Abstract
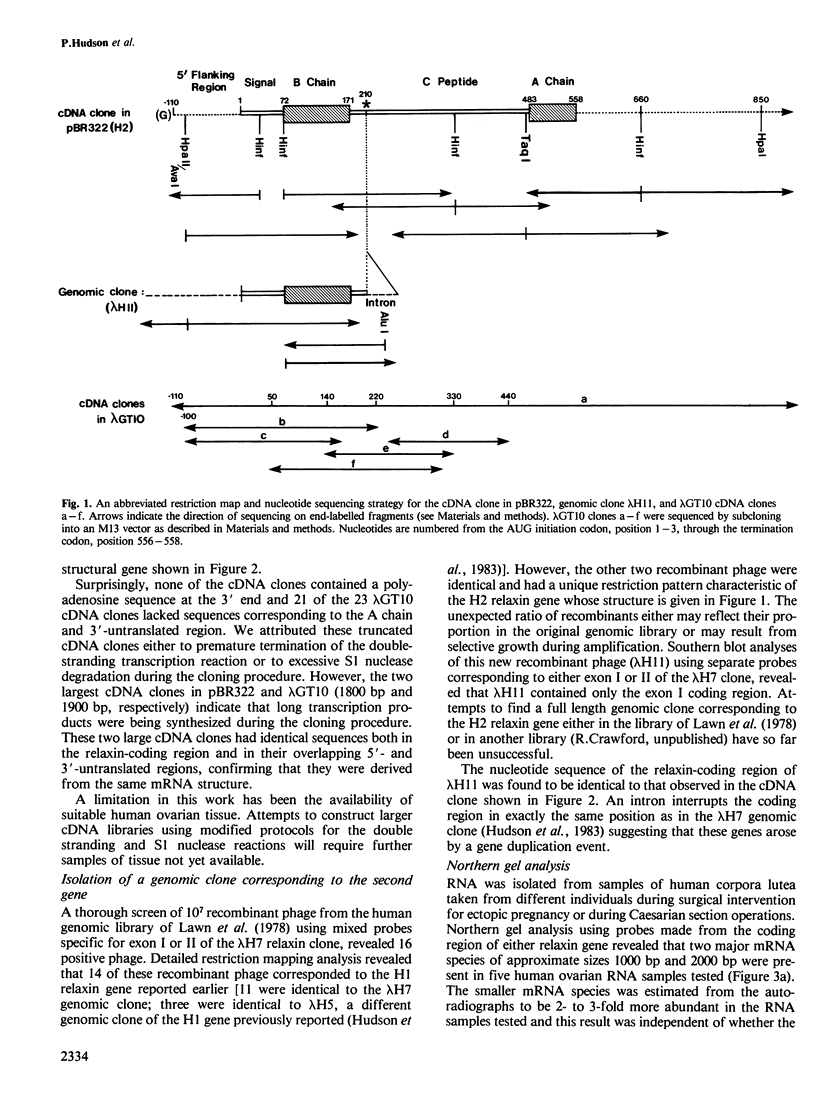
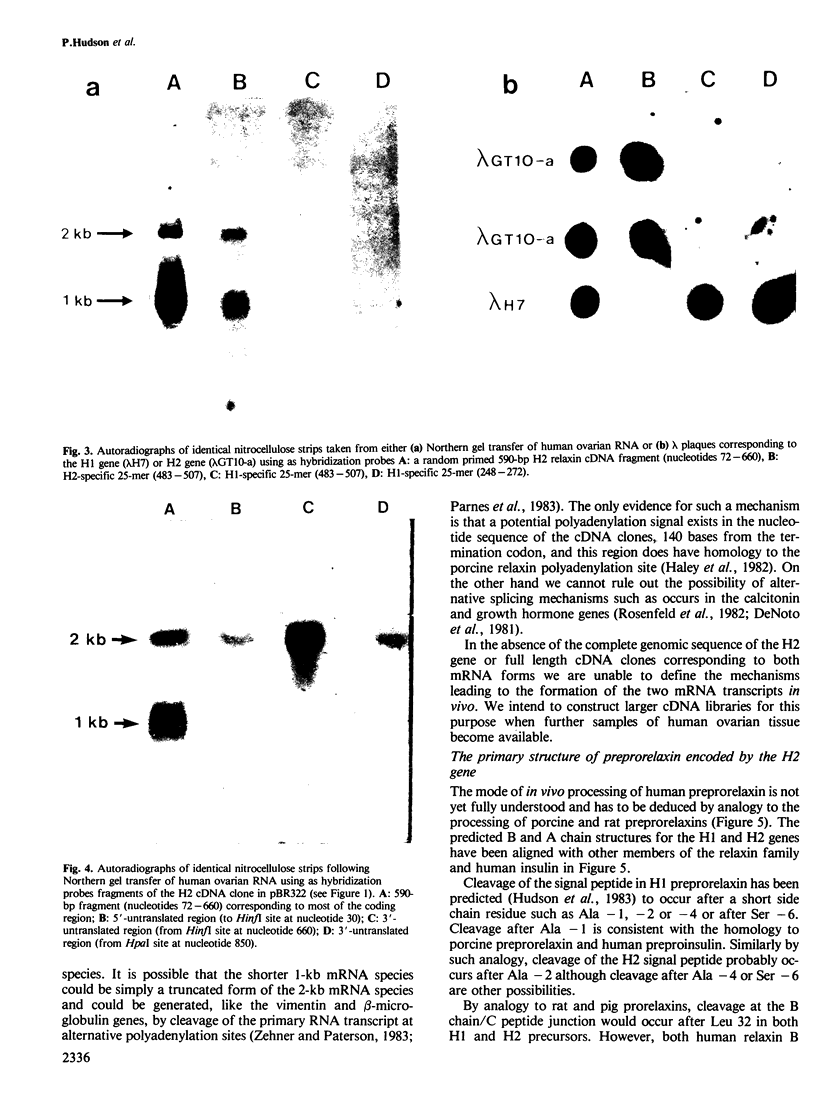
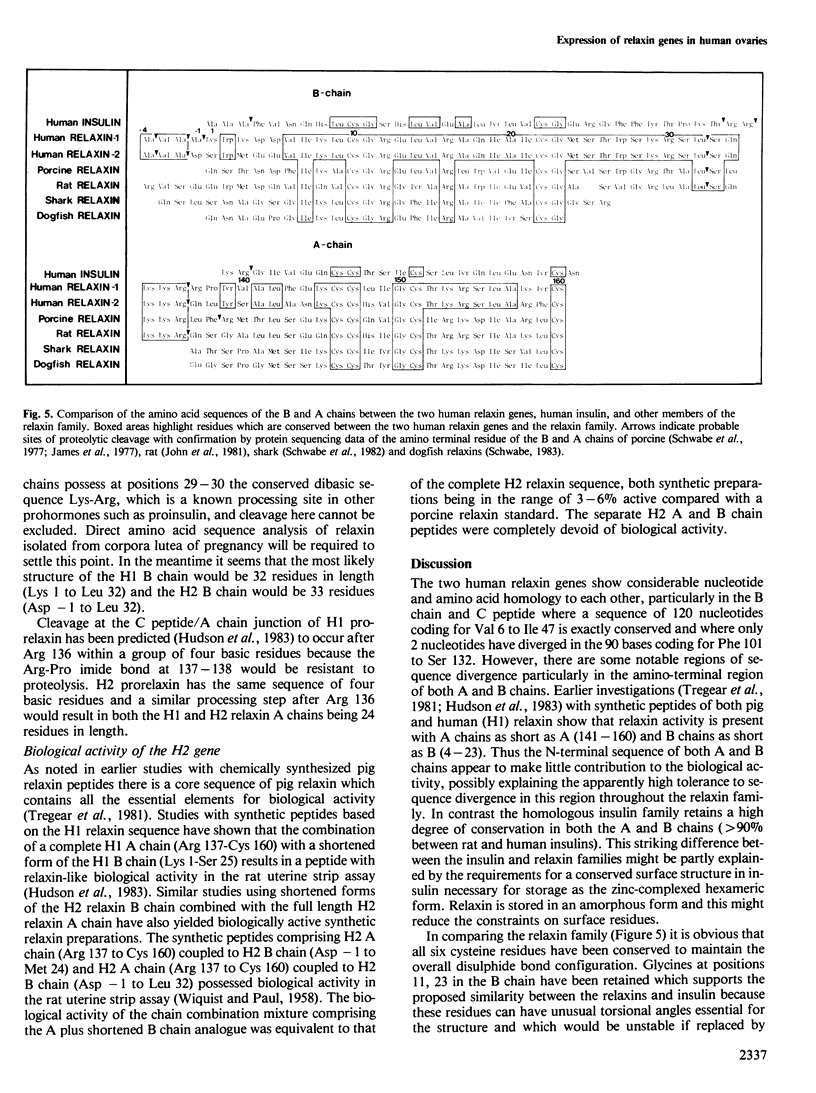
In earlier studies we identified in a human genomic library a gene (human relaxin gene H1) coding for a relaxin-related peptide. We now have evidence that the human genome possesses an additional relaxin-related gene (designated human relaxin gene H2) which appears to be selectively expressed in the ovary during pregnancy. Nucleotide sequence analysis revealed striking differences in the predicted structures of relaxin encoded by these two genes. Chemical synthesis of biologically active relaxin based on the sequence obtained from ovarian cDNA clones confirmed that the expressed gene (H2) encodes an authentic human relaxin. The expressed gene appears to be transcribed into two different sized mRNAs and preliminary evidence suggests that the mRNA transcripts possess different 3'-untranslated regions. There was no evidence for the expression of human relaxin gene H1 in the ovary and so far it is unclear whether gene H1 is expressed in another tissue or whether it represents a pseudogene. From the sequence data presented here it will now be possible to construct oligonucleotide probes and raise antibodies against synthetic peptides which could then be used to identify sites of relaxin biosynthesis and specifically quantitate the expression from either the H1 or H2 relaxin genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. L., Long J. A., Hayashida T. Immunofluorescence studies on the localization of relaxin in the corpus luteum of the pregnant rat. Biol Reprod. 1975 Dec;13(5):499–504. doi: 10.1095/biolreprod13.5.499. [DOI] [PubMed] [Google Scholar]
- Bedarkar S., Turnell W. G., Blundell T. L., Schwabe C. Relaxin has conformational homology with insulin. Nature. 1977 Dec 1;270(5636):449–451. doi: 10.1038/270449a0. [DOI] [PubMed] [Google Scholar]
- Bigazzi M., Nardi E., Bruni P., Petrucci F. Relaxin in human decidua. J Clin Endocrinol Metab. 1980 Oct;51(4):939–941. doi: 10.1210/jcem-51-4-939. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Nunberg J. H., Kaufman R. J., Erlich H. A., Schimke R. T., Cohen S. N. Phenotypic expression in E. coli of a DNA sequence coding for mouse dihydrofolate reductase. Nature. 1978 Oct 19;275(5681):617–624. doi: 10.1038/275617a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- DeNoto F. M., Moore D. D., Goodman H. M. Human growth hormone DNA sequence and mRNA structure: possible alternative splicing. Nucleic Acids Res. 1981 Aug 11;9(15):3719–3730. doi: 10.1093/nar/9.15.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P. A., Larkin L. H. Purification and immunohistochemical localization of relaxin in the human term placenta. J Clin Endocrinol Metab. 1981 Jan;52(1):79–85. doi: 10.1210/jcem-52-1-79. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Wallis J. Colony hybridization. Methods Enzymol. 1979;68:379–389. doi: 10.1016/0076-6879(79)68027-8. [DOI] [PubMed] [Google Scholar]
- Haley J., Hudson P., Scanlon D., John M., Cronk M., Shine J., Tregear G., Niall H. Porcine relaxin: molecular cloning and cDNA structure. DNA. 1982;1(2):155–162. doi: 10.1089/dna.1.1982.1.155. [DOI] [PubMed] [Google Scholar]
- Hudson P., Haley J., Cronk M., Shine J., Niall H. Molecular cloning and characterization of cDNA sequences coding for rat relaxin. Nature. 1981 May 14;291(5811):127–131. doi: 10.1038/291127a0. [DOI] [PubMed] [Google Scholar]
- Hudson P., Haley J., John M., Cronk M., Crawford R., Haralambidis J., Tregear G., Shine J., Niall H. Structure of a genomic clone encoding biologically active human relaxin. Nature. 1983 Feb 17;301(5901):628–631. doi: 10.1038/301628a0. [DOI] [PubMed] [Google Scholar]
- Hudson P., Penschow J., Shine J., Ryan G., Niall H., Coghlan J. Hybridization histochemistry: use of recombinant DNA as a "homing probe" for tissue localization of specific mRNA populations. Endocrinology. 1981 Jan;108(1):353–356. doi: 10.1210/endo-108-1-353. [DOI] [PubMed] [Google Scholar]
- Isaacs N., James R., Niall H., Bryant-Greenwood G., Dodson G., Evans A., North A. C. Relaxin and its structural relationship to insulin. Nature. 1978 Jan 19;271(5642):278–281. doi: 10.1038/271278a0. [DOI] [PubMed] [Google Scholar]
- James R., Niall H., Kwok S., Bryand-Greenwood G. Primary structure of porcine relaxin: homology with insulin and related growth factors. Nature. 1977 Jun 9;267(5611):544–546. doi: 10.1038/267544a0. [DOI] [PubMed] [Google Scholar]
- John M. J., Borjesson B. W., Walsh J. R., Niall H. D. Limited sequence homology between porcine and rat relaxins: implications for physiological studies. Endocrinology. 1981 Feb;108(2):726–729. doi: 10.1210/endo-108-2-726. [DOI] [PubMed] [Google Scholar]
- Karin M., Richards R. I. Human metallothionein genes--primary structure of the metallothionein-II gene and a related processed gene. Nature. 1982 Oct 28;299(5886):797–802. doi: 10.1038/299797a0. [DOI] [PubMed] [Google Scholar]
- Knoll B. J., Woo S. L., Beattie W., O'Malley B. W. Identification and sequence analysis of the 5' domain of the X and Y pseudo-ovalbumin genes. J Biol Chem. 1981 Aug 10;256(15):7949–7953. [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 1979;68:326–331. doi: 10.1016/0076-6879(79)68023-0. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Robinson R. R., Seidman J. G. Multiple mRNA species with distinct 3' termini are transcribed from the beta 2-microglobulin gene. 1983 Mar 31-Apr 6Nature. 302(5907):449–452. doi: 10.1038/302449a0. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Maniatis T. The structure of a human alpha-globin pseudogene and its relationship to alpha-globin gene duplication. Cell. 1980 Sep;21(2):537–544. doi: 10.1016/0092-8674(80)90491-2. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Lin C. R., Amara S. G., Stolarsky L., Roos B. A., Ong E. S., Evans R. M. Calcitonin mRNA polymorphism: peptide switching associated with alternative RNA splicing events. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1717–1721. doi: 10.1073/pnas.79.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schwabe C., Gowan L. K., Reinig J. W. Evolution, relaxin and insulin: a new perspective. Ann N Y Acad Sci. 1982;380:6–12. doi: 10.1111/j.1749-6632.1982.tb18023.x. [DOI] [PubMed] [Google Scholar]
- Schwabe C., McDonald J. K. Primary structure of the B-chain of porcine relaxin. Biochem Biophys Res Commun. 1977 Mar 21;75(2):503–510. doi: 10.1016/0006-291x(77)91070-1. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H. The human growth hormone gene family: nucleotide sequences show recent divergence and predict a new polypeptide hormone. DNA. 1982;1(3):239–249. doi: 10.1089/dna.1.1982.1.239. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- WIQVIST N., PAUL K. G. Inhibition of the spontaneous uterine motility in vitro as a bioassay of relaxin. Acta Endocrinol (Copenh) 1958 Sep;29(1):135–146. doi: 10.1530/acta.0.0290135. [DOI] [PubMed] [Google Scholar]
- Wilde C. D., Crowther C. E., Cowan N. J. Diverse mechanisms in the generation of human beta-tubulin pseudogenes. Science. 1982 Aug 6;217(4559):549–549. doi: 10.1126/science.6178164. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Kwok S. C., Greenwood F. C., Bryant-Greenwood G. D. Relaxin purification from human placental basal plates. J Clin Endocrinol Metab. 1981 Apr;52(4):601–604. doi: 10.1210/jcem-52-4-601. [DOI] [PubMed] [Google Scholar]
- Zehner Z. E., Paterson B. M. Characterization of the chicken vimentin gene: single copy gene producing multiple mRNAs. Proc Natl Acad Sci U S A. 1983 Feb;80(4):911–915. doi: 10.1073/pnas.80.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]





