Approximately 10,000 years ago, certain human groups began to rely on diets derived from domesticated plants and animals rather than acquiring wild sources of food via hunting, gathering, and foraging. This transition in subsistence economy occurred independently in several global regions, with particular starchy crops (e.g., wheat, barley, rice, maize, etc.) becoming staple food sources in different continents (1). The profound effects of the transition to agriculture on the biology of modern humans cannot be overstated. These effects include an increased tendency to stay in a single place for extended periods of time, changes in weaning practices, increases in the incidence of infectious disease, and increased fecundity, leading ultimately to an explosion in the global human population (2, 3). Without the development of horticultural and animal-rearing practices, it would not be possible to sustain the enormous population of humans alive on the planet today. Anthropologists have long been interested in the effects of this shift in subsistence strategy from a genetic, morphological, social, and medical perspective. Agricultural diets are, in general, less variable, higher in starch and sugars, and lower in protein compared with forager diets, resulting in a suite of health-related problems such as anemia, dental caries, vitamin deficiencies, and malnutrition (4, 5). Agricultural diets are also softer, on average, meaning that they are mechanically less demanding in terms of chewing than forager diets. Anthropologists have noted for some time that even prehistoric farmers had more gracile crania and lower jaws (mandibles) than foragers, which can be summarized by Carlson and Van Gerven’s (6) “masticatory-functional hypothesis.” This hypothesis explains the observed changes through time in Nubian cranial morphology (Fig. 1) in terms of reduced biomechanical stress from chewing softer agricultural foods (5, 7). Now, in PNAS, Katz et al. (8) add novel evidence in support of this hypothesis by explicitly quantifying the effects of eating a softer diet on the 3D form of the cranium and mandible. Drawing on an expansive global dataset and an innovative analytical approach (9), Katz et al. demonstrate small but consistent effects of a soft agricultural diet on skull morphology that relate directly to chewing anatomy.
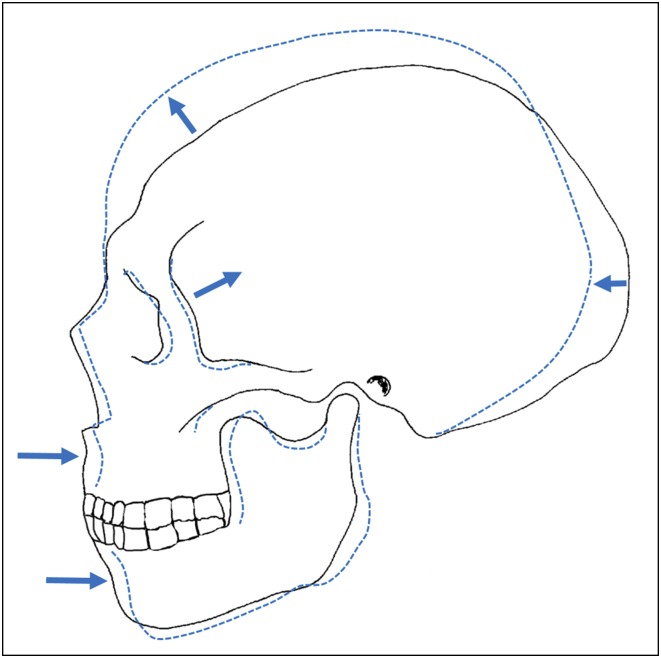
Fig. 1.
Summary of morphological changes observed in Nubian skulls through time from the Mesolithic (solid line) through to the Meroitic–Christian period (dashed line). These changes were proposed by Carlson and Van Gerven (6) to be caused by reduced masticatory stress associated with the transition from foraging to farming. Adapted from Carlson and Van Gerven (6) with permission from John Wiley and Sons.
One of the difficulties encountered when trying to study the relationship between morphology and dietary differences is the potentially confounding effect of population history. To illustrate the problem, imagine two human populations (one agricultural and one foraging) living in different geographic regions. We might observe systematic differences between them in terms of skull morphology and conclude that these differences reflect variation in chewing behavior. However, can we confidently assume the differences are indeed due to diet? Not really. The differences could be related to diet, but they could also reflect differences in the specific genetic population histories of the two groups. Disentangling the effects of population history from dietary factors is made all of the more difficult because geography mediates the evolutionary processes that are responsible for population history while subsistence economy is also, to some extent, related to geography (10, 11). So, for example, prehistoric farmers could spread relatively easily where there was an abundance of water, sunlight, and good soils for growing crops and raising animals, whereas some regions (such as deserts, dense rainforests, and the High Arctic) were difficult for farmers to settle for these reasons.
To circumvent the problem of distinguishing morphological signals of dietary change from those related to population history, anthropologists have either compared foragers and farmers from a single geographically localized area (6, 12–15) or have adopted methods that take global population history into consideration (11, 16). In general, these studies have shown a relatively potent effect of dietary change on the shape and size of the mandible and, in some cases, a commensurate effect on the form of the skull. It has sometimes been difficult to assess whether morphological signals are truly related to a shift in diet because additional factors, such as migration of new peoples into the area, could also have an impact on the observed patterns of morphology (15). Even in cases where the effects of population history have been statistically accounted for by controlling for genetic or geographic patterns, it has been difficult to accurately quantify the effects of dietary differences on skull morphology, both in terms of the intensity and the pattern of morphological change. The study by Katz et al. (8) overcomes these problems by using an innovative analytical approach. They adopt a mixed-effect model from quantitative genetics to characterize the effects of diet on the 3D shape of the skull and mandible for 25 globally distributed preindustrial forager and farming populations. Their model explicitly controls for other possible causative effects such as population history, sexual dimorphism, and climate. Their results are broadly consistent with the masticatory-functional hypothesis in showing that groups with softer diets show small, but definitive, differences in the size and shape of their skulls. These effects are strongest when comparing dairying populations with foragers. Moreover, the effects are much larger for the mandible than for the skull, and the regions most affected by diet are those related to chewing function such as the size of the chewing musculature, relative facial size, and the shape of the mandible (8, 11).
So what does this mean for the study of modern human morphological variation? There are two aspects that warrant further consideration. First, it is important to point out, as Katz et al. (8) do, that the effects of dietary changes are small when considered alongside other factors such as sexual dimorphism and population history (i.e., how groups are related). The majority of human cranial variation can be explained on the basis of a neutral (or stochastic) model of microevolutionary change (17–21). What this means is that most global human morphological diversity (irrespective of diet) was shaped by the past action of random mutations, population dispersals, and among-group gene flow. This basic pattern of human variation is then overlain by additional sources of variation, such as the effects of climatic selection to extreme cold climates (9, 10, 18, 22) and the effects of dietary changes related to the shift to agriculture (21). The study by Katz et al. (8) is timely and important in explicitly quantifying the relative importance of having a soft versus a harder diet on overall cranial form.
The second issue that warrants further investigation is what, at a proximate level, is driving these changes in skull morphology in response to a softer diet? In terms of biological mechanisms, there are two main options. One is that the differences are caused by
Drawing on an expansive global dataset and an innovative analytical approach, Katz et al. demonstrate small but consistent effects of a soft agricultural diet on skull morphology that relate directly to chewing anatomy.
natural selection having acted over the past few thousand years, generating genomic-level differences between farmers and foragers that manifest in terms of differing size and shape of the masticatory apparatus. The second option is that the differences are generated via phenotypic plasticity, or the tendency of bone tissue to remodel in response to biomechanical forces. This latter explanation is underwritten by a body of experimental animal studies (e.g., refs. 23–25) that demonstrate systematic changes in the morphology of the cranium in response to being fed either a hard or soft diet. The explanation of phenotypic plasticity also accords with the finding that farming populations tend to have a higher incidence of orthodontic problems such as malocclusions and dental crowding (7, 11). This is explained on the basis that the bony jaw responds to biomechanical forces whereas dental tissues do not. So if farmers are subjected to a less biomechanically challenging diet than foragers, their upper and lower jaw tissues may not be sufficiently stimulated to grow to the appropriate size and shape required for successful eruption of all adult teeth (26). However, some studies have found consistent differences related to chewing anatomy in young children (27, 28) indicating that forager–farmer differences arise early in development, and suggest that genetic differences underlie these morphological distinctions. Hence, there are many aspects of the relationship between cranial morphology and diet in humans that are still not clear. However, the study by Katz et al. (8) provides an important stimulus to think more carefully about these proximate mechanisms, and thereby gain a better understanding of the role of subsistence change in driving patterns of modern human cranial diversity.
Footnotes
The author declares no conflict of interest.
See companion article on page 9050.
References
- 1.Diamond J. Evolution, consequences and future of plant and animal domestication. Nature. 2002;418:700–707. doi: 10.1038/nature01019. [DOI] [PubMed] [Google Scholar]
- 2.Gignoux CR, Henn BM, Mountain JL. Rapid, global demographic expansions after the origins of agriculture. Proc Natl Acad Sci USA. 2011;108:6044–6049. doi: 10.1073/pnas.0914274108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinhasi R, Stock JT, editors. Human Bioarchaeology of the Transition to Agriculture. Wiley; London: 2011. [Google Scholar]
- 4.Larsen CS. The agricultural revolution as environmental catastrophe. Quat Int. 2006;150:12–20. [Google Scholar]
- 5.Larsen CS. Bioarchaeology: Interpreting Behavior from the Human Skeleton. 2nd Ed Cambridge Univ Press; Cambridge, UK: 2015. [Google Scholar]
- 6.Carlson DS, Van Gerven DP. Masticatory function and post-Pleistocene evolution in Nubia. Am J Phys Anthropol. 1977;46:495–506. doi: 10.1002/ajpa.1330460316. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman DE. The Evolution of the Human Head. Harvard Univ Press; Cambridge, MA: 2011. [Google Scholar]
- 8.Katz DC, Grote MN, Weaver TD. Changes in human skull morphology across the agricultural transition are consistent with softer diets in preindustrial farming groups. Proc Natl Acad Sci USA. 2017;114:9050–9055. doi: 10.1073/pnas.1702586114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz DC, Grote MN, Weaver TD. A mixed model for the relationship between climate and human cranial form. Am J Phys Anthropol. 2016;160:593–603. doi: 10.1002/ajpa.22896. [DOI] [PubMed] [Google Scholar]
- 10.Hubbe M, Hanihara T, Harvati K. Climate signatures in the morphological differentiation of worldwide modern human populations. Anat Rec (Hoboken) 2009;292:1720–1733. doi: 10.1002/ar.20976. [DOI] [PubMed] [Google Scholar]
- 11.von Cramon-Taubadel N. Global human mandibular variation reflects differences in agricultural and hunter-gatherer subsistence strategies. Proc Natl Acad Sci USA. 2011;108:19546–19551. doi: 10.1073/pnas.1113050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González-José R, et al. Functional-cranial approach to the influence of economic strategy on skull morphology. Am J Phys Anthropol. 2005;128:757–771. doi: 10.1002/ajpa.20161. [DOI] [PubMed] [Google Scholar]
- 13.Pinhasi R, Eshed V, Shaw P. Evolutionary changes in the masticatory complex following the transition to farming in the southern Levant. Am J Phys Anthropol. 2008;135:136–148. doi: 10.1002/ajpa.20715. [DOI] [PubMed] [Google Scholar]
- 14.Paschetta C, et al. The influence of masticatory loading on craniofacial morphology: A test case across technological transitions in the Ohio valley. Am J Phys Anthropol. 2010;141:297–314. doi: 10.1002/ajpa.21151. [DOI] [PubMed] [Google Scholar]
- 15.Galland M, Van Gerven DP, von Cramon-Taubadel N, Pinhasi R. 11,000 years of craniofacial and mandibular variation in Lower Nubia. Sci Rep. 2016;6:31040. doi: 10.1038/srep31040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noback ML, Harvati K. The contribution of subsistence to global human cranial variation. J Hum Evol. 2015;80:34–50. doi: 10.1016/j.jhevol.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Relethford JH. Global patterns of isolation by distance based on genetic and morphological data. Hum Biol. 2004;76:499–513. doi: 10.1353/hub.2004.0060. [DOI] [PubMed] [Google Scholar]
- 18.Roseman CC. Detecting interregionally diversifying natural selection on modern human cranial form by using matched molecular and morphometric data. Proc Natl Acad Sci USA. 2004;101:12824–12829. doi: 10.1073/pnas.0402637101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvati K, Weaver TD. Human cranial anatomy and the differential preservation of population history and climate signatures. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1225–1233. doi: 10.1002/ar.a.20395. [DOI] [PubMed] [Google Scholar]
- 20.Smith HF. Which cranial regions reflect molecular distances reliably in humans? Evidence from three-dimensional morphology. Am J Hum Biol. 2009;21:36–47. doi: 10.1002/ajhb.20805. [DOI] [PubMed] [Google Scholar]
- 21.von Cramon-Taubadel N. Evolutionary insights into global patterns of human cranial diversity: Population history, climatic and dietary effects. J Anthropol Sci. 2014;92:43–77. doi: 10.4436/jass.91010. [DOI] [PubMed] [Google Scholar]
- 22.Evteev A, Cardini AL, Morozova I, O’Higgins P. Extreme climate, rather than population history, explains mid-facial morphology of Northern Asians. Am J Phys Anthropol. 2014;153:449–462. doi: 10.1002/ajpa.22444. [DOI] [PubMed] [Google Scholar]
- 23.Corruccini RS, Beecher RM. Occlusal variation related to soft diet in a nonhuman primate. Science. 1982;218:74–76. doi: 10.1126/science.7123221. [DOI] [PubMed] [Google Scholar]
- 24.Lieberman DE, Krovitz GE, Yates FW, Devlin M, St Claire M. Effects of food processing on masticatory strain and craniofacial growth in a retrognathic face. J Hum Evol. 2004;46:655–677. doi: 10.1016/j.jhevol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Scott JE, McAbee KR, Eastman MM, Ravosa MJ. Teaching an old jaw new tricks: Diet-induced plasticity in a model organism from weaning to adulthood. J Exp Biol. 2014;217:4099–4107. doi: 10.1242/jeb.111708. [DOI] [PubMed] [Google Scholar]
- 26.Pinhasi R, Eshed V, von Cramon-Taubadel N. Incongruity between affinity patterns based on mandibular and lower dental dimensions following the transition to agriculture in the Near East, Anatolia and Europe. PLoS One. 2015;10:e0117301. doi: 10.1371/journal.pone.0117301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukase H, Suwa G. Growth-related changes in prehistoric Jomon and modern Japanese mandibles with emphasis on cortical bone distribution. Am J Phys Anthropol. 2008;136:441–454. doi: 10.1002/ajpa.20828. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez PN, Perez SI, Bernal V. Ontogeny of robusticity of craniofacial traits in modern humans: A study of South American populations. Am J Phys Anthropol. 2010;142:367–379. doi: 10.1002/ajpa.21231. [DOI] [PubMed] [Google Scholar]