3.8 |
| Eastern Baltic | ARK_4003 | 2012, May | Yes | 38 | 6.9 |
| Eastern Baltic | ARK_4006 | 2012, May | Yes | 34 | 6.3 |
| Eastern Baltic | ARK_4007 | 2012, May | Yes | 34 | 6.4 |
| Eastern Baltic | ARK_4008 | 2012, May | Yes | 47 | 8.8 |
| Eastern Baltic | ARK_4010 | 2012, May | Yes | 31 | 5.9 |
| Eastern Baltic | ARK_4011 | 2012, May | Yes | 49 | 9.1 |
| Eastern Baltic | ARK_4012 | 2012, May | Yes | 35 | 6.6 |
| Eastern Baltic | ARK_4013 | 2012, May | Yes | 37 | 6.9 |
| Eastern Baltic | ARK_4014 | 2012, May | Yes | 34 | 6.1 |
| Eastern Baltic | ARK_4015 | 2012, May | Yes | 44 | 8.3 |
| Eastern Baltic | ARK_4016 | 2012, May | Yes | 29 | 5.1 |
| Eastern Baltic | ARK_4017 | 2012, May | Yes | 45 | 8.5 |
| Eastern Baltic | ARK_4018 | 2012, May | Yes | 33 | 6.1 |
| Eastern Baltic | ARK_4019 | 2012, May | Yes | 47 | 8.8 |
| Eastern Baltic | ARK_4020 | 2012, May | Yes | 23 | 4.1 |
| Eastern Baltic | ARK_4021 | 2012, May | Yes | 57 | 10.6 |
| Eastern Baltic | ARK_4022 | 2012, May | Yes | 34 | 6.4 |
| Eastern Baltic | ARK_4024 | 2012, May | Yes | 31 | 5.8 |
| Eastern Baltic | ARK_4030 | 2012, May | Yes | 34 | 6.5 |
| Eastern Baltic | ARK_4032 | 2012, May | Yes | 91 | 17.2 |
| Eastern Baltic | ARK_4039 | 2012, May | Yes | 63 | 11.9 |
| Eastern Baltic | ARK_4043 | 2012, May | Yes | 68 | 12.8 |
| Eastern Baltic | BOR_90E | 2011, April | Yes | 44 | 8.3 |
| Eastern Baltic | BOR_91E | 2011, April | Yes | 47 | 9.0 |
| Eastern Baltic | BOR_74E | 2011, May | Yes | 45 | 8.6 |
| Eastern Baltic | BOR_79E | 2011, May | Yes | 50 | 9.6 |
| Eastern Baltic | BOR_60E | 2012, April | Yes | 46 | 8.7 |
| Eastern Baltic | BOR_611E | 2012, May | Yes | 45 | 8.5 |
| Eastern Baltic | BOR_613E | 2012, May | Yes | 43 | 8.2 |
| Eastern Baltic | BOR_614E | 2012, May | Yes | 42 | 7.9 |
| Eastern Baltic | BOR_615E | 2012, May | Yes | 42 | 8.0 |
| Eastern Baltic | BOR_617E | 2012, May | Yes | 41 | 7.8 |
| Eastern Baltic | BOR_621E | 2012, May | Yes | 41 | 7.8 |
| Eastern Baltic | BOR_624E | 2012, May | Yes | 42 | 8.1 |
| Eastern Baltic | BOR_630E | 2012, May | Yes | 40 | 7.5 |
| Eastern Baltic | BOR_655E | 2012, May | Yes | 73 | 13.8 |
| Eastern Baltic | BOR_659E | 2012, May | Yes | 59 | 11.1 |
| Eastern Baltic | BOR_664E | 2012, May | Yes | 66 | 12.5 |
| Eastern Baltic | BOR_AL713 | 2012, May | Yes | 44 | 8.4 |
| Eastern Baltic | BOR_739E | 2012, May | Yes | 57 | 10.8 |
| Eastern Baltic | BOR_749E | 2012, May | Yes | 59 | 11.2 |
| Eastern Baltic | BOR_760E | 2012, May | Yes | 6 | 1.1 |
| Eastern Baltic | BOR_AL724 | 2012, May | Yes | 49 | 9.3 |
| Eastern Baltic | BOR_AL736 | 2012, May | Yes | 44 | 8.4 |
| Eastern Baltic | BOR_AL741 | 2012, May | Yes | 46 | 8.7 |
| Eastern Baltic | BOR_AL777 | 2012, May | Yes | 43 | 8.3 |
| Iceland | I50_02 | 2003, April | Yes | 49 | 10.9 |
| Iceland | I50_03 | 2003, April | Yes | 43 | 9.7 |
| Iceland | I50_04 | 2003, April | Yes | 44 | 9.8 |
| Iceland | I50_05 | 2003, April | Yes | 46 | 10.3 |
| Iceland | I50_06 | 2003, April | Yes | 32 | 7.1 |
| Iceland | I50_07 | 2003, April | Yes | 42 | 9.4 |
| Iceland | I50_08 | 2003, April | Yes | 35 | 7.7 |
| Iceland | I50_09 | 2003, April | Yes | 51 | 11.2 |
| Iceland | I50_10 | 2003, April | Yes | 43 | 9.6 |
| Iceland | I50_11 | 2003, April | Yes | 50 | 11.1 |
| Iceland | I50_12 | 2003, April | Yes | 39 | 8.8 |
| Iceland | I50_13 | 2003, April | Yes | 48 | 10.7 |
| Iceland | I50_14 | 2003, April | Yes | 26 | 5.7 |
| Iceland | I50_15 | 2003, April | Yes | 41 | 9.3 |
| Iceland | I50_16 | 2003, April | Yes | 49 | 11.0 |
| Iceland | I50_17 | 2003, April | Yes | 43 | 9.5 |
| Iceland | I50_18 | 2003, April | Yes | 46 | 10.1 |
| Iceland | I50_19 | 2003, April | Yes | 52 | 11.4 |
| Iceland | I50_20 | 2003, April | Yes | 54 | 12.1 |
| Iceland | I50_23 | 2003, April | Yes | 58 | 12.9 |
| Iceland | I50_26 | 2003, April | Yes | 53 | 10.3 |
| Iceland | I50_38 | 2003, April | Yes | 64 | 12.3 |
| Iceland | I50_41 | 2003, April | Yes | 92 | 19.1 |
| Iceland | I50_42 | 2003, April | Yes | 52 | 11.6 |
| Lofoten | LOF_A_14_01 | 2014, August | No | 60 | 11.4 |
| Lofoten | LOF_A_14_03 | 2014, August | No | 54 | 10.2 |
| Lofoten | LOF_A_14_04 | 2014, August | No | 47 | 9.0 |
| Lofoten | LOF_A_14_05 | 2014, August | No | 47 | 8.9 |
| Lofoten | LOF_A_14_06 | 2014, August | No | 52 | 10.0 |
| Lofoten | LOF_A_14_08 | 2014, August | No | 43 | 8.2 |
| Lofoten | LOF_A_14_09 | 2014, August | No | 50 | 9.5 |
| Lofoten | LOF_A_14_10 | 2014, August | No | 48 | 9.1 |
| Lofoten | LOF_A_14_11 | 2014, August | No | 53 | 10.1 |
| Lofoten | LOF_A_14_16 | 2014, August | No | 54 | 10.1 |
| Lofoten | LOF_A_14_17 | 2014, August | No | 60 | 11.4 |
| Lofoten | LOF_A_14_18 | 2014, August | No | 52 | 9.9 |
| Lofoten | LOF_A_14_19 | 2014, August | No | 54 | 10.3 |
| Lofoten | LOF_A_14_20 | 2014, August | No | 43 | 8.2 |
| Lofoten | LOF_A_14_21 | 2014, August | No | 52 | 9.8 |
| Lofoten | LOF_A_14_22 | 2014, August | No | 48 | 9.1 |
| Lofoten | LOF_A_14_23 | 2014, August | No | 46 | 8.8 |
| Lofoten | LOF_A_14_24 | 2014, August | No | 49 | 9.3 |
| Lofoten | LOF_A_14_25 | 2014, August | No | 59 | 11.2 |
| Lofoten | LOF_A_14_26 | 2014, August | No | 51 | 9.7 |
| Lofoten | LOF_A_14_27 | 2014, August | No | 65 | 12.4 |
| Lofoten | LOF_A_14_28 | 2014, August | No | 49 | 9.3 |
| Lofoten | LOF_A_14_29 | 2014, August | No | 46 | 8.7 |
| Lofoten | LOF_A_14_30 | 2014, August | No | 44 | 8.3 |
| Lofoten | LOF_A_14_33 | 2014, August | No | 46 | 8.8 |
| Lofoten | LOF_A_14_41 | 2014, August | No | 45 | 8.6 |
| Lofoten | LOF_A_14_43 | 2014, August | No | 195 | 37.0 |
| North East Arctic | LOF_M_14_26 | 2014, March | Yes | 46 | 8.7 |
| North East Arctic | LOF_M_14_27 | 2014, March | Yes | 47 | 8.9 |
| North East Arctic | LOF_M_14_28 | 2014, March | Yes | 44 | 8.3 |
| North East Arctic | LOF_M_14_29 | 2014, March | Yes | 46 | 8.6 |
| North East Arctic | LOF_M_14_30 | 2014, March | Yes | 43 | 8.1 |
| North East Arctic | LOF_M_14_31 | 2014, March | Yes | 50 | 9.4 |
| North East Arctic | LOF_M_14_32 | 2014, March | Yes | 50 | 9.4 |
| North East Arctic | LOF_M_14_33 | 2014, March | Yes | 50 | 9.4 |
| North East Arctic | LOF_M_14_35 | 2014, March | Yes | 42 | 8.0 |
| North East Arctic | LOF_M_14_36 | 2014, March | Yes | 53 | 10.0 |
| North East Arctic | LOF_M_14_43 | 2014, March | Yes | 88 | 16.7 |
| North East Arctic | LOF_M_14_44 | 2014, March | Yes | 47 | 9.0 |
| North East Arctic | LOF_M_14_45 | 2014, March | Yes | 45 | 8.6 |
| North East Arctic | LOF_M_14_46 | 2014, March | Yes | 57 | 10.7 |
| North East Arctic | LOF_M_14_47 | 2014, March | Yes | 50 | 9.4 |
| North East Arctic | LOF_M_14_50 | 2014, March | Yes | 39 | 7.4 |
| North East Arctic | LOF_M_14_51 | 2014, March | Yes | 43 | 8.1 |
| North East Arctic | LOF_M_14_52 | 2014, March | Yes | 44 | 8.3 |
| North East Arctic | LOF_M_14_53 | 2014, March | Yes | 24 | 4.5 |
| North East Arctic | LOF_M_14_54 | 2014, March | Yes | 43 | 8.2 |
| North East Arctic | LOF_M_14_55 | 2014, March | Yes | 46 | 8.7 |
| North East Arctic | LOF_M_14_56 | 2014, March | Yes | 55 | 10.5 |
| North East Arctic | LOF_M_14_62 | 2014, March | Yes | 50 | 9.4 |
| North East Arctic | LOF_M_14_68 | 2014, March | Yes | 38 | 7.2 |
| Øresund | ORE_301 | 2012, March | Yes | 44 | 8.2 |
| Øresund | ORE_302 | 2012, March | Yes | 42 | 7.7 |
| Øresund | ORE_303 | 2012, March | Yes | 40 | 7.6 |
| Øresund | ORE_308 | 2012, March | Yes | 33 | 6.0 |
| Øresund | ORE_309 | 2012, March | Yes | 45 | 8.4 |
| Øresund | ORE_310 | 2012, March | Yes | 39 | 7.4 |
| Øresund | ORE_313 | 2012, March | Yes | 105 | 19.8 |
| Øresund | ORE_314 | 2012, March | Yes | 34 | 6.0 |
| Øresund | ORE_315 | 2012, March | Yes | 25 | 4.7 |
| Øresund | ORE_316 | 2012, March | Yes | 49 | 9.3 |
| Øresund | ORE_317 | 2012, March | Yes | 38 | 7.1 |
| Øresund | ORE_318 | 2012, March | Yes | 36 | 6.6 |
| Øresund | ORE_322 | 2012, March | Yes | 33 | 6.0 |
| Øresund | ORE_323 | 2012, March | Yes | 52 | 9.7 |
| Øresund | ORE_325 | 2012, March | Yes | 46 | 8.6 |
| Øresund | ORE_326 | 2012, March | Yes | 39 | 7.2 |
| Øresund | ORE_331 | 2012, March | Yes | 50 | 9.3 |
| Øresund | ORE_332 | 2012, March | Yes | 49 | 9.2 |
| Øresund | ORE_333 | 2012, March | Yes | 48 | 8.9 |
| Øresund | ORE_336 | 2012, March | Yes | 41 | 7.7 |
| Øresund | ORE_341 | 2012, March | Yes | 45 | 8.4 |
| North Sea | SOD_01 | 2002, March | Yes | 43 | 8.3 |
| North Sea | SOD_02 | 2002, March | Yes | 46 | 8.8 |
| North Sea | SOD_03 | 2002, March | Yes | 44 | 8.3 |
| North Sea | SOD_04 | 2002, March | Yes | 55 | 10.4 |
| North Sea | SOD_06 | 2002, March | Yes | 45 | 8.7 |
| North Sea | SOD_07 | 2002, March | Yes | 46 | 8.8 |
| North Sea | SOD_08 | 2002, March | Yes | 42 | 8.1 |
| North Sea | SOD_09 | 2002, March | Yes | 49 | 9.4 |
| North Sea | SOD_10 | 2002, March | Yes | 42 | 8.1 |
| North Sea | SOD_13 | 2002, March | Yes | 43 | 8.3 |
| North Sea | SOD_14 | 2002, March | Yes | 48 | 9.2 |
| North Sea | SOD_15 | 2002, March | Yes | 45 | 8.5 |
| North Sea | SOD_17 | 2002, March | Yes | 46 | 8.7 |
| North Sea | SOD_19 | 2002, March | Yes | 47 | 8.8 |
| North Sea | SOD_20 | 2002, March | Yes | 39 | 7.2 |
| North Sea | SOD_21 | 2002, March | Yes | 45 | 8.3 |
| North Sea | SOD_22 | 2002, March | Yes | 46 | 8.8 |
| North Sea | SOD_23 | 2002, March | Yes | 44 | 8.4 |
| North Sea | SOD_25 | 2002, March | Yes | 45 | 8.6 |
| North Sea | SOD_26 | 2002, March | Yes | 53 | 10.0 |
| North Sea | SOD_27 | 2002, March | Yes | 51 | 9.0 |
| North Sea | SOD_28 | 2002, March | Yes | 46 | 8.6 |
| North Sea | SOD_29 | 2002, March | Yes | 46 | 8.8 |
| North Sea | SOD_30 | 2002, March | Yes | 42 | 7.6 |
We report if sampling took place during spawning, the number of reads obtained, and the resulting fold coverage for the nuclear genome.
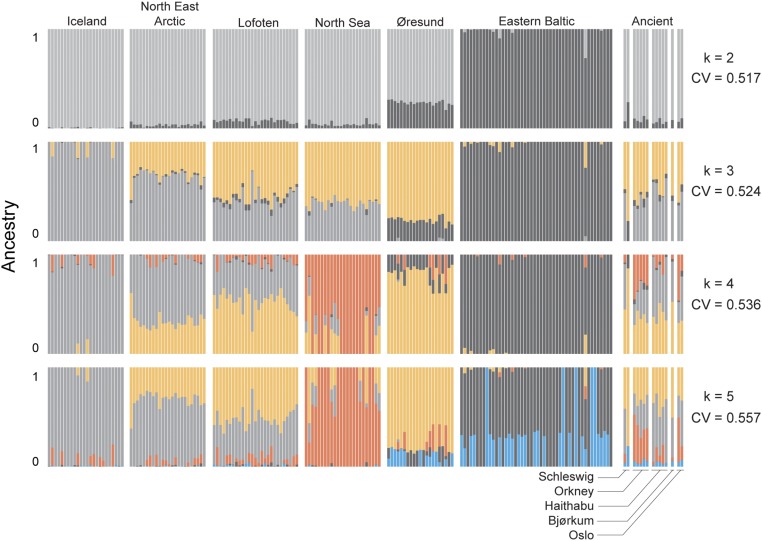
First, we compared the genome-wide diversity of ancient samples and modern samples using principal component (PCA) and ADMIXTURE analyses. We excluded the four chromosomes [linkage group (LG)01, 02, 07, and 12] that contain the large inversions (31–33), as these distort genetic analyses that assume linkage equilibrium. The PCA shows that three modern populations—North East Arctic (NEA), Lofoten and North Sea—form a large, overlapping group, with Iceland clustering near the NEA (Fig. 2A). The majority of the ancient samples cluster with this large group. The modern eastern Baltic and Øresund form two distinct groups, with one ancient specimen from Schleswig clustering with the Øresund. A lack of strong genetic population structure between most modern samples is supported by ADMIXTURE, for which the best fit [based on lowest cross-validation (CV) error] is two populations (k = 2). Here, Iceland and eastern Baltic have distinct ancestry, whereas the other modern populations show a more mixed ancestry (Fig. 2B). The Øresund has a higher level of Baltic ancestry than the other admixed populations. All ancient individuals have mixed ancestry, with the one Schleswig sample having a similar level of Baltic ancestry as the modern Øresund. At a lower optimal fit, ADMIXTURE estimates biologically plausible ancestry for k = 3 (identifying an Øresund component) and k = 4 (identifying a North Sea component). Nonetheless, for k = 5, the model separates the original eastern Baltic cluster rather than differentiating either NEA or Lofoten cod (Fig. S2). Overall, these analyses show that the ancient individuals are genetically most related to four modern populations: one Schleswig individual is genetically more similar to the Øresund and all other individuals clearly classify to the NEA, Lofoten, or North Sea population.
Fig. 2.
Genetic population structure in 183 Atlantic cod specimens. (A) PCA based on 99,819 SNPs. Ancient specimens (stars) were projected onto the first two principal components calculated by using individuals from modern populations (circles). (B) ADMIXTURE ancestry components (k = 2) for modern and ancient specimens. The width of the bar for ancient specimens is widened to aid visualization. LG01, 02, 07, 12, and unplaced scaffolds were excluded from these analyses (Results for explanation).
Fig. S2.
ADMIXTURE ancestry components for modern and ancient Atlantic cod specimens. Population structure was investigated by using models with a variable number of clusters (k). Model fit was assessed by calculating the CV error, with a lower CV error indicating a better fit. LG01, 02, 07, 12, and unplaced scaffolds were excluded from these analyses (see Results for explanation).
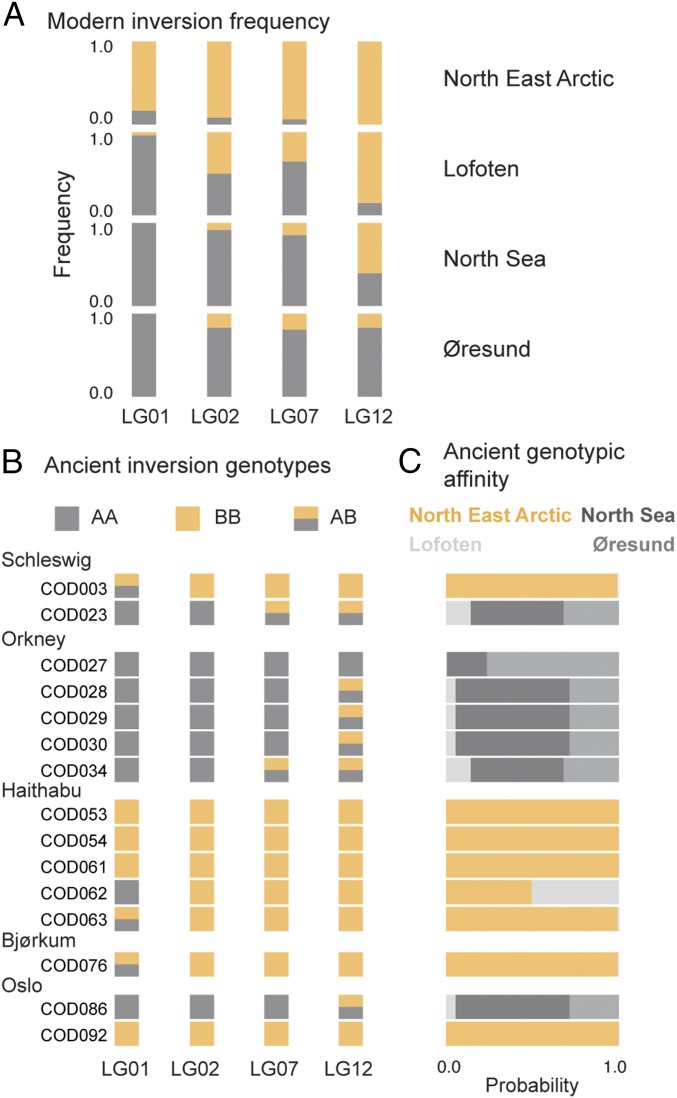
To further differentiate the origins of the ancient individuals, we determined their genotypes for the inversion loci on LG01, 02, 07, and 12. Modern populations can have divergent allele frequencies for these loci (31, 32), and the NEA population is set apart by having inverted alleles near fixation at all four loci (Fig. 3A). PCAs of these inversions for the four modern populations show the typical trimodal clustering of a biallelic locus with heterozygote genotypes clustering intermediate to the two divergent homozygotes (46) (Fig. S3). All ancient individuals cluster within this trimodel pattern, allowing for the decisive determination of their genotypes at each inversion locus (Fig. S3). Collinear alleles dominate in the Orkney samples, while inverted alleles dominate in Haithabu (Fig. 3B). The Bjørkum sample has a predominantly inverted composite genotype, whereas Schleswig and Oslo show a mixed pattern, with one specimen having a collinear and the other having a more inverted composite genotype, in each location, respectively.
Fig. 3.
Spatial genomic variation in megabase-scale inversions in Atlantic cod. (A) Allele frequency distribution of four inversions (on LG01, 02, 07, and 12) in four modern populations. The collinear allele (gray) and inverted allele (yellow) segregate as biallelic loci. (B) Individual inversion genotypes of ancient Atlantic cod. The collinear (gray, AA), inverted (yellow, BB), and heterozygote (yellow/gray, AB) genotypes segregate independently on four chromosomes. (C) Genotypic affinity of ancient specimens. The overall probability of obtaining the ancient individual’s composite genotype was calculated by binomial sampling of inversion genotypes from the respective allele frequency distributions of the four modern populations.
Fig. S3.
PCA of genomic inversions in Atlantic cod. Ancient specimens (stars) were projected onto the first two principal components calculated by using individuals from modern populations (circles). The first principal component (PCA 1) separates genomic variation within each of the 4-Mbp-long regions (LG01, 9.1–26.2 Mbp; LG02, 18.5–24 Mbp; LG07, 13.6–23 Mbp; LG12, 1.3–13.6 Mbp) into distinct clusters (gray dotted ovals) that reflect the biallelic segregation of the three major inversion genotypes (AA, collinear; AB, heterozygote; BB, inverted). The number of SNPs (n) used per region is indicated. Mean heterozygosity values per genotype (presented in gray under each genotype; estimated by calculating the inbreeding coefficient F using a method of moments as implemented in VCFTOOLS v0.1.14) show the marked decrease in F values for the AB genotypes due to heterozygote excess. Ancient samples follow the trimodal cluster pattern of the modern individuals.
Based on their inversion genotypes, we investigated if ancient individuals statistically differed in their resemblance toward a particular modern population. The probability of obtaining an inversion genotype follows a binomial distribution given the underlying allele frequency in a population. We can assume independence between loci since the inversions are located on different chromosomes. It is thus straightforward to calculate the overall probability of obtaining a composite ancient inversion genotype—based on the four modern populations’ respective allele frequencies—as a measure of an individual’s affinity toward a specific population. Based on this probability, we find that, apart from a single Haithabu specimen, the ancient samples show two types of affinity (Fig. 3C); individuals with a predominantly collinear composite genotype have a >99% probability of being drawn from the Lofoten, the North Sea, or the Øresund population. Conversely, individuals with a predominantly inverted composite genotype have a >99% probability of being drawn solely from the NEA population. The atypical Haithabu specimen has a >99% probability of coming from either the NEA or Lofoten population.
Discussion
By investigating genome-wide patterns of variation—including four megabase-scale inversions (31–34)—we show that ancient cod specimens from Viking Age (Haithabu and Bjørkum) and early medieval (Schleswig and Oslo) trading sites have a genomic affinity to the modern North East Arctic population. This finding has archaeological and evolutionary implications.
First, this study provides a unique genome-wide study of archaeological fish bone and demonstrates its potential as an archive of ancient DNA. We obtained short reads (<100 bp), with the typical fragmentation and cysteine deamination patterns expected after postmortem degradation (47–49). These results are therefore fully consistent with the extraction and analysis of authentic ancient Atlantic cod DNA. Not all types of animal bone preserve DNA equally well, and significantly higher proportions of endogenous DNA have been found in dense and heavy bone types such as the petrous bone (50–52). It is presumed that it is the high density of the bone that leads to reduced bacterial and chemical-mediated decay and improved DNA preservation (50, 53). This hypothesis would suggest that fish bones—that are porous, brittle and light—should be a poor source of DNA. Instead, we find surprisingly high levels (15–46%) of endogenous DNA preservation in 15 of 19 fish bone specimens up to 1,300 y old from five different archeological sites. Notwithstanding the use of a recently developed extraction protocol aimed to maximize endogenous DNA (54), this rate of success compares favorably to results from mammalian bones whereby the majority of samples—excluding petrous bones—typically yield a few percent endogenous DNA at most (55). Our positive results agree with studies using PCR-based methods that have reported successful amplification from fish bones (56–60) in some cases up to 10,000 y old (61). The observation that porous, light fish bones can yield whole genome shotgun libraries with high levels of endogenous DNA underscores our lack of understanding of DNA preservation in different types of animal bone. These results also illuminate the potential of the large reservoir of archaeological fish bone as a source for aDNA of sufficient quantity and quality to study long-term evolutionary processes in the marine environment.
Second, we identify polymorphic chromosomal inversions in ancient Atlantic cod specimens. Chromosomal inversions are expected to play a major role in ecological adaptation (62, 63). In cod, these regions contribute to elevated genomic diversification between modern populations and ecotypes—despite low overall levels of divergence (25, 31–34, 64)—and they have been suspected to be under selection (22, 28, 29). The high genomic divergence of these alleles, in combination with their wide geographic distribution, suggests that these have been maintained as a polymorphism for hundreds of thousands, if not millions of years (32, 33). Here, we directly observe the millennium-long maintenance of such inversions, which provides an opportunity to investigate their temporal stability. Although speculative due to the currently small samples sizes from Orkney (n = 5) and Haithabu (n = 5), our results suggest that the divergent frequency distributions of these inversions indeed have remained stable in their respective populations. Given the low overall genomic differentiation outside of these inversions in modern populations (31–33), it appears that this divergence is sustained despite ongoing gene flow, which further supports a hypothesis that these alleles are maintained as a balanced polymorphism (65).
Finally, we solve a long-standing hypothesis generated by history, archaeology, and stable isotope analysis that dried cod from northern Norway were transported during the Viking Age to Haithabu. Currently, the NEA cod feeds in the Barents Sea and its spawning grounds are restricted to the northern coasts of Norway, especially (although not exclusively) in the area around Lofoten (10, 11). One could argue that this population spawned in the Skagerrak or Kattegat during the Viking Age, but such a scenario is implausible; historical records of the Norwegian fisheries since the 12th century show that spatial fluctuations in the distribution of fishing effort targeting these spawning aggregations have been restricted (43, 66). Furthermore, our ancient data are also consistent with observed long-term spawning fidelity in the North Sea region (67, 68); the genetic affinity of the ancient Orkney population, a focus of fishing since the Viking Age (69), agrees with those of modern individuals living in the North Sea. Similarly, we observe the specific affinity of one ancient Schleswig specimen toward a distinct population in the Øresund (70). This observation also suggests local spawning fidelity and agrees with fine-scale population structure reported for this region (70–73). Overall, by identifying their most likely source population, we conclude that the ancient Haithabu cod were not caught locally, but around the Norwegian coast during historic spawning aggregations of NEA cod. Since climatic conditions restrict the production of dried cod without salt to the north of Norway (66), we can further constrain their source to this northern region, implying transportation over large spatial distances during the Viking Age. Salting of cod did not begin in Norway until the 1690s, when klipfish was introduced to compete with new international products from locations such as Newfoundland. Before that, Norwegian farmers, Sami hunters, and sometimes immigrant fishermen instead produced air-dried stockfish without salt, a practice limited to the north, especially Lofoten (43, 74). We cannot yet infer conclusions about the scale of cod transport and/or trade in the Viking Age, potentially ranging from providing travelers’ rations to supplying an urban staple, which—together with obtaining a more refined chronology—should be a subject of future research. Moreover, knowing the Haithabu results, it becomes important to ask whether cod bones from even earlier trading sites in the western Baltic region (e.g., Groß Strömkendorf) might also represent fish from a distant source (75).
Conclusion
Our discovery of distinctive genomic inversions in ancient cod specimens has made it possible to answer the long-standing question of whether dried cod was transported from northern Norway during the Viking Age (800–1066 CE), solving a mystery epitomized by the ninth-century account of an Arctic Norwegian chieftain’s voyage to Haithabu. Our findings suggest that distant requirements for Arctic protein had thus already begun to influence the economy and ecology of the north over the chronology under consideration. Our study highlights the potential of coupling modern genomics with ancient DNA to study the origins of historic trade routes in fish and other taxa.
Materials and Methods
The ancient samples (n = 19) are from the archives of excavations conducted at Haithabu (39), Schleswig (76), Bjørkum (77), Oslo (78), and Orkney (26) and are dated based on archaeological context. They come from waterlogged (Haithabu, Schleswig, and Oslo) and free-draining (Bjørkum and Orkney) deposits and have been stored dry and unfrozen in fluctuating ambient temperatures after excavation. Bones were morphologically identified as Atlantic cod and selected from different archaeological layers, from fish of differing size and/or from the same element to avoid multiple samples from individual fish (Table S1).
Extraction and Library Creation.
DNA from ancient samples was extracted in a dedicated aDNA laboratory at the University of Oslo by following strict precautions (79, 80) using a combined bleach and predigestion (BleDD2) protocol (54). Ancient DNA libraries were created by using a blunt-end ligation protocol (81) with minor adjustments (20) (SI Materials and Methods). DNA from modern samples (n = 168, Fig. 1 and Table S2) was extracted—in a separate laboratory from the ancient samples—using a DNeasy Blood & Tissue kit (Qiagen) and sheared to an approximate insert size of 350 bp (82). Modern libraries were created by using a TruSeq DNA PCR-Free Preparation Kit. All libraries were sequenced on an Illumina HiSeq 2500.
Data Processing.
The ancient read data were processed by using PALEOMIX (83). In short, forward and reverse reads were collapsed with AdapterRemoval v1.5 (84) and aligned to the Gadmor2 reference (85, 86) by using BWA aln v.0.7.5a-r405 (87). The modern data were aligned by using BWA mem. Reads that aligned with a minimum quality score (MapQ) of 25 used for subsequent analyses. aDNA damage patterns were investigated by using mapDamage v.2.0.6 (49).
SNP genotypes were obtained by using GATK v. 3.4.46 (88), after duplicate removal (Picard Tools v. 1.96) and indel realignment (GATKs IndelRealigner). Genotypes were jointly (GATKs Genotypecaller) called for modern and ancient samples separately with default settings, allowing a maximum of three alternate alleles. The modern data were filtered with BCFTOOLS v. 1.3 (89) by using filter -e “FS>60.0 || MQRankSum<-12.5 || ReadPosRankSum<-8.0 || QD<2.0 || MQ<40'–SnpGap 10” and VCFTOOLS v.0.1.14 (90), keeping biallelic loci with a maximum average read depth of 30 and a minimum MAF of 0.05. The filtered modern and ancient dataset were intersected (BCFTOOLS isec), after which genotypes with a quality below 15 and read depth below 3 were set as missing and all C > T and G > A SNPs were removed. The final dataset consisted of 156,695 SNPs.
Analyses.
For inferring genome-wide population structure, SNPS were pruned (–indep-pairwise 100 10 0.5) for linkage disequilibrium (LD) by using PLINK v1.90p (91) and LG01, 02, 07, and 12 were excluded. PCA was performed with smartPCA, EIGENSOFT v.6.1.4 (92) whereby ancient individuals were “projected” by using “lsqproject” to account for missing data. Model-based clustering was performed using ADMIXTURE v1.3 (93). PCA plots for the inverted regions (LG01, 9.1–26.2 Mbp; LG02, 18.5–24 Mbp; LG07, 13.6–23 Mbp; LG12, 1.3–13.6 Mbp) were generated without LD pruning (46). Finally, the probability of obtaining the ancient individual’s composite inversion genotype from the allele frequency distribution of the four modern populations was calculated by binomial sampling of genotypes and scaling these probabilities to one.
SI Materials and Methods
All extraction and library protocols for archaeological Atlantic cod samples were performed in a dedicated laboratory at the Department of Biosciences, University of Oslo, following strict aDNA precautions (79, 80). This is a laboratory that is physically separated from the modern DNA laboratories and in which no modern samples have ever been processed. Extraction of ancient Atlantic cod bones (n = 19) used a combined bleach and predigestion protocol (54). Each sample was exposed to UV for 10 min on each side, resulting in a total dosage of 4,800 J/m2 before being cut and milled to powder. Then, for each sample, two times 150–200 mg of bone powder (milled in a Retsch MM400) was incubated in 0.5% bleach solution for 15 min (94). The samples were subsequently washed with H2O, and the remaining bone powder was exposed to a 30-min predigestion treatment followed by an overnight, second digestion by using a freshly prepared digestion buffer (95). Following the second digestion, the two eluates were combined and concentrated (Amicon-30kDA Centrifugal Filter Units) after which DNA was extracted by using Qiagen Minelute columns according to manufacturer’s instructions. DNA was eluted in 60 μL of preheated (60 °C) EB buffer with a 15-min incubation at 37 °C. Negative controls were included in all extraction experiments. Blunt-end Illumina libraries were built (81) with minor adjustments (20), and ligated DNA was amplified by using sample-specific 7-bp indexes in the P7 primer to allow multiplexing. PCRs were done in 15 µL [2.5 U PfuTurbo Cx Hotstart DNA Polymerase (Agilent Technologies), 1× buffer, 0.2 mM per dNTP, 0.2 μM P7 index primer, 0.2 μM P5 IS4 primer, and 0.4 mg/mL BSA] for 13 cycles (2 min at 95 °C, 13 cycles of 30 s at 95 °C, 30 s at 60 °C, and 70 s at 72 °C with a final extension of 10 min at 72 °C). Amplified products were cleaned by using Agencourt AMPure XP beads at a 1:1.7 ratio, eluted in 30 µL in EB buffer and quantified by using a Bioanalyzer 2100 (Agilent). Libraries were sequenced on an Illumina HiSeq 2500 at the Norwegian Sequencing Centre (125 bp paired-end) and demultiplexed allowing zero mismatches in the index tag.
Acknowledgments
We thank M. Skage, S. Kollias, A. Tooming-Klunderud, and H. Rydbeck from the Norwegian Sequencing Centre for sequencing and processing of samples; and M. H. Hansen for extracting modern Atlantic cod specimens. P. R. Berg, M. Malmstrøm, and K. Berndt assisted in sampling modern cod from Norway and the Øresund, respectively; U. Schmölcke kindly facilitated sampling at the Centre for Baltic and Scandinavian Archaeology; and M. Ramstad of the University of Bergen provided access to the Bjørkum assemblage. This work was supported by Research Council of Norway Projects “Tracking Viking-assisted dispersal using ancient DNA (230821/F20), “Fisheries induced evolution in Atlantic cod investigated by ancient and historic samples” (203850/E40), “The Aqua Genome Project” (221734/O30), and Leverhulme Trust Project “Northern Journeys: Reimagining the Medieval Revolution and its Aftermath” (MRF-2013-065).
Footnotes
The authors declare no conflict of interest.
Database deposition: All ancient read data are available at the European Nucleotide Archive, www.ebi.ac.uk/ena, (accession no. PRJEB20524 and PRJEB15516). The filtered SNP dataset is available at Dryad, (datadryad.org; doi:10.5061/dryad.81ps2).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1710186114/-/DCSupplemental.
References
- 1.Lenzen M, et al. International trade drives biodiversity threats in developing nations. Nature. 2012;486:109–112. doi: 10.1038/nature11145. [DOI] [PubMed] [Google Scholar]
- 2.Frachetti MD, Smith CE, Traub CM, Williams T. Nomadic ecology shaped the highland geography of Asia’s Silk Roads. Nature. 2017;543:193–198. doi: 10.1038/nature21696. [DOI] [PubMed] [Google Scholar]
- 3.Barbier EB. Scarcity and Frontiers: How Economies Have Developed Through Natural Resource Exploitation. Cambridge Univ Press; Cambridge, UK: 2010. [Google Scholar]
- 4.Hoffmann R. An Environmental History of Medieval Europe. Cambridge Univ Press; Cambridge, UK: 2014. [Google Scholar]
- 5.Hoffmann R. Carp, cods and connections: New fisheries in the medieval European economy and environment. In: Henninger-Voss MJ, editor. Animals in Human Histories: The Mirror of Nature and Culture. Univ of Rochester Press; Rochester, NY: 2002. pp. 3–55. [Google Scholar]
- 6.Perdikaris S, McGovern TH. Codfish and kings, seals and subsistence: Norse marine resource use in the North Atlantic. In: Rick T, Erlandson J, editors. Human Impacts on Ancient Marine Ecosystems: A Global Perspective. Univ of California Press; Berkeley: 2008. pp. 187–214. [Google Scholar]
- 7.Barrett JH, et al. Interpreting the expansion of sea fishing in medieval Europe using stable isotope analysis of archaeological cod bones. JAS. 2011;38:1516–1524. [Google Scholar]
- 8.Orton DC, et al. Stable isotope evidence for late medieval (14th–15th C) origins of the eastern Baltic cod (Gadus morhua) fishery. PLoS ONE. 2011;6:e27568. doi: 10.1371/journal.pone.0027568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Göhring A, Mauder M, Kröger P, Grupe G. Using Gaussian Mixture Model clustering for multi-isotope analysis of archaeological fish bones for palaeobiodiversity studies. Rapid Commun Mass Spectrom. 2016;30:1349–1360. doi: 10.1002/rcm.7573. [DOI] [PubMed] [Google Scholar]
- 10.Sundby S, Nakken O. Spatial shifts in spawning habitats of Arcto-Norwegian cod related to multidecadal climate oscillations and climate change. ICES J Mar Sci. 2008;65:953–962. [Google Scholar]
- 11.Opdal AF. Fisheries change spawning ground distribution of northeast Arctic cod. Biol Lett. 2010;6:261–264. doi: 10.1098/rsbl.2009.0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nedkvitne A. The development of the Norwegian long-distance stockfish trade. In: Barrett JH, Orton DC, editors. Cod and Herring: The Archaeology and History of Medieval Sea Fishing. Oxbow Books; Oxford: 2016. pp. 50–59. [Google Scholar]
- 13.Pauly D. Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol Evol. 1995;10:430. doi: 10.1016/s0169-5347(00)89171-5. [DOI] [PubMed] [Google Scholar]
- 14.Jackson JB, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–637. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 15.Barrett JH, Locker AM, Roberts CM. The origins of intensive marine fishing in medieval Europe: The English evidence. Proc Biol Sci. 2004;271:2417–2421. doi: 10.1098/rspb.2004.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotze HK, Worm B. Historical baselines for large marine animals. Trends Ecol Evol. 2009;24:254–262. doi: 10.1016/j.tree.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen R, et al. Tracing the peopling of the world through genomics. Nature. 2017;541:302–310. doi: 10.1038/nature21347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arndt A, et al. Roman trade relationships at Sagalassos (Turkey) elucidated by ancient DNA of fish remains. JAS. 2003;30:1095–1105. [Google Scholar]
- 19.Boessenkool S, Star B, Scofield RP, Seddon PJ, Waters JM. Lost in translation or deliberate falsification? Genetic analyses reveal erroneous museum data for historic penguin specimens. Proc Biol Sci. 2009;277:1057–64. doi: 10.1098/rspb.2009.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroeder H, et al. Genome-wide ancestry of 17th-century enslaved Africans from the Caribbean. Proc Natl Acad Sci USA. 2015;112:3669–3673. doi: 10.1073/pnas.1421784112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kayser M, de Knijff P. Improving human forensics through advances in genetics, genomics and molecular biology. Nat Rev Genet. 2011;12:179–192. doi: 10.1038/nrg2952. [DOI] [PubMed] [Google Scholar]
- 22.Bradbury IR, et al. Genomic islands of divergence and their consequences for the resolution of spatial structure in an exploited marine fish. Evol Appl. 2013;6:450–461. doi: 10.1111/eva.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knutsen H, et al. Are low but statistically significant levels of genetic differentiation in marine fishes ‘biologically meaningful’? A case study of coastal Atlantic cod. Mol Ecol. 2010;20:768–83. doi: 10.1111/j.1365-294X.2010.04979.x. [DOI] [PubMed] [Google Scholar]
- 24.Selkoe KA, et al. A decade of seascape genetics: Contributions to basic and applied marine connectivity. MEPS. 2016;554:1–19. [Google Scholar]
- 25.Hutchinson WF, et al. The globalization of naval provisioning: Ancient DNA and stable isotope analyses of stored cod from the wreck of the Mary Rose, AD 1545. R Soc Open Sci. 2015;2:150199. doi: 10.1098/rsos.150199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harland JF, Barrett JH. The maritime economy: Fish bone. In: Barrett JH, editor. Being an Islander: Production and Identity at Quoygrew, Orkney, AD 900–1600. McDonald Inst Archaeol Res; Cambridge, UK: 2012. pp. 115–138. [Google Scholar]
- 27.Barrett JH. Medieval sea fishing AD 500–1550: Chronology, causes and consequences. In: Barrett JH, Orton DC, editors. Cod and Herring: The Archaeology and History of Medieval Sea Fishing. Oxbow Books; Oxford: 2016. pp. 250–271. [Google Scholar]
- 28.Bradbury IR, et al. Parallel adaptive evolution of Atlantic cod on both sides of the Atlantic Ocean in response to temperature. Proc Biol Sci. 2010;277:3725–3734. doi: 10.1098/rspb.2010.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemmer-Hansen J, et al. FishPopTrace Consortium A genomic island linked to ecotype divergence in Atlantic cod. Mol Ecol. 2013;22:2653–2667. doi: 10.1111/mec.12284. [DOI] [PubMed] [Google Scholar]
- 30.Berg P, et al. Adaptation to Low Salinity Promotes Genomic Divergence in Atlantic Cod. Genome Biol Evol. 2015;7:1644–1663. doi: 10.1093/gbe/evv093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sodeland M, et al. “Islands of Divergence” in the Atlantic cod genome represent polymorphic chromosomal rearrangements. Genome Biol Evol. 2016;8:1012–1022. doi: 10.1093/gbe/evw057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berg PR, et al. Three chromosomal rearrangements promote genomic divergence between migratory and stationary ecotypes of Atlantic cod. Sci Rep. 2016;6:23246. doi: 10.1038/srep23246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirubakaran TG, et al. Two adjacent inversions maintain genomic differentiation between migratory and stationary ecotypes of Atlantic cod. Mol Ecol. 2016;25:2130–2143. doi: 10.1111/mec.13592. [DOI] [PubMed] [Google Scholar]
- 34.Barney BT, Munkholm C, Walt DR, Palumbi SR. Highly localized divergence within supergenes in Atlantic cod (Gadus morhua) within the Gulf of Maine. BMC Genomics. 2017;18:271. doi: 10.1186/s12864-017-3660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bately J, Stanley EG. In: The Source. Ohthere’s Voyages: A Late 9th-Century Account of Voyages Along the Coasts of Norway and Denmark and Its Cultural Context. Bately J, Englert A, editors. The Viking Ships Mus; Roskilde, Denmark: 2007. pp. 10–58. [Google Scholar]
- 36.Hilberg V, Kalmring S. Viking Age Hedeby and its relations with Iceland and the North Atlantic: Communications, long-distance trade, and production. In: Zori D, Byock J, editors. Viking Archaeology in Iceland: Mosfell Archaeological Project. Brepols; Turnhout, Belgium: 2014. pp. 221–241. [Google Scholar]
- 37.Nedkvitne A. The German Hansa and Bergen 1100-1600. Böhlau; Köln, Germany: 2014. [Google Scholar]
- 38.Lepiksaar J, Heinrich D. Berichte über die Ausgrabungen in Haithabu. Vol 10. Wachholtz; Neumünster, Germany: 1977. Untersuchungen an Fischresten aus der frühmittelalterlichen Siedlung Haithabu; pp. 7–122. [Google Scholar]
- 39.Heinrich D. Berichte über die Ausgrabungen in Haithabu. Vol 35. Wachholtz; Neumünster, Germany: 2006. Die Fischreste aus dem Hafen von Haithabu - Handaufgelesene Funde; pp. 157–193. [Google Scholar]
- 40.Schmölcke U, Heinrich D. Berichte über die Ausgrabungen in Haithabu. Vol 35. Wachholtz; Neumünster, Germany: 2006. Die Tierknochen aus dem Hafen von Haithabu - Schlämmfunde; pp. 195–239. [Google Scholar]
- 41.Barrett J, et al. Detecting the medieval cod trade: A new method and first results. JAS. 2008;35:850–861. [Google Scholar]
- 42.Orton DC, Morris J, Locker A, Barrett JH. Fish for the city: Meta-analysis of archaeological cod remains and the growth of London’s northern trade. Antiquity. 2014;88:516–530. [Google Scholar]
- 43.Nielssen AR. Early commercial fisheries and the interplay among farm, fishing station and fishing village in North Norway. In: Barrett JH, Orton DC, editors. Cod and Herring: The Archaeology and History of Medieval Sea Fishing. Oxbow Books; Oxford: 2016. pp. 42–49. [Google Scholar]
- 44.Wickler S, Narmo L. Tracing the development of fishing settlement from the Iron Age to the Modern Period in northern Norway: A case study from Borgvær in the Lofoten Islands. J Island Coast Archaeol. 2014;9:72–87. [Google Scholar]
- 45.Nielssen AR. Kysten - overgangen fra vikingtid til middelalder. In: Nielssen AR, editor. Fangstmenn, fiskerbønder og værfolk: Fram til 1720. Vol 1. Fagbokforlaget; Bergen, Norway: 2014. pp. 187–208. [Google Scholar]
- 46.Ma J, Amos CI. Investigation of inversion polymorphisms in the human genome using principal components analysis. PLoS One. 2012;7:e40224. doi: 10.1371/journal.pone.0040224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Briggs AW, et al. Patterns of damage in genomic DNA sequences from a Neandertal. Proc Natl Acad Sci USA. 2007;104:14616–14621. doi: 10.1073/pnas.0704665104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Green RE, et al. The Neandertal genome and ancient DNA authenticity. EMBO J. 2009;28:2494–2502. doi: 10.1038/emboj.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jónsson H, Ginolhac A, Schubert M, Johnson PLF, Orlando L. mapDamage2.0: Fast approximate Bayesian estimates of ancient DNA damage parameters. Bioinformatics. 2013;29:1682–1684. doi: 10.1093/bioinformatics/btt193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gamba C, et al. Genome flux and stasis in a five millennium transect of European prehistory. Nat Comm. 2014;5:5257. doi: 10.1038/ncomms6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinhasi R, et al. Optimal ancient DNA yields from the inner ear part of the human petrous bone. PLoS One. 2015;10:e0129102. doi: 10.1371/journal.pone.0129102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hansen HB, et al. Comparing ancient DNA preservation in petrous bone and tooth cementum. PLoS One. 2017;12:e0170940. doi: 10.1371/journal.pone.0170940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prinz M, et al. International Society for Forensic Genetics DNA Commission of the International Society for Forensic Genetics (ISFG): Recommendations regarding the role of forensic genetics for disaster victim identification (DVI) Forensic Sci Int Genet. 2007;1:3–12. doi: 10.1016/j.fsigen.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Boessenkool S, et al. Combining bleach and mild predigestion improves ancient DNA recovery from bones. Mol Ecol Resour. 2017;17:742–751. doi: 10.1111/1755-0998.12623. [DOI] [PubMed] [Google Scholar]
- 55.Carpenter ML, et al. Pulling out the 1%: Whole-genome capture for the targeted enrichment of ancient DNA sequencing libraries. Am J Hum Genet. 2013;93:852–864. doi: 10.1016/j.ajhg.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Speller CF, et al. High potential for using DNA from ancient herring bones to inform modern fisheries management and conservation. PLoS One. 2012;7:e51122. doi: 10.1371/journal.pone.0051122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moss ML, Judd KG, Kemp BM. Can salmonids (Oncorhynchus spp.) be identified to species using vertebral morphometrics? A test using ancient DNA from Coffman Cove, Alaska. J Archaeol Sci. 2014;41:879–889. [Google Scholar]
- 58.Ólafsdóttir GÁ, Westfall KM, Edvardsson R, Pálsson S. Historical DNA reveals the demographic history of Atlantic cod (Gadus morhua) in medieval and early modern Iceland. Proc Biol Sci. 2014;281:20132976. doi: 10.1098/rspb.2013.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grealy A, et al. Tropical ancient DNA from bulk archaeological fish bone reveals the subsistence practices of a historic coastal community in southwest Madagascar. JAS. 2016;75:82–88. [Google Scholar]
- 60.Nikulina EA, Schmölcke U. Reconstruction of the historical distribution of sturgeons (Acipenseridae) in the eastern North Atlantic based on ancient DNA and bone morphology of archaeological remains: Implications for conservation and restoration programmes. Divers Distrib. 2016;22:1036–1044. [Google Scholar]
- 61.Moss ML, Rodrigues AT, Speller CF, Yang DY. The historical ecology of Pacific herring: Tracing Alaska Native use of a forage fish. JAS. 2016;8:504–512. [Google Scholar]
- 62.Hoffmann AA, Sgrò CM, Weeks AR. Chromosomal inversion polymorphisms and adaptation. Trends Ecol Evol. 2004;19:482–488. doi: 10.1016/j.tree.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 63.Kirkpatrick M. How and why chromosome inversions evolve. PLoS Biol. 2010;8:e1000501. doi: 10.1371/journal.pbio.1000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hutchinson WF, Carvalho GR, Rogers SI. Marked genetic structuring in localised spawning populations of cod Gadus morhua in the North Sea and adjoining waters, as revealed by microsatellites. MEPS. 2001;223:251–260. [Google Scholar]
- 65.Árnason E, Halldórsdóttir K. Nucleotide variation and balancing selection at the Ckma gene in Atlantic cod: Analysis with multiple merger coalescent models. PeerJ. 2015;3:e786. doi: 10.7717/peerj.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sørheim H. The birth of commercial fisheries and the trade of stockfish in the Borgundfjord, Norway. In: Barrett JH, Orton DC, editors. Cod and Herring: The Archaeology and History of Medieval Sea Fishing. Oxbow Books; Oxford: 2016. pp. 60–70. [Google Scholar]
- 67.Wright P, Galley E, Gibb I, Neat F. Fidelity of adult cod to spawning grounds in Scottish waters. Fish Res. 2006;77:148–158. [Google Scholar]
- 68.Skjæraasen JE, Meager JJ, Karlsen Ø, Hutchings JA, Fernö A. Extreme spawning-site fidelity in Atlantic cod. ICES J Mar Sc. 2011;68:1472–1477. [Google Scholar]
- 69.Barrett JH, Nicholson RA, Cerón-Carrasco R. Archaeo-ichthyological evidence for long-term socioeconomic trends in northern Scotland: 3500 BC to AD 1500. JAS. 1999;26:353–388. [Google Scholar]
- 70.Barth JMI, et al. Genome architecture enables local adaptation of Atlantic cod despite high connectivity. Mol Ecol. 2017 doi: 10.1111/mec.14207. [DOI] [PubMed] [Google Scholar]
- 71.Knutsen H, Jorde PE, André C, Stenseth NC. Fine-scaled geographical population structuring in a highly mobile marine species: The Atlantic cod. Mol Ecol. 2003;12:385–394. doi: 10.1046/j.1365-294x.2003.01750.x. [DOI] [PubMed] [Google Scholar]
- 72.Jorde PE, Knutsen H, Espeland SH, Stenseth NC. Spatial scale of genetic structuring in coastal cod Gadus morhua and geographic extent of local populations. Mar Ecol Prog Ser. 2007;343:229–237. [Google Scholar]
- 73.Neuenfeldt S, et al. Analysing migrations of Atlantic cod Gadus morhua in the north-east Atlantic Ocean: Then, now and the future. J Fish Biol. 2013;82:741–763. doi: 10.1111/jfb.12043. [DOI] [PubMed] [Google Scholar]
- 74.Nielssen AR. Norwegian fisheries c.1100-1850. In: Starkey DJ, Thór JT, Heidbrink I, editors. A History of the North Atlantic Fisheries Volume 1: From Early Times to the Mid-Nineteenth Century. H. M. Hauschild GmbH; Bremen, Germany: 2009. [Google Scholar]
- 75.Schmölcke U. Nutztierhaltung, Jagd und Fischfang: Zur Nahrungsmittelwirtschaft des frühgeschichtlichen Handelsplatzes von Groß Strömkendorf, Landkreis Nordwestmecklenburg. Lübstorf; Germany: 2004. [Google Scholar]
- 76.Heinrich D. 1987. Untersuchungen an mittelalterlichen Fischresten aus Schleswig: Ausgrabung Schild 1971-1975. Ausgrabungen in Schleswig. Berichte und Studien. (Wachholtz, Neumünster, Germany), Vol 6, pp 87–126.
- 77.Barrett JH, Hufthammer AK, Bratbakk O. Animals and Animal Products at the Late Iron Age Settlement of Bjørkum, Lærdal: The Zooarchaeological Evidence. Univ Mus of Bergen; Bergen, Norway: 2015. [Google Scholar]
- 78.Lie RW. Animal bones. In: Schia E, editor. De Arkeologiske Utgravninger I Gamlebyen, Oslo Bind 5: “Mindets Tomt” - “Søndre Felt” Animal Bones, Moss-, Plant-, Insect- and Parasite Remains. Alvheim & Eide Akademisk Forlag; Øvre Ervik, Norway: 1988. pp. 153–195. [Google Scholar]
- 79.Cooper A, Poinar HN. Ancient DNA: Do it right or not at all. Science. 2000;289:1139. doi: 10.1126/science.289.5482.1139b. [DOI] [PubMed] [Google Scholar]
- 80.Gilbert MT, Bandelt H-J, Hofreiter M, Barnes I. Assessing ancient DNA studies. Trends Ecol Evol. 2005;20:541–544. doi: 10.1016/j.tree.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 81.Meyer M, Kircher M. Illumina sequencing library preparation for highly multiplexed target capture and sequencing. Cold Spring Harb Protoc. 2010;2010:pdb.prot5448. doi: 10.1101/pdb.prot5448. [DOI] [PubMed] [Google Scholar]
- 82.Star B, et al. Genomic characterization of the Atlantic cod sex-locus. Sci Rep. 2016;6:31235. doi: 10.1038/srep31235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schubert M, et al. Characterization of ancient and modern genomes by SNP detection and phylogenomic and metagenomic analysis using PALEOMIX. Nat Protoc. 2014;9:1056–1082. doi: 10.1038/nprot.2014.063. [DOI] [PubMed] [Google Scholar]
- 84.Lindgreen S. AdapterRemoval: Easy cleaning of next-generation sequencing reads. BMC Res Notes. 2012;5:337. doi: 10.1186/1756-0500-5-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Star B, et al. The genome sequence of Atlantic cod reveals a unique immune system. Nature. 2011;477:207–210. doi: 10.1038/nature10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tørresen OK, et al. An improved genome assembly uncovers a prolific tandem repeat structure in Atlantic cod. BMC Genomics. 2017;18:95. doi: 10.1186/s12864-016-3448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McKenna A, et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li H, et al. 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Danecek P, et al. 1000 Genomes Project Analysis Group The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Purcell S, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Korlević P, et al. Reducing microbial and human contamination in DNA extractions from ancient bones and teeth. Biotechniques. 2015;59:87–93. doi: 10.2144/000114320. [DOI] [PubMed] [Google Scholar]
- 95.Damgaard PB, et al. Improving access to endogenous DNA in ancient bones and teeth. Sci Rep. 2015;5:11184. doi: 10.1038/srep11184. [DOI] [PMC free article] [PubMed] [Google Scholar]