Abstract
Obligate endosymbiosis is operationally defined when loss or removal of the endosymbiont from the host results in the death of both. Whereas these relationships are typically viewed as mutualistic, molecular and cellular analysis reveals numerous instances in which these symbiotic relationships are established by alternative, nonmutualistic strategies. The endosymbiont usurps or integrates into core host processes, creating a need where none previously existed. Here I discuss examples of these addictive symbiotic relationships and how they are a likely outcome of all complex evolving systems.
Images of a plover bird inside the open jaws of an alligator nonchalantly pecking at the reptile’s razor-sharp teeth provide a striking example of a mutualistic form of symbiosis: food for the plover, and dental hygiene for the alligator. Coiled at the other extreme of the symbiosis spectrum is the parasitic tapeworm living in the intestine, getting first dibs on meals and eventually producing anemia in its human host. A third form of symbiosis, commensalism, refers to the situation in which one organism benefits while the other is unaffected. The classic example is the remora fish receiving free transportation and protection through its association with sharks. Often, the nature of the symbiotic relationship is not clear. For example, the remora may provide a net benefit to its host by removing skin parasites. One of the most intimate forms of symbiosis is that in which one organism lives within another. In its most extreme form, known as endosymbiosis, the symbiont lives inside the cells of its host. Endosymbionts also exhibit the spectrum of relationships from parasitism to mutualism. A striking example of the former is Listeria monocytogenes, a bacterium that resides inside mammalian cells and is the cause of many food-borne illnesses. Listeria is famous for forming a jet-like tail by polymerizing host actin, propelling it within and between cells (Pizarro-Cerda et al., 2017).
Many endosymbionts form an obligate mutualistic relationship with their host. That is, loss or removal of the endosymbiont from the host results in the death of both. In a number of cases, metabolic complementation is the basis of this obligate relationship. This refers to situations in which the host and its symbiont both lack different steps in a biochemical pathway but together possess the enzymes for the complete pathway. Basically, this is a natural version of Beadle and Tatum’s famous metabolic complementation experiments in Neurospora. For example, formation of select amino acids in the pea aphid requires intermediate products from its bacterial endosymbiont, Buchnera (Gerardo and Wilson, 2011). Buchnera carries out the initial steps in the biochemical pathway, and the aphid provides the enzyme to carry out the final transaminase step, resulting in the formation of branched-chain amino acids. In another example, Wolbachia, a bacterial endosymbiont, provides vitamin B to its bedbug host, which feeds on mammalian blood (Hosokawa et al., 2010). Although rich in many nutrients, blood is deficient in vitamin B. In accord with this finding, analysis of the bedbug and Wolbachia genomes reveals that only the latter contains the genetic pathway required for vitamin B synthesis (Nikoh et al., 2014). There are numerous examples of metabolic complementation, and with the increasing ease of sequencing host and endosymbiont genomes, many more examples of obligate mutualism based on metabolic complementation will be forthcoming.
These examples leave us with a comforting mutualistic kumbaya view of obligate endosymbiosis. This perspective is reinforced by the widely accepted idea that mitochondria and chloroplasts originated as endosymbionts establishing a mutualistic relationship with their ancestral host (Martin et al., 2001). Thus it is of interest to ask whether obligate symbiotic relationships might be established by alternative, nonmutualistic strategies. A wonderful series of laboratory experiments probing the molecular basis of endosymbiotic relationships make a compelling case for nonmutualistic origins (Jeon and Ahn, 1978). Free-living, single-celled amebas were infected with a strain of bacteria that decimated the ameba population. However, rare surviving amebas were recovered. Examination of these survivors revealed that the invading bacteria established a stable presence in their cytoplasm. Particularly striking, during the course of this experiment, the amebas had become dependent on the bacteria for their survival. This evolution-in-a-test-tube experiment was repeated with the same result: an obligate endosymbiont relationship was quickly established between the pathogenic bacteria and the amebas. The invading bacteria resulted in the suppression of the ameba’s S-adenosylmethionine synthetase (SAMS) gene product, and survival of the ameba now required the bacteria for production of the SAMS gene product (Jeon and Jeon, 2003). Thus the free-living amebas had become addicted to the presence of originally pathogenic bacteria. It is unlikely that the presence of the bacteria improved the fitness of the ameba, making it difficult to explain the establishment of this symbiosis from a classic Darwinian perspective. This form of symbiosis has been termed addictive symbiosis (Douglas, 2010).
These experiments raise a series of intriguing questions. Does addictive symbiosis occur in nature? If so, is it frequent or rare? Are there different forms of addictive symbiosis? Can the concept of addictive symbiosis be more broadly applied toward the evolution of core molecular machinery of the cell? Answering the first question is difficult without knowing the functionality of the host immediately before invasion by the symbiont. However, studies exploring the cellular and developmental basis of the obligate mutualism may provide insight. For example, if the symbiont is required for proper functioning of highly conserved core cellular or developmental events, this suggests an addictive symbiotic relationship in which the endosymbiont created a need where none previously existed. That is, conserved host processes rooted deep in the tree of life are likely to have been present before the introduction of the symbiont.
The leafhopper Euscelis plebejus and its maternally inherited ball of bacterial endosymbionts provides a wonderful example of an endosymbiont becoming essential for early insect development. Removal of the endosymbiont through antibiotic curing results in the production of embryos lacking abdominal segments (Sander, 1968). In addition, partial reduction of the symbiont ball results in a partial reduction in abdominal segments. Similar results were found with the maternally inherited yeast-like endosymbiont of the brown planthopper: removal of the endosymbiont produces defects in abdominal segmentation (Schwemmler, 1974). Thus these symbionts are essential for normal segmentation in their insect host. It is likely that these highly conserved core mechanisms of axis formation and segmentation were established in the ancestral leafhopper and planthopper well before endosymbiont acquisition. This suggests that during the eons of coexistence, leafhoppers and planthoppers must have compensated for the presence of a large, maternally inherited endosymbiont positioned at a key time and place during insect segment establishment. Consequently, early embryogenesis became dependent on the presence of this endosymbiont. Loss of the endosymbiont disrupts this balance: without the symbiont, the host compensatory mechanisms disrupt embryonic development. The leafhopper and the endosymbiont exist in an obligate mutualistic relationship, but it is likely based on addiction rather than functional complementation between the two organisms.
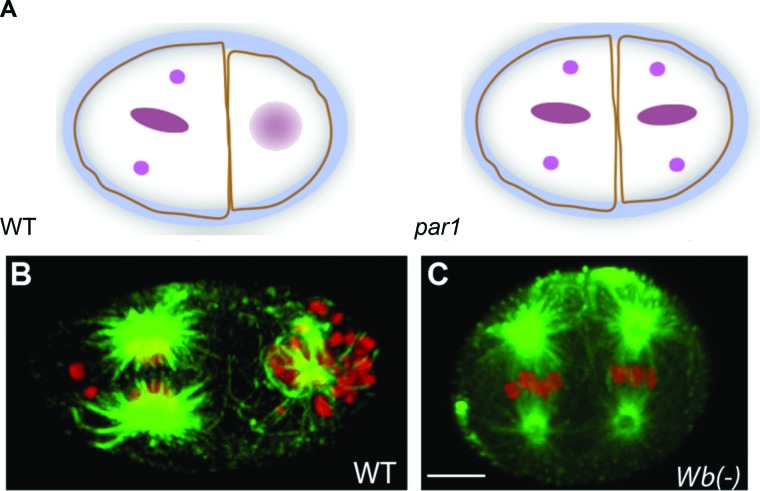
Another example of addictive symbiosis occurs between Wolbachia, a bacterial endosymbiont that occupies the germline of its filarial nematode host. It is well established that Wolbachia is required for the survival and fertility of filarial nematodes, but the basis of the obligate symbiotic relationship remained unresolved (Desjardins et al., 2013). Insight came from antibiotic curing experiments in order to generate nematodes lacking Wolbachia (Landmann et al., 2014). Surprisingly, embryos derived from these nematodes exhibit distinct anterior-posterior (A-P) polarity defects identical to the phenotype of Caenorhabditis elegans embryos lacking the Par-1 polarity gene product (Figure 1). This result suggests that among the nematode lineages, Wolbachia has coevolved with its filarial nematode host such that it has become an essential component of the core conserved machinery driving axis formation.
FIGURE 1:
Wolbachia is required for filarial nematode A-P axis formation. (A) Nematode par-1 mutants disrupt A-P axis formation, as evidenced by failed spindle rotation at the two-cell stage. (B) Wild-type and (C) Wolbachia-depleted filarial nematode two-cell stage embryos exhibit normal and failed spindle rotation, respectively. Wolbachia depletion phenocopies par-1 mutant disruption of A-P axis formation. DNA (red) and tubulin (green). Modified from Landmann et al. (2014).
Wolbachia is also essential for proper regulation of host apoptosis. Loss of Wolbachia in the moth Asobara tabida results in a dramatic increase in apoptosis and infertility in the host oocytes (Dedeine et al., 2001; Pannebakker et al., 2007). Apoptosis is part of the normal process in insect oogenesis. Through the establishment of Wolbachia as an essential component of normal apoptotic regulation in A. tabida, an addictive symbiotic relationship has been formed. Wolbachia is also essential for normal regulation of apoptosis in filarial nematodes. Loss of Wolbachia results in a dramatic increase in apoptosis in both the germline and somatic tissues of the nematode (Landmann et al., 2011) In both organisms, coevolution has resulted in the outsourcing of host apoptosis regulation to the endosymbiont. Remarkably, recent studies demonstrate that embedded in the Wolbachia genome is a bacteriophage required for spread of Wolbachia through insect populations (Beckmann et al., 2017; LePage et al., 2017). Thus Wolbachia itself may be the victim of addictive symbiosis.
Addictive symbiosis creates a need where none previously existed. Whereas adaptive processes and selection may have driven initial establishment of the symbiosis, subsequent nonadaptive processes result in the endosymbiont becoming an essential component of core host cellular and developmental processes. As a result, the host and endosymbiont become locked into an obligate mutualistic relationship. Subsequent gene transfer from the endosymbiont to the host nucleus would ensure irreversibility of the symbiosis (Keeling et al., 2015).
One can argue that addictive symbiotic relationships are a likely outcome in all complex evolving systems. In fact, societal systems such as language, business, administration, and technology provide many examples of addictive symbiosis. Although not directly applicable to biological symbiosis, they illustrate the ease in which addictive relationships are established. For example, you may have the impression that there are fewer drinking fountains in public venues. This is not an illusion. The increasing popularity of bottled water largely driven by aggressive industry marketing has lessened the demand for drinking fountains. Whereas drinking fountains were once a signature of an advanced society, the omnipresence of bottled water is making them unnecessary, such that fewer are designed into new buildings and current ones are not being maintained (Pierre-Louis, 2015). The 2015 edition of the plumber’s building code has reduced the number of required number of drinking fountains by half. Even more dramatic, Florida Central University recently built a football stadium without fountains. The university saved on construction costs and generated additional income by selling bottled water. Although bottled water clearly has some advantages over fountains, the loss of fountains enforces a lucrative but unnecessary dependence on bottled water. The notorious introduction of baby formula in underdeveloped nations provides a more consequential example of additive symbiosis (Kent, 2015). Nestle’s aggressive promotion and marketing of baby formula in place of breast-feeding changed cultural norms, reduced lactation in mothers, and ultimately resulted in an artificial dependence on formula.
Conceptual insight into how an addictive symbiotic relationship might be established is provided by exploring the evolution of core eukaryotic cellular processes. For example, the complexities of RNA processing, splicing, and editing and its associated enzymatic machinery never cease to amaze. The spliceosome alone is endowed with >100 proteins (Papasaikas and Valcarcel, 2016). Even for RNA-world experts, viewing the evolution of this complexity from a Darwinian adaptive perspective is a daunting task. In addition, although the primary function of prokaryotic and eukaryotic ribosomes is translation, the latter is much more complex, possessing an additional RNA and many more proteins (Ban et al., 2014). Harry Noller (personal communication) likens the prokaryotic ribosome to a Ferrari and the eukaryotic ribosome to a Winnebago lumbering along with assorted camping gear strapped on top. This analogy is in accord with the finding that a number of eukaryotic ribosome proteins provide key functions distinct from their role in the ribosome (Wool, 1996). One could imagine that these proteins originally evolved with functions independent of the ribosome but serendipitously possessed domains that bound ribosomal proteins. As the eukaryotic cell evolved, these proteins became an integral component of the eukaryotic ribosome. Others have made a compelling case that similar nonadaptive “rachet-like” mechanisms may account for many aspects of cellular complexity (Lynch, 2007; Gray et al., 2010; Koonin, 2016). For example, Gray et al. (2010) describe a process in which fortuitous interactions between cellular components led to a functional dependence requiring both components. In addition, studies of the eukaryotic V-ATPase proton pump document how gene duplications followed by mutations that degrade complimentary regions of each gene result in increased complexity of a protein complex, with no obvious adaptive advantage (Finnigan et al., 2012). Similarly, it has been shown that complementary patterns of gene inactivation have expanded single obligatory endosymbionts into two distinct lineages, both essential for host survival (Van Leuven et al., 2014). Again, this reveals increased complexity through nonadaptive processes.
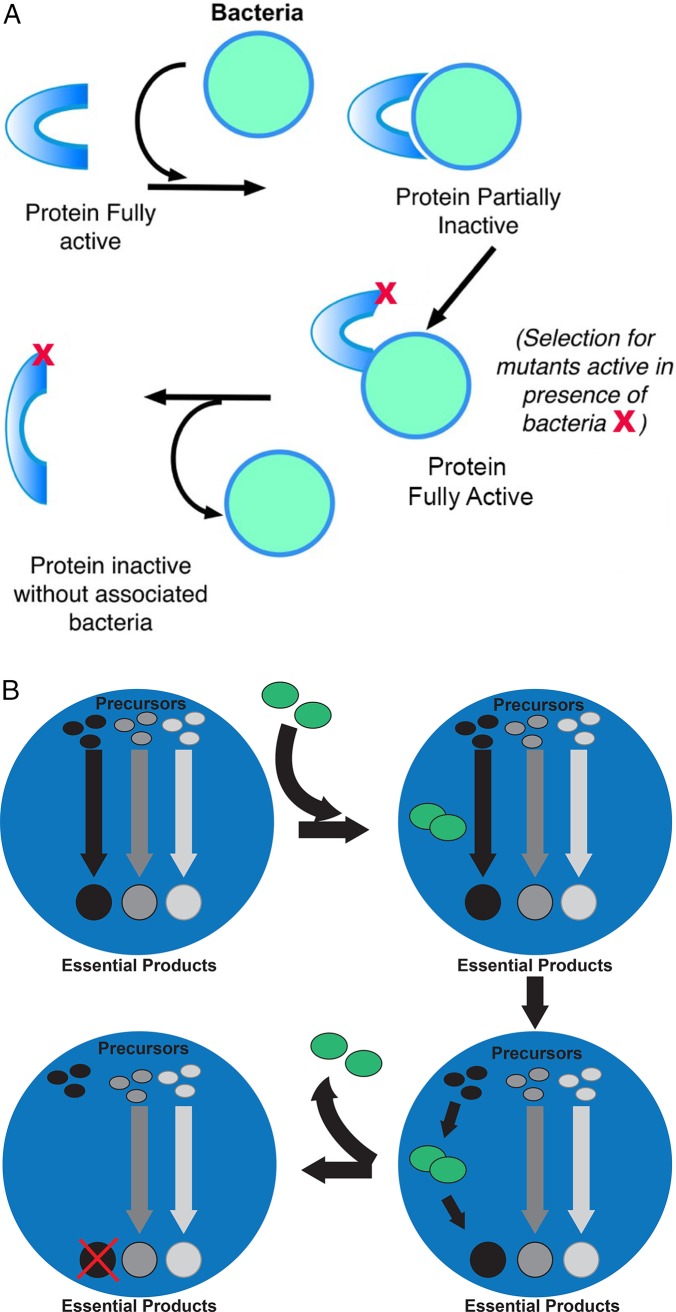
It is possible that obligate functional dependences between an endosymbiont and its host could evolve in a manner analogous to the rachet-like mechanisms proposed by Gray et al. (2010). For example, initial interaction between the endosymbiont and a key host protein complex may slightly compromise the function of the complex. Unable to rid itself of the endosymbiont, a host’s compensatory mutations may restore the endosymbiont/protein complex to full function. However, proper function of the complex now requires the presence of the endosymbiont (Figure 2A). A second mechanism by which addictive symbiotic relationships are established is illustrated by the bacterial/ameba experiments described earlier. Whereas the bacterial infection decimated the ameba population, outsourcing of key metabolic functions occurred in the rare ameba that survived infection, resulting in an additive symbiotic relationship. One can imagine a similar scenario playing out in many natural obligate endosymbiont relationships (Figure 2B). That is, initial contact is a prokaryotic invasion of a eukaryote causing significant stress to one or both (Burns et al., 2017). The outcome is uncertain but likely results in the death of either the nascent symbiont or the host (Keeling et al., 2015). In rare survivors, the endosymbiont is maintained by integrating and becoming essential to core host cellular or developmental processes.
FIGURE 2:
Potential origins of addictive symbiosis. (A) Initial interaction: Symbiont compromises function of key host enzyme. Host response: Compensatory host mutations restore the endosymbiont/enzyme complex to full function. However, proper function of the complex now requires the presence of the endosymbiont. This analogous to the evolutionary rachet mechanism proposed by Gray et al. (2010). (B) Symbiont invades host and is maintained by integrating and becoming essential for a core host cellular or developmental process. By outsourcing essential processes, the host becomes dependent on the presence of the endosymbiont.
I would argue that many obligate endosymbiotic relationships are more akin to a big-box store locating in a town: much outcry and displacement, but ultimately a dependence on its presence. Simply by its presence, the box store created a need where none previously existed. Both in society and in biology, addictive symbiosis may be the rule rather than the exception.
Acknowledgments
I thank Barton Slatko and Richard Mitchell for stimulating initial conversations. I also acknowledge the reviewers, Robert Pollie, Harry Noller, and members of the Sullivan lab for helpful comments. Special thanks to Sandy Johnson for insights and pointing me to the relevant literature. This work was supported by National Science Foundation Grant 81279.
Abbreviations used:
- A-P
anterior-posterior
- Wb
Wolbachia.
Footnotes
REFERENCES
- Ban N, Beckmann R, Cate JH, Dinman JD, Dragon F, Ellis SR, Lafontaine DL, Lindahl L, Liljas A, Lipton JM, et al. A new system for naming ribosomal proteins. Curr Opin Struct Biol. 2014;24:165–169. doi: 10.1016/j.sbi.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JF, Ronau JA, Hochstrasser M. A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat Microbiol. 2017;2:17007. doi: 10.1038/nmicrobiol.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JA, Zhang H, Hill E, Kim E, Kerney R. Transcriptome analysis illuminates the nature of the intracellular interaction in a vertebrate-algal symbiosis. Elife. 2017;6, e22054 doi: 10.7554/eLife.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedeine F, Vavre F, Fleury F, Loppin B, Hochberg ME, Bouletreau M. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc Natl Acad Sci USA. 2001;98:6247–6252. doi: 10.1073/pnas.101304298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins CA, Cerqueira GC, Goldberg JM, Dunning Hotopp JC, Haas BJ, Zucker J, Ribeiro JM, Saif S, Levin JZ, Fan L, et al. Genomics of Loa loa, a Wolbachia-free filarial parasite of humans. Nat Genet. 2013;45:495–500. doi: 10.1038/ng.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas AE. The Symbiotic Habit. Princeton, NJ: Princeton University Press; 2010. [Google Scholar]
- Finnigan GC, Hanson-Smith V, Stevens TH, Thornton JW. Evolution of increased complexity in a molecular machine. Nature. 2012;481:360–364. doi: 10.1038/nature10724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerardo NM, Wilson AC. The power of paired genomes. Mol Ecol. 2011;20:2038–2040. doi: 10.1111/j.1365-294x.2011.05103.x. [DOI] [PubMed] [Google Scholar]
- Gray MW, Lukes J, Archibald JM, Keeling PJ, Doolittle WF. Cell biology. Irremediable complexity. Science. 2010;330:920–921. doi: 10.1126/science.1198594. [DOI] [PubMed] [Google Scholar]
- Hosokawa T, Koga R, Kikuchi Y, Meng XY, Fukatsu T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci USA. 2010;107:769–774. doi: 10.1073/pnas.0911476107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon KW, Ahn TI. Temperature sensitivity: a cell character determined by obligate endosymbionts in amoebas. Science. 1978;202:635–637. doi: 10.1126/science.202.4368.635. [DOI] [PubMed] [Google Scholar]
- Jeon TJ, Jeon KW. Characterization of sams genes of Amoeba proteus and the endosymbiotic X-bacteria. J Eukaryot Microbiol. 2003;50:61–69. doi: 10.1111/j.1550-7408.2003.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Keeling PJ, McCutcheon JP, Doolittle WF. Symbiosis becoming permanent: survival of the luckiest. Proc Natl Acad Sci USA. 2015;112:10101–10103. doi: 10.1073/pnas.1513346112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent G. Global infant formula: monitoring and regulating the impacts to protect human health. Int Breastfeed J. 2015;10:6. doi: 10.1186/s13006-014-0020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin EV. Splendor and misery of adaptation, or the importance of neutral null for understanding evolution. BMC Biol. 2016;14:114. doi: 10.1186/s12915-016-0338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann F, Foster JM, Michalski ML, Slatko BE, Sullivan W. Co-evolution between an endosymbiont and its nematode host: Wolbachia asymmetric posterior localization and AP polarity establishment. PLoS Negl Trop Dis. 2014;8:e3096. doi: 10.1371/journal.pntd.0003096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann F, Voronin D, Sullivan W, Taylor MJ. Anti-filarial activity of antibiotic therapy is due to extensive apoptosis after Wolbachia depletion from filarial nematodes. PLoS Pathog. 2011;7:e1002351. doi: 10.1371/journal.ppat.1002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LePage DP, Metcalf JA, Bordenstein SR, On J, Perlmutter JI, Shropshire JD, Layton EM, Funkhouser-Jones LJ, Beckmann JF, Bordenstein SR. Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature. 2017;543:243–247. doi: 10.1038/nature21391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M. The frailty of adaptive hypotheses for the origins of organismal complexity. Proc Natl Acad Sci USA. 2007;104(Suppl 1):8597–8604. doi: 10.1073/pnas.0702207104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W, Hoffmeister M, Rotte C, Henze K. An overview of endosymbiotic models for the origins of eukaryotes, their ATP-producing organelles (mitochondria and hydrogenosomes), and their heterotrophic lifestyle. Biol Chem. 2001;382:1521–1539. doi: 10.1515/BC.2001.187. [DOI] [PubMed] [Google Scholar]
- Nikoh N, Hosokawa T, Moriyama M, Oshima K, Hattori M, Fukatsu T. Evolutionary origin of insect-Wolbachia nutritional mutualism. Proc Natl Acad Sci USA. 2014;111:10257–10262. doi: 10.1073/pnas.1409284111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannebakker BA, Loppin B, Elemans CP, Humblot L, Vavre F. Parasitic inhibition of cell death facilitates symbiosis. Proc Natl Acad Sci USA. 2007;104:213–215. doi: 10.1073/pnas.0607845104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasaikas P, Valcarcel J. The Spliceosome: the ultimate RNA chaperone and sculptor. Trends Biochem Sci. 2016;41:33–45. doi: 10.1016/j.tibs.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Pierre-Louis K. We don’t trust drinking fountains anymore, and that’s bad for our health. 2015. Washington Post. July 8. Avalailable at https://www.washingtonpost.com/opinions/we-dont-trust-drinking-fountains-anymore-and-thats-bad-for-our-health/2015/07/02/24eca9bc-15f0-11e5-9ddc-e3353542100c_story.html?utm_term=.7b2bfe1aef6c.
- Pizarro-Cerda J, Chorev DS, Geiger B, Cossart P. The diverse family of Arp2/3 complexes. Trends Cell Biol. 2017;27:93–100. doi: 10.1016/j.tcb.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander K. Developmental physiological studies on embryonal mycetoma of Euscelis plebejus F. (Homoptera, Cicadina). I. Removal of and abnormal combinations of unicellular components of symbiotic systems. [In German.] Dev Biol. 1968;17:16–38. doi: 10.1016/0012-1606(68)90087-0. [DOI] [PubMed] [Google Scholar]
- Schwemmler W. Endosymbionts: factors on egg pattern formation. J Insect Physiol. 1974;20:1467–1474. doi: 10.1016/0022-1910(74)90078-x. [DOI] [PubMed] [Google Scholar]
- Van Leuven JT, Meister RC, Simon C, McCutcheon JP. Sympatric speciation in a bacterial endosymbiont results in two genomes with the functionality of one. Cell. 2014;158:1270–1280. doi: 10.1016/j.cell.2014.07.047. [DOI] [PubMed] [Google Scholar]
- Wool IG. Extraribosomal functions of ribosomal proteins. Trends Biochem Sci. 1996;21:164–165. [PubMed] [Google Scholar]