Forest expansion into Brazilian savanna due to fire suppression causes precipitous species loss.
Abstract
Tropical savannas have been increasingly viewed as an opportunity for carbon sequestration through fire suppression and afforestation, but insufficient attention has been given to the consequences for biodiversity. To evaluate the biodiversity costs of increasing carbon sequestration, we quantified changes in ecosystem carbon stocks and the associated changes in communities of plants and ants resulting from fire suppression in savannas of the Brazilian Cerrado, a global biodiversity hotspot. Fire suppression resulted in increased carbon stocks of 1.2 Mg ha−1 year−1 since 1986 but was associated with acute species loss. In sites fully encroached by forest, plant species richness declined by 27%, and ant richness declined by 35%. Richness of savanna specialists, the species most at risk of local extinction due to forest encroachment, declined by 67% for plants and 86% for ants. This loss highlights the important role of fire in maintaining biodiversity in tropical savannas, a role that is not reflected in current policies of fire suppression throughout the Brazilian Cerrado. In tropical grasslands and savannas throughout the tropics, carbon mitigation programs that promote forest cover cannot be assumed to provide net benefits for conservation.
INTRODUCTION
Carbon mitigation programs that protect and expand tropical forests are widely embraced as a win-win situation for conservation because they presumably enhance species diversity (1, 2). This presumption requires closer scrutiny in the seasonally dry tropics, where large areas of natural grasslands and savannas could be converted to forest through afforestation or fire suppression (3–5). In these biomes, it cannot be assumed that increased tree cover will generate net benefits for biodiversity because savannas contain many endemic light-demanding species that can be displaced or eliminated by forest cover (3, 6, 7); thus, species-rich savannas may undergo a net loss of species when replaced by forest. For this reason, it is important to rigorously quantify the impacts on biodiversity of management practices that seek to maximize carbon sequestration by promoting forest cover.
Where savanna management is not aimed specifically at carbon sequestration, other factors may lead to the same outcome of increased tree density and ecosystem carbon stocks. Tree densities have been increasing in many savannas across the tropics (8), likely because of fire suppression and increasing atmospheric CO2 (9–12). In the savannas of the Brazilian Cerrado, governmental regulations severely restrict the use of fire, and fire suppression is actively practiced in most reserves and parks (13) because of widespread perception that fire is detrimental to biodiversity. This is compounded by expansion of crops, pasture, and forest plantations at alarming rates (14, 15), resulting in highly fragmented landscapes. Because such fragmentation impedes fire spread, natural ignitions alone are unable to maintain fire frequencies that were once possible (16).
We assessed the impact of 30 years of fire suppression and forest encroachment in savannas in the Brazilian Cerrado, a biodiversity hotspot (17) and putatively the most species-rich savanna region in the world (18, 19). To understand the long-term impact of current fire policies, we quantified changes in biodiversity and ecosystem carbon stocks at three savanna-forest boundaries actively undergoing forest encroachment due to fire suppression. At each site, we measured soil and vegetation carbon stocks and surveyed plant communities across a network of 30 plots ranging from open savanna (mean tree basal area of 3.1 m2 ha−1) to recently formed forest (mean basal area of 21.5 m2 ha−1). Aboveground-foraging ant communities were also surveyed, serving as a representative animal group. We used a 30-year series of Landsat satellite images to quantify rates of vegetation change and to hindcast carbon sequestration and the resulting species loss.
RESULTS
The study area has undergone a steady increase in tree cover over the past 30 years. Over this time, there was a continuous increase in the enhanced vegetation index (EVI; Fig. 1), a satellite-derived metric that is strongly and positively correlated with tree basal area, leaf area index (LAI), and ecosystem carbon stocks across our study sites (figs. S1 and S2). As inferred from historical EVI, all plots that are now forest were occupied by savanna or grassland in 1986, corroborating observations by reserve personnel working at the site during this period.
Fig. 1. Historical changes in EVI over 30 years of fire suppression.
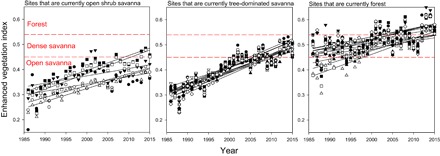
Historical changes in the EVI over 30 years of fire suppression, as determined by Landsat images. Each study plot is denoted by a different symbol and a separate regression line.
In 2015, ecosystem carbon stocks (plants + surface soils) ranged from a mean of 20.8 Mg ha−1 in open savanna sites to 83.5 Mg ha−1 in forest. The gradient in carbon stocks can be attributed primarily to a 14-fold increase in tree biomass and, to a lesser extent, a 2-fold increase in surface soil stocks (0 to 20 cm) over the vegetation gradient (Fig. 2A). The annual increase in ecosystem carbon storage was 1.19 Mg ha−1 year−1 over the past three decades, as estimated by combining the time series of EVI (Fig. 1) with the empirical relationship between EVI and ecosystem carbon (fig. S2).
Fig. 2. Carbon stocks and species richness due to forest encroachment.
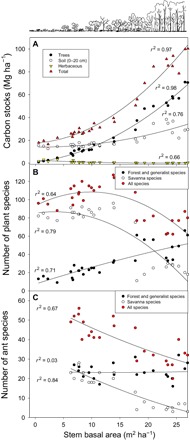
Changes in (A) carbon stocks, (B) plant species richness, and (C) ant species richness over gradients of forest encroachment. All variables were significantly correlated with basal area (P < 0.0001) except for the number of forest and generalist ants (P = 0.42).
The increase in tree biomass over the vegetation gradient was accompanied by decreases in the richness of both plant and ant species, with particularly large losses in savanna specialists (Fig. 2). The loss of savanna species was most acute when vegetation reached a stem basal area of approximately 15 m2 ha−1 and an LAI of 2.5, a threshold that corresponds roughly to the transition between savanna and forest as indicated by the disappearance of C4 grasses (20, 21). Forest sites had 69% fewer savanna plant species and 74% fewer savanna ant species, compared to dense savanna. This decline in savanna species was partially offset by an increase in the number of forest specialists (Fig. 2) thus, when all species were considered, forest sites had 33% fewer plant species and 34% fewer ant species than dense savanna. Because species richness of each group was strongly correlated with EVI (r2 = 0.65 to 0.86, P < 0.0001; fig. S2), we used historical changes in EVI (Fig. 1) to estimate changes in species diversity since 1986. In the sites that are now forest, we estimate that 27% of plant species and 35% of ant species have been lost during 30 years of fire suppression, whereas the loss of savanna specialists was 67 and 86% for plants and ants, respectively. The loss of plant species involved primarily shrubs and herbaceous species, whereas the number of tree species increased (fig. S3).
These concomitant changes in diversity and ecosystem properties resulted in a strong trade-off between ecosystem carbon stocks and species richness across the savanna-forest gradients (Fig. 3). Over the ranges of tree densities studied, each 1% increase in ecosystem carbon resulted in a 0.27% decrease in total plant species richness and a 0.43% decrease in ant species richness (Fig. 3), as determined from the sensitivity coefficient. When only savanna species were considered, each 1% increase in ecosystem carbon corresponds to decreases of 0.98 and 1.38% for plants and ants, respectively (Fig. 3). Together, forest and generalist plant species increased by 0.76% for each 1% increase in carbon, but there was no detectable change for ants.
Fig. 3. Carbon-biodiversity trade-offs across savanna-forest transitions.
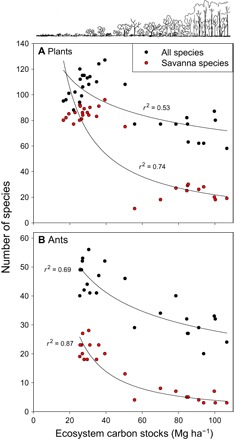
Carbon-biodiversity trade-offs across savanna-forest transitions. Relationships between ecosystem carbon stocks and species richness of (A) plants and (B) ants. All relationships are statistically significant (P < 0.0001). Fitted relationships: all plants, y = 245.88x−0.271; savanna plants, y = 1953.4x−0.975; all ants, y = 198.68x−0.426; savanna ants, y = 2258.8x−1.380.
Stem basal area in 2015 was significantly correlated with properties of surface soils (0 to 20 cm). Sites with higher basal area had greater silt, clay, and organic matter; higher cation exchange capacity (CEC); higher availabilities of P, K, B, and S (fig. S4); and higher concentrations of P, K, B, and S (fig. S4). Higher basal area was associated with lower pH, sand, and Cu (fig. S4).
DISCUSSION
Previous studies in the Cerrado have suggested that forest encroachment into open savanna results in a net increase in plant species richness (6, 22–24), but these studies have been limited to trees and therefore overlook the high diversity of shrubs and herbaceous plants in savannas. Here, diversity of tree species increased with forest encroachment, whereas diversity of shrubs and herbaceous plants declined markedly (fig. S3). The decline in these groups is particularly noteworthy because shrubs and herbaceous plants comprise 75 to 80% of plant species in the Cerrado region (25) and 58% of species at the study sites (table S1). Of these nontree species, 79% are savanna specialists and are therefore particularly sensitive to forest encroachment. Herbaceous communities are vastly understudied in the Cerrado, compared to trees, but deserve greater attention considering their high diversity and vulnerability to fire suppression.
In sites encroached by forest, a few savanna plant species still persist at low abundances, and these are nearly certain to continue declining if fire is not reintroduced into the system. Some species may depend directly on fire for reproduction (26, 27), but more generally, plant species adapted to savanna environments cannot tolerate the low light availability under a forest canopy (5, 28, 29). These species mostly persist in forest as occasional moribund individuals with few leaves. A few robust savanna trees remain in the forests, but these are rare individuals that are sufficiently tall to access ample light. Even these species are unlikely to persist beyond the life span of these individuals because their seedlings are nearly absent from sites encroached by forest. In short, we expect continued loss of savanna specialist species in forest-encroached sites, and the results presented here should be considered an underestimate of the eventual impact of fire suppression.
Similar responses to forest encroachment are expected for animal communities, represented here by ants because they are a diverse group that lend themselves to systematic sampling. As for plants, ant communities are strongly dependent on vegetation structure (30, 31); consequently, forest encroachment results in a notable loss of ant species adapted to open vegetation (Fig. 2C). These savanna-dependent species have evolved within most or all major groups of animals of the Cerrado region, including reptiles (32), amphibians (33), birds (19), and mammals (34). Among these are species of particular conservation concern, such as the maned wolf (35), Pampas deer (36), and the greater rhea (37). The interrelationships between vegetation, fauna, and fire are complex (38), but generally, animal species specialized to savanna and grassland should be considered vulnerable to fire suppression and forest encroachment.
Forest encroachment at this site was made possible by long-term fire suppression, although increasing atmospheric CO2 may have contributed by accelerating tree establishment and growth (9–12). However, encroachment was not uniform across the study area, with substantial variation in both initial density and subsequent rate of increase (Fig. 1). Previous fire history likely contributed to the initial differences, because fires were common before the 1980s and would not have burned uniformly across the area. However, during the 30-year study period, only four of the most open plots had burned, and only one was burned more than once in these three decades. Fire is not likely to be the only factor responsible for this variation because encroachment was greatest in soils that have greater water retention capacity (high silt and clay and low sand; fig. S4) and higher concentrations of P, K, B, and S (fig. S4). These soil variables were measured in the top 20 cm of the soil profile, where vegetation can have large influences on soil chemistry (39); thus, causality is difficult to ascertain. Nevertheless, the gradient in sand content reveals preexisting soil differences that likely influenced rates of forest encroachment under fire suppression. Regardless of the role that soils and nutrients may play in controlling the rate of forest expansion (40–43), tree encroachment has continued steadily in all savanna sites (Fig. 1).
If the current trajectory is not interrupted, we expect most or all high-quality savanna to be lost from the study area within a decade or two, with obvious negative consequences for biodiversity. Reintroduction of fire would reverse the process of tree encroachment, but to avoid catastrophic loss of biodiversity, it is important to restore a natural fire regime well before the canopy becomes dense enough to substantially impact the herbaceous layer. Once the diversity of the herbaceous community has been substantially depleted, it is unlikely to recover readily, even after the vegetation is opened. Restoring “old-growth” grasslands such as these is notoriously difficult (44), particularly in the Cerrado, where receding forest is typically replaced by aggressive exotic grasses (21, 45) that preclude the recovery of native herbaceous species (45).
Implications for policy and management
These findings challenge the widespread assumption that promoting forest cover has universal co-benefits for carbon sequestration and biodiversity (1, 2). Forest encroachment resulted in increased ecosystem carbon at the expense of reduced species diversity, an outcome that is likely repeated wherever forest encroaches on the Cerrado and other species-rich savannas and grasslands in the world. Under these conditions, a carbon-centered conservation strategy may be inappropriate (46, 47), except in excessively burned savannas, where modest reductions in fire frequency or intensity benefit both biodiversity and carbon storage (48).
The results presented here are particularly alarming in light of the current policy of fire suppression throughout the Cerrado region (13). Policies of fire suppression in Brazil are exacerbated by ongoing land-use change and vegetation fragmentation, which restrict fire spread and thereby reduce fire frequency (16). In large, unbroken expanses of the Cerrado, lightning fires can sustain frequent burning (49), but this becomes increasingly improbable as vegetation is fragmented (16). Where lightning fires are not replaced with sufficient human-initiated fires, biodiversity losses on protected lands, as observed here, will be increasingly common throughout the Cerrado. Even greater losses should be expected in the core of the Cerrado region, which supports greater diversity of savanna species than the study area (50), which lies near the southeastern limit of the Cerrado. To date, the consequences of excluding fire are much more evident in the study area (51) because of its longer history of habitat fragmentation and protection from fire. This history offers important lessons that are relevant throughout the Cerrado and other tropical savanna regions.
Environmental legislation in Brazil advanced a step in the right direction by legalizing prescribed fires for conservation of protected areas in the Cerrado (Law number 12651, Article 38, in effect since May 2012), but to date, controlled fires have been used only in a few reserves whose management plans specifically permit prescribed burning. However, burning cerrado vegetation outside formal reserves remains illegal (13). This situation could change if the expected National Fire Policy mandated by law were implemented (Law number 12651, Article 40), regulating the use of fire in natural vegetation throughout the Cerrado ecoregion. Nevertheless, the overall perception that fire is destructive can be an obstacle even more difficult to surpass because managers of protected areas hesitate to prescribe fire in fear of formal punishment or social condemnation (13).
Negative attitude toward fire is difficult to overcome; however, our results offer important direct evidence of biodiversity loss due to woody encroachment when fire is eliminated in the Cerrado. The expansion of forests and trees in savanna ecosystems may be a global phenomenon, attributed to a complex of different factors in each region (8, 52), but there is no question that fire is an effective tool to prevent it. Our results indicate that, on any Cerrado land managed for conserving savanna landscapes and biota, burning should occur with sufficient frequency and intensity to prevent tree densities from approaching the point at which light-demanding species fail to thrive (that is, basal area of approximately 15 m2 ha−1). This should be considered the absolute maximum acceptable tree density for avoiding catastrophic loss of savanna species, and even this density may exceed the point at which prescribed burning can safely and effectively reverse tree encroachment. Ideally, consideration should be given to maintaining the mosaic of grassland (campo limpo), shrub savanna (campo sujo), and tree savanna (cerrado sensu stricto) that may have historically occurred at a site. Ultimately, fire management in the Cerrado should evolve to consider the ecological role of fire, such as stimulating flowering in herbaceous plants and influencing exotic species, but this requires observations that can accrue only if land managers and scientists are allowed to use prescribed fire as a management tool. Until this information is available for the Cerrado, managers can use knowledge from long-term fire experiments in Africa and Australia (53, 54), as well as traditional knowledge on fire management in the Cerrado (55). Meanwhile, it is urgent to reformulate the current timid policies that favor fire suppression over all other options.
MATERIALS AND METHODS
Experimental design
Study area
The Santa Barbara Ecological Station (SBES) is located in the municipality of Águas de Santa Bárbara, São Paulo State, Brazil, with an area of 2715 ha (56). It lies within 22°46′33″ and 22°50′33″ S and 49°10′27″ and 49°15′36″ W, near the southeastern edge of the Cerrado region, at altitudes between 600 and 680 m above sea level. The climate in the area is Köppen Cwa-type (56), with monthly mean temperatures varying between 16° and 24°C. The annual mean rainfall is between 1100 and 1300 mm, with dry winters and rainy summers. The soils of the study area are deep oxisols with high sand content, low nutrient content, and high saturation of aluminum (57).
Data collection
The study was undertaken at three savanna-forest transitions located at distances of 2 to 5 km from each other. A network of 30 plots was distributed across these transitions to include 12 in recently formed forest, 12 in tree-dominated savanna, and 6 in shrub-dominated savanna. These vegetation classes correspond to cerradão, cerrado sensu stricto, and campo sujo of the Brazilian classification system (25). Each plot was 20 m × 50 m (0.1 ha), within which the stem diameter of all trees greater than 5 cm were measured. All trees and shrubs with stem diameters between 1 and 5 cm were measured within 10 5-m × 5-m subplots distributed regularly throughout each plot. Tree seedlings and the herbaceous community were sampled within 40 1-m × 1-m subplots distributed in a grid within each 0.1-ha plot. All individuals sampled within these plots were identified to species in the field or collected for subsequent identification.
Plant species were categorized into eight growth forms: (i) grass; (ii) sedge; (iii) forb, that is, nongraminoid plant without a persistent woody stem; (iv) subshrub, that is, woody plant typically less than 75 cm tall as adults; (v) shrub; (vi) palm; (vii) treelet; and (viii) tree. On the basis of literature (58, 59), species were also classified according to habitat preference into three groups: (i) savanna specialists, (ii) forest specialists, and (iii) generalists, that is, species that are commonly found in both savanna and forest habitats (table S1). Some species of the latter group may be true generalists that persist indefinitely in either environment, but most are best considered as transitional species typically found in forest but readily colonize savanna under fire suppression.
The aboveground-foraging ant community was characterized for each of the 0.1-ha plots placed in forest or tree-dominated savanna; the six plots in shrub-dominated savanna were omitted. Five 2.5-m × 2.5-m grids were established along the borders of each plot, keeping a minimum distance of 20 m between any two grids. Four pitfall traps were set in each grid, totaling 20 traps per plot. Pitfall traps consisted of a small plastic cup (250 ml, 8.5 cm high, and 7.8 cm in diameter) partially filled with water and detergent. The traps remained in operation for 48 hours. All ant specimens collected were sorted to morphospecies and, whenever possible, identified to species using available taxonomic keys or through comparison with specimens previously identified by experts and deposited at the Zoological Collection of the Federal University of Uberlândia, Brazil.
Each ant species or morphospecies was classified as savanna specialist, forest specialist, or habitat generalist (table S2). Because the habitat preference of some ant species could not be determined from existing literature sources, these species were classified on the basis of abundance at the site. A species was considered a specialist if it occurred exclusively, or nearly so, in either savanna or forest and was considered a generalist if it occurred at similar frequencies in both habitats. To eliminate the possibility that this post hoc classification might introduce spurious relationships when testing for vegetation impact on species richness within the functional types, we also applied an alternate approach to classifying species using subsets of the data. For this, abundance data from two of the savanna-forest boundaries were used to classify species, and then this classification scheme was used to determine the number of savanna, forest, and generalist species at the third boundary. By using successive subsets of the data in this way, the test for vegetation response was independent of the data used to classify the species. Fewer species could be classified as habitat specialists in this manner, but in relative terms, results were stronger than when analyzed in the previous manner. Specifically, forest encroachment was found to result in an 87% decline in the mean number of savanna ant species per plot (3.0 in forest versus 22.3 in savanna) but a 3.5-fold increase in the number of forest species (12.3 versus 3.7). Consequently, we used the classification scheme based on the full data set for all subsequent analyses because this allowed a greater number of species to be classified.
In each of the 30 vegetation plots, the LAI of the overstory was measured with hemispherical canopy photographs taken at each of the 40 subplots used to characterize the ground layer community. To ensure conditions of diffuse light, all photos were taken before sunrise, after sunset, or under homogeneous overcast skies. A tripod was used to position the camera (Canon EF 8-15mm fisheye lens) at a height of 1 m, and the top of the camera was oriented relative to the north. Photos were taken with an underexposure of one f stop (60), and the color images were converted to black and white using Hemisfer 2.12 (61, 62) and using maximum blue contrast (63). The images were then analyzed with Hemisfer 2.12 using an automatic threshold for closed-canopy vegetation and with a supervised manual threshold under open canopies. The LAI values were averaged over the 40 subplots to obtain a single value for each 0.1-ha plot.
Ecosystem carbon and soil properties
Samples of surface soil (0- to 20-cm depth) were collected for each of the 30 plots. A composite soil sample was made with five subsamples from surface soil collected immediately adjacent to the plot at intervals of 10 m along its longest axis. The soil samples were submitted to a commercial laboratory to quantify coarse sand, fine sand, silt, and clay fractions; extractable Ca, Mg, K, B, Cu, Mn, Fe, and Zn; available P; exchangeable Al; pH and organic matter; and the derived variables: potential acidity (H + Al), CEC, exchangeable acidity, and base saturation and aluminum saturation. A subset of 16 plots was sampled to determine carbon stocks in litter, duff, and herbaceous plant pools. These were sampled in four or five 0.25-m2 subplots adjacent to each plot. In each subplot, the ground layer biomass was collected and divided into grass, sedge, and dicot fractions. Litter was divided into leaf and wood fractions. When a discernable organic horizon (duff) was present, this was also collected in a 0.0625-m2 portion of the subplot. All these components were weighed after drying for at least 5 days at 60°C. Each dried duff sample was thoroughly mixed, and a subsample was weighed before ashing in a muffle furnace to determine the fraction of organic matter. The carbon content of the lost mass was calculated using a conversion factor of 0.58 kg C kg−1 organic matter. The combined carbon content of litter, duff, and herbaceous plants was summed after scaling each to similar units (Mg ha−1). Because this combined pool accounted for a small fraction of the ecosystem carbon pools (mean, 13%; range, 9 to 21%) and was strongly correlated to tree basal area (r2 = 0.70), we used regression to estimate the size of this pool for the 14 plots for which this was not measured directly.
Total tree biomass was estimated for each plot using allometric equations developed for trees in a range of Cerrado physiognomies and including both root and shoot biomass (64) as follows
| (1) |
| (2) |
where M is the predicted dry biomass (in kilograms), d is the stem diameter (in centimeters), and h is the height (in meters). Mass of carbon was calculated using a conversion factor of 0.5 kg C kg−1 biomass (65, 66).
Remote sensing
To quantify vegetation change over 30 years of fire suppression, we used vegetation indices derived from Landsat satellite images and obtained from the USGS (United States Geological Survey) portal (https://espa.cr.usgs.gov/). We first evaluated the performance of four vegetation indices, normalized difference vegetation index (NDVI), enhanced vegetation index (EVI), soil-adjusted vegetation index (SAVI), and modified soil-adjusted vegetation index (MSAVI) calculated from surface reflectances derived from Landsat 8. Of these four indices, the EVI was most strongly correlated with tree basal area (r2 = 0.75) and LAI (r2 = 0.89; fig. S2); thus, all further analyses were undertaken with this index. To determine long-term vegetation trends, we used all available images from 1986 to 2016 that were acquired by Landsat 5–8 under cloudless conditions and during the wet season months of November through May. For each image, EVI was spatially averaged for each study plot. This resulted in multiple values for each calendar year, which were then composited by determining the yearly maximum.
Statistical analysis
Analyses were performed with the base stats package in R (67). The lm function was used to fit linear and quadratic models, and the package nlstools (68) was used for fitting nonlinear models.
To characterize the relative change in species richness (r) for a relative change in ecosystem carbon (C), we calculated the coefficient of sensitivity, , also known as elasticity in economics and demography (69). This was calculated as the slope of the relationship between ln(r) and ln(C).
We estimated past changes in carbon stocks and species number by using a space-for-time substitution. We first used nonlinear regression to obtain predictive relationships for estimating ecosystem carbon stocks and species richness as a function of current EVI (fig. S2). These regression equations were then used to estimate carbon and richness based on EVI values for 1986 and 2015. To avoid potential artifacts resulting from year-to-year variation in the composite EVI values, we used linear trend lines fitted to each plot (Fig. 1) to interpolate the EVI values for these 2 years.
Acknowledgments
We thank M. S. Suganuma, M. G. B. Cava, G. B. de Assis, É. R. Silveira, F. M. de Souza, J. Maravalhas, and E. Koch for assistance with data collection and the employees of the Instituto Florestal for logistical assistance. This work was performed under COTEC Technical-Scientific Commission of the Forestry Institute research license 26108-008.476/2014. Funding: This material is based on work supported by the NSF under grant number DEB1354943 to W.A.H. H.L.V. was funded by CNPq (National Council for Scientific and Technological Development) grant #457407/2012-3, N.A.P. was funded by FAPESP (São Paulo Research Foundation) grant #2016/17888-2, D.R.R. was funded by CNPq grant #301589/2015-1, and G.D. was funded by CNPq grant #303179/2016-3. Author contributions: W.A.H. and G.D. designed the study. R.C.R.A., W.A.H., N.A.P., and G.D. contributed to the vegetation fieldwork, analysis, and manuscript writing. H.L.V. performed the work related to ant biodiversity and analysis and contributed to writing. W.A.H. performed the remote sensing analysis. D.R.R. contributed to manuscript writing and project logistics. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/8/e1701284/DC1
fig. S1. Relationships between the EVI and vegetation structure.
fig. S2. Relationships used to hindcast historical vegetation change.
fig. S3. Response of plant growth forms to vegetation structure.
fig. S4. Relationships between tree basal area and characteristics of surface soils (0 to 20 cm) in 2015.
table S1. Plant species recorded at SBES, Brazil, in 2015.
table S2. Ant species sampled at SBES, Brazil, in 2015.
table S3. Details of the 30 study plots in this study.
REFERENCES AND NOTES
- 1.Phelps J., Webb E. L., Adams W. M., Biodiversity co-benefits of policies to reduce forest-carbon emissions. Nat. Clim. Change 2, 497–503 (2012). [Google Scholar]
- 2.Gilroy J. J., Woodcock P., Edwards F. A., Wheeler C., Baptiste B. L. G., Uribe C. A. M., Haugaasen T., Edwards D. P., Cheap carbon and biodiversity co-benefits from forest regeneration in a hotspot of endemism. Nat. Clim. Change 4, 503–507 (2014). [Google Scholar]
- 3.Veldman J. W., Overbeck G. E., Negreiros D., Mahy G., Le Stradic S., Fernandes G. W., Durigan G., Buisson E., Putz F. E., Bond W. J., Where tree planting and forest expansion are bad for biodiversity and ecosystem services. Bioscience 65, 1011–1018 (2015). [Google Scholar]
- 4.Staver A. C., Archibald S., Levin S. A., The global extent and determinants of savanna and forest as alternative biome states. Science 334, 230–232 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Parr C. L., Lehmann C. E. R., Bond W. J., Hoffmann W. A., Andersen A. N., Tropical grassy biomes: Misunderstood, neglected, and under threat. Trends Ecol. Evol. 29, 205–213 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Pellegrini A. F. A., Socolar J. B., Elsen P. R., Giam X., Trade-offs between savanna woody plant diversity and carbon storage in the Brazilian Cerrado. Glob. Chang. Biol. 22, 3373–3382 (2016). [DOI] [PubMed] [Google Scholar]
- 7.de Abreu R. C. R., Durigan G., Changes in the plant community of a Brazilian grassland savannah after 22 years of invasion by Pinus elliottii Engelm. Plant Ecol. Divers. 4, 269–278 (2011). [Google Scholar]
- 8.Stevens N., Lehmann C. E. R., Murphy B. P., Durigan G., Savanna woody encroachment is widespread across three continents. Glob. Chang. Biol. 23, 235–244 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Oliveras I., Malhi Y., Many shades of green: The dynamic tropical forest–savannah transition zones. Philos. Trans. R. Soc. B Biol. Sci. 371, 20150308 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moncrieff G. R., Scheiter S., Bond W. J., Higgins S. I., Increasing atmospheric CO2 overrides the historical legacy of multiple stable biome states in Africa. New Phytol. 201, 908–915 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Buitenwerf R., Bond W. J., Stevens N., Trollope W. S. W., Increased tree densities in South African savannas: >50 years of data suggests CO2 as a driver. Glob. Chang. Biol. 18, 675–684 (2012). [Google Scholar]
- 12.Franco A. C., Rossatto D. R., de Carvalho Ramos Silva L., da Silva Ferreira C., Cerrado vegetation and global change: The role of functional types, resource availability and disturbance in regulating plant community responses to rising CO2 levels and climate warming. Theor. Exp. Plant Physiol. 26, 19–38 (2014). [Google Scholar]
- 13.Durigan G., Ratter J. A., The need for a consistent fire policy for Cerrado conservation. J. Appl. Ecol. 53, 11–15 (2016). [Google Scholar]
- 14.Strassburg B. B. N., Brooks T., Feltran-Barbieri R., Iribarrem A., Crouzeilles R., Loyola R., Latawiec A. E., Oliveira Filho F. J. B., Scaramuzza C. A. M., Scarano F. R., Soares Filho B., Balmford A., Moment of truth for the Cerrado hotspot. Nat. Ecol. Evol. 1, 1–3 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Beuchle R., Grecchi R. C., Shimabukuro Y. E., Seliger R., Eva H. D., Sano E., Achard F., Land cover changes in the Brazilian Cerrado and Caatinga biomes from 1990 to 2010 based on a systematic remote sensing sampling approach. Appl. Geogr. 58, 116–127 (2015). [Google Scholar]
- 16.Archibald S., Staver A. C., Levin S. A., Evolution of human-driven fire regimes in Africa. Proc. Natl. Acad. Sci. U.S.A. 109, 847–852 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers N., Mittermeier R. A., Mittermeier C. G., da Fonseca G. A. B., Kent J., Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Murphy B. P., Andersen A. N., Parr C. L., The underestimated biodiversity of tropical grassy biomes. Philos. Trans. R. Soc. B Biol. Sci. 371, 20150319 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardoso da Silva J. M., Bates J. M., Biogeographic patterns and conservation in the South American Cerrado: A tropical savanna hotspot. Bioscience 52, 225–234 (2002). [Google Scholar]
- 20.Hoffmann W. A., Geiger E. L., Gotsch S. G., Rossatto D. R., Silva L. C. R., Lau O. L., Haridasan M., Franco A. C., Ecological thresholds at the savanna-forest boundary: How plant traits, resources and fire govern the distribution of tropical biomes. Ecol. Lett. 15, 759–768 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Silvério D. V., Brando P. M., Balch J. K., Putz F. E., Nepstad D. C., Oliveira-Santos C., Bustamante M. M. C., Testing the Amazon savannization hypothesis: Fire effects on invasion of a neotropical forest by native cerrado and exotic pasture grasses. Philos. Trans. R. Soc. B Biol. Sci. 368, 20120427 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roitman I., Felfili J. M., Rezende A. V., Tree dynamics of a fire-protected cerrado sensu stricto surrounded by forest plantations, over a 13-year period (1991–2004) in Bahia, Brazil. Plant Ecol. 197, 255–267 (2008). [Google Scholar]
- 23.Marimon B. S., Marimon-Junior B. H., Feldpausch T. R., Oliveira-Santos C., Mews H. A., Lopez-Gonzalez G., Lloyd J., Franczak D. D., de Oliveira E. A., Maracahipes L., Miguel A., Lenza E., Phillips O. L., Disequilibrium and hyperdynamic tree turnover at the forest–cerrado transition zone in southern Amazonia. Plant Ecol. Divers. 7, 281–292 (2014). [Google Scholar]
- 24.Morandi P. S., Marimon-Junior B. H., de Oliveira E. A., Reis S. M., Valadão M. B. X., Forsthofer M., Passos F. B., Marimon B. S., Vegetation sucession in the Cerrado-Amazonian forest transition zone of Mato Grasso State, Brazil. Edinburgh J. Bot. 73, 83–93 (2016). [Google Scholar]
- 25.T. S. Filgueiras, Herbaceous plant communities, in The Cerrados of Brazil, P. Oliveira, R. J. Marquis, Eds. (Columbia Univ. Press, 2002), pp. 121–139. [Google Scholar]
- 26.L. M. Coutinho, Fire in the ecology of the Brazilian Cerrado, in Fire in the Tropical Biota, J. Goldammer Ed. (Springer, 1990), pp. 82–105. [Google Scholar]
- 27.Fidelis A., Blanco C., Does fire induce flowering in Brazilian subtropical grasslands? Appl. Veg. Sci. 17, 690–699 (2014). [Google Scholar]
- 28.de Abreu R. C. R., de Assis G. B., Frison S., Aguirre A., Durigan G., Can native vegetation recover after slash pine cultivation in the Brazilian Savanna? For. Ecol. Manage. 262, 1452–1459 (2011). [Google Scholar]
- 29.Lemos-Filho J. P., Barros C. F. A., Dantas G. P. M., Dias L. G., Mendes R. S., Spatial and temporal variability of canopy cover and understory light in a Cerrado of Southern Brazil. Braz. J. Biol. 70, 19–24 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Pacheco R., Vasconcelos H. L., Habitat diversity enhances ant diversity in a naturally heterogeneous Brazilian landscape. Biodivers. Conserv. 21, 797–809 (2012). [Google Scholar]
- 31.Parr C. L., Gray E. F., Bond W. J., Cascading biodiversity and functional consequences of a global change-induced biome switch. Divers. Distrib. 18, 493–503 (2012). [Google Scholar]
- 32.Nogueira C., Colli G. R., Martins M., Local richness and distribution of the lizard fauna in natural habitat mosaics of the Brazilian Cerrado. Austral Ecol. 34, 83–96 (2009). [Google Scholar]
- 33.Brasileiro C. A., Sawaya R. J., Kiefer M. C., Martins M., Amphibians of an open cerrado fragment in southeastern Brazil. Biota Neotrop. 5, 1–17 (2005). [Google Scholar]
- 34.Mares M. A., Ernest K. A., Gettinger D. D., Small mammal community structure and composition in the Cerrado Province of Central Brazil. J. Trop. Ecol. 2, 289–300 (1986). [Google Scholar]
- 35.de A. Jácomo A. T., Silveira L., Diniz-Filho J. A. F., Niche separation between the maned wolf (Chrysocyon brachyurus), the crab-eating fox (Dusicyon thous) and the hoary fox (Dusicyon vetulus) in central Brazil. J. Zool. 262, 99–106 (2004). [Google Scholar]
- 36.Duarte J. M. B., Vogliotti A., dos Santos Zanetti E., de Oliveira M. L., Tiepolo L. M., Rodrigues L. F., de Almeida L. B., Braga F. G., Avaliação do Risco de Extinção do Veado-campeiro Ozotoceros bezoarticus Linnaeus, 1758, no Brasil. Biodiversidade Bras. 2, 20–32 (2012). [Google Scholar]
- 37.de Azevedo C. S., Ferraz J. B., Tinoco H. P., Young R. J., Rodrigues M., Time–activity budget of greater rheas (Rhea americana, Aves) on a human-disturbed area: The role of habitat, time of the day, season and group size. Acta Ethol. 13, 109–117 (2010). [Google Scholar]
- 38.Bowman D. M. J. S., Perry G. L. W., Higgins S. I., Johnson C. N., Fuhlendorf S. D., Murphy B. P., Pyrodiversity is the coupling of biodiversity and fire regimes in food webs. Philos. Trans. R. Soc. London B Biol. Sci. 371, 20150169 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jobbágy E. G., Jackson R. B., The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 10, 423–436 (2000). [Google Scholar]
- 40.Bond W. J., Do nutrient-poor soils inhibit development of forests? A nutrient stock analysis. Plant Soil. 334, 47–60 (2010). [Google Scholar]
- 41.Silva L. C. R., Hoffmann W. A., Rossatto D. R., Haridasan M., Franco A. C., Horwath W. R., Can savannas become forests? A coupled analysis of nutrient stocks and fire thresholds in central Brazil. Plant Soil. 373, 829–842 (2013). [Google Scholar]
- 42.Lloyd J., Lloyd J., Domingues T. F., Schrodt F., Ishida F. Y., Feldpausch T. R., Saiz G., Quesada C. A., Schwarz M., Torello-Raventos T., Gilpin M., Marimon B., Marinon B. H. Jr., Ratter J. A., Grace J., Naradoto G. B., Veenendaal E. M., Arroyo L., Villarroel D., Killeen T., Steininger M., Phillips O. L., Edaphic, structural and physiological contrasts across Amazon Basin forest–savanna ecotones suggest a role for potassium as a key modulator of tropical woody vegetation structure and function. Biogeosciences 12, 6529–6571 (2015). [Google Scholar]
- 43.Pellegrini A. F. A., Nutrient limitation in tropical savannas across multiple scales and mechanisms. Ecology 97, 313–324 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Bond W. J., Ancient grasslands at risk. Science 351, 120–122 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Veldman J. W., Putz F. E., Grass-dominated vegetation, not species-diverse natural savanna, replaces degraded tropical forests on the southern edge of the Amazon Basin. Biol. Conserv. 144, 1419–1429 (2011). [Google Scholar]
- 46.Gardner T. A., Burgess N. D., Aguilar-Amuchastegui N., Barlow J., Berenguer E., Clements T., Danielsen F., Ferreira J., Foden W., Kapos V., Khan S. M., Lees A. C., Parry L., Roman-Cuesta R. M., Schmitt C. B., Strange N., Theilade I., Vieira I. C. G., A framework for integrating biodiversity concerns into national REDD+ programmes. Biol. Conserv. 154, 61–71 (2012). [Google Scholar]
- 47.Phelps J., Friess D. A., Webb E. L., Win–win REDD+ approaches belie carbon–biodiversity trade-offs. Biol. Conserv. 154, 53–60 (2012). [Google Scholar]
- 48.Russell-Smith J., Yates C. P., Edwards A. C., Whitehead P. J., Murphy B. P., Lawes M. J., Deriving multiple benefits from carbon market-based savanna fire management: An Australian example. PLOS ONE 10, e0143426 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramos-Neto M. B., Pivello V. R., Lightning fires in a Brazilian savanna national park: Rethinking management strategies. Environ. Manage. 26, 675–684 (2000). [DOI] [PubMed] [Google Scholar]
- 50.J. A. Ratter, S. Bridgewater, J. F. Ribeiro, Biodiversity patterns of the woody vegetation of the Brazilian Cerrado, in Neotropical Savannas and Seasonally Dry Forests: Plant Diversity, T. Pennington, G. P. Lewis, J. A. Ratter, Eds. (CRC Press, 2006), pp. 31–66. [Google Scholar]
- 51.Durigan G., Ratter J. A., Successional changes in cerrado and cerrado/forest ecotonal vegetation in western São Paulo State, Brazil, 1962–2000. Edinburgh J. Bot. 63, 119–130 (2006). [Google Scholar]
- 52.Lehmann C. E. R., Anderson T. M., Sankaran M., Higgins S. I., Archibald S., Hoffmann W. A., Hanan N. P., Williams R. J., Fensham R. J., Felfili J., Hutley L. B., Ratnam J., San Jose J., Montes R., Franklin D., Russell-Smith J., Ryan C. M., Durigan G., Hiernaux P., Haidar R., Bowman D. M. J. S., Bond W. J., Savanna vegetation-fire-climate relationships differ among continents. Science 343, 548–552 (2014). [DOI] [PubMed] [Google Scholar]
- 53.van Wilgen N., Govender B. W., Biggs H. C., The contribution of fire research to fire management: A critical review of a long-term experiment in the Kruger National Park, South Africa. Int. J. Wildl. Fire 16, 519–530 (2007). [Google Scholar]
- 54.A. Andersen, G. D. Cook, R. J. Williams, Fire in Tropical Savannas: The Kapalga Experiment (Spriner-Verlag, 2003). [Google Scholar]
- 55.Pivello V. R., The use of fire in the Cerrado and Amazonian Rainforests of Brazil: Past and present. Fire Ecol. 7, 24–39 (2011). [Google Scholar]
- 56.Meira Neto J. A., Martins F. R., Valente G. E., Composição florística e espectro biológico na Estação Ecológica de Santa Bárbara, Estado de São Paulo, Brasil. Rev. Árvore 31, 907–922 (2007). [Google Scholar]
- 57.A. C. G. Melo, G. Durigan, Plano de Manejo da Estação Ecológica de Santa Bárbara (SEMA, 2010).
- 58.G. Durigan, M. F. Siqueira, G. A. D. C. Franco, W. A. Contieri, A flora arbustivo‑arbórea do Médio Paranapanema: Base para a restauração dos ecossistemas naturais, in Pesquisas em Conservação e Recuperação Ambiental no Oeste Paulista: Resultados da Cooperação Brasil/Japão, O. Vilas Bôas, G. Durigan, Eds. (Páginas & Letras, ed. 1, 2004), pp. 199–239. [Google Scholar]
- 59.R. C. Mendonça, J. M. Felfili, B. M. T. Walter, M. C. Silva Júnior, A. V. Rezende, T. S. Filgueiras, P. E. Nogueira, C. W. Fagg, in Flora Vascular do Bioma Cerrado Checklist com 12.356 Espécies, S. M. Sano, S. P. Almeida, J. F. Ribeiro, Eds. (Embrapa Cerrados, 2008), pp. 423–1279. [Google Scholar]
- 60.Macfarlane C., Ryu Y., Ogden G. N., Sonnentag O., Digital canopy photography: Exposed and in the raw. Agric. For. Meteorol. 197, 244–253 (2014). [Google Scholar]
- 61.Thimonier A., Sedivy I., Schleppi P., Estimating leaf area index in different types of mature forest stands in Switzerland: A comparison of methods. Eur. J. For. Res. 129, 543–562 (2010). [Google Scholar]
- 62.Schleppi P., Conedera M., Sedivy I., Thimonier A., Correcting non-linearity and slope effects in the estimation of the leaf area index of forests from hemispherical photographs. Agric. For. Meteorol. 144, 236–242 (2007). [Google Scholar]
- 63.Nobis M., Hunziker U., Automatic thresholding for hemispherical canopy-photographs based on edge detection. Agric. For. Meteorol. 128, 243–250 (2005). [Google Scholar]
- 64.E. D. S. Pinheiro, Análises ecológicas e sensoriamento remoto aplicados à estimativa de fitomassa de cerrado na Estação Ecológica de Assis, SP. Ph.D. thesis, Universidade de São Paulo (2008). [Google Scholar]
- 65.Clark D. A., Brown S., Kicklighter D. W., Chambers J. Q., Thomlinson J. R., Ni J., Measuring net primary production in forests: Concepts and field methods. Ecol. Appl. 11, 356–370 (2001). [Google Scholar]
- 66.Malhi Y., Baker T. R., Phillips O. L., Almeida S., Alvarez E., Arroyo L., Chave J., Czimczik C. I., Di Fiore A., Higuchi N., Killeen T. J., Laurance S. G., Laurance W. F., Lewis S. L., Montoya L. M. M., Monteagudo A., Neill D. A., Vargas P. N., Patino S., Pitman N. C. A., Quesada C. A., Salomao R., Silva J. N. M., Lezama A. T., Martinez R. V., Terborgh J., Vinceti B., Lloyd J., The above-ground coarse wood productivity of 104 Neotropical forest plots. Glob. Chang. Biol. 10, 563–591 (2004). [Google Scholar]
- 67.R Core Team, R: A Language and Environment for Statistical Computing (2016).
- 68.Baty F., Ritz C., Charles S., Brutsche M., Flandrois J.-P., Delignette-Muller M.-L., A toolbox for nonlinear regression in R : The package nlstools. J. Stat. Softw. 66, 1773 (2015). [Google Scholar]
- 69.H. Caswell, Matrix Population Models (Sinauer Associates, 2001), 722 pp. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/8/e1701284/DC1
fig. S1. Relationships between the EVI and vegetation structure.
fig. S2. Relationships used to hindcast historical vegetation change.
fig. S3. Response of plant growth forms to vegetation structure.
fig. S4. Relationships between tree basal area and characteristics of surface soils (0 to 20 cm) in 2015.
table S1. Plant species recorded at SBES, Brazil, in 2015.
table S2. Ant species sampled at SBES, Brazil, in 2015.
table S3. Details of the 30 study plots in this study.


