Chemical communication is the primordial and possibly most efficient way of transmitting messages between living units (1). It has reached its apex in the “superorganisms” (2), for example in colonies of eusocial insects, such as honey bees (3). Colony survival and reproductive success rely on the chemical communication channel to maintain an advanced social organization characterized by high levels of cooperation and low levels of conflicts (1, 4, 5). Eusocial bees and ants are model organisms for understanding social chemical communication; hence, recent research has focused on the identification of chemoreceptors (6). A new study by Slone et al. (7) uses the ant Harpegnathos saltator to investigate the molecular mechanisms underlying chemoreception of socially relevant semiochemicals.
Chemoreceptors can be differentiated into olfactory receptors (ORs), gustatory receptors, and ionotropic receptors, as well as several other receptor classes (8). ORs are transmembrane proteins representing the interface between animals and their olfactory environment for detecting food sources or, in a social context, nestmates or sexual partners. They are expressed in olfactory receptor neurons (ORNs) in the insect antenna; after binding odors, information is transferred to the brain, eventually inducing behavioral responses. It has long been believed that perception of “general” odors (food and flower scent) is separated from that of “social” odors (e.g., pheromones), and that these are detected by different ORs. Recent research contradicts such strict partition: even highly specialized ORNs (sex pheromone ORNs) may respond to ordinary odors (9). In ants, colony-mate recognition relies on cuticular hydrocarbons (CHCs), which also inform about caste and reproductive status (1, 10). Being primarily a barrier against desiccation and pathogens, CHCs have been co-opted to serve as a multicomponent cue/signal (11), first in solitary species for reproduction behavior (e.g., species recognition), then in social species, where they serve at least two functions (signature mixtures and pheromones) at different levels (individual, within colony, between colonies). However, ambiguity remains about the mechanisms by which chemoreceptors regulate social behavior. Recent data indicate that ORs underwent a huge expansion in ants; based on their sequence, OR genes were classified in different subfamilies, with the 9-exon OR subfamily thought responsible for CHC-detection (12).
Are CHCs exclusively detected by ORs of the 9-exon subfamily? With an elegant approach, Slone et al. (7) functionally characterize a set of 25 distinct ORs (across nine OR subfamilies) by using heterologous expression of ant ORs in Drosophila melanogaster ORNs, testing a large panel of social (CHCs) and more general odors.
CHCs Are Not Uniquely Detected by the 9-Exon OR Gene Subfamily
Slone et al. (7) show that ORs belonging to several different OR subfamilies respond to both CHCs, some of which are pheromones (1, 10), and general odorants. Consequently, CHCs are not exclusively detected by one OR subfamily (9-exon). This novel finding does not align with the currently held hypotheses of OR subfamily odor coding. The fact that many ORs showed a strong response bias toward long-chain alkanes, notable for this ant species’ CHC profile (10), fits also with other ant studies focusing on behavioral and chemical data (e.g., ref. 13). Furthermore, a distinct group of male-specific ORs showed a significant sensitivity to CHCs, indicating that these not only regulate social interactions between female ants, but also between sexual partners. This finding is in line with studies suggesting that CHCs are used in sexual communication in social hymenoptera (14) and solitary insects [e.g., Drosophila (15)]. Slone et al. (7) confirm recent findings that there are no absolute odor-coding boundaries for ant-OR subfamilies in relation to pheromone and nonpheromone stimuli (Fig. 1). In moths, a model insect taxon for sex-pheromone communication, some receptors belonging to the pheromone receptor subfamily do not exclusively bind sex-pheromone components but also plant volatiles (e.g., ref. 16). Accordingly, plant odors evoke neural responses in the pheromone coding part of the antennal lobe in Agrotis moths (9).
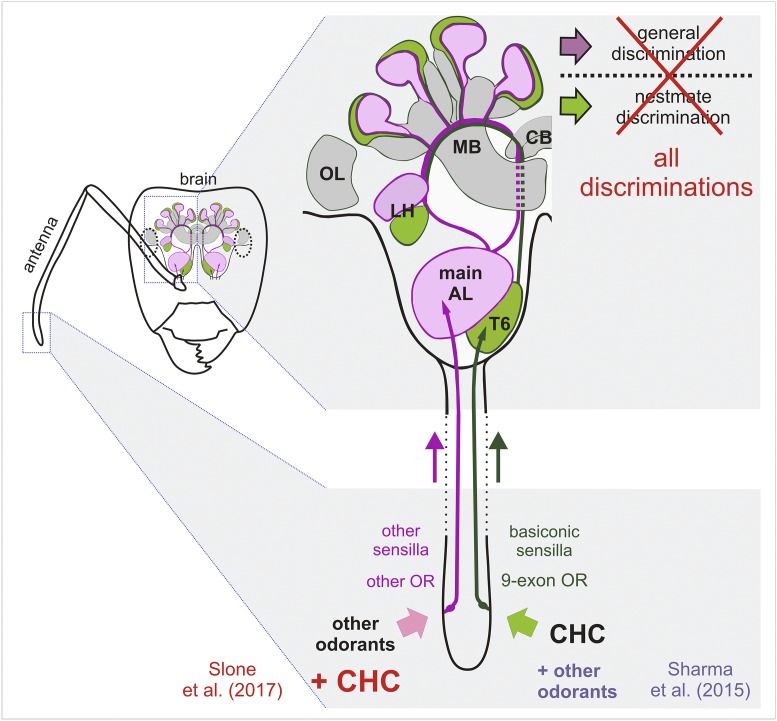
Fig. 1.
Olfactory information is treated by two subsystems: signals from basiconic sensilla are processed in the T6 glomerular cluster, other olfactory cues within the main AL. Under the previous hypothesis, CHCs were specifically detected by 9-exon ORs (within basiconic sensilla), while other odorants (non-CHCs) were detected by non–9-exon ORs. Together with previous work (23), the new study by Slone et al. (7) shows that these are not exclusive categories and favors a combined model of ant social odor processing in which both subsystems process all odorants. Ant brain designed after Nishikawa et al. (24). CB, central body; LH, lateral horn; MB, mushroom bodies; OL, optic lobe.
Consequences for Neuro-Ethological Models of Insect Social Communication
The evolution of cooperation (and of altruism) is a persisting conundrum for evolutionary biologists and scientists in general: How can a Darwinian selfish unit (e.g., an individual organism) forego its own reproduction for “the good of the society”? Social insects provide an answer, deep-rooted in more than a century of kin-selection theory-inspired theoretical and empirical work (17, 18). A sensory system allowing detection of kinship (originally equal to colony membership) (5) would be a key preadaptation facilitating the emergence of sociality by preventing altruistic acts toward unrelated individuals. In social insects, CHCs play a crucial role in colony-mate discrimination processes (1, 13); yet, several hypotheses have been proposed to explain how ants can discern between nestmates and nonnestmates. In one scenario, ants would endure sensory adaptation (receptor level) or habituation (antennal lobe level) via repeated contacts with nestmates and would thus not perceive their own colony profile: only alien profiles induce aggression (19, 20). This means that the neural substrate for the nestmate recognition template would not be localized in the higher-order brain centers, but rather at the periphery (antennal receptors) or in the first brain relay (antennal lobes, see below). Other accounts suggest that ants’ discrimination abilities allow exquisite odor representation of CHCs, both of colony members and alien individuals (21, 22); ants would thus learn a “template colony profile” (stored in higher-order brain centers) and use it when making behavioral decisions at the nest entrance (1, 21, 22). The work by Slone et al. (7) does not allow directly choosing between these alternative models, but appears to support the latter by showing that many ant ORs can detect CHCs, even outside the greatly expanded 9-exon subfamily. Thus, ants’ investment in CHC detection may be greater than suspected.
The study by Slone et al. (7) also sheds new light on the issue of whether the ant brain uses a specialized subsystem for nestmate discrimination (Fig. 1). The first brain relay of the insect olfactory pathway is the antennal lobe (AL), made of individual functional units (glomeruli). The AL processes olfactory signals detected at the periphery and transmits them to higher-order brain centers: mushroom bodies (learning and memory) and the lateral horn (innate behavior). Each AL glomerulus receives all ORNs carrying a given OR protein. In line with their expanded repertoire of OR types, ants’ ALs contain >400 glomeruli, organized in different clusters. Based on its exclusive presence in female ants and some neuroanatomical peculiarities (ventral location of antennal basiconic sensilla), it was proposed that one specific cluster of glomeruli (termed T6) is responsible for the processing of CHC
A new study by Slone et al. uses the ant Harpegnathos saltator to investigate the molecular mechanisms underlying chemoreception of socially relevant semiochemicals.
information and thus nestmate recognition (12, 22, 23). T6 does not exchange local connections with other parts of the AL and its projections to higher-order centers are largely separated: it represents a segregated and specialized olfactory subsystem for CHCs (12, 22–25).
In the ant AL, T6 comprises about one-third of all glomeruli, similarly to the number of expressed members of the 9-exon OR subfamily (12). There was a fair consensus that 9-exon ORs detect CHCs (6) and that this information is processed within the T6 cluster. Still, functional calcium imaging and two-photon microscopy measurements in the whole ant AL revealed CHC-evoked activity in many glomeruli (21). Although the T6 cluster could not be precisely located, this work suggested that many ORs respond to CHCs, including those outside the 9-exon OR subfamily. Discriminating between complex mixtures of CHCs is a very difficult sensory task, which might require using the whole capacity of the ant olfactory system, not just one-third (the T6). The ant brain may thus use parallel processing of CHC information, different parts of the olfactory system extracting different pieces of information from the CHC profile, which would then be bound together again within higher-order centers to make behavioral decisions (21, 26).
A key contribution of the work by Slone et al. (7) is that it clearly challenges the established model of a dedicated CHC nestmate recognition subsystem. It was recently shown (22) that ant basiconic sensilla (harboring ORNs feeding into T6) do respond to CHCs but also respond to general odorants. Now, Slone et al. (7) provide the missing element that non-T6 related ORs respond to CHCs in addition to general odorants. Both studies bring down the walls that were carefully elevated between the two parts of ants’ olfactory system and support the idea of parallel processing both for nestmate cues and general odors. However, the fact that one subsystem can be activated by a class of compounds does not inevitably mean that downstream pathways (in mushroom bodies and the lateral horn) will read out and use this information for shaping behavioral responses. It is not because non–9-exon ORs respond to some CHCs that the ant brain will use this information for nestmate recognition. Pathways involved in discrimination behavior might read out neural activity only from the T6 subsystem. Proving that information from both subsystems is indeed relevant for nestmate discrimination will require using lesion/neural block approaches. Transgenic methods and the possibility to use genome-editing tools (CRISPR/Cas9) could prove decisive to this goal. We believe that Slone et al.’s (7) work will stimulate proponents of the “dedicated subsystem theory” to come forward with compelling new data, bringing the field closer to a deep understanding of the neuro-ethology of social communication.
Acknowledgments
We thank the CNRS. The authors are supported by the French National Research Agency through Project PheroMod ANR-14-CE18-003 (to P.d. and N.D.) and the Bee-o-CHOC Project (J.C.S.).
Footnotes
The authors declare no conflict of interest.
See companion article on page 8586 in issue 32 of volume 114.
References
- 1.d’Ettorre P, Moore AJ. Chemical communication and the coordination of social interactions in insects. In: d’Ettorre P, Hughes DP, editors. Sociobiology of Communication. Oxford Univ Press; Oxford: 2008. pp. 81–96. [Google Scholar]
- 2.Wheeler WM. The ant-colony as an organism. J Morphol. 1911;22:307–325. [Google Scholar]
- 3.Sandoz JC, Deisig N, de Brito Sanchez MG, Giurfa M. Understanding the logics of pheromone processing in the honeybee brain: From labeled-lines to across-fiber patterns. Front Behav Neurosci. 2007;1:5. doi: 10.3389/neuro.08.005.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strassmann JE, Queller DC. The social organism: Congresses, parties, and committees. Evolution. 2010;64:605–616. doi: 10.1111/j.1558-5646.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- 5.Boomsma JJ. Kin selection versus sexual selection: Why the ends do not meet. Curr Biol. 2007;17:R673–R683. doi: 10.1016/j.cub.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 6.Pask GM, et al. Specialized odorant receptors in social insects that detect cuticular hydrocarbon cues and candidate pheromones. Nat Commun. 2017 doi: 10.1038/s41467-017-00099-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slone JD, et al. Functional characterization of odorant receptors in the ponerine ant, Harpegnathos saltator. Proc Natl Acad Sci USA. 2017;114:8586–8591. doi: 10.1073/pnas.1704647114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph RM, Carlson JR. Drosophila chemoreceptors: A molecular interface between the chemical world and the brain. Trends Genet. 2015;31:683–695. doi: 10.1016/j.tig.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rouyar A, et al. Unexpected plant odor responses in a moth pheromone system. Front Physiol. 2015;6:148. doi: 10.3389/fphys.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liebig J, Peeters C, Oldham NJ, Markstädter C, Hölldobler B. Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator? Proc Natl Acad Sci USA. 2000;97:4124–4131. doi: 10.1073/pnas.97.8.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wyatt TD. Pheromones and Animal Behavior. 2nd Ed Cambridge Univ Press; Cambridge, UK: 2014. [Google Scholar]
- 12.McKenzie SK, Fetter-Pruneda I, Ruta V, Kronauer DJC. Transcriptomics and neuroanatomy of the clonal raider ant implicate an expanded clade of odorant receptors in chemical communication. Proc Natl Acad Sci USA. 2016;113:14091–14096. doi: 10.1073/pnas.1610800113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Zweden JS, Heinze J, Boomsma JJ, d’Ettorre P. Ant queen egg-marking signals: Matching deceptive laboratory simplicity with natural complexity. PLoS One. 2009;4:e4718. doi: 10.1371/journal.pone.0004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wenseleers T, van Zweden JS. Sensory and cognitive adaptations to social living in insect societies. Proc Natl Acad Sci USA. 2017;114:6424–6426. doi: 10.1073/pnas.1707141114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bontonou G, Wicker-Thomas C. Sexual communication in the Drosophila genus. Insects. 2014;5:439–458. doi: 10.3390/insects5020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bengtsson JM, et al. A predicted sex pheromone receptor of codling moth Cydia pomonella detects the plant volatile peer ester. Front Ecol Evol. 2016;2:Article 33. [Google Scholar]
- 17.Boomsma JJ, d’Ettorre P. Nice to kin and nasty to non-kin: Revisiting Hamilton’s early insights on eusociality. Biol Lett. 2013;9:20130444. doi: 10.1098/rsbl.2013.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boomsma JJ, Gawne R. Superorganismality and caste differentiation as points of no return: How the major evolutionary transitions were lost in translation. Biological Reviews. May 15, 2017 doi: 10.1111/brv.12330. [DOI] [PubMed] [Google Scholar]
- 19.Ozaki M, et al. Ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science. 2005;309:311–314. doi: 10.1126/science.1105244. [DOI] [PubMed] [Google Scholar]
- 20.Guerrieri FJ, et al. Ants recognize foes and not friends. Proc Biol Sci. 2009;276:2461–2468. doi: 10.1098/rspb.2008.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandstaetter AS, Kleineidam CJ. Distributed representation of social odors indicates parallel processing in the antennal lobe of ants. J Neurophysiol. 2011;106:2437–2449. doi: 10.1152/jn.01106.2010. [DOI] [PubMed] [Google Scholar]
- 22.Sharma KR, et al. Cuticular hydrocarbon pheromones for social behavior and their coding in the ant antenna. Cell Reports. 2015;12:1261–1271. doi: 10.1016/j.celrep.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 23.Nakanishi A, Nishino H, Watanabe H, Yokohari F, Nishikawa M. Sex-specific antennal sensory system in the ant Camponotus japonicus: glomerular organizations of antennal lobes. J Comp Neurol. 2010;518:2186–2201. doi: 10.1002/cne.22326. [DOI] [PubMed] [Google Scholar]
- 24.Nishikawa M, Watanabe H, Yokohari F. Higher brain centers for social tasks in worker ants, Camponotus japonicus. J Comp Neurol. 2012;520:1584–1598. doi: 10.1002/cne.23001. [DOI] [PubMed] [Google Scholar]
- 25.Couto A, Mitra A, Thiéry D, Marion-Poll F, Sandoz JC. Hornets have it: A conserved olfactory subsystem for social recognition in hymenoptera? Front Neuroanat. 2017;11:48. doi: 10.3389/fnana.2017.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bos N, d’Ettorre P. Recognition of social identity in ants. Front Psychol. 2012;3:83. doi: 10.3389/fpsyg.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]