Significance
Several forms of the oxytocin neurohormone have been found in New World monkeys (NWMs). Previous research has suggested an association between these forms and behaviors typical of this primate branch, including paternal care and monogamy. Our study provides genetic, pharmacological, behavioral, and in silico evidence supporting this connection. Rats treated intranasally with two NWM oxytocin variants showed an increase in some parental care behaviors. The same two variants were found to elicit different cell-signaling profiles in cell-based assays compared with ancestral oxytocin. Our findings highlight how mutations in the OXT DNA sequence coding for a nonapeptide result in distinct signaling profiles that may be linked to the emergence of novel adaptive traits, in this case, paternal care and monogamy.
Keywords: paternal care, biased agonism, primates, oxytocin variants, social behaviors
Abstract
The neurohormone oxytocin is a key player in the modulation of reproductive and social behavioral traits, such as parental care. Recently, a correlation between different forms of oxytocin and behavioral phenotypes has been described in the New World Monkeys (NWMs). Here, we demonstrate that, compared with the Leu8OXT found in most placental mammals, the Cebidae Pro8OXT and Saguinus Val3Pro8OXT taxon-specific variants act as equi-efficacious agonists for the Gq-dependent pathway but are weaker agonists for the β-arrestin engagement and subsequent endocytosis toward the oxytocin receptor (OXTR). Upon interaction with the AVPR1a, Pro8OXT and the common Leu8OXT yielded similar signaling profiles, being equally efficacious on Gq and β-arrestin, while Val3Pro8OXT showed reduced relative efficacy toward β-arrestin. Intranasal treatment with either of the variants increased maternal behavior and also promoted unusual paternal care in rats, as measured by pup-retrieval tests. We therefore suggest that Val3Pro8OXT and Pro8OXT are functional variants, which might have been evolutionarily co-opted as an essential part of the adaptive genetic repertoire that allowed the emergence of taxon-specific complex social behaviors, such as intense parental care in the Cebidae and the genus Saguinus.
In placental mammals, the nonapeptides oxytocin (Leu8OXT) and arginine vasopressin (AVP) differ by two amino acid residues at positions 3 and 8 (OXT: Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Leu-Gly; AVP: Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-Gly). Both act as hormones and neurotransmitters and play a key role in the modulation of reproductive and social behavioral traits, such as parental care and bonding between sexual partners. It is thought that the oxytocin system plays a greater role in females, while the AVP system primarily influences bonding and parental care in males (1–4).
Until a few years ago, it was thought that the nine amino acid sequence of oxytocin, known since the 1950s (5), was strictly conserved across all placental mammals. Recently, this paradigm was challenged when several taxon-specific forms of oxytocin were identified in New World monkeys (NWMs). In a first instance, Lee et al. (6) reported a Leu-to-Pro substitution at position 8 (Pro8OXT) in four species. Later, four more NWM forms of oxytocin, Ala8OXT, Thr8OXT, Phe2OXT, and Val3Pro8OXT were identified by our group (7) and by Ren et al. (8). On the other hand, the AVP amino acid sequence was shown to be conserved in species expressing the novel oxytocin forms (9, 10).
The Pro8OXT variant emerged at least ∼20 Mya (10) and is expressed by all genera of the Cebidae family. In the genus Saguinus (family Cebidae, subfamily Callitrichinae) (11), an additional mutation led to the substitution of Ile to Val at position 3 (Val3Pro8OXT). These two variants might form an essential part of the genetic repertoire which allowed certain NWM to evolve behavioral traits that are otherwise rare among mammals, such as social monogamy (8) and intense caregiving behavior by males (7). These behaviors are typical of the Cebidae family, which is also characterized by small body size and, in some subfamilies such as Callitrichinae, twin births. Furthermore, a clear positive selection signal was detected for the Pro8OXT variant in the Cebidae (7). Based on these findings, it seems plausible that these recently described taxon-specific oxytocin variants might have relevant functional and evolutionary implications. In this context, it is noteworthy that an independent Leu-to-Pro substitution at position 8 occurred ∼11 Mya in the family Atelidae, leading to the convergent occurrence of the Pro8OXT variant in the genus Ateles (10).
The activity of the oxytocin and vasopressin systems depends on the interaction of these neuropeptides with their respective G protein-coupled receptors (GPCRs), namely, OXTR, AVPR1a, AVPR1b, and AVPR2, although it is known that some level of cross-interaction can occur among them (12, 13). The GPCRs are the largest family of signal transducers. The classical GPCR activation model posits that, after agonist binding, a signaling cascade is started by the interaction with, and thereby activation of, heterotrimeric G proteins. Under this classical model, GPCRs are phosphorylated by GPCR kinases after activation, promoting the recruitment of β-arrestin proteins to the receptor, which in turn evokes receptor internalization and termination of G-protein signaling (14, 15). More recently, β-arrestins were also shown to serve as scaffolds for other effectors, such as kinases (16, 17), and several groups have reported that some compounds can trigger β-arrestin–mediated cellular responses even in the absence of G-protein activation (18–21). The finding that different molecules can selectively or preferentially trigger different pathways after interacting with the same receptor led to the concept of biased agonism or functional selectivity (22). As a result, ligands are now often referred to as “β-arrestin–biased” or “G protein-biased” agonists to indicate their preferential triggering of β-arrestin–dependent or G protein-dependent signaling pathways, respectively.
The correlation between taxon-specific oxytocin variants and behavioral phenotypes suggested by us and others (7, 8), paired with the current knowledge of GPCR signaling diversity (22), prompted us to hypothesize that the Val3Pro8OXT and Pro8OXT variants might display distinct signaling profiles, which could be correlated to the complex social behaviors observed in some NWM clades, such as paternal care, which are unusual among primates and mammals in general.
In the present study, we explored the functional selectivity of the Cebidae Pro8OXT and Saguinus Val3Pro8OXT variants by performing cell-based pharmacological characterization and in silico analyses. Additionally, we evaluated the role of Val3Pro8OXT and Pro8OXT in parental behavior in male and female rats using intranasal spray treatment. The distinct in vivo behaviors observed are well correlated with the signaling profiles and the in silico analyses, reinforcing the evidence that Val3Pro8OXT and Pro8OXT are functionally distinct variants that may underlie the unusual caregiving behaviors observed in some NWM clades.
Results
Pro8OXT and Val3Pro8OXT Are Full Agonists of the G-Protein Pathway but Are Less Efficacious Toward β-Arrestin at the OXTR.
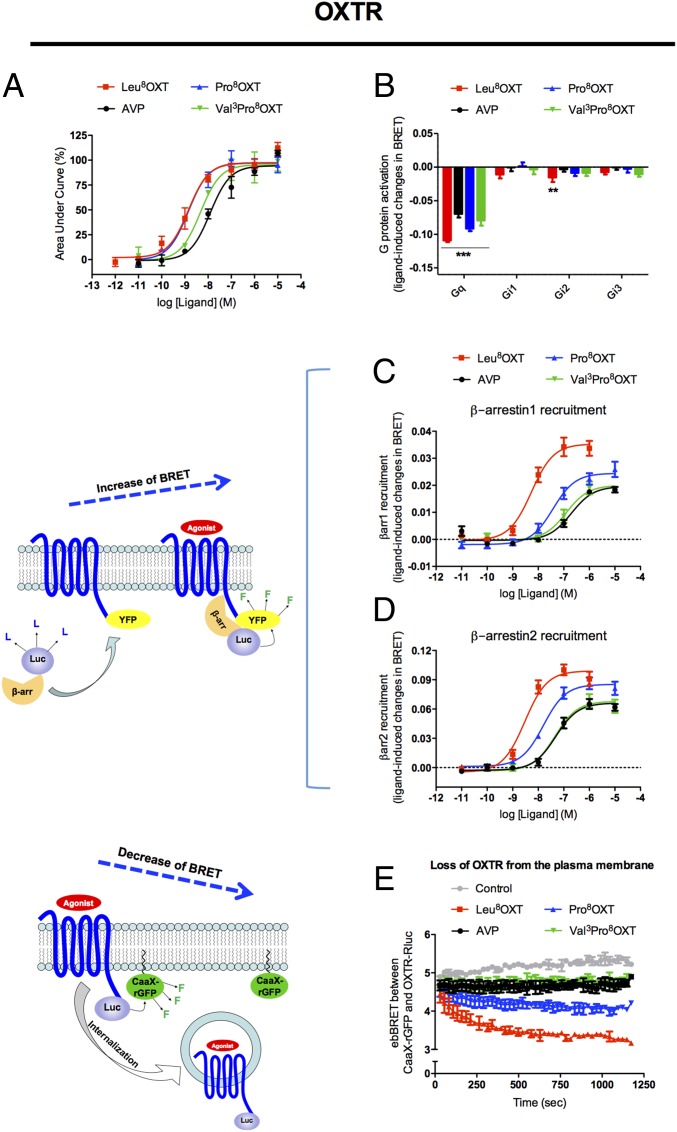
To functionally characterize the NWM oxytocin forms, we investigated the abilities of the Saguinus Val3Pro8OXT and Cebidae Pro8OXT variants to trigger distinct signaling cascades when binding to the OXTR and to AVPR1a. In an initial approach, we used HEK293T cells expressing the human OXTR to evaluate Gq coupling, the classical pathway triggered by OXTR, through the intracellular mobilization of calcium. Fig. 1A shows that both variants behave as full agonists, yielding the same maximal response as the ancestral Leu8OXT, with potencies similar to that of Leu8OXT (1.3 nM, 1.7 nM, and 5.6 nM, respectively, for Leu8OXT, Pro8OXT, and Val3Pro8OXT) (SI Appendix, Table S1). To evaluate if the variants could promote OXTR coupling to other G proteins, we also evaluated the activation profiles of Gi1, Gi2, Gi3, and Gq using bioluminescence resonance energy transfer (BRET)-based G-protein activation assays (23, 24). Fig. 1B shows that the variants behave similarly to Leu8OXT and are mainly coupled to Gq (P < 0.001).
Fig. 1.
Pro8OXT and Val3Pro8OXT are better agonists toward the calcium signaling than the arrestin pathway at the OXTR. The different OXT forms and AVP were functionally analyzed for calcium mobilization (A); Gq, Gi1, Gi2, and Gi3 coupling (B); β-arrestin1 (C) and β-arrestin2 (D) recruitment; and OXTR internalization kinetics (E) induced by 100 nM of ligands. β-Arrestin recruitment and receptor internalization were monitored by BRET as shown by the diagram on the left side of C and D, respectively).
We also addressed β-arrestin engagement by measuring the OXT variants-promoted recruitment of β-arrestin1 and 2 to the OXTR by BRET (25). As shown in Fig. 1 C and D, Leu8OXT induced robust β-arrestin1 and 2 recruitment to the OXTR, resulting in clear dose–response curves with potencies of 4.8 nM and 3 nM, respectively. Interestingly, the Pro8OXT variant yielded decreased potencies for recruitment of β-arrestin1 (33.9 nM, P < 0.01) and 2 (15.8 Nm, P < 0.05) as well as reduced maximal response for β-arrestin1 (72% of the response induced by Leu8OXT, P < 0.05). The Val3Pro8OXT-induced arrestin recruitment was even more reduced, yielding lower potencies for β-arrestin1 (128.8 nM, P < 0.001) and β-arrestin2 (45.7 nM, P < 0.001) as well as smaller maximal responses (62%, P < 0.01 and 70%, P < 0.05, respectively) (Fig. 1 C and D and SI Appendix, Table S1). To confirm this observation, we monitored the kinetics of β-arrestin recruitment to the plasma membrane using an enhanced bystander BRET-based assay (ebBRET) (26). In agreement with the results described in Fig. 1 C and D, the NWM Pro8OXT and Val3Pro8OXT variants are clearly less efficacious than Leu8OXT in promoting β-arrestin1 and 2 recruitment to the plasma membrane (SI Appendix, Fig. S1 A and B). One of the first consequences of arrestin engagement is the desensitization of G-protein signaling followed by receptor internalization. As illustrated in Fig. 1E, Leu8OXT induces a robust and time-dependent loss of plasma membrane receptor as assessed by ebBRET (26) that reflects agonist-promoted internalization. In contrast, Pro8OXT evokes only a moderate decrease of the plasma membrane receptor, and Val3Pro8OXT and AVP induce virtually no detectable internalization (Fig. 1E), consistent with their lower propensity to recruit β-arrestins.
In the calcium mobilization assay, the Val3Pro8OXT and Pro8OXT variants presented a maximal response similar to that of Leu8OXT, whereas for arrestin recruitment they showed significantly decreased maximal responses and potencies. Taken together, these data provide evidence that Val3Pro8OXT and Pro8OXT variants display a clear, distinct functional profile, acting as biased agonists toward the Gq and calcium signaling pathway at OXTR. Because the assays were performed using different biosensor cellular systems, a possible source of the differences observed in the efficacies and potencies could be different levels of receptor expression. To rule out this possibility, calcium responses and β-arrestin recruitment assays were carried out in the same cells, eliminating any possible contribution of different expression levels. As shown in SI Appendix, Fig. S2, both Pro8OXT, Val3Pro8OXT showed similar propensities to promote calcium mobilization but showed reduced β-arrestin recruitment compared with Leu8OXT.
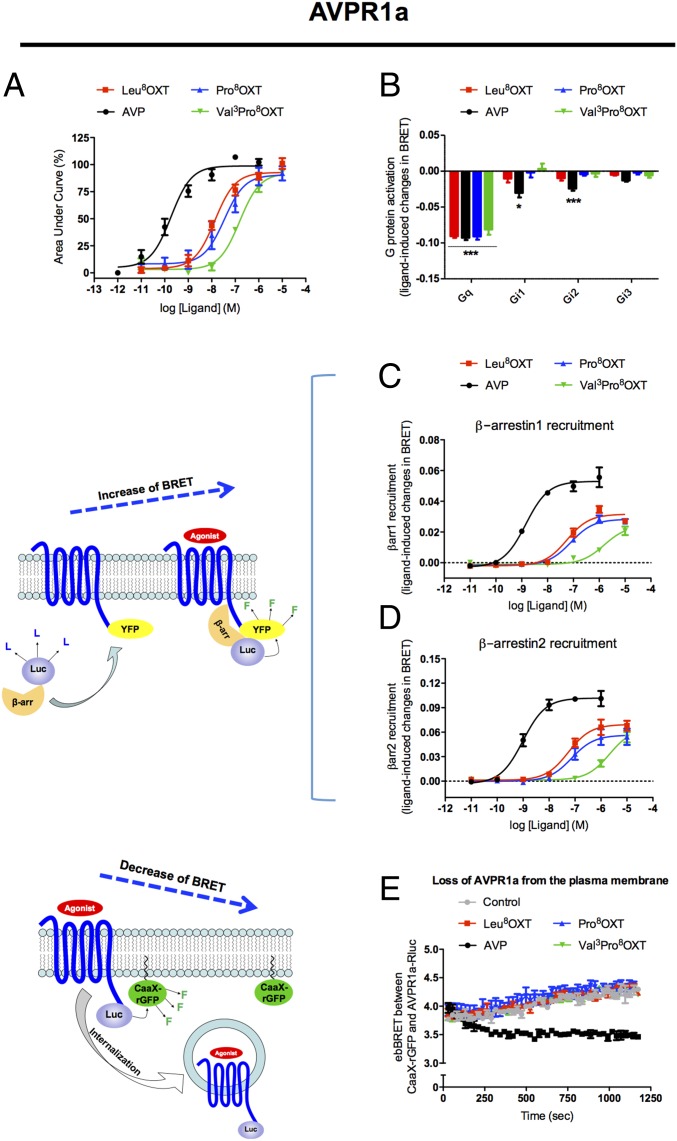
Pro8OXT Signaling Through AVPR1a Is Similar to Leu8OXT, While Val3Pro8OXT Presents an Altered Activation Profile.
AVPR1a is the main receptor through which AVP promotes pair bonding and paternal care (1, 2, 27). Based on the results described above, which show that Val3Pro8OXT and Pro8OXT variants display a more efficacious profile toward the calcium-signaling pathway at the OXTR receptor, and on studies that have reported that Leu8OXT can bind to AVPR1a (12, 13), we evaluated the signaling profiles of these NWM variants at the AVPR1a. As shown in Fig. 2A, native Leu8OXT and the Pro8OXT NWM variant yielded clear dose–response curves in intracellular calcium-mobilization assays, reaching maximal responses similar to that induced by AVP but with lower potency values (15.1 nM, P < 0.001 and 39.8 nM, P < 0.001 for Leu8OXT and Pro8OXT, respectively, compared with 0.2 nM obtained for AVP). Val3Pro8OXT also acted as a full agonist, inducing a maximal response similar to AVP, but displayed the lowest potency (151.3 nM, P < 0.001), showing that the presence of a valine at position 3 might have added novel functional characteristics to the nonapeptide.
Fig. 2.
The Pro8OXT signaling profile when activating the AVPR1a is similar to Leu8OXT, while Val3Pro8OXT presents decreased potencies of activation. The different OXT forms and AVP were functionally analyzed for calcium mobilization (A); Gq, Gi1, Gi2, and Gi3 coupling (B); β-arrestin1 (C) and β-arrestin2 (D) recruitment; and AVPR1a internalization kinetics (E) induced by 100 nM of ligands. β-Arrestin recruitment and receptor internalization were monitored by BRET as shown by the diagram on the left side of C and D, respectively).
To evaluate if the variants could induce coupling of AVPR1a to other G proteins, we evaluated the activation profiles of Gi1, Gi2, Gi3, and Gq. Fig. 2B shows that the major signaling pathway triggered by AVP, as well as by Leu8OXT, Pro8OXT, and Val3Pro8OXT, is Gq (P < 0.001). AVP was also found to promote moderate activation of Gi1 (P < 0.05) and Gi2 (P < 0.001). Dose–response experiments for assessment of β-arrestin1 and 2 recruitment to AVPR1a (Fig. 2 C and D) showed that only AVP was able to induce a maximal response, also with high potency values (both 1.4 nM). Native Leu8OXT and the NWM variants Pro8OXT and Val3Pro8OXT presented smaller maximal responses (respectively 64%, P < 0.05; 59%, P < 0.01; and 46%, P < 0.01 of AVP-promoted responses for β-arrestin1 and 65%, P < 0.05; 56%, P < 0.05; and 65%, P < 0.05 for β-arrestin2), as well as lower potencies (respectively 63.1 nM, P < 0.001; 123 nM, P < 0.001; and 3,548 nM, P < 0.001 for β-arrestin1 and 50.1 nM, P < 0.001; 74.1 nM, P < 0.001; and 2,291 nM, P < 0.001 for β-arrestin2) (SI Appendix, Table S1). Kinetic analyses of β-arrestin1 and 2 translocation to the plasma membrane using ebBRET confirmed that all OXT variants had reduced ability to promote β-arrestin translocation compared with AVP, with Val3Pro8OXT having the lowest ability (SI Appendix, Fig. S1 C and D). Agonist-promoted internalization as assessed by the loss of plasma membrane receptor using ebBRET was detected only for AVP stimulation (Fig. 2E), consistent with AVP having lowest propensity of the OXT variants to promote β-arrestin recruitment.
Intranasal Treatment with Val3Pro8OXT and Pro8OXT Induced Changes in Rat Parental Behaviors.
The distinct pharmacological profiles of Pro8OXT and Val3Pro8OXT compared with Leu8OXT, as described above, prompted us to explore the possible functional role of the NWM variants in vivo through behavioral assays (SI Appendix, Fig. S3). To this end, we conducted tests using rats (Rattus norvegicus; Wistar strain) subjected to intranasal treatment with either one of the two NWM variants or the ancestral Leu8OXT.
We tested whether the NWM oxytocin forms could trigger changes in paternal and maternal behaviors (SI Appendix, Tables S2 and S3). Fourteen behaviors related to female–pup, male–pup, and male–female interactions were evaluated and scored. We used generalized linear models to compare group means, selecting the appropriate distribution for each outcome evaluated (SI Appendix, Tables S4 and S5). Statistical significances of the differences were then assessed by a Dunn’s test for multiple comparisons (SI Appendix, Tables S6 and S7). To perform statistical analyses, the scores obtained for individual variables were clustered into three major sets of variables: parental care, couple bonding, and aggression/dominance (SI Appendix, Table S3). We found no significant differences between treatment groups in maternal care, couple bonding, or aggressive behavior toward male partners (SI Appendix, Table S4).
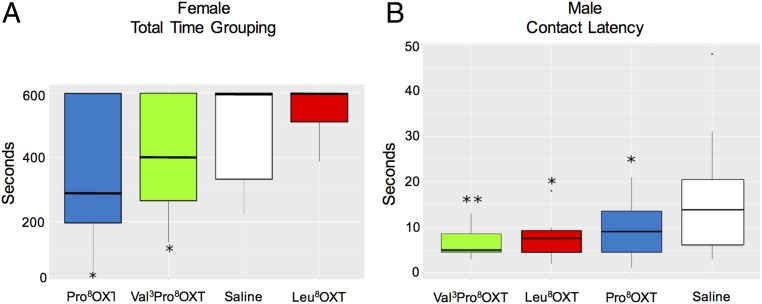
Caregiving behavior was additionally evaluated in males and females by measuring their motivation to recover their own pups using a known pup-retrieval test (28). Females treated with Val3Pro8OXT and Pro8OXT showed faster pup retrieval (total time to grouping) than females treated with Leu8OXT (Fig. 3A and SI Appendix, Tables S6 and S7). Although paternal care has been observed in mice under certain circumstances (29, 30), it has never been described in rats. In our study, males contacted their pups significantly faster when treated with Leu8OXT or with Pro8OXT and Val3Pro8OXT, compared with those treated with saline, indicating increased motivation to contact pups (contact latency, Fig. 3B and SI Appendix, Tables S6 and S8). Females showed a shorter time to group the litter (total time to grouping, Fig. 3A and SI Appendix, Tables S6 and S7).
Fig. 3.
(A) Female total time to group all pups at nest; *P < 0.05 for Dunn comparisons with Leu8OXT. (B) Time latency of male contact with first pup; *P < 0.05, **P < 0.01 for Dunn comparisons with saline treatment.
Physicochemical Analysis and Molecular Dynamics Simulation of OXT Forms.
To investigate possible physicochemical or conformational properties of OXT variants that could correlate to the observed different functional profiles, we analyzed their sequences and molecular dynamics compared with those of other nonapeptides. Our molecular dynamics analysis revealed only subtle conformational changes, indicating that the NWM OXT forms preserve conformational features similar to those of the ancestral Leu8OXT. On the other hand, physicochemical analysis of peptide sequences showed that the introduction of a proline residue in position 8 and valine in position 3 of Leu8OXT clearly altered their hydrophilicity indexes (SI Appendix, Fig. S4). Therefore, it is seems plausible to infer that the adaptive taxon-specific traits discussed in this study might be at least partially related to the altered hydropathic properties of the OXT forms.
Discussion
One of the greatest challenges of biological systems is unveiling the complex connection between molecular findings and phenotypic traits. Recently, our group and others have reported the discovery of the oxytocin variants Pro8OXT in the Cebidae and Val3Pro8OXT in the Saguinus genus (Cebidae, subfamily Callitrichinae) (11), which are characterized by complex social behaviors such as intense maternal care, male parental care, and social monogamy (7, 8). At least two of these behaviors (male parental care and social monogamy) are rare in mammals but are evolutionarily relevant in contexts where reproduction bears a high energetic cost. For example, in the Callitrichinae, the birth weight of twin infants is ∼30% of the female’s body weight (31, 32). As a result, the investment in caregiving behaviors by both parents, as well as sibs, increases the infant survival rate in the Callitrichinae (31).
Leu8OXT is a known agonist of the GPCR OXTR. Based on the current knowledge of the intricate and diversified signaling profiles triggered through GPCRs (22), we decided to explore if the taxon-specific oxytocin variants Pro8OXT and Val3Pro8OXT could lead to different signaling profiles, which might play a role in the complex behavioral traits described above. To that end, we synthesized both oxytocin variants and investigated their signaling profiles upon the activation of OXTR or AVPR1a with a particular focus on Gq and β-arrestin pathways.
The cell-based characterization showed that both Pro8OXT and Val3Pro8OXT are equi-efficacious to Leu8OXT for the Gq-dependent pathway (Fig. 1 A and B) while being significantly less efficacious than Leu8OXT in inducing β-arrestin recruitment to the OXTR (Fig. 1 C and D). As would be expected from the role of β-arrestins in agonist-promoted endocytosis, both Pro8OXT and Val3Pro8OXT showed significantly reduced ability to promote time-dependent loss of cell-surface OXTR (Fig. 1E), therefore possibly maintaining high receptor levels at the plasma membrane and allowing extended signaling of Gq-dependent pathways. Upon interaction with the AVPR1a, Pro8OXT yielded a signaling profile similar to that obtained for Leu8OXT, but the Saguinus Val3Pro8OXT showed changes in its activation profile (Fig. 2 C and D and SI Appendix, Fig. S1 C and D). These data suggest that the adaptive behavioral phenotypes observed in monkeys that present a proline at position 8 of their OXT amino acid sequences could be related to taxon-specific altered signaling pathways mainly triggered downstream of OXTR, while an additional change at position 3 (Val) also modified signaling pathways triggered downstream of AVPR1a.
The relative hydrophilicity of the oxytocin residues in positions 3 and 8 could determine the type of ligand–receptor interaction (7, 10, 33), thereby affecting their downstream activation pathways. In this context, it is of interest that Busnelli et al. (34) have described two synthetic oxytocin analogs that share the same critical change at position 8 that increases hydrophilicity. Like the NWM variants discussed here, these variants have low performance for β-arrestin recruitment and OXTR internalization, supporting the importance of the position 8 (Leu in the ancestral OXT) in selectively triggering β-arrestin recruitment. We also detected that valine at position 3 in Saguinus has a possible implication for β-arrestin recruitment at AVPR1a (Fig. 2 C and D). Thus, two successive mutations in the OXT gene over the past ∼20 My led to amino acid changes with key functional alterations concerning β-arrestin recruitment. From an evolutionary perspective, they were likely selected to allow the emergence of successive behavioral adaptive novelties in Cebid monkeys without influencing this neurohormone’s basic functions in placental mammals, such as lactation and uterine contractions.
Adding to these findings, there is evidence for a coevolutionary process between oxytocin variants and their receptors in NWMs (7). In Saguinus, for instance, the OXTR Leu368 residue and the oxytocin Val3 residue seem to be the result of coevolution (7). From a functional perspective, the replacement of Ser368 by Leu in a Ser cluster in the OXTR C terminus disrupts an important phosphorylation site that has been reported to contribute to β-arrestin recruitment in many GPCRs (13). Our current results and the above-described data reveal an important evolutionary tendency in the NWM OXT–OXTR system, moving toward reduced β-arrestin recruitment and a possible loss of G-protein desensitization by altering key residues in the ligand peptides (e.g., Pro8OXT and Val3Pro8OXT), in the receptor (e.g., OXTR-Leu368 in Saguinus), or in both. Of note, the experiments reported here were performed with human OXTR, in which the C-terminal Ser cluster is preserved. Our work therefore highlights that alterations in ligand peptide structure seem to be sufficient to significantly decrease β-arrestin engagement and diminish OXTR internalization. Taken together, our results suggest that molecular changes in the OXT–OXTR system in Cebidae and Saguinus could produce a sustained G-protein–activated system due to their lower capacity to promote OXTR internalization. This hypothesis is further supported by earlier experiments in rats, in which synthetic oxytocin analogs characterized by Val3 (35) or Pro8 (36) substitutions were found to lead to a significant increase in the peptide’s half-life in in vivo assays compared with ancestral oxytocin. In the context of our work, such observations suggest that Pro8OXT and Val3Pro8OXT might also have an increased half-life, representing a complementary way to lead to sustained activation of Gq-protein–dependent signaling pathways.
Kosfeld et al. (37) suggested that synthetic Leu8OXT administered by intranasal delivery increased interpersonal confidence in humans. Although these and other results regarding this kind of treatment have been controversial (10), several studies have since reported an effect of intranasal oxytocin administration on a range of complex social behaviors in humans and other mammalian species (38–40). Black-tufted marmosets (Callithrix penicillata) subjected to intranasal treatment with Pro8OXT showed reduced altruistic food sharing with opposite-sex strangers, while altruistic food sharing with their long-term pair-mates was unaffected (10). A recent study by Calcagnoli et al. (41) in male rats demonstrated that intranasal administration of Leu8OXT was effective in stimulating prosocial behavior, while parental care was not addressed.
Interestingly, we were able to show that rats treated intranasally with Pro8OXT or Val3Pro8OXT changed their behavior in a pup-retrieval test. Mothers treated with these variants showed faster pup retrieval (total time to grouping) than mothers treated with Leu8OXT (Fig. 3A). It is important to note that after delivery and during lactation females already bear high endogenous levels of Leu8OXT. As shown in Fig. 1 C and D, Leu8OXT is able to efficiently induce β-arrestin1 and 2 recruitment and internalization of OXTR (Fig. 1E). We therefore believe that intranasal treatment with additional Leu8OXT might result in a desensitization effect, decreasing pup retrieval. In contrast, treatment with the NWM variants, which induce less efficacious β-arrestin recruitment (Fig. 1 C and D) and decreased (Pro8OXT) or no (Val3Pro8OXT) OXTR internalization (Fig. 1E), did not have the same effect. This suggests that this difference in the signaling profile could be related to the observed maternal care behavior. In this context, it is important to note that rats treated with the NWM variants produce endogenous Leu8OXT, which can attenuate the variants’ effects. We can therefore speculate that in Cebidae and Saguinus, which express only Pro8OXT and Val3Pro8OXT, respectively, the behavioral effects could be even more pronounced.
When investigating the effect of the NWM oxytocin variants on rat male parental care behavior, we found that fathers contacted their pups significantly faster after treatment with Leu8OXT, Pro8OXT, or Val3Pro8OXT than after treatment with saline (Fig. 3B). Unlike lactating females, the OXT-OXTR system in males is not over-activated, meaning that it is not desensitized by treatment with exogenous Leu8OXT. Additionally, when rat males were treated with Val3Pro8OXT, the variant with the most altered pharmacological profile, they contacted their pups faster than those with any other intranasal treatment (P < 0.01).
Importantly, as discussed before, the effect of oxytocin on this particular kind of behavior seems to be related to Gq activation followed by calcium mobilization, a pathway efficaciously triggered by all ligands studied here. Recent research on genetically modified animals has shown a clear link between calcium-dependent signaling pathways and behavioral modulation. For instance, mice lacking the Itpka gene involved in inositol 1,4,5-trisphosphate (IP3) regulation show sustained high calcium levels in central amygdala and display alterations in control of fear and anxiety (42). Calcium signaling has also been reported to be involved in presynaptic degranulation and neurotransmitter release, including oxytocin, thereby contributing to prolonged OXTR stimulation in postsynaptic neurons (43). Although the effectors, pathways, and the behaviors described above are different from the ones investigated here, we believe that they highlight the importance of calcium signaling in behavior modulation, in agreement our hypothesis that the reduced ability to desensitize OXTR may be a key mechanism by which the NWM oxytocin variants are involved in the origin of specific adaptive behaviors. It is noteworthy that although Val3Pro8 OXT promotes a weaker recruitment of β-arrestins to AVPR1a (Fig. 2 C and D), it is unable to promote receptor internalization as assessed by the loss of plasma membrane receptor using ebBRET (Fig. 2E). One of the possible explanations for this result is that, although arrestins can reach the AVPR1a, they could be stabilized in distinct conformations (44, 45) that might not be suitable for allowing receptor internalization. Taking these findings together, we can hypothesize that AVPR1a, when activated by Val3Pro8OXT, might also play a role in the parental behaviors considered in this study.
Nevertheless, it is clear that a range of other molecular and biological processes is involved in the modulation of social complex behaviors. For example, OXT and OXTR present distinct brain distribution patterns in different mammal species (2, 10, 27, 46). By extension, genetic and molecular changes involving biological systems and signaling pathways beyond those discussed here may play an important role in these complex behavioral traits. Interestingly, the NWM Callicebus (family Pitheciidae), which is characterized by social monogamy and paternal care and which expresses the native (ancestral) Leu8OXT, has a large number of progesterone-response elements in the OXTR promoter region. This is similar to what is found in Cebidae (47) and might be another way to contribute to increased caregiving behaviors. On the other hand, Pro8OXT is present in the large-bodied spider monkey (genus Ateles; family Atelidae). Although the males of this species are not involved in raising the offspring, it is interesting that females present the longest breast-feeding time among all NWMs.
Finally, despite of the contribution of other elements and processes such as gene regulation and epigenetic mechanisms, it is likely that the Cebidae Pro8OXT and Saguinus Val3Pro8OXT variants represent key components of a genetic repertoire that allowed the emergence of taxon-specific complex behavioral phenotypes.
Materials and Methods
Peptide Synthesis and Cell-Based Pharmacological Characterization.
The Pro8OXT and Val3Pro8OXT variants were synthesized by solid-phase peptide synthesis and purified by HPLC. The efficiency of synthesis was assessed by amino acid composition analysis. HEK293T cells were cultured in supplemented DMEM and transfected with OXTR- or AVPR1a-encoding plasmids using polyethylenimine. After transfection, cells were transferred to specific plates for development of different cell-based functional assays.
In Vivo Tests.
Forty-two pairs of Rattus norvegicus were mated randomly, and pregnancy was confirmed on the day after mating by the presence of sperm in vaginal smears. Pairs were assigned to four experimental groups and received intranasal treatment with saline or the Leu8OXT, Pro8OXT, or Val3Pro8OXT variants. A total of 14 behaviors related to female–pup, male–pup, or male–female interactions were scored. Maternal and paternal motivations were evaluated in the pup-retrieval test. Results were analyzed by a pairwise comparison (Dunn’s test).
In Silico Analyses.
The starting structure of oxytocin was obtained from the Protein Data Bank (48). Molecular Dynamics simulations were performed for Leu8OXT, Pro8OXT, Val3Pro8OXT, Ala8OXT, Thr8OXT, Phe2OXT, and AVP, and their structural and physicochemical parameters were compared using Shapiro–Wilk, Kruskal–Wallis, and Dunn’s tests, as well as Principal Component Analysis.
Ethics.
All experimental procedures were approved by the Ethics Committee on Animal Use of the Universidade Federal do Rio Grande do Sul (Approval no. 28542) and are in accordance with Brazilian Law 11.794/2008, which regulates these procedures in Brazil. A complete description of the methods can be found in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by Fundação de Pesquisa do Estado de São Paulo (FAPESP) Grant 2012/20148-0; by Canadian Institute for Health Research Grant FDN-148431; and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Conselho Nacional de Desenvolvimento Científico e Tecnológico, and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Rio Grande do Sul. M.B. holds a Canada Research Chair in in Signal Transduction and Molecular Pharmacology and is a Fellow of the Canadian Institute for Advanced Research. C.M.C.-N. and M.B. are recipients of Joint International Cooperation Grant SPRINT 2015/50086-4 funded by the FAPESP.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1711687114/-/DCSupplemental.
References
- 1.Wang Z, Ferris CF, De Vries GJ. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proc Natl Acad Sci USA. 1994;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 3.Goodson JL. Nonapeptides and the evolutionary patterning of sociality. Prog Brain Res. 2008;170:3–15. doi: 10.1016/S0079-6123(08)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee H-J, Macbeth AH, Pagani JH, Young WS., 3rd Oxytocin: The great facilitator of life. Prog Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du Vigneaud V, Ressler C, Trippett S. The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin. J Biol Chem. 1953;205:949–957. [PubMed] [Google Scholar]
- 6.Lee AG, et al. A novel form of oxytocin in New World monkeys. Biol Lett. 2011;7:584–587. doi: 10.1098/rsbl.2011.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vargas-Pinilla P, et al. Evolutionary pattern in the OXT-OXTR system in primates: Coevolution and positive selection footprints. Proc Natl Acad Sci USA. 2015;112:88–93. doi: 10.1073/pnas.1419399112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren D, et al. Genetic diversity in oxytocin ligands and receptors in New World monkeys. PLoS One. 2015;10:e0125775. doi: 10.1371/journal.pone.0125775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren D, Chin KR, French JA. Molecular variation in AVP and AVPR1a in New World monkeys (Primates, Platyrrhini): Evolution and implications for social monogamy. PLoS One. 2014;9:e111638. doi: 10.1371/journal.pone.0111638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.French JA, Taylor JH, Mustoe AC, Cavanaugh J. Neuropeptide diversity and the regulation of social behavior in New World primates. Front Neuroendocrinol. 2016;42:18–39. doi: 10.1016/j.yfrne.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perelman P, et al. A molecular phylogeny of living primates. PLoS Genet. 2011;7:e1001342. doi: 10.1371/journal.pgen.1001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slusarz MJ, Sikorska E, Slusarz R. Interactions of vasopressin and oxytocin receptors with vasopressin analogues substituted in position 2 with 3,3′-diphenylalanine–a molecular docking study. J Pept Sci. 2013;19:118–126. doi: 10.1002/psc.2485. [DOI] [PubMed] [Google Scholar]
- 13.Zingg HH, Laporte SA. The oxytocin receptor. Trends Endocrinol Metab. 2003;14:222–227. doi: 10.1016/s1043-2760(03)00080-8. [DOI] [PubMed] [Google Scholar]
- 14.Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Barak LS, Winkler KE, Caron MG, Ferguson SS. A central role for beta-arrestins and clathrin-coated vesicle-mediated endocytosis in beta2-adrenergic receptor resensitization. Differential regulation of receptor resensitization in two distinct cell types. J Biol Chem. 1997;272:27005–27014. doi: 10.1074/jbc.272.43.27005. [DOI] [PubMed] [Google Scholar]
- 16.Luttrell LM, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 17.Luttrell LM, et al. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci USA. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azzi M, et al. Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci USA. 2003;100:11406–11411. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker JG, Hall IP, Hill SJ. Agonist and inverse agonist actions of beta-blockers at the human beta 2-adrenoceptor provide evidence for agonist-directed signaling. Mol Pharmacol. 2003;64:1357–1369. doi: 10.1124/mol.64.6.1357. [DOI] [PubMed] [Google Scholar]
- 20.Wei H, et al. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA. 2003;100:10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gbahou F, et al. Protean agonism at histamine H3 receptors in vitro and in vivo. Proc Natl Acad Sci USA. 2003;100:11086–11091. doi: 10.1073/pnas.1932276100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costa-Neto CM, Parreiras-e-Silva LT, Bouvier M. A pluridimensional view of biased agonism. Mol Pharmacol. 2016;90:587–595. doi: 10.1124/mol.116.105940. [DOI] [PubMed] [Google Scholar]
- 23.Galés C, et al. Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat Struct Mol Biol. 2006;13:778–786. doi: 10.1038/nsmb1134. [DOI] [PubMed] [Google Scholar]
- 24.Quoyer J, et al. Pepducin targeting the C-X-C chemokine receptor type 4 acts as a biased agonist favoring activation of the inhibitory G protein. Proc Natl Acad Sci USA. 2013;110:E5088–E5097. doi: 10.1073/pnas.1312515110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angers S, et al. Detection of beta 2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET) Proc Natl Acad Sci USA. 2000;97:3684–3689. doi: 10.1073/pnas.060590697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Namkung Y, et al. Monitoring G protein-coupled receptor and β-arrestin trafficking in live cells using enhanced bystander BRET. Nat Commun. 2016;7:12178. doi: 10.1038/ncomms12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koshimizu TA, et al. Vasopressin V1a and V1b receptors: From molecules to physiological systems. Physiol Rev. 2012;92:1813–1864. doi: 10.1152/physrev.00035.2011. [DOI] [PubMed] [Google Scholar]
- 28.Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proc Natl Acad Sci USA. 2008;105:17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang M, et al. Pairmate-dependent pup retrieval as parental behavior in male mice. Front Neurosci. 2014;8:186. doi: 10.3389/fnins.2014.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H-X, et al. Displays of paternal mouse pup retrieval following communicative interaction with maternal mates. Nat Commun. 2013;4:1346. doi: 10.1038/ncomms2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cleveland J, Snowdon CT. Social development during the first twenty weeks in the cotton-top tamarin (Saguinus O. Oedipus) Anim Behav. 1984;32:432–444. [Google Scholar]
- 32.Cavanaugh J, French JA. Post-partum variation in the expression of paternal care is unrelated to urinary steroid metabolites in marmoset fathers. Horm Behav. 2013;63:551–558. doi: 10.1016/j.yhbeh.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koehbach J, Stockner T, Bergmayr C, Muttenthaler M, Gruber CW. Insights into the molecular evolution of oxytocin receptor ligand binding. Biochem Soc Trans. 2013;41:197–204. doi: 10.1042/BST20120256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Busnelli M, Bulgheroni E, Manning M, Kleinau G, Chini B. Selective and potent agonists and antagonists for investigating the role of mouse oxytocin receptors. J Pharmacol Exp Ther. 2013;346:318–327. doi: 10.1124/jpet.113.202994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith MW, Ginsburg M. Fate of synthetic oxytocin analogues in the rat. Br Pharmacol Chemother. 1961;16:244–252. doi: 10.1111/j.1476-5381.1961.tb01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gazis D. Plasma half-lives of vasopressin and oxytocin analogs after iv injection in rats. Proc Soc Exp Biol Med. 1978;158:663–665. doi: 10.3181/00379727-158-40269. [DOI] [PubMed] [Google Scholar]
- 37.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 38.Carter GG, Wilkinson GS. Intranasal oxytocin increases social grooming and food sharing in the common vampire bat Desmodus rotundus. Horm Behav. 2015;75:150–153. doi: 10.1016/j.yhbeh.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Lieberwirth C, Wang Z. Social bonding: Regulation by neuropeptides. Front Neurosci. 2014;8:171. doi: 10.3389/fnins.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veening JG, Olivier B. Intranasal administration of oxytocin: Behavioral and clinical effects, a review. Neurosci Biobehav Rev. 2013;37:1445–1465. doi: 10.1016/j.neubiorev.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calcagnoli F, Kreutzmann JC, de Boer SF, Althaus M, Koolhaas JM. Acute and repeated intranasal oxytocin administration exerts anti-aggressive and pro-affiliative effects in male rats. Psychoneuroendocrinology. 2015;51:112–121. doi: 10.1016/j.psyneuen.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 42.Chung S, et al. The role of inositol 1,4,5-trisphosphate 3-kinase A in regulating emotional behavior and amygdala function. Sci Rep. 2016;6:23757. doi: 10.1038/srep23757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 44.Zimmerman B, et al. Differential β-arrestin-dependent conformational signaling and cellular responses revealed by angiotensin analogs. Sci Signal. 2012;5:ra33. doi: 10.1126/scisignal.2002522. [DOI] [PubMed] [Google Scholar]
- 45.Santos GA, et al. Comparative analyses of downstream signal transduction targets modulated after activation of the AT1 receptor by two β-arrestin-biased agonists. Front Pharmacol. 2015;6:131. doi: 10.3389/fphar.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freeman SM, Young LJ. Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: Translational implications. J Neuroendocrinol. 2016;28:1–22. doi: 10.1111/jne.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vargas-Pinilla P, et al. Progesterone response element variation in the OXTR promoter region and paternal care in New World monkeys. Behav Genet. 2017;47:77–87. doi: 10.1007/s10519-016-9806-2. [DOI] [PubMed] [Google Scholar]
- 48.Rose JP, Wu CK, Hsiao CD, Breslow E, Wang BC. Crystal structure of the neurophysin-oxytocin complex. Nat Struct Biol. 1996;3:163–169. doi: 10.1038/nsb0296-163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.