Significance
The endosomal sorting of integral proteins is essential for controlling signaling pathways at the plasma membrane. Posttranslational modification by ubiquitin is key to proper degradation of plasma membrane proteins as the ubiquitylated transmembrane proteins are recognized by multiple ubiquitin adaptor proteins and trafficked to the vacuole for degradation. Although plants lack orthologs of the yeast and metazoan endosomal sorting complex required for transport-0 heterodimer that functions as an ubiquitin adaptor, plants appear to have evolved other strategies to recognize and concentrate ubiquitylated proteins that have been endocytosed. Here, we report the SH3P2 protein as a yet-unknown ubiquitin adaptor protein in Arabidopsis and its molecular function in regulating the endocytic transport and degradation.
Keywords: ubiquitin, ESCRT, DUB, clathrin, Arabidopsis
Abstract
Clathrin-mediated endocytosis of plasma membrane proteins is an essential regulatory process that controls plasma membrane protein abundance and is therefore important for many signaling pathways, such as hormone signaling and biotic and abiotic stress responses. On endosomal sorting, plasma membrane proteins maybe recycled or targeted for vacuolar degradation, which is dependent on ubiquitin modification of the cargos and is driven by the endosomal sorting complexes required for transport (ESCRTs). Components of the ESCRT machinery are highly conserved among eukaryotes, but homologs of ESCRT-0 that are responsible for recognition and concentration of ubiquitylated proteins are absent in plants. Recently several ubiquitin-binding proteins have been identified that serve in place of ESCRT-0; however, their function in ubiquitin recognition and endosomal trafficking is not well understood yet. In this study, we identified Src homology-3 (SH3) domain-containing protein 2 (SH3P2) as a ubiquitin- and ESCRT-I–binding protein that functions in intracellular trafficking. SH3P2 colocalized with clathrin light chain-labeled punctate structures and interacted with clathrin heavy chain in planta, indicating a role for SH3P2 in clathrin-mediated endocytosis. Furthermore, SH3P2 cofractionates with clathrin-coated vesicles (CCVs), suggesting that it associates with CCVs in planta. Mutants of SH3P2 and VPS23 genetically interact, suggesting that they could function in the same pathway. Based on these results, we suggest a role of SH3P2 as an ubiquitin-binding protein that binds and transfers ubiquitylated proteins to the ESCRT machinery.
Plants are constantly facing environmental changes, and are therefore forced to readily respond and adapt to the environment for optimal growth and reproduction. Plasma membrane receptors and transporters play an essential role in the coordination of extracellular signals and intracellular responses. Regulation of the activity and abundance of signaling molecules at both transcriptional and posttranscriptional levels is therefore key to all important signaling pathways, such as phytohormone signaling and biotic and abiotic stress responses (1–3). The clathrin-mediated endosomal sorting and degradation pathway is responsible for the degradation of many plasma membrane proteins and is conserved in eukaryotes (4–6). The binding and polymerization of clathrin triskelia in concert with adaptor protein complexes at the plasma membrane result in the budding and release of plasma membrane cargo protein-containing clathrin-coated vesicles (CCVs) that deliver their cargo to the trans-Golgi network (TGN)/early endosomal compartment (EE) (5). From there, the internalized plasma membrane proteins can either be transported by intracellular vesicle trafficking to the vacuole for degradation or recycled back to the plasma membrane (3).
Vacuolar degradation of plasma membrane proteins is dependent on posttranslational modification by ubiquitin, or ubiquitylation. Ubiquitin can be conjugated into different chain types, and depending on the type of linkage, these chain types target the modified proteins to different cellular degradation pathways (7). Ubiquitin chains linked at the internal lysine 63 (K63) can serve as a transport signal for endocytic trafficking and selective autophagy (7, 8). In plants, degradation of membrane proteins, such as the auxin efflux carrier PIN-FORMED 2 (PIN2) and the brassinosteroid receptor BRASSINOSTEROID INSENSITIVE 1 (BRI1), was shown to depend on K63-linked ubiquitin chain modification (9, 10). The abundance of the plasma membrane transporters IRON-REGULATED TRANSPORTER 1 (IRT1) and REQUIRES HIGH BORON 1 (BOR1) is also controlled by ubiquitin-dependent endocytic degradation in response to nutrient availability in the environment (11, 12). Some pathogens also use ubiquitylation as a mechanism to remove host defense proteins. The bacterial effector AvrPtoB has ubiquitin ligase activity and ubiquitylates the host FLAGELLIN-SENSITIVE (FLS) 2 and CHITIN ELICITOR RECEPTOR KINASE (CERK) 1, the removal of which is advantageous for the bacteria (13, 14).
Ubiquitin-mediated sorting of cargo proteins from the plasma membrane is driven by the endosomal sorting complexes required for transport (ESCRTs); ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III (3, 15). The ESCRT machinery is highly conserved in eukaryotes, and, besides playing a central role in intracellular trafficking (16), it was shown in other organisms to be involved in viral budding (17, 18) and membrane reformation and repair (19–23). The ESCRT machinery was shown to be important in many physiological aspects of plant biology (3). Of the four types of ESCRT machinery (ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III), plants lack ESCRT-0. ESCRT-0, a heterodimer of two ubiquitin-binding proteins, Vps27p/Hrs and Hse1/signal-transducing adaptor molecule (STAM), is important for concentrating and trapping ubiquitylated membrane cargos and passing them to ESCRT-I. ESCRT-I and ESCRT-II also contain ubiquitin-binding subunits, which are important for keeping the ubiquitylated cargos on the endosomal membranes en route to the vacuole for degradation (24). ESCRT-III interacts with ESCRT-II and is responsible for the scission of membranes and the formation of intraluminal vesicles during the maturation of multivesicular bodies (MVBs) (16).
Ubiquitin recognition is key for the ESCRT-dependent intracellular trafficking pathway. All ESCRTs, except for ESCRT-III, and many ESCRT-related proteins contain ubiquitin-binding domains, and many of these proteins can interact with each other, thereby increasing their affinity toward ubiquitylated cargos (16, 25). Additional protein families that mediate recognition of ubiquitylated cargo proteins have been identified and shown to direct ubiquitylated cargos to the ESCRT machinery (24). The ubiquitin adaptor proteins, Golgi-localized, γ-ear–containing ADP ribosylation factor-binding proteins (GGAs), are present in both mammals and yeast, but not in plants, whereas TARGET OF MYB (TOM) 1 proteins appear in metazoans and have homologs in plants (24, 26). GGAs and TOM1 interact with ESCRT-I and send the cargo to the ESCRT-mediated protein degradation route (27, 28).
Although plants lack GGA homologs (3, 15), nine TOM-LIKEs (TOLs) are encoded in the Arabidopsis genome (26). TOLs were shown to bind ubiquitylated proteins and to localize to the plasma membrane and intracellular punctate structures, and they are necessary for endocytosis and recycling of the auxin efflux carrier PIN2 (26). Fab1, YOTB, Vac1, and EEA (FYVE) domain-containing protein 1 (FYVE1)/FYVE DOMAIN PROTEIN REQUIRED FOR ENDOSOMAL SORTING 1 (FREE1) is another ubiquitin-binding protein in Arabidopsis that interacts and colocalizes with ESCRT-I, and is important for intracellular trafficking and degradation of ubiquitylated proteins (29–32). For a better understanding as to how the recognition and condensation of ubiquitylated cargo proteins work in plant cells, it is necessary to uncover the molecular framework in which ubiquitin-binding proteins and ESCRT-I function.
In this work, we demonstrate that the Arabidopsis Src homology-3 (SH3) domain-containing protein 2 (SH3P2) can act as an ubiquitin-binding protein and functions together with ESCRT-I and the deubiquitylating enzyme (DUB) associated molecule with the SH3 domain of STAM3 (AMSH3). Arabidopsis has three homologs that are named SH3P1, SH3P2, and SH3P3. Besides the C-terminal SH3 domain, the SH3Ps contain a putative N-terminal BIN-amphiphysin-RVS (BAR) domain (33), a domain present in membrane-associated proteins that are involved in clathrin-mediated endocytosis. SH3Ps bind phosphoinositides, and SH3P1 and SH3P2 localize on clathrin-labeled structures, indicating that these proteins are involved in intracellular trafficking (32–35). Our data show that SH3P2 interacts with the ESCRT-I subunit VPS23 and binds K63-linked ubiquitin chains, and also the DUB AMSH3. SH3P2, VPS23, and the DUB AMSH3 all localize to punctate structures labeled by the clathrin marker. Moreover, VPS23 and SH3P2 show genetic interactions, suggesting that they could function together. Based on these results, we propose a role for SH3P2 in the recognition of ubiquitinated membrane cargos and in passing them to the ESCRT machinery for degradation.
Results
SH3P2 Binds K63-Linked Ubiquitin Chains.
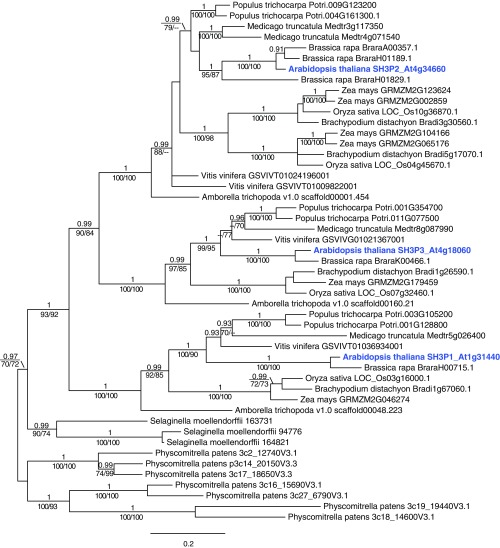
To establish whether there are other ubiquitin adaptors in the plant endocytosis pathway, we investigated proteins that were reported to localize on the plasma membrane or CCVs for putative ubiquitin-binding motifs. We focused on the SH3Ps since SH3 domains were previously reported to bind ubiquitin (36–38). There are three homologs of SH3Ps (SH3P1, SH3P2, and SH3P3) that seem to be distributed early in evolution of dicots (Fig. S1). Of these three homologs, SH3P1 and SH3P2 were reported to localize on clathrin-positive vesicles (32, 34).
Fig. S1.
Phylogenetic analysis of SH3Ps. A Bayesian tree was deduced from CDS homologous to Arabidopsis SH3P1, SH3P2, and SH3P3. Values above branches indicate posterior probabilities (≥0.9) calculated by Bayesian analysis, and those below branches indicate bootstrap values of ML (Left) and MP (Right) (≥70). Bold blue font indicates the SH3P genes of Arabidopsis thaliana.
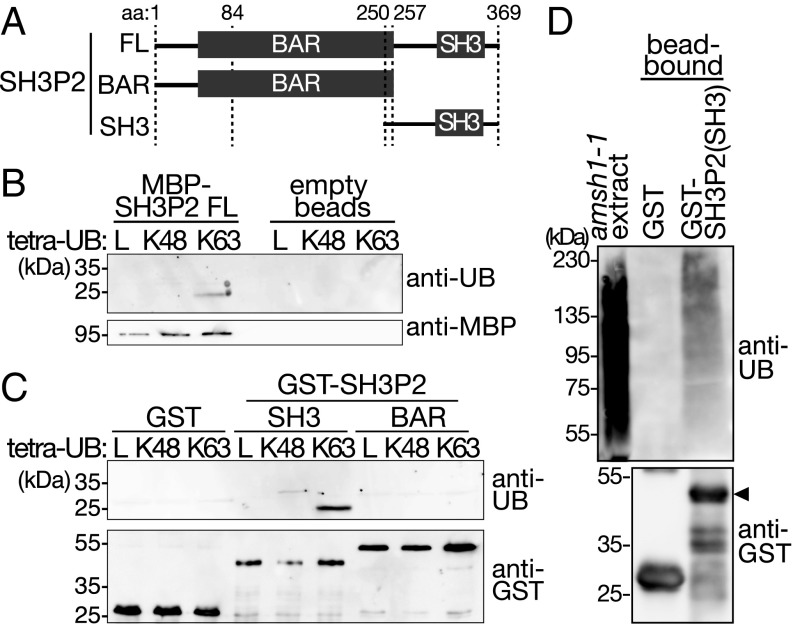
We first examined whether the SH3 domain of SH3P2 has the ability to bind ubiquitin and ubiquitin conjugates, and examined full-length SH3P2 and fragments of SH3P2 with and without the SH3 domain (Fig. 1A) in an ubiquitin-binding assay. Full-length SH3P2 binds to K63-linked ubiquitin chains but does not bind linear or K48-linked tetra-ubiquitin (Fig. 1B). Further experiments show that an SH3P2 fragment containing the SH3 domain binds K63-linked tetra-ubiquitin, whereas a fragment deleted of the SH3 domain did not show binding (Fig. 1C). GST-SH3P2(SH3) also bound to ubiquitin conjugates from plant total extracts, whereas GST alone did not (Fig. 1D). These data suggest that the SH3 domain of SH3P2 can bind ubiquitin and has a preference toward the K63-linkage type.
Fig. 1.
SH3P2 binds ubiquitin conjugates. (A) Schematic presentation of full-length SH3P2(FL) and truncation constructs SH3P2(BAR) and SH3P2(SH3) used in C and D. (B) Tetra-ubiquitin (UB)–binding assay with recombinant MBP-SH3P2. Equal amounts of bead-bound MBP-SH3P2(FL) and linear (L), K48-linked (K48), or K63-linked (K63) tetra-UB were incubated together. After washing, the binding of tetra-UB to MBP-SH3P2(FL) was analyzed by immunoblotting with anti-UB (P4D1) and anti-MBP antibodies. Empty amylose beads were used as a negative control. (C) Tetra-UB–binding assay with GST-SH3P2(SH3) and GST-SH3P2(BAR). Equal amounts of bead-bound GST-SH3P2(SH3), GST-SH3P2(BAR), and GST were incubated with L, K48, or K63 tetra-UB. After washing, the binding of tetra-UB to the GST-fusion proteins was analyzed by immunoblotting with anti-UB (P4D1) and anti-GST antibodies. (D) UB-binding assay with GST-SH3P2(SH3) and GST. Bead-bound recombinant GST-SH3P2(SH3) and GST were incubated with crude extracts of amsh1-1. After washing, bead-bound materials were subjected to immunoblotting with anti-UB (P4D1) and anti-GST antibodies. The arrowhead indicates the position of GST-SH3P2(SH3).
SH3P2 Interacts with ESCRT-I.
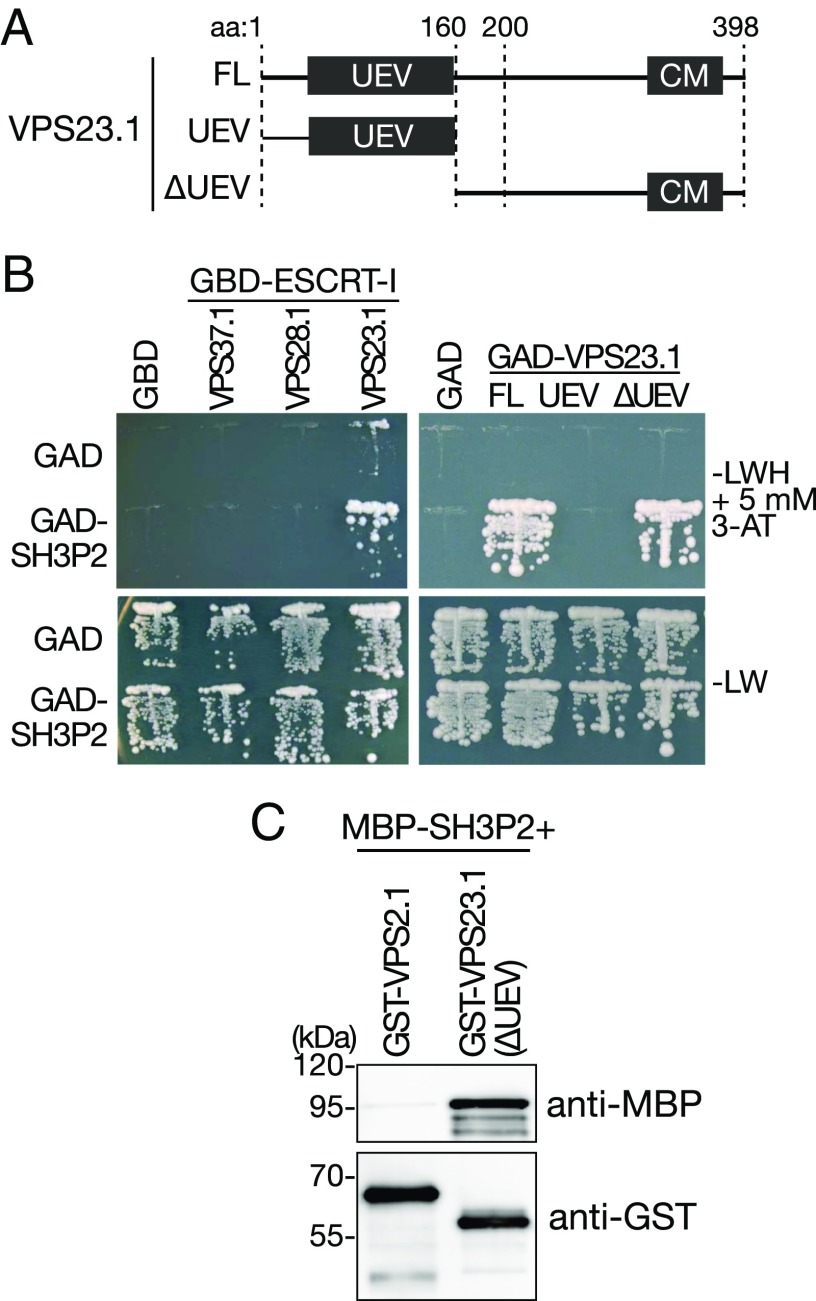
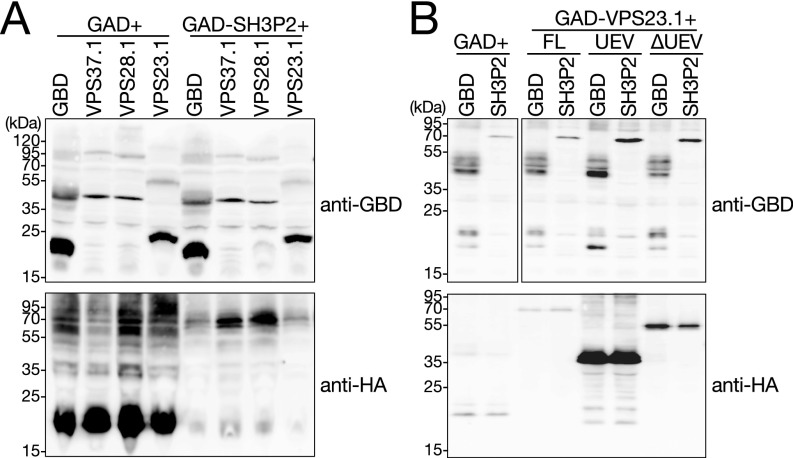
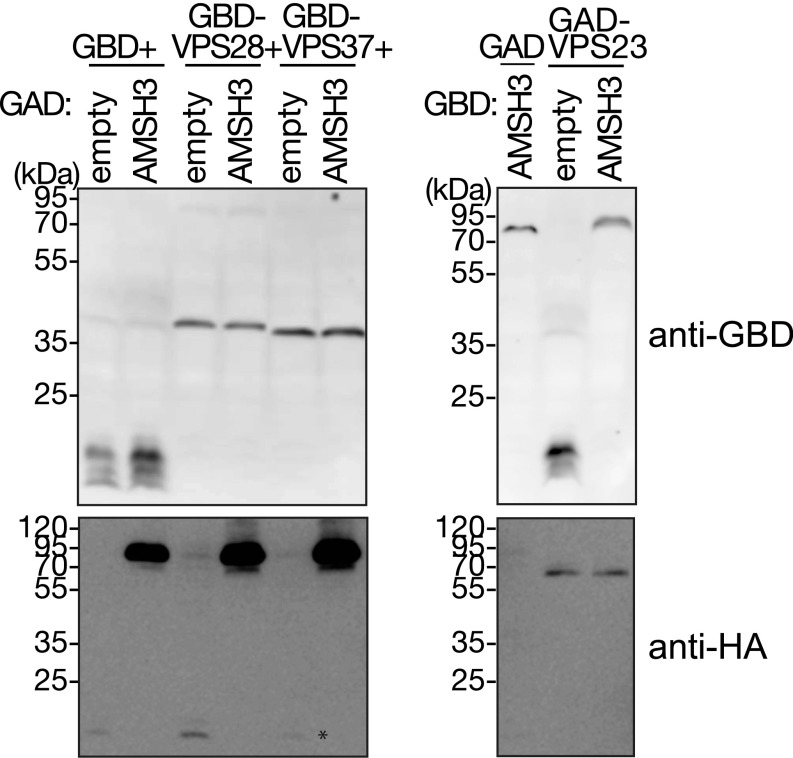
The function of ubiquitin adaptors in endocytosis is to concentrate ubiquitinated cargos and to transfer them to the ESCRT machinery, especially ESCRT-I. We therefore investigated whether SH3P2 interacts with ESCRT-I components. ESCRT-I consists of three core subunits, VPS37, VPS28, and VPS23, and Arabidopsis has two homologs for each subunit. We tested one of each of the homologs and examined the interaction of GAD-SH3P2 against GBD-fused VPS23.1, VPS28.1, and VPS37.1 in a yeast two-hybrid (YTH) analysis. VPS23.1 has an N-terminal ubiquitin E2 variant (UEV) domain that is a conserved ubiquitin-binding domain and a C-terminal core motif (Fig. 2A). SH3P2 showed YTH interaction with only VPS23.1 (Fig. 2B and Fig. S2A). A targeted YTH analysis and an in vitro-binding assay have shown that the UEV domain is dispensable for this interaction (Fig. 2 B and C and Fig. S2B).
Fig. 2.
SH3P2 interacts directly with VPS23.1. (A) Schematic presentation of full-length VPS23.1(FL) and truncated constructs VPS23.1(UEV) and VPS23.1(∆UEV) used for YTH and in vitro assays in B and C. (B) YTH analysis of GAD-SH3P2 with GBD-fusions of ESCRT-I subunits GBD-VPS37.1, GBD-VPS28.1, and GBD-VPS23.1, and YTH analysis of GBD-SH3P2 with GAD-fused VPS23.1(FL), VPS23.1(UEV), and VPS23.1(∆UEV). Yeast transformants were grown on media lacking leucine and tryptophan (−LW) or leucine, tryptophan, and histidine (−LWH) supplemented with 5 mM 3-amino-1,2,4-triazole (3-AT) to test their auxotrophic growth. Empty vectors, GAD and GBD, were used as negative controls. The expression of all fusion proteins was verified by immunoblotting shown in Fig. S2 A and B. (C) In vitro-binding assay of SH3P2 with VPS23.1(∆UEV). The ESCRT-III subunit VPS2.1 was used as a negative control. Bead-bound GST-VPS2.1 and GST- VPS23.1(∆UEV) were incubated with equal amounts of full-length MBP-SH3P2. After intensive washing, bead-bound materials were subjected to immunoblotting using anti-MBP and anti-GST antibodies.
Fig. S2.
Expression of YTH constructs. (A and B) Total proteins were extracted from yeast cells used for YTH analyses in Fig. 2B and subjected to immunoblotting using anti-GAL4BD and an anti-HA antibody. The anti-HA antibody was used for the detection of GAD-fusion proteins that also contain an HA-tag.
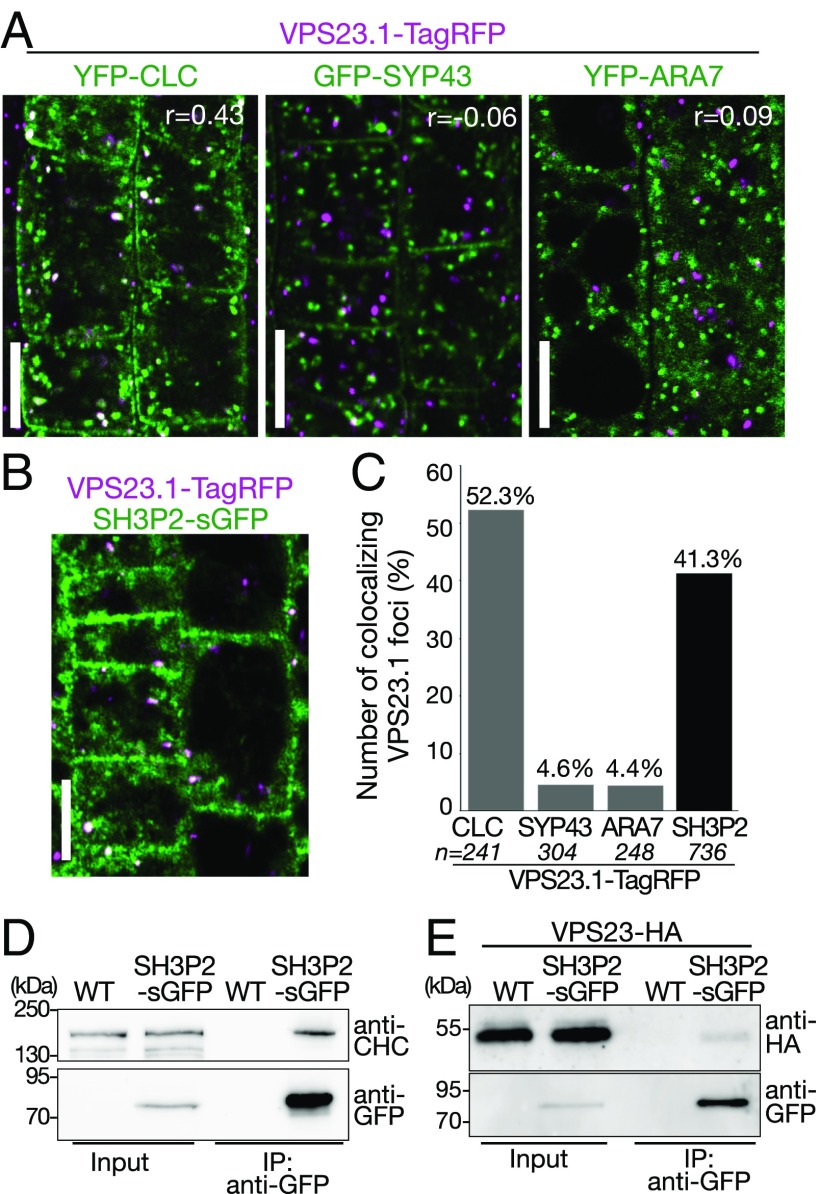
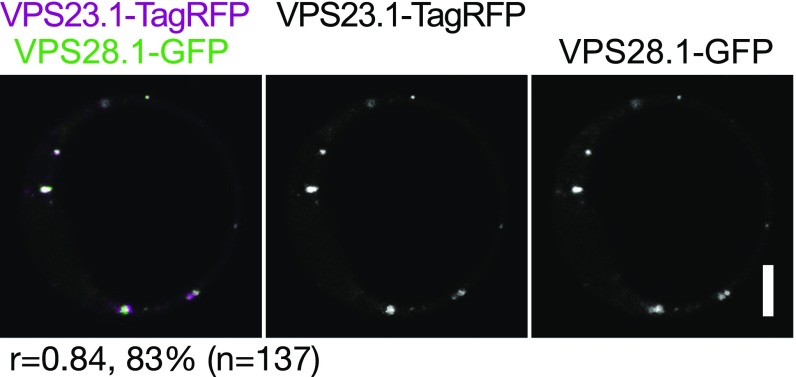
Immunoelectron microscopic analysis has previously shown that SH3P1, a homolog of SH3P2, localizes on the plasma membrane, the TGN, and the CCVs associated with them (34). We have previously shown that SH3P2 localizes on clathrin light chain (CLC)-labeled punctate structures and on late-endosome–associated aggregates that appear upon blockage of ESCRT-III disassembly (32). The ESCRT-I subunit VPS28 was previously demonstrated by immunoelectron microscopic analysis to localize on the TGN (39). However, colocalization of ESCRT-I subunits with clathrin markers has not been established in planta. We therefore generated plants expressing VPS23.1-TagRFP under the endogenous promoter and analyzed the localization with endosomal markers, including YFP-CLC (clathrin), GFP-SYP43 (TGN/EE) (40), and YFP-ARA7 (PVC/MVBs) (41) (Fig. 3A), as well as with SH3P2-superfolder GFP (sGFP) (32) (Fig. 3B). VPS23.1-TagRFP localized on punctate structures and colocalized with another ESCRT-I subunit, GFP-VPS28.1, when both were expressed under their native promoters (Fig. S3). Colocalization efficiency analysis showed that VPS23.1-TagRFP punctate structures showed the highest level of colocalization (52.3%, n = 241) with YFP-CLC (Fig. 3 A and C), whereas only 4.6% (n = 304) and 4.4% (n = 248) of the VPS23.1-TagRFP vesicles showed colocalization with the GFP-SYP43 and ARA7-labeled early and late endosomes, respectively (Fig. 3C). We could not detect localization of VPS23.1-TagRFP on the plasma membrane or on cell plates. When crossed with an SH3P2-sGFP line, 41.3% (n = 736) of the VPS23.1-TagRFP foci colocalized with SH3P2-sGFP–positive foci (Fig. 3 B and C).
Fig. 3.
VPS23.1-TagRFP colocalizes with SH3P2-sGFP and YFP-CLC in planta. (A) Colocalization analysis of VPS23.1-TagRFP with endosomal markers. Colocalization of VPS23.1-TagRFP with the clathrin marker YFP-CLC (Left), the early endosome marker GFP-SYP43 (Center), and the late endosome marker YFP-ARA7 (Right) was analyzed with a confocal microscope in root epidermis cells of 7-d-old Arabidopsis seedlings. r, Pearson’s correlation coefficient. (Scale bars: 10 μm.) (B) Colocalization of VPS23.1-TagRFP and SH3P2-sGFP. The localization of VPS23.1-TagRFP and SH3P2-sGFP in root epidermis cells of 7-d-old seedlings was analyzed under a confocal microscope. (Scale bar: 10 μm.) (C) Percentage of VPS23.1-TagRFP–positive foci that colocalize with endosomal markers or SH3P2-sGFP shown in A and B. n, total numbers of analyzed VPS23.1-TagRFP foci. (D) CHC coimmunoprecipitates with SH3P2-sGFP. Total extracts of 7-d-old wild-type (WT) and SH3P2-sGFP–expressing seedlings were incubated with anti-GFP antibody-immobilized agarose. After intensive washing, bead-bound materials [immunoprecipitated (IP) material: anti-GFP] and total extracts (input) were subjected to immunoblotting using anti-CHC and anti-GFP antibodies. (E) VPS23.1-HA coimmunoprecipitates with SH3P2-sGFP. Total extracts of WT and SH3P2-sGFP seedlings expressing VPS23.1-HA were incubated with anti-GFP antibody-immobilized agarose. After intensive washing, the IP material (anti-GFP) was subjected to immunoblots together with the total extracts (input) using anti-HA and anti-GFP antibodies.
Fig. S3.
VPS23-TagRFP colocalizes together with VPS28-sGFP on vesicles. Coexpression analysis in Arabidopsis root cell-derived protoplast of VPS23:VPS23-TagRFP and VPS28:VPS28-sGFP. The percentage of VPS23.1-TagRFP–positive foci that colocalize with VPS28.1-sGFP is indicated below the picture. n, total numbers of analyzed VPS23.1-TagRFP foci; r, Pearson’s correlation coefficient. (Scale bar: 10 μm.)
Next, we investigated whether SH3P2 and ESCRT-I can interact in vivo. Total extracts were prepared from wild-type and SH3P2pro:SH3P2-sGFP seedlings and mixed with GFP-trap beads. Immunoblots using anti-GFP and anti-clathrin heavy chain (CHC) antibodies showed that the endogenous CHC was coimmunoprecipitated with SH3P2-sGFP (Fig. 3D). Similarly, VPS23.1-HA was also specifically coimmunoprecipitated with SH3P2-sGFP (Fig. 3E). Taken together, these data show that both VPS23.1 and SH3P2 colocalize with CLC, suggesting that they can function together on intracellular clathrin-positive organelles in planta. The fact that SH3P2-sGFP interacts with both CHC and VPS23.1-HA in vivo suggests that these proteins can function together in the intracellular trafficking pathway.
SH3P2 Is Associated with CCVs.
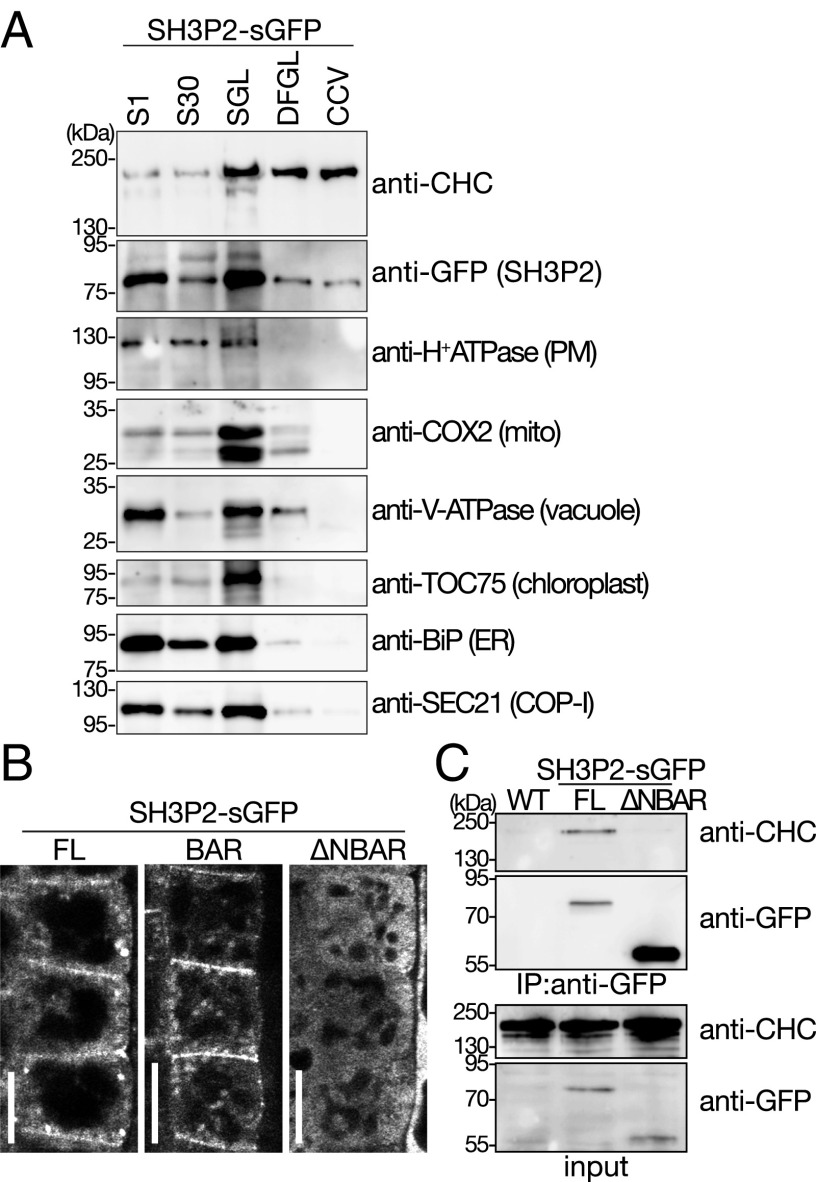
To further establish whether SH3P2 is localized on CCVs, we purified CCVs from 7-d-old seedlings expressing SH3P2-GFP under the native promoter using a tandem fractionation scheme based on differential and density centrifugation (42). SH3P2-GFP cofractionated with CHC, and was retained in the purified CCV fraction, whereas other organelle or compartment markers did not (Fig. 4A), indicating that SH3P2 is associated with CCVs.
Fig. 4.
SH3P2 fractionates with the CCV fraction. (A) CCVs were prepared from total plant extract of 7-d-old seedlings. Samples were collected during the procedure of CCV purification and subjected to Western blot analyses using antibodies against CHC, GFP, and various subcellular organelle marker proteins. CCV, CCV-containing fraction; DFGL, linear deuterium oxide/Ficoll gradient load; S1, supernatant after 1,000 × g centrifugation; S30, supernatant after 30,000 × g centrifugation; SGL, sucrose step gradient load. The following antibodies were used as organelle- or compartment-specific markers: anti–H+-ATPase [plasma membrane (PM)], anti-COX2 [mitochondria (mito)], anti–V-ATPase (vacuole), TOC75 (chloroplast), anti-BiP [endoplasmic reticulum (ER)], and anti-SEC21 [COP-I vesicle (COP-1)]. (B) Localization of full-length SH3P2-sGFP(FL), SH3P2-sGFP(BAR), and SH3P2-sGFP(∆NBAR). Localization was analyzed in the root epidermis cells of 7-d-old seedlings by confocal microscopy. Whereas the GFP-fused SH3P2-sGFP(FL) and SH3P2-sGFP(BAR) localized to the PM and on intracellular punctate structures, SH3P2-sGFP(∆NBAR) signals are dispersed in the cytosol. (Scale bars: 10 μm.) (C) Immunoprecipitation of full-length SH3P2-sGFP(FL) and SH3P2-sGFP(∆NBAR). Full-length SH3P2-sGFP(FL) and SH3P2-sGFP(∆NBAR) were immunoprecipitated from total extracts prepared from 8-d-old seedlings expressing the respective constructs. The bead-bound material (IP material: anti-GFP) and total extracts (input) was subjected to immunoblotting using anti-GFP and anti-CHC antibodies.
SH3P2 was previously reported to bind phospholipids, and it was shown that the BAR domain alone was sufficient for this binding (33–35). Accordingly, we observed that the truncated protein without the complete BAR domain SH3P2(∆NBAR: amino acids 179–369)-sGFP did not localize on the plasma membrane or on intracellular punctate structures, whereas the localization of SH3P2(BAR: amino acids 1–257)-sGFP was indistinguishable from that of the full-length fusion protein (FL) (Fig. 4B). SH3P2(∆NBAR)-sGFP also lost its interaction with CHC (Fig. 4C), indicating that the N-terminal region containing the BAR domain is indispensable for the interaction with membrane structures containing clathrin and for the localization of SH3P2 on the plasma membrane and on clathrin-positive foci.
SH3P2 Interacts with the DUB AMSH3.
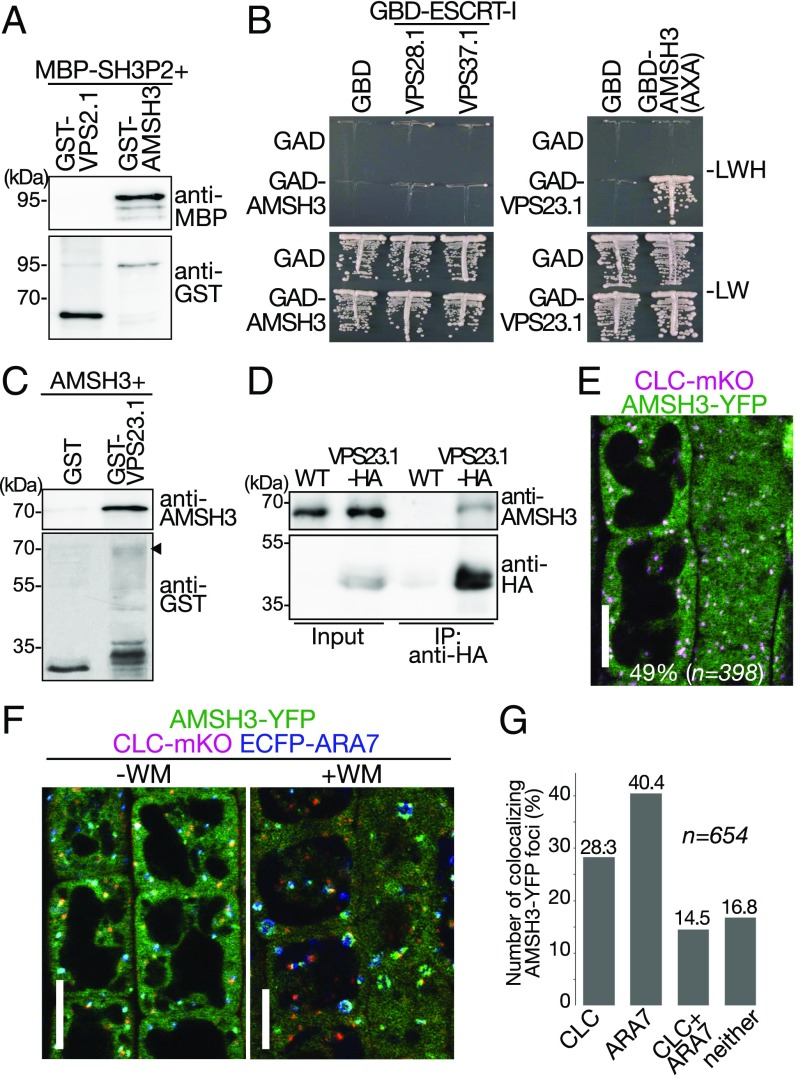
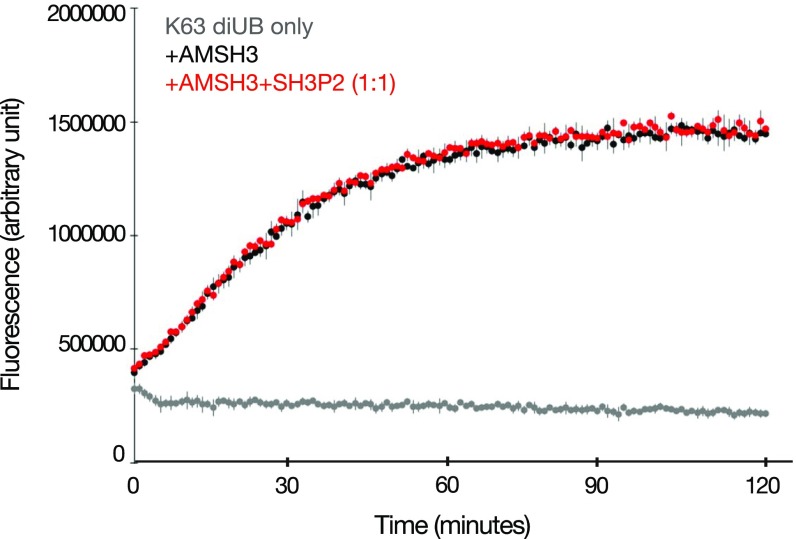
Since the DUB AMSH was initially identified as a protein that interacts with the SH3 domain of STAM, an ESCRT-0 subunit in mammals (43), we investigated whether the Arabidopsis AMSH3 and SH3P2 can directly interact. As shown in Fig. 5A, MBP-SH3P2 bound to GST-AMSH3 but not to GST-VPS2.1, an ESCRT-III subunit that served as a negative control, showing that AMSH3 interacts with SH3P2 in vitro (Fig. 5A). However, the addition of SH3P2 in a DUB assay using AMSH3 did not show altered activity of AMSH3 (Fig. S4), indicating that SH3P2 does not influence the in vitro DUB activity of AMSH3.
Fig. 5.
DUB AMSH3 interacts directly with SH3P2 and VPS23.1 and localizes on both CLC- and ARA7-positive endosomes. (A) In vitro-binding assay of SH3P2 with AMSH3. Bead-bound GST-AMSH3 and the negative control GST-VPS2.1 were incubated with equal amounts of full-length MBP-SH3P2. After intensive washing, bead-bound materials were analyzed by immunoblotting with anti-MBP and anti-GST antibodies. (B) YTH analysis of AMSH3 with ESCRT-I subunits. GAD-AMSH3 or GBD-AMSH3(AXA) was coexpressed with ESCRT-I subunits GBD-VPS28.1, GBD-VPS37.1, and GAD-VPS23.1. Yeast transformants expressing both GAD- and GBD-fusions were grown on media lacking leucine and tryptophan (−LW) or leucine, tryptophan, and histidine (−LWH) to test their auxotrophic growth. Empty vectors were used as negative controls. The expression of fusion proteins was verified by immunoblotting, as shown in Fig. S5. (C) In vitro-binding assay of AMSH3 with VPS23.1. Bead-bound GST-VPS23.1 was incubated with an equal amount of AMSH3. After intensive washing, bead-bound materials were analyzed by immunoblotting with anti-AMSH3 and anti-GST antibodies. The arrowhead indicates the position of GST-VPS23.1. (D) AMSH3 coimmunoprecipitates with VPS23.1-HA. Total extracts of wild-type (WT) and VPS23.1-HA seedlings were incubated with anti-HA antibody-immobilized agarose. After intensive washing, the immunoprecipitated (IP) material (anti-HA) was subjected to immunoblotting together with the total extracts (input) using anti-HA and anti-AMSH3 antibodies. (E) Colocalization of AMSH3-YFP and CLC-mKO. The localization of AMSH3-YFP and CLC-mKO in root epidermis cells of 7-d-old seedlings was analyzed under a confocal microscope. (Scale bar: 10 μm.) (F) Colocalization of AMSH3-YFP with CLC-mKO and ECFP-ARA7. The localization was analyzed under a confocal microscope in root epidermis cells of 7-d-old seedlings expressing AMSH3-YFP with CLC-mKO and ECFP-ARA7 without (Left) or with 60 min of WM treatment (Right). (Scale bars: 10 μm.) (G) Percentage of AMSH3-YFP foci that colocalize with CLC only, ARA7 only, with both CLC and ARA7, or with neither marker (without WM treatment). n, total number of analyzed AMSH3-YFP foci.
Fig. S4.
SH3P2 does not affect AMSH3 DUB activity. DUB assay using K63-linked diubiquitin-TAMRA as a substrate. Recombinant AMSH3 was incubated with diubiquitin-TAMRA with or without preincubation with an equimolar amount of SH3P2. Fluorescence was measured every minute (black, diubiquitin-TAMRA with AMSH3; red, diubiquitin-TAMRA with AMSH3 and SH3P2; gray, K63-linked diubiquitin-TAMRA only).
To establish whether AMSH3 has a function in clathrin-mediated endocytosis, we explored the interaction of AMSH3 with other proteins reported to function in the pathway. Like SH3P2, AMSH3 was found to interact with the ESCRT-I subunit VPS23.1 in an YTH assay (Fig. 5B and Fig. S5) and in an in vitro-binding assay (Fig. 5C). In addition, VPS23.1-HA and AMSH3 were coimmunoprecipitated from total protein extracts of Arabidopsis seedlings (Fig. 5D), suggesting that these proteins function together in vivo. AMSH3 was previously shown to localize on ARA6- and ARA7-labeled late endosomes (44). To analyze whether AMSH3 can also localize together with VPS23.1 on clathrin-labeled foci, we analyzed the colocalization between AMSH3-YFP and CLC-monomeric Kusabira Orange (mKO). Forty-nine percent of AMSH3-YFP foci colocalized with CLC-mKO (n = 398; Fig. 5E). To investigate whether AMSH3-YFP foci showed distinct localization with CLC- and ARA7-labeled endosomes, seedlings expressing AMSH3-YFP, CLC-mKO, and ECFP-ARA7 were examined. A total of 40.4% of AMSH3-YFP signals colocalized with endosomes labeled with ECFP-ARA7, 28.3% of AMSH3-YFP signals colocalized with CLC-mKO, and 14.5% of the AMSH3-YFP signals colocalized with both markers (Fig. 5 F and G). These results indicate that CLC-mKO and ARA7-YFP predominantly reside on separate compartments and that AMSH3-YFP is associated with both CLC- and ARA7-labeled endosomes. To verify the independence of the CLC-mKO and ECFP-ARA7 in this line, we treated seedlings with the PI3K/PI4K inhibitor Wortmannin (WM). ECFP-ARA7 localized to the typical swollen late endosomal compartments induced by WM, whereas CLC-mKO did not (Fig. 5F), indicating the two markers maintain largely distinct localization patterns even upon coexpression of AMSH3-YFP. Taken together, these data show that VPS23.1, AMSH3, and SH3P2 all localize on CLC-positive punctate structures and likely function together in plants.
Fig. S5.
Expression of YTH constructs. Total proteins were extracted from yeast cells used for YTH analysis in Fig. 4B and subjected to immunoblotting using anti-GAL4BD and an anti-HA antibody. The anti-HA antibody was used for the detection of GAD-fusion proteins that also contain an HA-tag.
SH3P2 Shows Genetic Interactions with the ESCRT-I Subunit VPS23.1.
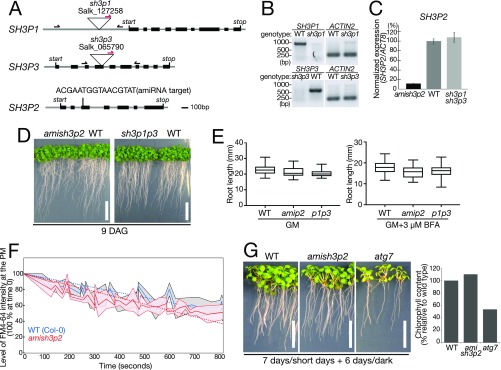
To investigate the physiological role of SH3P2 in Arabidopsis, we examined transfer-DNA (T-DNA) insertion lines of SH3P1, SH3P2, and SH3P3 and identified null mutant alleles designated sh3p1 and sh3p3 (Fig. S6 A and B). For SH3P2, we could not identify any T-DNA insertion lines that affected the level of SH3P2 transcript, and therefore generated an artificial microRNA (amiRNA) line designated amish3p2, which has a 90% reduction in the level of SH3P2 mRNA relative to wild type and the sh3p1sh3p3 double mutant (Fig. S6C).
Fig. S6.
Mutants of sh3p2 and sh3p1sh3p3 do not show apparent phenotypes. (A) Schematic presentation of the T-DNA insertion sites in sh3p1 and sh3p3 and position of the amiRNA target sequence of amish3p2. Gray lines indicate untranslated regions, and introns and black boxes indicate exons. Positions of start and stop codons are indicated in the figure. Binding sites of the forward and reverse primers and the T-DNA left border primer used for genotyping are indicated by arrows. (B) PCR analysis of cDNA generated from total RNA of sh3p1 and sh3p3 homozygous mutants. Expression of SH3P1 and SH3P3 in wild-type (WT), sh3p1, and sh3p3 seedlings was analyzed using SH3P1-, SH3P3-, and ACTIN2-specific primers. (C) qRT-PCR analysis of amish3p2. Expression of SH3P2 in amish3p2-, WT, and sh3p1sh3p3 double-homozygous seedlings was analyzed using gene-specific primers of SH3P2 and normalized against transcript levels of ACT8. (D) Photographs of 9-d-old WT, amish3p2, and sh3p1sh3p3 seedlings. Seedlings were grown on GM under short-day conditions. (Scale bars: 2 cm.) (E) Measurement of the root length of 6-d-old WT and mutant seedlings grown on GM or GM supplemented with 3 μM BFA. (F) Measurement of FM4-64 uptake at the plasma membrane in root epidermis cells of 7-d-old WT (blue line) and amish3p2 (red line) seedlings. Seedlings were incubated for 5 min in liquid GM with 2 μM FM4-64 and washed twice in liquid GM before the analysis under a confocal microscope. FM4-64 signals were imaged using the same settings over a period of 15 min. Signal intensities were analyzed using Olympus Fluoview software. The signal intensity at 0 min was set to 100%. (G, Left) Photographs of WT, amish3p2, and atg7 seedlings after a 6-d dark treatment. (Scale bars: 1 cm.) (G, Right) Total chlorophyll content in the seedlings was analyzed. Seedlings were grown for 7 d on half-strength MS under long-day conditions before being transferred into the dark. Note that starvation-induced chlorosis is not enhanced in amish3p2 compared with the autophagy mutant atg7. The value of the WT was set to 100%.
Neither the sh3p1sh3p3 double mutant nor amish3p2 showed apparent developmental phenotypes (Fig. S6D) even in the presence of the ARF-GEF inhibitor Brefeldin A (BFA) (Fig. S6E). In addition, uptake of the endocytosis tracer FM4-64 in amish3p2 was comparable to wild-type seedlings (Fig. S6F). As SH3P2 was reported to be involved in autophagy, we next tested the amish3p2 for dark-induced autophagy phenotypes. The mutants remained indistinguishable from the wild type even after prolonged dark treatment, whereas atg7, a bona fide autophagy defective mutant, displayed pale green leaves and significantly reduced chlorophyll content (Fig. S6G). Repeated attempts to generate a sh3p1sh3p3amish3p2 triple mutant failed, as the amiRNA construct did not express in the sh3p1sh3p3 double-mutant background, and thus we cannot rule out the existence of redundancy between the three SH3Ps.
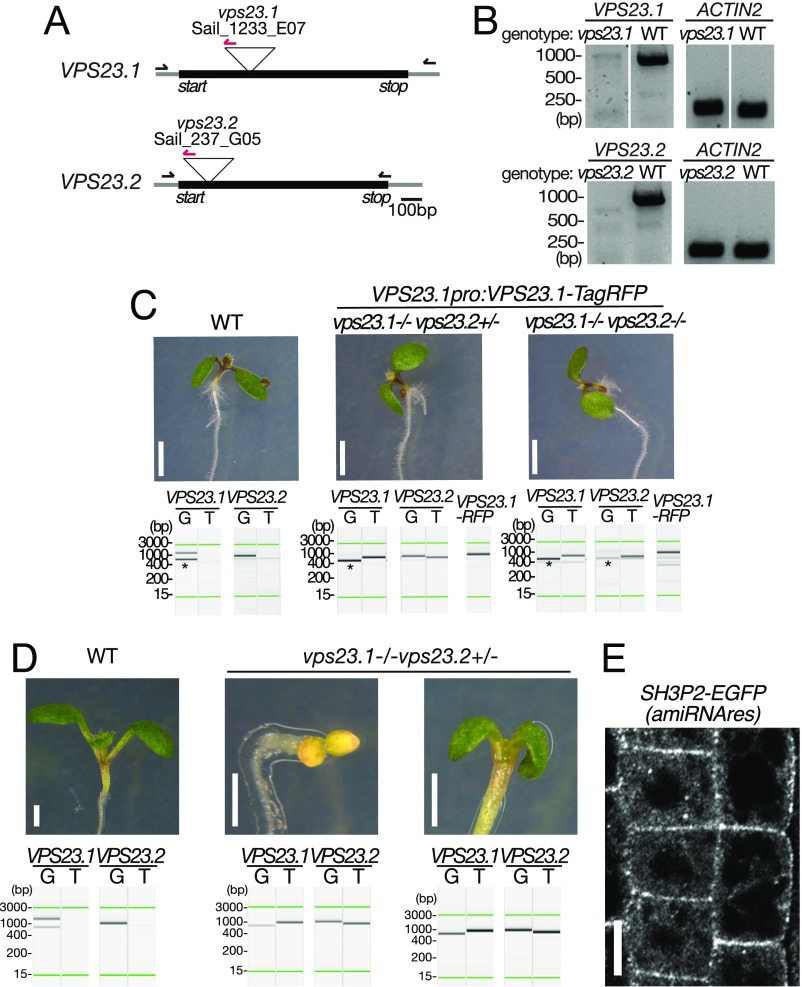
To test whether mutants of vps23 and sh3p2 show genetic interactions, we first established mutants of vps23. Arabidopsis has two VPS23 homologs, VPS23.1/VPS23A/ELCH and VPS23.2/VPS23B/ELCH-LIKE. We isolated T-DNA insertion mutants of VPS23.1 and VPS23.2 that we named vps23.1-3 and vps23.2-1, both of which were null mutants (Fig. S7 A and B). We then crossed them with each other to generate double mutants. We could not recover double-homozygous mutants among the progeny of a double-heterozygous plant, suggesting that the double-homozygous mutants are not viable. The inviable phenotype of the double homozygotes was rescued by the expression of VPS23.1:VPS23.1-TagRFP (Fig. S7C), indicating that the VPS23 function is essential in Arabidopsis and that the VPS23.1-TagRFP construct used for the localization studies was functional.
Fig. S7.
VPS23.1:VPS23.1-RFP complements seedling lethality of the vps23.1-3vps23.2-1 double-homozygous mutant. (A) Schematic presentation of the T-DNA insertion sites in vps23.1-3 and vps23.2-1. Gray lines indicate untranslated regions, and introns and black boxes indicate exons. The positions of start and stop codons are indicated in the figure. Binding sites of the forward and reverse primers and the T-DNA left border primer used for genotyping are indicated by arrows. (B) Expression of VPS23.1 and VPS23.2 in wild-type (WT), vps23.1-3, and vps23.2-1 seedlings was analyzed with VPS23.1-, VPS23.2-, and ACTIN2-specific primers. (C) Photographs of 7-d-old vps23.1-3vps23.2-1 seedlings. Photographs of WT (Left), vps23.1-3−/−vps23.2-1+/− (Center), and vps23.1-3−/−vps23.2-1−/− (Right) seedlings complemented with VPS23.1-TagRFP. PCR-based genotyping was conducted to verify the genotypes. Lanes G, genomic fragments; lanes T, T-DNA insertion-specific products. The green lines represent the 15-bp and 3,000-bp marker lines, respectively. The presence of the VPS23.1-TagRFP transgene was verified using construct-specific PCR primers. (Scale bars: 2 mm.) (D) Photographs of 7-d-old vps23.1-3−/−vps23.2-1+/− seedlings. Photographs of WT (Left) and vps23.1-3−/−vps23.2-1+/− (Center and Right) are shown. PCR-based genotyping was conducted to verify the genotypes, shown below the pictures. (Scale bars: 1 mm.) (E) Expression and localization of the amiRNA-resistant SH3P2:SH3P2(amiRNAres)-EGFP was observed under a confocal microscope. (Scale bar: 10 μm.)
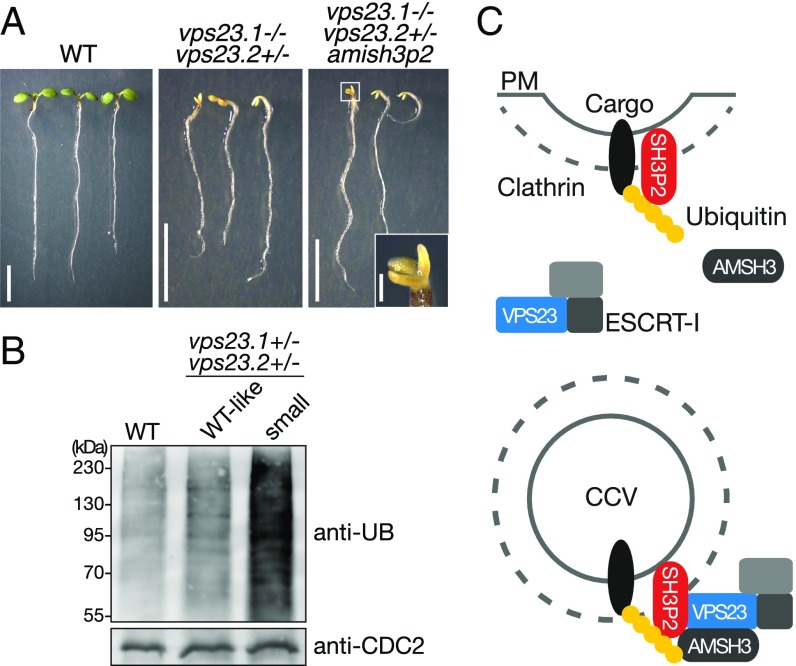
We also observed that 9.6% of the total progenies of vps23 double-heterozygous plants were smaller than their siblings and had small cotyledons. PCR-based genotyping identified them as vps23.1-3−/−vps23.2-1+/− (Fig. 6A, Table 1, and Fig. S7D). The phenotypes could be further divided according to the color of the cotyledons. A total of 8.6% of progenies had small but green cotyledons (Table 1 and Fig. S7D), while 1% of progenies remained white even when grown in light, suggesting that these mutants have defects in greening in addition to developmental defects. In progenies from a wild-type plant, none of the seedlings showed this phenotype (Table 1). In the progeny of a vps23.1-3+/−vps23.2-1+/− line harboring the amish3p2 construct, the number of seedlings with small white cotyledons significantly increased to 4.7% (Fig. 6A and Table 1), while the number of seedlings with small green cotyledons decreased to 5.8%, indicating that the presence of amish3p2 increased the number of vps23.1-3−/−vps23.2-1+/− seedlings with greening defects. To verify that this shift is caused by amish3p2, we generated an amiRNA-resistant variant of SH3P2-sGFP named SH3P2(amiRNAres)-EGFP, and introduced it into the vps23.1-3+/−vps23.2-1+/−amish3p2 background. SH3P2(amiRNAres)-EGFP restored the segregation of seedling phenotypes (Table 1), confirming the effect of amish3p2 on the vps23 mutants. The expression of SH3P2(amiRNAres)-EGFP was verified under confocal microscopy (Fig. S7E).
Fig. 6.
The vps23.1-3−/−vps23.2-1+/− seedlings show severe developmental defects and accumulate ubiquitylated proteins. (A) Phenotypes of vps23.1-3−/−vps23.2-1+/− seedlings. Photographs of 7-d-old wild type (WT; Left), vps23.1-3−/−vps23.2-1+/− (Center), and vps23.1-3−/−vps23.2-1+/− harboring the amish3p2 construct (Right) are shown. (Right Inset) Among the vps23.1-3−/−vps23.2-1+/−amish3p2 seedlings, seedlings with tricotyledons were also found. (Scale bars: 5 mm; Inset, 0.5 mm.) (B) Accumulation of ubiquitin (UB) conjugates in vps23 mutants. Total protein extracts of 7-d-old WT, vps23.1-3−/−vps23.2-1+/− WT-looking progenies (WT-like siblings), and aberrant vps23.1-3−/−vps23.2-1+/− (small) seedlings were subjected to immunoblotting using an anti-UB antibody. Note that vps23.1-3−/−vps23.2-1+/− (small) seedlings accumulate ubiquitylated proteins compared with the WT seedlings. Anti-CDC2 antibody was used as a loading control. (C) Possible function of SH3P2 on CCVs. SH3P2 can bind to the K63-linked ubiquitin chain of a cargo protein at the plasma membrane (PM) and, through its BAR domain, can bind to the membrane during clathrin-mediated endocytosis of ubiquitylated cargo proteins. SH3P2 could recruit ESCRT-I to the CCV via the interaction with the subunit VPS23. VPS23 binds the ubiquitin chain of the endocytosed cargo and leads the cargo to the downstream ESCRT machinery. The DUB AMSH3 can bind both the SH3P2 and the ESCRT-I subunit VPS23 and could be recruited by both proteins to the CCVs. Deubiquitylated membrane cargos might be recycled back to the PM.
Table 1.
Segregation of vps23 mutant progenies
| Genotype of mother plant | Normal seedlings, % | Small seedlings | n | χ2 P* | |
| Green, % | White, % | ||||
| Wild type (Col-0) | 100.0 | 0 | 0 | 292 | 0.0050 |
| vps23.1-3+/−vps23.2-1+/− | 90.5 | 8.6 | 1.0 | 629 | — |
| vps23.1-3+/−vps23.2-1+/−amish3p2 | 89.5 | 5.8 | 4.7 | 551 | 0.0007 |
| vps23.1-3+/−vps23.2-1+/−amish3p2 | 87.1 | 10.9 | 2.0 | 248 | 0.4184 |
| SH3P2-sGFP(amiRNAres) | |||||
Col-0, Columbia-0.
Compared with vps23.1-3+/−vps23.2-1+/−.
Recently, it has been reported that VPS23.1 is involved in the ubiquitin-dependent degradation of the abscisic acid (ABA)-receptor PYR1-like 4 (PYL4) in Arabidopsis (45). Upon treatment with ABA, a null mutant of vps23.1 (vps23a) accumulated ubiquitylated proteins, while the level of ubiquitin conjugates was indistinguishable from the wild type when untreated. To test whether the vps23.1-3−/−vps23.2-1+/− mutant shows defects in ubiquitin-mediated protein degradation, we examined the ubiquitin profile of mutant seedlings. Seven-day-old seedlings with mutant phenotypes accumulated ubiquitinated proteins at a high level (Fig. 6B), indicating that VPS23 is required for the removal of ubiquitin conjugates also in ABA-independent processes.
Taken together, previous data (34, 35) and the data presented in this study suggest that Arabidopsis ESCRT-I VPS23.1, AMSH3, and SH3P2 localize and function together on clathrin-labeled endomembrane structures. SH3P2 can bind K63-linked ubiquitin chains of membrane proteins in close proximity to the plasma membrane or on/in CCVs. We propose a model (Fig. 6C) in which SH3P2 binds ubiquitylated cargos at the plasma membrane and during clathrin-mediated endocytosis. Alternatively, SH3P2 could be also recruited separately to the plasma membrane and to CCVs. SH3P2 could enrich ubiquitylated cargos at the endosomal membrane and, by interacting with VPS23, could pass them to the ESCRT machinery. On CCVs, SH3P2 could function together with the ESCRT-I subunit VPS23 and the DUB AMSH3. Since SH3P2 localizes to the plasma membrane, whereas VPS23 and AMSH3 do not, SH3P2 could also have an ESCRT-I– and DUB-independent function at the plasma membrane. Deubiquitylation of cargos by DUBs at this stage might rescue the cargo from being sorted into the ESCRT-dependent degradation pathway.
Discussion
SH3 domains can be found in all eukaryotes, and whereas more than 100 SH3 domain-containing proteins regulate diverse signaling pathways in mammals (46, 47), Arabidopsis has only five SH3 domain proteins, suggesting a different regulatory mechanism by SH3 domain proteins in animals and plants. In this work, we have demonstrated that the plant-specific SH3 domain-containing protein SH3P2 from Arabidopsis binds preferentially K63-linked ubiquitin chains and interacts in vitro and in vivo with the ESCRT-I subunit VPS23.1. SH3P2 interacts also with the DUB AMSH3. SH3P2, VPS23.1, and AMSH3 all localize on clathrin-positive intracellular structures. Based on these results, we propose a role for SH3P2 in the recognition of ubiquitinated cargos that are to be sorted into the ESCRT pathway.
Among proteins involved in membrane trafficking of plants, FYVE1/FREE1, ALG-2-interacting protein X (ALIX), TOLs, VPS23.1, and the ESCRT-II subunit VPS36 were shown to have affinity toward ubiquitin and/or ubiquitin chains in Arabidopsis (26, 30, 44, 48, 49). The lack of any obvious phenotypes in the sh3p mutants, despite the well-established importance of the ESCRT-mediated degradation pathway, could be due to the genetic redundancy between members of the SH3P family as well as other plant ubiquitin adaptor proteins. SH3P2 was recently reported to interact with the ubiquitin-binding FYVE1/FREE1 protein (30, 32), suggesting that ubiquitin-binding proteins may interact and function together. This may increase their affinity to ubiquitin chains and could enhance the efficiency of sorting the cargos into the ESCRT-dependent endocytic degradation pathway.
Polyubiquitylation of membrane proteins by ubiquitylating enzymes is key for the determination of protein stability (50, 51). By hydrolyzing the ubiquitin chains, DUBs could rescue the target proteins from degradation and recycle part of the cargos back to the plasma membrane. The recycling of membrane proteins can fine-tune the regulation of protein abundance at the plasma membrane and could also enable a faster reaction upon environmental changes without the cost for de novo protein synthesis.
Some DUBs require interacting proteins for their enzymatic activation (52). AMSH proteins, however, display DUB activity in vitro as monomers, although human AMSH proteins were reported to show higher activity in the presence of the interactor of AMSH, STAM (52). STAM is an SH3 domain-containing protein and ESCRT-0 subunit. The interaction between AMSH and SH3 domain-containing proteins seems to be conserved in plants, albeit the absence of ESCRT-0 and the structural differences between SH3P2 and STAM. Ubiquitin-binding proteins could facilitate the access of DUBs to the ubiquitin chain by simultaneously binding both the ubiquitin chain and the DUBs. In a quantitative in vitro DUB assay, however, preincubation of AMSH3 with SH3P2 did not accelerate the DUB activity of AMSH3. The acceleration of DUB activity may require different substrates or additional factors. AMSH3 lacks recognizable membrane-binding motifs, and thus is probably dependent on binding partners, such as ALIX, for its recruitment to endosomes (44). SH3P2 binds phospholipids (34, 35) and could also play a role in stabilizing the association of AMSH3 with endosomal membranes and the ESCRT machinery.
To date, only single mutants of ESCRT-I components have been analyzed in plants. The analyzed mutants of VPS23.1/VPS23A/ELCH, VPS28.1, and VPS37.1 were all viable, but were defective in cytokinesis, endosomal sorting, and degradation of membrane-localized proteins and showed an altered phytohormone or pathogen response (45, 48, 53). The fact that the double-null mutant vps23.1-3−/−vps23.2-1−/− displayed embryo lethality, shows that ESCRT-I, like ESCRT-III, is essential for plant growth and development. Similar to previously reported ESCRT-III mutants or ESCRT-III–related mutants, including fyve1 and amsh3 (30, 32, 44, 54), part of vps23.1-3vps23.2-1 seedlings are small and have white cotyledons. Although the exact molecular mechanisms are unknown, a previous report has shown that mutants defective in membrane trafficking or plants treated with inhibitors of membrane trafficking have defects in plastid development (55). The mutant phenotypes observed in this study suggest that defects in ESCRT-I function may also have a similar effect on plastid development.
Localization studies have revealed similar but distinct localization patterns of ubiquitin-binding proteins in Arabidopsis. TOLs are predominantly localized to the plasma membrane, with a fraction on punctate structures in the cytosol (26), whereas FYVE1 and ALIX were not found at the plasma membrane, but were shown to localize on punctate structures that were colabeled with late endosomal markers (30, 32, 44). Our data, together with a previous report (34), demonstrate that the SH3Ps are associated with CCVs and localize on the plasma membrane, cell plate, and clathrin-positive foci. These localization studies suggest that ubiquitin adaptors, including SH3P2, can be involved in vesicle trafficking at diverse membrane compartments. Clathrin has been shown to form non-triskelia flat plaques on endosomal membranes, where it is assumed to be involved in the recruitment of ubiquitylated cargos together with ubiquitin-binding proteins (56–58). Whether SH3P2 could also interact and function together with clathrin on similar structures on the endosomes has yet to be elucidated.
While this paper was in preparation, Ahn et al. (35) reported that SH3P2 localizes to the tubulovesicular network at the cell plate and could function together with DYNAMIN RELATED PROTEIN (DRP) 1A during cell plate formation. It was also shown that SH3P2 binds phosphoinositides through its N-terminal BAR domain and that the BAR domain of SH3P2 can cause tubulation of liposomes (35). Future studies should reveal whether SH3P2 is also involved in membrane bending and/or scission events at the plasma membrane together with the DRPs and how the dynamics of SH3P2, ESCRT-I, and other ubiquitin-binding proteins are regulated during endocytosis.
Materials and Methods
Biological Material.
All experiments were performed with Arabidopsis thaliana Columbia-0 (Col-0) background, except for the ELCH-HA line, which is in Wassilewskija-2 (Ws2) background (59). T-DNA insertion mutants sh3p1 (Salk_127258), sh3p3 (Salk_065790), vps23.1-3 (Sail_1233E07_2-4) and vps23.2-1 (Sail_237G05_3-4) were obtained from the Nottingham Arabidopsis Stock Centre.
Plant transformations were performed using the floral dip method (60). Plants were grown in continuous light or under long-day (16-h light/8-h dark) conditions with 100–120 μmol⋅m−2⋅s−1 light on Murashige and Skoog medium (MS; Duchefa Biochemie) supplemented with 1% sucrose germination media (GM). For root length measurements, seedlings were grown 6 d on GM supplemented with 3 μM BFA. For artificial starvation, 7-d-old seedlings grown on half-strength MS were transferred to the dark for 6 d.
Protein Purification, in Vitro-Binding Assays, and Ubiquitin-Binding Assay.
GST- or MBP-fused proteins were expressed in the Escherichia coli Rosetta (DE3), Rosetta-gami 2, or Rosetta-gami B strains (all from Merck Millipore) and purified using Pierce Glutathione Magnetic Beads (Thermo Scientific), ProtinoGlutathione Agarose 4B (Macherey–Nagel), or Amylose Resin (New England Biolabs), depending on the fusion protein.
For in vitro-binding assays, Pierce Glutathione Magnetic Beads saturated with 25, 50, or 65 pmol of GST-fusion proteins were incubated with an equimolar amount of another protein or tetra-ubiquitin chains (Enzo Life Science) in 400 μL of cold buffer (50 mM Tris⋅HCl at pH 7.5, 150 mM NaCl, 10 mM MgCl2, 0.05% Tween-20) under rotation at 4 °C. The beads were then washed four times with cold buffer, proteins were eluted with 40 mM glutathione, and bead-bound materials were subjected to SDS/PAGE and analyzed by immunoblotting.
For the ubiquitin-binding assay with plant total extracts, 6 g of Arabidopsis seedlings was ground and homogenized in 10 mL of lysis buffer A (50 mM Tris⋅HCl at pH 7.5, 100 mM NaCl, 10% glycerol), supplemented with 0.2% Triton X-100 and 1× protease inhibitor mixture (Roche), and clarified by centrifugation for 15 min at 13,000 × g. The supernatant was mixed with 400 μg of bead-bound GST or GST-SH3P2(SH3) and incubated for 6 h at 4 °C with gentle rotation. Samples were washed four times in buffer A and eluted by boiling in 1× SDS sample buffer. Samples were separated by SDS/PAGE and analyzed by immunoblotting.
Immunoprecipitations.
One gram of 7-d-old seedlings was ground and homogenized in 2 mL of buffer A (50 mM Tris⋅HCl at pH 7.5, 100 mM NaCl, 10% glycerol) supplemented with 0.2% Nonidet P-40 and 1× protease inhibitor mixture. Cell lysates were clarified by centrifugation for 15 min at 13,000 × g, diluted 10-fold with buffer A, and incubated with GFP-TRAP Magnetic Agarose Beads (ChromoTek) for 1.5 h at 4 °C while rotating. Samples were washed four times in buffer A and eluted by boiling in 30 μL of 1× SDS sample buffer before being subjected to SDS/PAGE and immunoblotting analysis.
SI Materials and Methods
Cloning Procedures.
All primers used for cloning are listed in Table S1.
Table S1.
Primers used in this study
| Primer name | Sequence |
| AG47 At5g13860 rv | GTATCACGAATGCAACCTAGCTGCAATGGAAG |
| AG49 At3g12400 fw | AACTTTGGACTTTGGAACATGTCCACTCCTTACA |
| AG51 At3g12400 rv | AGGAACCATCATCACACGCATAGCCACAG |
| EI1 GW adaptor fw | GGGGACAAGTTTGTACAAAAAAGCAGGCT |
| EI2 GW adaptor rv | GGGGACCACTTTGTACAAGAAAGCTGGGT |
| EI223 CLC GW fw | GGGGACAAGTTTGTACAAAAAAGCAGGCTTGTCTGCCTTTGAAGACGA |
| EI224 CLC GW rv | GGGGACCACTTTGTACAAGAAAGCTGGGTACACAGATGTAAGCAACGAA |
| FA121 TagRFP rv EcoRI | CGCGAATTCTCAATTAAGTTTGTGCC |
| FA169 Sail_237_G05 fw | TCGTCATCTGTCGTCGTCTTCAAG |
| FA212 SH3P2 fw | GAATACATGTACTAACGGAAATGTTTTAACACGGGCT |
| FA213 SH3P2 rv | AGCCCGTGTTAAAACATTTCCGTTAGTACATGTATTC |
| FA214 SH3P2 ins fw | TGTCCGAAGTTTTCAAGCGGTGGGCGCGCCGA |
| FA215 SH3P2 ins rv | TCGGCGCGCCCACCGCTTGAAAACTTCGGACA |
| KK101 ARA7 attB1 | AAAAAGCAGGCTTGGCTGCAGCTGGA |
| KK102 ARA7 attB2 | AGAAAGCTGGGTCTAAGCACAACAAGATGAGCTC |
| LBb1.3 mod | ATTTTGCCGATTTCGGAACCACCATC |
| LB3Sail | AATTTCATAACCAATCTCGATACAC |
| MN91 SALK127258 fw | ACACAACCGTTGTTGGTAT |
| MN92b SALK127258 rv | CCTCAACAATCCTATCTACCC |
| MN93 SALK065790 fw | TGTCACTTTCAGGAGTTCC |
| MN 99 At1g31440 fw | GGACCTCTTACAAGTCGG |
| MN100 At1g31440 rv | GAATCATTCTGGCTGAGAA |
| MN103 At4g18060 fw | AGTCAGAACATTGATGAGAATA |
| MN104 At4g18060 rv | GGATGGCTGCTATTCTTAAG |
| MN146 At2g36680 fw EcoRI | AAGGGAATTCATGTTCAATTTCTGGGGAT |
| MN147 At2g36680 rv SalI | AAGGGTCGACTCAGCCAATAGATGAAGTTTT |
| MN148 At4g05000 fw EcoRI | AAGGGAATTCATGATGGAGGTCAAATTATG |
| MN149 At4g05000 rv SalI | AAGGGTCGACTTAATTACCAGCTTTAGGCA |
| MN150 At3g12400 fw EcoRI | AAGGGAATTCATGGTTCCCCCGCCG |
| MN151 At3g12400 rv SalI | AAGGGTCGACTCATGAATGTAACCTACCTGCGATG |
| MN156-2 At4g34660 rv NotI | AAGGGCGGCCGCTCAGAAAACTTCGGACAC |
| MN157 SH3P2(c250) fw EcoRI | AAGGGAATTCCAGCTCGAAGGAGAGATG |
| MN158 SH3P2(c179) fw EcoRI | AAGGGAATTCGAGTCTCAAGGTAATCCTGAC |
| MN174 At4g34660 left | TTGATCAGCTCGAAGGAGAGA |
| MN175 At4g34660 right | GCAAACACTCCATTAGCTTCCT |
| MN185 VPS23 genomic Pro fw | AAGGACAAGTTTGTACAAAAAAGCAGGCTGAGATCATTTTTGTGTCACCA |
| MN186 VPS23 without Stop rv | AAGGACCACTTTGTACAAGAAAGCTGGGTTTGAATGTAACCTACCTGCG |
| MN198 VPS23(N1-159) rv SalI | AAGGGTCGACCTAATAAAGAGGCGGATCACG |
| MN199 VPS23(C160-stop) fw EcoRI | AAGGGAATTCATGTCTCGACGCCGTCCT |
| MN201 VPS23(C200-stop) fw EcoRI | AAGGGAATTCATGGGAGGAGTAAGTAGGGTGC |
| MN216 SH3P2(N1-257) rv NotI | AAGGGCGGCCGCTCATACCATCTCTCCTTCGAG |
| MN229 SH3P2(N1-257) fw TOPO | CACCATGGATGCAATTAGAAAACA |
| MN230 SH3P2(N1-257) rv TOPO | TACCATCTCTCCTTCGAG |
| MN231 SH3P2(c179-*) fw TOPO | CACCATGGAGTCTCAAGGTAATCCTGAC |
| MN232 SH3P2(c179-*) rv TOPO | GAAAACTTCGGACACTTTG |
| MN262 ProVPS23a (EcoRI) fw | AAGGGAATTCGAGATCATTTTTGTGTCACCA |
| MN263 Pro:VPS28a fw EcoRI | AAGGGAATTCTAATAGACCCGATCTGATCC |
| MN264 Pro:VPS28a rv NcoI | AAGGCCATGGATTACCAGCTTTAGGCAAAG |
| MN265 GFP fw NcoI | AAGGCCATGGATGGTGAGCAAGGGC |
| MN269 NOS-Term rv BamHI | AAGGGGATCCAGTTAGCTCACTCATTAGGCA |
| MS11 SH3P2 fw EcoRI | GGAAGAATTCAGTGATGCAATTAGAAAACA |
| Oligo dT | GACTCGAGTCGACATCGATTTTTTTTTTTTTTTTT |
fw, forward; rv, reverse.
Cloning of GBD-SH3P2, GBD-AMSH3(AXA), and GAD-AMSH3 was previously described (32, 61). For GAD-SH3P2, the ORF of SH3P2 was cloned between the EcoRI and SalI sites of pGADT7 (Clontech). To obtain GAD-VPS23.1, GBD-VPS23.1, GBD-VPS28.1, and GBD-VPS37.1, the ORFs were amplified with primers MN150-MN151 (VPS23.1), MN148-MN149 (VPS28.1), and MN146-MN147 (VPS37.1) and cloned between the EcoRI and XhoI sites of pGADT7 or EcoRI and SalI sites of pGBKT7. VPS23 fragments were amplified with MN150-MN198 to obtain GAD-VPS23(UEV) and with MN199-MN151 to obtain GAD-VPS23(∆UEV). To generate GST-SH3P2(FL), GST-SH3P2(SH3), GST-SH3P2(BAR), and GST-SH3P2(∆NBAR), gene fragments of SH3P2 were amplified using primers MS11-MN156-2, MN157-MN156-2, MS11-MN216, and MN158-MN156-2, respectively, and cloned between the EcoRI and NotI sites of pGEX-6P-1 (GE Healthcare Life Science). For MBP-SH3P2(FL), SH3P2 was cloned between the EcoRI and NotI sites of pMAL-p2T, a gift from the laboratory of Keiji Tanaka, Tokyo Metropolian Institute of Medical Science, Tokyo. VPS23 was digested with EcoRI and XhoI from GBD-VPS23 construct and cloned between the EcoRI and SalI sites of pGEX-6P-1. The C terminus of VPS23 was amplified using primers MN199-MN151 and cloned between the EcoRI and SalI sites of pGEX-6P-1 to generate GST-VPS23.1(∆UEV). GST-VPS2.1 and GST-AMSH3 were described previously (54, 61). To generate VPS23.1:VPS23.1-TagRFP, VPS23.1 was amplified together with a 1,989-bp promoter region with primers MN185-MN186 and cloned into pGWB558 (62). Using this construct as a template, VPS23.1:VPS23.1-TagRFP was amplified with primers MN262-MN269 and cloned into pUC119 for expression in protoplasts. VPS28.1 was amplified together with a 1,001-bp promoter region with primers MN263-MN264 from genomic DNA and sGFP and the nopaline synthase terminator (NOSterm) were amplified from the pGWB404 (62) vector using primer combination MN265-MN269. Both gene fragments were together cloned into pUC119 vector to obtain VPS28.1:VPS28.1-sGFP. The 35S:SH3P2(BAR)-sGFP and 35S:SH3P2(∆NBAR)-sGFP were generated using primers MN229-MN230 and MN231-MN232, respectively, and cloned into pFAST-R05 (63).
For the amiRNA construct amish3p2, the target sequence ACGAATGGTAACGTAT was cloned into pGWB502 (62). To generate an amiRNA-resistant SH3P2:SH3P2-EGFP(amiRNAres) construct, the target sequence of the amiRNA was mutated with primers FA212-FA213 by PCR-based mutagenesis and cloned into pFAST-R07 (63) with a one-base insertion using primers FA214-FA215. The 35S:YFP-CLC was cloned using primers EI223-EI224 and cloned into pExtag-YFP, a gift from J. Parker, Max Planck Institute Cologne, Cologne, Germany, and for ECFP-ARA7, primers KK101-KK102 were used and the gene was cloned into pUBN-CFP-DEST (64). SH3P2:SH3P2-sGFP, CLC:CLC-mKO, AMSH3:AMSH3-YFP, SYP43:SYP43-GFP, UBQ10:YFP-ARA7, and TOL6:TOL6-mCherry were described elsewhere (26, 32, 40, 41, 61, 65).
Molecular Phylogeny.
Coding sequences used for phylogenetic analysis of SH3P1, SH3P2, and SH3P3 homologs in angiosperms (Physcomitrella patens, Selaginella moellendorffii, Amborella trichopoda, Brachypodium distachyon, Zea mays, Vitis vinifera, Medicago truncatula, Populus trichocarpa, and Brassica rapa) were obtained using a BLAST search in Phytozome v11.0. The alignment was performed with MAFFT v.7, and, subsequently, unreliably aligned regions were trimmed with Gblocks 0.91b, allowing half-gap positions. The phylogenetic trees were constructed with Bayesian analysis using MrBayes 3.1.2, and with maximum likelihood (ML) and maximum parsimony (MP) analyses using PAUP* 4.0b10. Bases that were not identified were treated as unknown, and gaps were treated as missing data. For Bayesian analysis, the nucleotide model, a general time-reversible model with gamma distributed rate variation among sites and a proportion of invariant sites (GTR + I + G), was selected by Akaike’s information criterion (AIC) with MrModeltest 2.3. Bayesian searches were conducted using Markov chain Monte Carlo with two independent sets of four chains, each run with 10 million generations and sampling every 1,000 generations. Tracer was used to check that the runs had reached stationarity, and the effective sample size of all of the parameters was high (n > 200). The first 2.5 million generations were discarded as burn-in. For MP analysis, all characters were equally weighed and heuristic searches were conducted with 1,000 random addition replicates involving tree bisection-reconnection (TBR) branch swapping. Bootstrap values were calculated with 1,000 pseudoreplicates with 100 random additions. In the ML method, jModelTest 2.1.10 was used to determine the nucleotide substitution model. The GTR + I + G model were specified by AIC. ML analysis was implemented using PAUP 4.0b10 as heuristic searches involving nearest-neighbor interchange (NII) branch swapping with 100 random addition sequence replicates. Bootstrap values were calculated with 100 pseudoreplicates with 10 random additions. The coding DNA sequences (cDNAs) of P. patens were used as outgroups.
Genotyping PCR.
Genotyping was performed based on PCR with the following primer combinations: vps23.1-3, AG49-AG51 (genomic), and AG51-LB3Sail (T-DNA); vps23.2-1, AG47-FA169 (genomic) and AG47-LB3Sail (T-DNA); sh3p1, MN91-MN92b (genomic) and MN91-LBb1.3 (T-DNA); and sh3p3, MN93-MN104 (genomic) and MN93-Lb1.3 (T-DNA). The presence of the VPS23.1:VPS23.1-RFP and amish3p2 construct was verified with primer pairs MN201-FA121 and EI1-EI2, respectively.
RT-PCR and qRT-PCR.
Total RNA was extracted with a NucleoSpin RNA plant kit (Machery–Nagel), and 1 μg of total RNA was reverse-transcribed with an oligo(dT) primer and M-MulV reverse transcriptase (Fermentas) following the manufacturer’s instructions. Primer pairs used for detection of SH3P1 and SH3P3 transcripts are MN99-MN100 and MN103-MN104, respectively. Primers for ACTIN2 were described previously (61). A qRT-PCR analysis was performed using iQ SYBR Green Supermix (Bio-Rad) in a CFX96 real-time system cycler (Bio-Rad). A 45-cycle, two-step amplification protocol (10 s at 95 °C, 25 s at 60 °C) was used for all measurements. Primers MN174-MN175 were used to amplify SH3P2. Primers for ACTIN8 were previously described (66).
Protein Extraction and Immunoblotting.
To prepare total protein extracts from Arabidopsis seedlings, frozen seedlings were homogenized with the TissueLyser-II (Qiagen), using glass beads. Pulverized tissue was mixed with extraction buffer A [50 mM Tris⋅HCl at pH 7.5, 100 mM NaCl, 10% glycerol, 0.4% Triton X-100, protease inhibitor mixture (Roche)] and subsequently boiled in 5× SDS sample buffer. The mixture was centrifuged for 10 min at 13,000 × g, and the supernatant was used for further experiments. SDS/PAGE, Coomassie Brilliant Blue staining, and immunoblotting were performed according to the standard protocols. Antibodies used for immunoblotting were anti-AMSH3 (54), anti-GAL4BD (Santa Cruz Biotechnology), anti-CDC2 PSTAIRE (Santa Cruz Biotechnology), anti-UGPase (Agrisera), anti–H+-ATPase (Agrisera), anti-BiP2 (Agrisera), anti-COX2 (Agrisera), anti-Sec21 (Agrisera), anti–V-ATPase (Agrisera), anti-Toc75 (Agrisera), anti-GST (GE Healthcare), anti-HA (Sigma–Aldrich), anti-MBP (New England Biolabs), anti-CHC (Agrisera), anti-GFP (Roche), and anti-ubiquitin P4D1 (Santa Cruz Biotechnology).
YTH Assays.
GAD- and GBD-fusion constructs were cotransformed into yeast Y8800 (based on PJ69-4; MATa, leu2-3,112 trp1-901 his3-200 ura3-52 gal4Δ gal80Δ GAL2-ADE2 LYS2::GAL1-HIS3 MET2::GAL7-lacZ cyh2R). Transformants were selected after 3 d on synthetic complete (SC) medium lacking leucine and tryptophan (−LW) at 30 °C. To examine reporter gene expression, transformants were grown on solid SC medium lacking leucine, tryptophan, and histidine (−LWH) supplemented with 5 mM 3-amino-1,2,4-triazole for 2 d at 30 °C. Yeast total proteins were extracted as described previously (67), and expression of constructs was analyzed by immunoblotting using anti-GBD and anti-HA (GAD) antibodies.
DUB Assay.
For diubiquitin-tetramethylrhodamin (TAMRA) assay, 0.4 μM diubiquitin (K63-linked) FRET TAMRA position 3 (R&D Systems) was incubated with 50 nM recombinant AMSH3 in TAMRA DUB buffer [50 mM Tris⋅HCl at pH 7.5, 100 mM NaCl, 0.1% Pluronic F-127, 1 mM Tris (2-carboxyethyl) phosphine] in a volume of 100 μL. To examine the effect of SH3P2 on AMSH3 activity, AMSH3 was preincubated for 30 min with 50 nM recombinant MBP-SH3P2 before the addition of diubiquitin-TAMRA. Fluorescence was measured every minute with a Synergy 2 Multi-Mode Microplate Reader (BioTek) equipped with filters for excitation at 530 nm and emission at 590 nm.
Microscopy.
WM (Sigma–Aldrich) treatments were performed at a concentration of 33 μM WM for 90 min at room temperature. ECFP-, sGFP-, YFP-, and RFP (TagRFP-, mCherry-, and mKO)-fused proteins were analyzed with an FV-1000/IX81 confocal laser scanning microscope (Olympus) equipped with GaAsP detectors (Olympus) and a UPlanSApo 60×/1.20 (Olympus) objective using the 458-, 488-, 515-, or 559-nm laser line, respectively. Images were processed and analyzed using FluoView (Olympus), Fiji (68), and Photoshop CS6 (Adobe). Pearson’s correlation coefficient (r) was analyzed using Fiji (68) with automated Costes-threshold. Transformation of Arabidopsis cell culture-derived protoplasts was performed as described previously (61).
CCV Purification.
Ten grams of 7-d-old seedlings was ground using a mortar and pestle and subjected to fractionation to purify CCVs as described previously (42). Volumes of the buffers were adjusted to the amount of material. Samples collected during the purification and the final CCV fraction were analyzed by immunoblot analyses using antibodies specific to organelles/cellular compartments.
Acknowledgments
We thank Akihiko Nakano, Takashi Ueda, Tomohiro Uemura, and Masaru Fujimoto (The University of Tokyo) for the endosomal marker lines and Sven Schellmann and Martin Hülskamp (University of Cologne) for the VPS23.1-HA (ELCH-HA) line. We thank Stefan Helfrich and the Bioimaging Center (University of Konstanz) for support in image analysis. We thank the Nottingham Arabidopsis Stock Center (NASC) for providing Arabidopsis T-DNA insertion lines. This work was supported by Grants IS 221/2-1, IS 221/4-1, and SFB924(A6) from the German Science Foundation (to E.I.); European cooperation in science and technology (COST) Action BM1307 [COST-proteostasis (to E.I.)]; Grant 1121998 from the National Science Foundation (to S.Y.B.); and Grant 16H05068 from the Ministry of Education, Culture, Sports, Science and Technology Japan (to M.H.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1710866114/-/DCSupplemental.
References
- 1.Vierstra RD. The expanding universe of ubiquitin and ubiquitin-like modifiers. Plant Physiol. 2012;160:2–14. doi: 10.1104/pp.112.200667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben Khaled S, Postma J, Robatzek S. A moving view: Subcellular trafficking processes in pattern recognition receptor-triggered plant immunity. Annu Rev Phytopathol. 2015;53:379–402. doi: 10.1146/annurev-phyto-080614-120347. [DOI] [PubMed] [Google Scholar]
- 3.Paez Valencia J, Goodman K, Otegui MS. Endocytosis and endosomal trafficking in plants. Annu Rev Plant Biol. 2016;67:309–335. doi: 10.1146/annurev-arplant-043015-112242. [DOI] [PubMed] [Google Scholar]
- 4.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 5.Baisa GA, Mayers JR, Bednarek SY. Budding and braking news about clathrin-mediated endocytosis. Curr Opin Plant Biol. 2013;16:718–725. doi: 10.1016/j.pbi.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Robinson MS. Forty years of clathrin-coated vesicles. Traffic. 2015;16:1210–1238. doi: 10.1111/tra.12335. [DOI] [PubMed] [Google Scholar]
- 7.Komander D, Rape M. The ubiquitin code. Annu Rev Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 8.Lauwers E, Jacob C, André B. K63-linked ubiquitin chains as a specific signal for protein sorting into the multivesicular body pathway. J Cell Biol. 2009;185:493–502. doi: 10.1083/jcb.200810114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leitner J, et al. Lysine63-linked ubiquitylation of PIN2 auxin carrier protein governs hormonally controlled adaptation of Arabidopsis root growth. Proc Natl Acad Sci USA. 2012;109:8322–8327. doi: 10.1073/pnas.1200824109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martins S, et al. Internalization and vacuolar targeting of the brassinosteroid hormone receptor BRI1 are regulated by ubiquitination. Nat Commun. 2015;6:6151. doi: 10.1038/ncomms7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barberon M, et al. Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants. Proc Natl Acad Sci USA. 2011;108:E450–E458. doi: 10.1073/pnas.1100659108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasai K, Takano J, Miwa K, Toyoda A, Fujiwara T. High boron-induced ubiquitination regulates vacuolar sorting of the BOR1 borate transporter in Arabidopsis thaliana. J Biol Chem. 2011;286:6175–6183. doi: 10.1074/jbc.M110.184929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Göhre V, et al. Plant pattern-recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Curr Biol. 2008;18:1824–1832. doi: 10.1016/j.cub.2008.10.063. [DOI] [PubMed] [Google Scholar]
- 14.Gimenez-Ibanez S, et al. AvrPtoB targets the LysM receptor kinase CERK1 to promote bacterial virulence on plants. Curr Biol. 2009;19:423–429. doi: 10.1016/j.cub.2009.01.054. [DOI] [PubMed] [Google Scholar]
- 15.Winter V, Hauser MT. Exploring the ESCRTing machinery in eukaryotes. Trends Plant Sci. 2006;11:115–123. doi: 10.1016/j.tplants.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Strack B, Calistri A, Craig S, Popova E, Göttlinger HG. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell. 2003;114:689–699. doi: 10.1016/s0092-8674(03)00653-6. [DOI] [PubMed] [Google Scholar]
- 18.von Schwedler UK, et al. The protein network of HIV budding. Cell. 2003;114:701–713. doi: 10.1016/s0092-8674(03)00714-1. [DOI] [PubMed] [Google Scholar]
- 19.Olmos Y, Hodgson L, Mantell J, Verkade P, Carlton JG. ESCRT-III controls nuclear envelope reformation. Nature. 2015;522:236–239. doi: 10.1038/nature14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vietri M, et al. Spastin and ESCRT-III coordinate mitotic spindle disassembly and nuclear envelope sealing. Nature. 2015;522:231–235. doi: 10.1038/nature14408. [DOI] [PubMed] [Google Scholar]
- 21.Denais CM, et al. Nuclear envelope rupture and repair during cancer cell migration. Science. 2016;352:353–358. doi: 10.1126/science.aad7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raab M, et al. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science. 2016;352:359–362. doi: 10.1126/science.aad7611. [DOI] [PubMed] [Google Scholar]
- 23.Gong YN, et al. ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell. 2017;169:286–300.e216. doi: 10.1016/j.cell.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shields SB, Piper RC. How ubiquitin functions with ESCRTs. Traffic. 2011;12:1306–1317. doi: 10.1111/j.1600-0854.2011.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren X, Hurley JH. VHS domains of ESCRT-0 cooperate in high-avidity binding to polyubiquitinated cargo. EMBO J. 2010;29:1045–1054. doi: 10.1038/emboj.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korbei B, et al. Arabidopsis TOL proteins act as gatekeepers for vacuolar sorting of PIN2 plasma membrane protein. Curr Biol. 2013;23:2500–2505. doi: 10.1016/j.cub.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 27.Puertollano R, Bonifacino JS. Interactions of GGA3 with the ubiquitin sorting machinery. Nat Cell Biol. 2004;6:244–251. doi: 10.1038/ncb1106. [DOI] [PubMed] [Google Scholar]
- 28.Puertollano R. Interactions of TOM1L1 with the multivesicular body sorting machinery. J Biol Chem. 2005;280:9258–9264. doi: 10.1074/jbc.M412481200. [DOI] [PubMed] [Google Scholar]
- 29.Belda-Palazon B, et al. FYVE1/FREE1 interacts with the PYL4 ABA receptor and mediates its delivery to the vacuolar degradation pathway. Plant Cell. 2016;28:2291–2311. doi: 10.1105/tpc.16.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao C, et al. A unique plant ESCRT component, FREE1, regulates multivesicular body protein sorting and plant growth. Curr Biol. 2014;24:2556–2563. doi: 10.1016/j.cub.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Gao C, et al. Dual roles of an Arabidopsis ESCRT component FREE1 in regulating vacuolar protein transport and autophagic degradation. Proc Natl Acad Sci USA. 2015;112:1886–1891. doi: 10.1073/pnas.1421271112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolb C, et al. FYVE1 is essential for vacuole biogenesis and intracellular trafficking in Arabidopsis. Plant Physiol. 2015;167:1361–1373. doi: 10.1104/pp.114.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhuang X, et al. A BAR-domain protein SH3P2, which binds to phosphatidylinositol 3-phosphate and ATG8, regulates autophagosome formation in Arabidopsis. Plant Cell. 2013;25:4596–4615. doi: 10.1105/tpc.113.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lam BC, Sage TL, Bianchi F, Blumwald E. Role of SH3 domain-containing proteins in clathrin-mediated vesicle trafficking in Arabidopsis. Plant Cell. 2001;13:2499–2512. doi: 10.1105/tpc.010279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahn G, et al. SH3P2 plays a crucial role at the step of membrane tubulation during cell plate formation in plants. Plant Cell. 2017;29:1388–1405. doi: 10.1105/tpc.17.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ortega Roldan JL, et al. Distinct ubiquitin binding modes exhibited by SH3 domains: Molecular determinants and functional implications. PLoS One. 2013;8:e73018. doi: 10.1371/journal.pone.0073018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stamenova SD, et al. Ubiquitin binds to and regulates a subset of SH3 domains. Mol Cell. 2007;25:273–284. doi: 10.1016/j.molcel.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willige BC, et al. The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. Plant Cell. 2007;19:1209–1220. doi: 10.1105/tpc.107.051441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheuring D, et al. Multivesicular bodies mature from the trans-Golgi network/early endosome in Arabidopsis. Plant Cell. 2011;23:3463–3481. doi: 10.1105/tpc.111.086918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uemura T, et al. Qa-SNAREs localized to the trans-Golgi network regulate multiple transport pathways and extracellular disease resistance in plants. Proc Natl Acad Sci USA. 2012;109:1784–1789. doi: 10.1073/pnas.1115146109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geldner N, et al. Rapid, combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. Plant J. 2009;59:169–178. doi: 10.1111/j.1365-313X.2009.03851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds GD, August B, Bednarek SY. Preparation of enriched plant clathrin-coated vesicles by differential and density gradient centrifugation. Methods Mol Biol. 2014;1209:163–177. doi: 10.1007/978-1-4939-1420-3_13. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka N, et al. Possible involvement of a novel STAM-associated molecule “AMSH” in intracellular signal transduction mediated by cytokines. J Biol Chem. 1999;274:19129–19135. doi: 10.1074/jbc.274.27.19129. [DOI] [PubMed] [Google Scholar]
- 44.Kalinowska K, et al. Arabidopsis ALIX is required for the endosomal localization of the deubiquitinating enzyme AMSH3. Proc Natl Acad Sci USA. 2015;112:E5543–E5551. doi: 10.1073/pnas.1510516112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu F, et al. ESCRT-I component VPS23A affects ABA signaling by recognizing ABA receptors for endosomal degradation. Mol Plant. 2016;9:1570–1582. doi: 10.1016/j.molp.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Mayer BJ. SH3 domains: Complexity in moderation. J Cell Sci. 2001;114:1253–1263. doi: 10.1242/jcs.114.7.1253. [DOI] [PubMed] [Google Scholar]
- 47.Zarrinpar A, Bhattacharyya RP, Lim WA. The structure and function of proline recognition domains. Sci STKE. 2003;2003:RE8. doi: 10.1126/stke.2003.179.re8. [DOI] [PubMed] [Google Scholar]
- 48.Spitzer C, et al. The Arabidopsis elch mutant reveals functions of an ESCRT component in cytokinesis. Development. 2006;133:4679–4689. doi: 10.1242/dev.02654. [DOI] [PubMed] [Google Scholar]
- 49.Wang HJ, et al. VPS36-dependent multivesicular bodies are critical for plasmamembrane protein turnover and vacuolar biogenesis. Plant Physiol. 2017;173:566–581. doi: 10.1104/pp.16.01356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu D, et al. Direct ubiquitination of pattern recognition receptor FLS2 attenuates plant innate immunity. Science. 2011;332:1439–1442. doi: 10.1126/science.1204903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shin LJ, et al. IRT1 degradation factor1, a ring E3 ubiquitin ligase, regulates the degradation of iron-regulated transporter1 in Arabidopsis. Plant Cell. 2013;25:3039–3051. doi: 10.1105/tpc.113.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCullough J, et al. Activation of the endosome-associated ubiquitin isopeptidase AMSH by STAM, a component of the multivesicular body-sorting machinery. Curr Biol. 2006;16:160–165. doi: 10.1016/j.cub.2005.11.073. [DOI] [PubMed] [Google Scholar]
- 53.Spallek T, et al. ESCRT-I mediates FLS2 endosomal sorting and plant immunity. PLoS Genet. 2013;9:e1004035. doi: 10.1371/journal.pgen.1004035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Isono E, et al. The deubiquitinating enzyme AMSH3 is required for intracellular trafficking and vacuole biogenesis in Arabidopsis thaliana. Plant Cell. 2010;22:1826–1837. doi: 10.1105/tpc.110.075952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hummel E, Osterrieder A, Robinson DG, Hawes C. Inhibition of Golgi function causes plastid starch accumulation. J Exp Bot. 2010;61:2603–2614. doi: 10.1093/jxb/erq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raiborg C, et al. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat Cell Biol. 2002;4:394–398. doi: 10.1038/ncb791. [DOI] [PubMed] [Google Scholar]
- 57.Raiborg C, Wesche J, Malerød L, Stenmark H. Flat clathrin coats on endosomes mediate degradative protein sorting by scaffolding Hrs in dynamic microdomains. J Cell Sci. 2006;119:2414–2424. doi: 10.1242/jcs.02978. [DOI] [PubMed] [Google Scholar]
- 58.Sachse M, Urbé S, Oorschot V, Strous GJ, Klumperman J. Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol Biol Cell. 2002;13:1313–1328. doi: 10.1091/mbc.01-10-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spitzer C, et al. The ESCRT-related CHMP1A and B proteins mediate multivesicular body sorting of auxin carriers in Arabidopsis and are required for plant development. Plant Cell. 2009;21:749–766. doi: 10.1105/tpc.108.064865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clough SJ, Bent AF. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 61.Katsiarimpa A, et al. The Arabidopsis deubiquitinating enzyme AMSH3 interacts with ESCRT-III subunits and regulates their localization. Plant Cell. 2011;23:3026–3040. doi: 10.1105/tpc.111.087254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakagawa T, et al. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng. 2007;104:34–41. doi: 10.1263/jbb.104.34. [DOI] [PubMed] [Google Scholar]
- 63.Shimada TL, Shimada T, Hara-Nishimura I. A rapid and non-destructive screenable marker, FAST, for identifying transformed seeds of Arabidopsis thaliana. Plant J. 2010;61:519–528. doi: 10.1111/j.1365-313X.2009.04060.x. [DOI] [PubMed] [Google Scholar]
- 64.Grefen C, et al. A ubiquitin-10 promoter-based vector set for fluorescent protein tagging facilitates temporal stability and native protein distribution in transient and stable expression studies. Plant J. 2010;64:355–365. doi: 10.1111/j.1365-313X.2010.04322.x. [DOI] [PubMed] [Google Scholar]
- 65.Ito E, et al. Dynamic behavior of clathrin in Arabidopsis thaliana unveiled by live imaging. Plant J. 2012;69:204–216. doi: 10.1111/j.1365-313X.2011.04782.x. [DOI] [PubMed] [Google Scholar]
- 66.Katsiarimpa A, et al. The deubiquitinating enzyme AMSH1 and the ESCRT-III subunit VPS2.1 are required for autophagic degradation in Arabidopsis. Plant Cell. 2013;25:2236–2252. doi: 10.1105/tpc.113.113399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kushnirov VV. Rapid and reliable protein extraction from yeast. Yeast. 2000;16:857–860. doi: 10.1002/1097-0061(20000630)16:9<857::AID-YEA561>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 68.Schindelin J, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]