Abstract
The RNA polymerase II (pol II) transcription complex undergoes a structural transition around registers 20–25, as indicated by ExoIII footprinting analyses. We have employed a highly purified system to prepare pol II complexes stalled at very precise positions during the initial stage of transcript elongation. Using potassium permanganate we analyzed the open region (‘transcription bubble’) of complexes stalled between registers 15 and 35. We found that from register 15 up to 25 the transcription bubble expands concomitantly with RNA synthesis. At registers 26 and 27 the bubble has a high tendency to retract at the leading edge. Addition of transcription elongation factor TFIIS re-extends the bubble to the stall site, resulting in complexes competent for transcript elongation. These findings are discussed in the light of the recently determined structures for RNA polymerases.
INTRODUCTION
DNA transcription by RNA polymerases proceeds through several steps before reaching productive elongation: preinitiation complex assembly, promoter opening, transcription initiation and promoter escape. These steps are remarkably similar in general outline between eukaryotic nuclear RNA polymerases and the bacterial enzyme (for reviews see 1–4). Prior to transcription initiation a promoter segment of 10–12 bp predominantly upstream of the start site converts to the single-stranded state. This marks the closed to open complex transition. Transcription complexes synthesize RNA in abortive mode until register 9–12. After this, RNA synthesis continues in productive elongation mode. The mechanistic similarities most likely originate from structural similarities between the transcription complexes. Indeed, recent crystallographic studies showed that a 10 subunit RNA polymerase II (pol II) from yeast (5) is topologically very similar to the three subunit RNA polymerase (RNAP) from the bacterium Thermus aquaticus (6). However, important differences also exist between transcription systems.
In bacteria one protein, the σ-factor, suffices to guide the enzyme through the above-described steps, but the pol II system requires at least 15 proteins distributed over five basal transcription factors (for reviews see 7–9). TBP (TATA box-binding protein, also designated TFIID), TFIIB and TFIIF allow promoter recognition by pol II. TFIIE and TFIIH are required for opening of pol II promoters. In contrast to other systems, transcription initiation by pol II depends on a β–γ bond hydrolyzable form of ATP (ATP or dATP) as a cofactor (10). The ATP cofactor requirement is coupled to the transcriptional requirement for TFIIH (11,12). TFIIH consists of nine subunits and is the only basal factor with enzymatic activities, which depend on ATP hydrolysis (for reviews see 13,14). These comprise two presumed DNA helicases (XPB and XPD) and a protein kinase (cdk7/cyclin H). It has been shown that the XPB subunit of TFIIH and ATP hydrolysis are essential for open complex formation (15–18). Recent crosslinking data suggest that XPB induces opening by twisting promoter DNA (19) and argues against a classical DNA helicase model (discussed in 4). Additionally, TFIIH, by virtue of its XPB subunit, can stimulate the transition from abortive to productive transcription under certain conditions (20–22). The cdk7 subunit can phosphorylate the C-terminal domain (CTD) of the largest subunit of pol II. It has been proposed that this modification triggers the transition from initiation to elongation mode. However, transcriptional dependence on CTD kinase is promoter-specific (23–26) and it depends on the composition of the pol II transcription system (27).
Many protein–protein interactions are involved in establishing a functional pol II preinitiation complex. Transcription elongation complexes lack basal factors (with the exception of TFIIF, which is also an elongation factor). Therefore, these contacts need to be rearranged or disrupted during initiation, which may mark structural transitions within the transcription complex. An early study indicated that TFIIB and TFIIE dissociate during abortive transcription (28). Using the adenovirus major late promoter (AdMLP) it was noted that transcription becomes independent of TFIIH and of ATP hydrolysis after register 4, which constitutes the second transition (29,30). Nevertheless, TFIIH is still associated at this stage, as it was found to dissociate between +30 and +68 (28). Transition from abortive to productive transcription occurs around register 10, with concomitant collapse of the upstream (–9/–2) open region (30). This coincides with attaining a mature RNA–DNA hybrid, which spans 8–9 bp. Within bacterial RNAP a rudder-like structure has been proposed to split off the 5′-end of the RNA (6) and direct it to an RNA-binding site on the enzyme. This binding site extends 10–20 nt from the catalytic site (discussed in 5,6). This implies that RNA emanates from the polymerase when it is >20 nt long. Interestingly, Samkurashvili and Luse proposed that pol II complexes pass through a major structural transition around register 25 (31). They found that stalled complexes with 20–25 nt RNAs have an increased tendency to slide back and become arrested. Similarly, bacterial RNAP complexes move discontinuously between register 23 and 27 and are increasingly sensitive to transcript cleavage induced by GreB (32).
Studies from bacterial RNAP have indicated that a mature transcription bubble is 14–22 nt in size (33). Previously we reported that productive transcription complexes at register 11 contain a bubble of 9–12 nt (30). Clearly, this is not a mature sized transcription bubble. In this paper we address the fate of the open region during the early phase of productive elongation by pol II. Employing a highly purified pol II system we stalled transcription complexes by incorporation of an RNA chain terminator between registers 15 and 35. Single-stranded regions in promoter DNA were detected by potassium permanganate modification of DNA. We observe that the transcription bubble of pol II complexes stalled at +26, +27 and +31 is highly unstable. Collapse of the bubble at the leading edge indicates back-tracking by pol II. Interestingly, a functional transcription bubble can be rescued by elongation factor TFIIS.
MATERIALS AND METHODS
Materials
Nucleotides were obtained from Roche Molecular Biochemicals. Radioactive nucleotides were obtained from Amersham or NEN/DuPont. Restriction enzymes, Klenow polymerase and calf intestine phosphatase were purchased from Pharmacia. KMnO4 was from Jansen Chimica and piperidine from Fluka. Oligonucleotides were from Pharmacia. The DNA constructs were verified using the ABI Prism Big Dye Terminator Cycle Sequencing Ready Reaction Kit in a GeneAmp PCR System 2400 and analyzed with an ABI Prism 310 Genetic Analyzer (all PE Applied Biosystems). The QiaQuick gel extraction kit was purchased from Qiagen. Quantification by phosphorimager analysis was performed on a Storm 820 gel scanner from Molecular Dynamics using ImageQuaNT software v.4.2.
Purification of the transcription factors
Pol II was affinity purified from calf thymus using the 8WG16 antibody as described previously (34). Recombinant histidine-tagged human TBP, recombinant human TFIIB and recombinant human TFIIE were expressed in Escherichia coli and purified as described previously (12,16,34). Recombinant δTFIIS (130–301) was a gift from Dr C. M. Kane (University of California Berkeley). Recombinant TFIIF (human RAP74/rat RAP30) was purified from baculovirus-infected insect cells as described previously (16). TFIIH was purified from HeLa cell extracts with the following modification of the protocol described in Holstege et al. (16). The phenyl-Sepharose column was replaced by an isopropyl-Sepharose column (Pharmacia CL 4B, 100 × 16 mm). The column was developed with a linear gradient from 1.2 to 0 M (NH4)2SO4 in T buffer (20 mM Tris–HCl, pH 8.0, 20% glycerol, 1 mM EDTA, 1 mM DTT) containing 50 mM KCl. TFIIH activity eluted between 800 and 700 mM (NH4)2SO4.
Preparations of TBP, TFIIB, TFIIE and TFIIF were homogeneous and TFIIH and pol II preparations were highly purified as judged on silver stained protein gels. None of the purified proteins contained nuclease, topoisomerase or other basal factor activity.
Template DNA
The AdML promoter constructs were derived from pDN-AdMLmut (16). The pDN-AdML+17G, +19G, +21G, +23G, +25G, +26G, +27G, +28G, +29G, +31G, +33G and +35G plasmids were constructed by inserting the appropriate PCR fragment into EcoRI- and XbaI-digested plasmid pDN-AdMLmut. PCR fragments were obtained using the M13reverse primer (Stratagene), which anneals in the pDN-AdMLmut plasmid upstream of the AdML promoter between bases 108 and 128, and an oligonucleotide that anneals between bases 30 and 50, introducing extra DNA sequence directly upstream of the XbaI site. The XbaI site creates the first G in the transcribed sequence. All constructs were verified by DNA sequence analysis. For KMnO4 sensitivity assays EcoRI- and HindIII-digested fragments were labeled at the HindIII site using [α-32P]dATP and the Klenow fragment of E.coli DNA polymerase I following standard procedures (35). For transcription assays, promoter DNA fragments were isolated from the appropriate pDN-AdMLmut derivative by restriction enzyme digestion with PvuII. The fragments were recovered using a QiaQuick gel extraction kit.
KMnO4 sensitivity assay
Radiolabeled probe (0.2–0.4 ng) was incubated at 30°C for 30 min with 25 ng TBP, 25 ng TFIIB, 80 ng TFIIF, 40 ng pol II, 30 ng TFIIE, saturating amounts of TFIIH and 12.5 ng δTFIIS (where indicated) in a 20 µl reaction that contained 12 mM HEPES–KOH, pH 7.9, 60 mM KCl, 5 mM MgCl2, 3 mM (NH4)2SO4, 0.6 mM EDTA, 0.4 mM DTT and 90 µg/ml BSA. Nucleotides were added for 10 min at the final concentrations as indicated in the figure legends. The permanganate modification of the DNA fragments was restricted to 20 s but otherwise performed as described previously (30).
Transcription assay
Template DNA fragments (5 ng PvuII fragments) were incubated at 30°C for 30 min with 25 ng TBP, 25 ng TFIIB, 80 ng TFIIF, 40 ng pol II, 30 ng TFIIE, saturating amounts of TFIIH and 12.5 ng δTFIIS (when indicated) in a 20 µl reaction that contained 12 mM HEPES–KOH, pH 7.9, 60 mM KCl, 5 mM MgCl2, 3 mM (NH4)2SO4, 0.6 mM EDTA, 0.7 mM DTT and 90 µg/ml BSA. After assembly, nucleotides were added to the concentrations indicated in the figure legends and incubated at 30°C for the appropriate time as indicated. The reactions were stopped and processed as described previously (36). Short RNA products (up to 33 nt) were analyzed by electrophoresis on 21% polyacrylamide (acrylamide:bisacrylamide 19:2)–7 M urea gels. Longer RNAs were analyzed on 7 or 10% polyacrylamide (acrylamide:bisacrylamide 19:1)–8.3 M urea gels.
RESULTS
Progression of the open region within pol II transcription complexes (the ‘transcription bubble’) was analyzed in our reconstituted basal transcription system. Previously we found that in the early phase of initiation (RNA products up to 15 nt) the leading edge of the bubble coincides with the site of NMP addition (37). The trailing half of the bubble, the –9/–2 region opened by TFIIH helicase, collapses when pol II reaches register 11. The current study analyzes RNA product formation and the transcription bubble of pol II complexes stalled between registers 15 and 35. To this end, we constructed a set of AdML promoter derivatives with their first G residue at +17 up to +35 (Fig. 1). As indicated in Figure 1, the DNA sequences beyond the stall site are identical in all constructs. This is important as recent structural studies indicate that 15 bp of duplex DNA downstream of the active site are tightly clamped by pol II (5). To prevent read-through we found that it is essential to include the RNA chain terminator 3′-O-methyl-GTP (3′-OMeGTP) in the reactions. Simply omitting GTP from the reactions results in a heterogeneous population of RNAs extending well beyond the expected stall site (data not shown). We believe that the longer RNAs are caused by pol II read-through, as inclusion of elongation factor TFIIS reverses these larger than expected RNAs (data not shown and see also Fig. 7). This effect of TFIIS excludes the possibility that read-through is caused by GTP contamination of the system. Previously, misincorporation had led to the false impression that formation of the first phosphodiester bond results in a dramatic expansion of the bubble (16,37). The templates were used in reactions containing homogeneous preparations of recombinant TBP, TFIIB, TFIIE and TFIIF and highly purified HeLa cell-derived TFIIH and calf thymus pol II. The DNA templates were preincubated for 30 min with saturating amounts (except for pol II) of these factors to allow preinitiation complex assembly. Transcription was initiated by addition of nucleotides and stopped after 10 min incubation. Figure 2 depicts a representative experiment, which shows that RNA synthesis in the presence of 3′-OMeGTP results in a homogeneous population of RNA products of the expected size. Variability in the signals (Fig. 2, for example compare lanes 3 and 5) is due to variation in the efficiency of ethanol precipitation of such short RNAs and does not depend on the particular template.
Figure 1.
DNA sequence of the non-template strand of the pDN-AdML+nG plasmids. The natural transcription start site (also depicted by the arrow) and the first G residue in the RNA chain are indicated in bold. The templates are identical outside the indicated region. Residues transcribed into RNA are depicted as capitals. The asterisk identifies the position of the radiolabel in the fragments used for footprinting.
Figure 7.
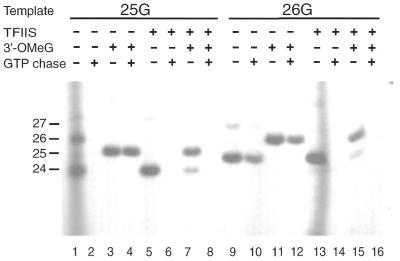
Only part of the RNA products formed by the 26G complex can be chased into longer products. Initiation complexes were assembled on AdML+25G and AdML+26G templates in the presence and absence of δTFIIS as indicated at the top of the figure. Reactions included nucleotides (60 µM ATP, 10 µM UTP and 2 µM [α-32P]CTP). Lanes 4, 5, 7, 8, 11, 12, 15 and 16 included 120 µM 3′-OMeGTP in addition. After 10 min incubation at 30°C, GTP was added at 600 µM to the reactions of the even numbered lanes and incubation was continued for another 10 min. Subsequently, RNA products were processed and analyzed on a 21% denaturing polyacrylamide gel as described in Materials and Methods. To the left are indicated the lengths of the RNA products.
Figure 2.
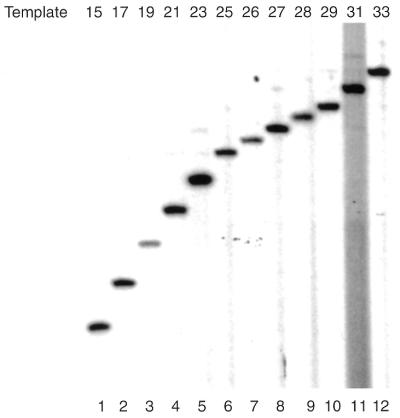
Analysis of the RNA products formed with the mutant AdMLP templates. Transcription initiation complexes were assembled on mutant AdML templates as indicated at the top of the figure. Nucleotide triphosphates (60 µM ATP, 10 µM UTP, 2 µM [α-32P]CTP and 120 µM 3′-OMeGTP) were added for 10 min. Subsequently, RNA products were processed and analyzed by denaturing PAGE as described in Materials and Methods.
Open complex progression in elongating pol II complexes
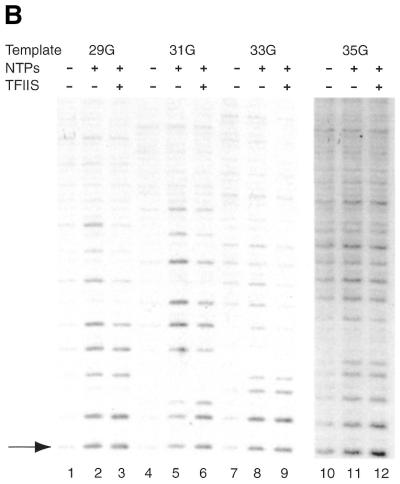
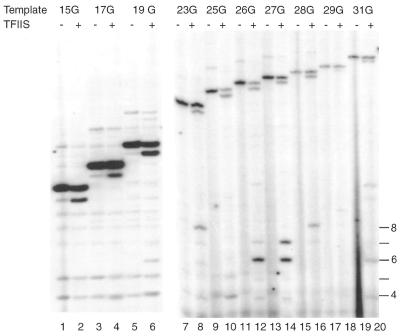
Having established conditions that yield a homogeneous population of RNA products we analyzed the transcription bubble of pol II complexes stalled at the different positions. Transcription complexes were assembled on DNA fragments with a radiolabeled non-template strand under conditions for RNA analysis. Transcription complexes stalled by 3′-OMeGTP were briefly treated with potassium permanganate, which preferentially modifies single-stranded thymidines. Figure 3 shows the results of this permanganate analysis using the different DNA templates. The position of the first G residue is indicated to the left of the figure. In accordance with our earlier study (30), stalling pol II at register 15 by inclusion of 3′-OMeGTP in the nascent mRNA leads to increased sensitivity to potassium permanganate from position +3T to +13T (Fig. 3, lanes 1 and 2). Moving pol II to register 17 extends the sensitivity to +15T. As expected the transcription bubble also expands further downstream when transcription is stalled at registers 19, 21, 23 and 25. Surprisingly, the transcription bubble seems to be retracted in complexes stalled at registers 26 and 27. Permanganate sensitivities can be observed from +5T to +19T and +5T to +22T, respectively. At registers 28 and 29 this retraction is not observed and, additionally, sensitivity at positions +3 and +5 is no longer apparent (Fig. 3, lanes 18 and 20; see also Fig. 5A and B). Careful quantitation of multiple independent experiments indicates that the sensitivity of these positions is gradually lost when complexes are stalled beyond register 28. Table 1 gives a summary of the analysis of the open regions of stalled pol II complexes from between three and five independent experiments. Please note that complexes stalled at register 31 also show a retracted transcription bubble (see also Fig. 5B).
Figure 3.
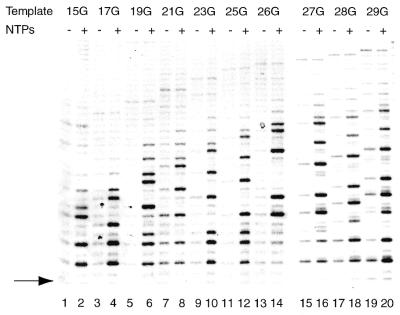
Permanganate sensitivity of pol II complexes stalled at different positions. Transcription complexes were assembled with AdML+15G (lanes 1 and 2), AdML+17G (lanes 3 and 4), AdML+19G (lanes 5 and 6), AdML+21G (lanes 7 and 8), AdML+23G (lanes 9 and 10), AdML+25G (lanes 11 and 12), AdML+26G (lanes 13 and 14), AdML+27G (lanes 15 and 16), AdML+28G (lanes 17 and 18) and AdML+29G (lanes 19 and 20). Reactions included nucleotides (60 µM ATP, 10 µM UTP, 10 µM CTP and 120 µM 3′-OMe-GTP) for 10 min at 30°C. After this, potassium permanganate was added for 20 s and the reactions were processed as described in Materials and Methods. The G residue creating the stall site is indicated by the arrow. Please note that the distance between stall site and labeling site is identical for all DNA template fragments (see also Fig. 1).
Figure 5.
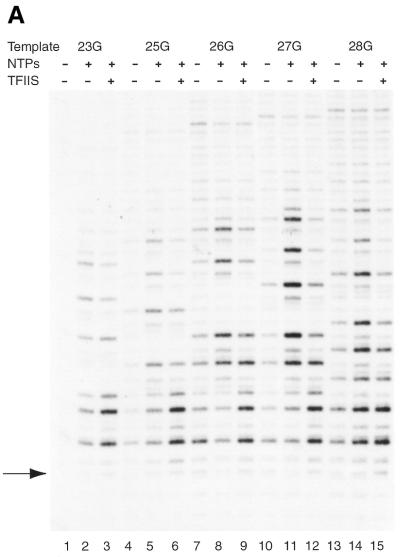
Template opening in the presence and absence of δTFIIS. Transcription initiation complexes were assembled in the presence and absence of δTFIIS on radiolabeled mutant AdML templates as indicated at the top of the figure. (A) Initiation complexes were assembled with AdML+23G (lanes 1–3), AdML+25G (lanes 4–6), AdML+26G (lanes 7–9), AdML+27G (lanes 10–12) and AdML+28G (lanes 13–15). The G residue creating the stall site is indicated by the arrow. (B) Initiation complexes were assembled with AdML+29G (lanes 1–3), AdML+31G (lanes 4–6), AdML+33G (lanes 7–9), AdML+35G (lanes 10–12). The reactions included nucleotides (60 µM ATP, 10 µM UTP, 10 µM CTP and 120 µM 3′-OMeGTP) at 30°C for 10 min. After this, potassium permanganate was added for 20 s and the reactions were processed as described in Materials and Methods. The last T residue before the stall site is indicated by the arrow (see also Fig. 1).
Table 1. Borders of the transcription bubble of stalled complexes.
| Template |
Opening –TFIIS |
Opening +TFIIS |
| 15G | 3–15 | nd |
| 17G | 3–17 | nd |
| 19G | 3–19 | nd |
| 21G | 3–21 | nd |
| 23G | 3–23 | 3–23 |
| 25G | 3–25 | 8–25 |
| 26G | 5–19 | 8–26 |
| 27G | 5–20 | 11–27 |
| 28G | 7–28 | 18–28 |
| 29G | 7–29 | 18–29 |
| 31G | 7/8–22 | 13–31 |
| 33G | 14–33 | 22–33 |
| 35G | 14–35 | 20–35 |
This table summarizes the data obtained in the permanganate sensitivity assays using the mutated AdML templates. The numbers indicate the registers which form the borders of the open region. For the most downstream position we used the register of the stall site. Only positions with a permanganate sensitivity of twice the background were scored as sensitive. The assay was performed at least three times for each template. Complexes stalled at registers 25, 26, 27 and 28 were assayed more than five times. nd, not determined.
From these experiments it is clear that the transcription bubble expands in a continuous fashion in the downstream direction. This process is concomittant with NMP addition to the nascent RNA chain. In most cases the downstream border of the transcription bubble corresponds to the length of the RNA product. At certain positions the bubble retracts under the conditions of the assay, although the synthesized mRNAs had the expected size. The upstream part (+3 to +7) of the bubble remains sensitive to permanganate. Around register 28 the upstream part gradually starts to re-adopt the double-stranded conformation and the bubble reached a size of 20–22 nt.
Effects of elongation factor TFIIS on stalled transcription complexes
We decided to further investigate the retracted complexes stalled at registers 26, 27 and 31. The observation that RNA products from these templates had the expected sizes indicates that after reaching their respective stall sites these transcription complexes may have adopted a transcriptional arrest conformation. A hallmark of transcriptional arrest is that the 3′-end of the nascent RNA becomes disengaged from the active site. Elongation factor TFIIS can re-activate arrested complexes by promoting reiterative cleavage and re-extension of nascent transcripts (for reviews see 38–40). In this process cleavage products ranging from 2 to 7 nt are released. In our system one would expect that TFIIS would induce accumulation of these short RNAs of complexes stalled at registers 26, 27 and 31.
To test this hypothesis we compared RNA products formed in the presence and absence of human TFIIS from our set of AdML templates. In these experiments we used a shortened version of human TFIIS (residues 130–301; kindly provided by Dr C. M. Kane, which is functionally indistinguishable from the full-length protein (41). In the absence of TFIIS (Fig. 4, odd numbered lanes) RNAs of the expected size are obtained. In the presence of TFIIS (Fig. 4, even numbered lanes) two major RNAs are obtained from each template. In addition to the expected size, RNA molecules 1 nt shorter are observed. This agrees with the observation that incorporation of 3′-OMeGTP in nascent RNA is much slower compared to incorporation of natural ribonucleotides (data not shown), but also suggests that TFIIS induces continual removal of the terminal 3′-OMeGMP residue. In the case of the 26G, 27G and 31G complexes high levels of small RNAs ranging in size between 4 and 8 nt can be observed in reactions that received TFIIS (Fig. 4, lanes 12, 14 and 20). In contrast, small RNAs do not accumulate to such high levels with other complexes, although significant levels can be observed with the 23G and 28G complexes. Upon comparison of the yields of the full-length RNAs versus small released RNAs we estimate that efficiency of cleavage is >5-fold more efficient for the 26G and 27G complexes than for the 31G complex and >20-fold more efficient than for non-retracted complexes like the 23G complex. In combination with the results from the permanganate sensitivity experiments (Fig. 3), the RNA product analysis strongly suggests that the transcription complexes stalled at registers 26, 27 and 31 have a high tendency to back-track, resembling the transcriptional arrest state.
Figure 4.
Analysis of the RNA products formed with the mutant AdMLP templates in the presence and absence of δTFIIS. Transcription initiation complexes were assembled in the presence and absence of δTFIIS on mutant AdML templates as indicated at the top of the figure. The odd numbered reactions received no TFIIS while the even numbered reactions received δTFIIS protein. Initiation complexes were assembled with AdML+15G (lanes 1 and 2), AdML+17G (lanes 3 and 4), AdML+19G (lanes 5 and 6), AdML+23G (lanes 7 and 8), AdML+25G (lanes 9 and 10), AdML+26G (lanes 11 and 12), AdML+27G (lanes 13 and 14), AdML+28G (lanes 15 and 16), AdML+29G (lanes 17 and 18) and AdML+31G (lanes 19 and 20). The reactions included nucleotide triphosphates (60 µM ATP, 10 µM UTP, 2 µM [α-32P]CTP and 120 µM 3′-OMeGTP) for 10 min. Subsequently, RNA products were processed and analyzed on a 21% denaturing polyacrylamide gel as described in Materials and Methods.
Next, the effect of TFIIS on the transcription bubble of stalled complexes was analyzed. We expect that TFIIS addition will reverse bubble retraction. Figure 5 shows the results of the permanganate analyses using different AdML mutant templates (AdML 23G up to 35G) in the absence and presence of TFIIS. The borders of the transcription bubble are not affected by TFIIS when the transcription complexes were stalled at register 23 (Fig. 5A, lanes 1–3). Interestingly, the sensitivities of 18T, 19T and 21T were enhanced by TFIIS. Such alterations are not observed in the +3/+14 region. This result suggests that TFIIS alters the conformation of the transcription bubble but not its location. Alternatively, TFIIS may alter the distribution of transcription complexes over distinct functional states (see below). The effect of TFIIS on the complex stalled at register 25 is quite similar to that on the 23G complex (Fig. 5A, lanes 4–6). In contrast, dramatic effects of TFIIS on the transcription bubble were observed when pol II was stalled at registers 26, 27 and 31. TFIIS reverses retraction of the transcription bubble of the complex stalled at register 26 (Fig. 5A, compare lanes 8 and 9) and the +7/+11 region becomes less susceptible. These results are fully compatible with the hypothesis that the 26G complex has become arrested. Transcription bubble retraction is also reversed by TFIIS on the 27G and 31G templates (Fig. 5A, compare lanes 11 and 12; Fig. 5B, compare lanes 5 and 6). In the presence of TFIIS, transcription complexes are no longer retracted and the leading edge of the transcription bubble corresponds to the expected position. In addition, we observed that in most cases the position of the upstream border of the open transcription complexes changes. The upstream border seems to shift 2–3 nt further downstream (for a summary of the permanganate analysis see Table 1). Inclusion of TFIIS during preincubation was not needed to observe these effects. Similar results were obtained when TFIIS was added just before the NTPs (data not shown). From these results we conclude: (i) TFIIS reverses arrest of transcription complexes stalled at registers 26, 27 or 31; (ii) TFIIS affects the permanganate sensitivities of single-stranded thymidine residues in the transcription bubble such that the leading half of the bubble becomes more susceptible; (iii) TFIIS seems to restrict the size of the transcription bubble.
Transcription bubble collapse is influenced by 3′-OMeGTP and correlates with inefficient chase of RNA products
We investigated whether registers 26 and 27 also represent sites with a higher probability of transcriptional arrest or pausing during the normal elongation cycle. This was examined in standard run-off experiments by lowering the concentration of UTP, CTP or ATP beyond the apparent Km value. Also, we shortened transcription elongation times and lowered the reaction temperature. None of these conditions led to an accumulation of products, which would be indicative of stalling or arrest at the expected registers (data not shown). Apparently, even under these limiting conditions transcript elongation is favored over transcriptional stalling or arrest. Next, we examined whether transcription bubble retraction is influenced by incorporation of 3′-OMeGTP into the nascent mRNA. The Hawley group showed that unusual nucleotides like ITP slow down the kinetics of mRNA synthesis and can induce pausing (42). Even at saturating concentrations 3′-OMeGTP is incorporated at a slower rate in nascent mRNA than the standard nucleotides (Fig. 4 and data not shown). In the experiment shown in Figure 6 we examined the effect of 3′-OMeGTP on transcriptional arrest in the permanganate sensitivity assay. When transcription was performed on the 27G template with ATP, CTP and UTP but without 3′-OMeGTP most of the complexes will stall at register 26 and some of them will read through (∼20–40%; see Fig. 7 and data not shown). In the permanganate sensitivity experiments we observed template opening spanning +5T to +25T (Fig. 6, lane 2). Addition of TFIIS in the absence of 3′-OMeGTP results in a more efficient modification of single-stranded thymidine residues in the leading half of the transcription bubble (Fig. 6, lane 3). When reactions were performed in the presence of 3′-OMeGTP a more pronounced retraction of the transcription bubble was observed (Fig. 6, compare lanes 2 and 4), which can be reversed by TFIIS (Fig. 6, lane 5). The notion that 3′-OMeGTP enhances bubble retraction is also supported by comparison of 27G complexes formed in the absence of 3′-OMeGTP (Fig. 6, lane 2) with 26G complexes in the presence of 3′-OMeGTP (Fig. 3, lane 14; Fig. 5A, lane 8). The majority of both complexes are stalled at register 26, but bubble retraction is more pronounced in 3′-OMeGTP-stalled 26G complexes. It seems likely that this is related to the reduced stability of 3′-OMeGMP-capped RNAs in the catalytic site of pol II. However, the difference could also be caused by a portion of the transcription complexes reading through the stall site in the absence of 3′-OMeGTP (Fig. 7 and data not shown). However, in that case one would expect to observe sensitivity at +29T, which is not detected (Fig. 6, lane 2).
Figure 6.

Arrest at register 27 is enhanced by 3′-OMeGTP. Initiation complexes were assembled on the AdML+27G template in the presence and absence of δTFIIS as indicated at the top of the figure. After 30 min preincubation at 30°C the reactions received no nucleotides (lane 1), 60 µM ATP, 10 µM CTP and 10 µM UTP for 10 min (lanes 2 and 3) or 60 µM ATP, 10 µM CTP, 10 µM UTP and 120 µM 3′-OMeGTP for 10 min (lanes 4 and 5). After this time potassium permanganate was added for 20 s and the reactions were processed as described in Materials and Methods. The G residue creating the stall site is indicated by the arrow.
Together our analyses indicate that pol II complexes stalled by 3′-OMeGTP at registers 26, 27 and 31 resemble arrested transcription complexes. Such complexes do not resume transcription upon addition of ‘missing’ nucleotides, which is in contrast to stalled complexes. Our stalling strategy precludes a direct discrimination between stalling and arrest. While it yields homogeneous populations of RNAs, the 3′-OMeGMP cap precludes further extension into longer products. We therefore stalled complexes in the absence or presence of 3′-OMeGTP and tested whether addition of TFIIS was required to allow extension into longer products in the following way. Subsequent to the 10 min incubation with nucleotides, excess GTP was added to stalled complexes in the absence or presence of TFIIS for 10 min and RNA products were analyzed. Figure 7 shows the results for the 25G and 26G transcription complexes. Whereas all RNA products of the 25G complex can be chased by GTP, a significant portion of 26G products fails to become extended (Fig. 7, compare lanes 1 and 2 with lanes 9 and 10). Importantly, addition of TFIIS to the 26G complex allows extension of these products (Fig. 7, lanes 13 and 14). This TFIIS dependence indicates that this portion of the 26G products requires re-alignment with the catalytic center of pol II. As expected, inclusion of 3′-OMeGTP prevents the GTP chase in the absence of TFIIS but not in its presence. Products formed by the 27G complex behaved similarly to 26G products (data not shown).
In conclusion, the GTP chase analysis of Figure 7 also supports the conclusion that transcription complexes stalled around register 26 have a high probability of entering a transcriptional arrest state.
DISCUSSION
Preinitiation complex assembly involves a multitude of interactions between basal factors, pol II and DNA. Many of these interactions need to be broken during the early phase of transcription. When transcription converts from abortive to productive mode between registers 9–11 (37), several basal factors have already dissociated (28). However, at this stage the transcription bubble has yet to achieve its mature size.
In this study we have followed the structure of the DNA template during the early phase of pol II transcription. Using potassium permanganate we have probed for the single-stranded region of transcription complexes stalled up to register 35. Complexes were assembled in a reconstituted sytem with purified pol II and the minimal set of basal transcription factors. First we discuss our findings from experiments lacking TFIIS. We found that between registers 11 and 25 the bubble expands up to 22 nt in size. This is consistent with the reported size of the bacterial RNAP bubble of 14–22 nt (33). The registers at which pol II attains sliding clamp mode appear to be between 25 and 28 (Table 1). It is tempting to speculate that the bubble retraction we observed with the 26G and 27G complexes (Fig. 3) is related to conversion to the sliding clamp mode of transcription elongation. However, the observation that the bubble of the 31G complex (Fig. 5) is also retracted argues against a strong causal relationship. Bubble retraction is not solely dependent on the distance from the transcription start site but is also influenced by the sequence of the DNA template. Alteration of template bases at registers 21 and 22 affects bubble retraction of 3′-OMeGTP-stalled 27G complexes (data not shown). In addition, if registers 25–28 mark a soft spot in early initiation, one would expect that part of the complexes would pause or arrest at these sites. We failed to observe accumulation of RNA products of 25–28 nt during normal elongation (see also Fig. 2), even under conditions of low NTP concentrations, low temperature or restricted elongation times (data not shown). Neverthless, our results are in accordance with observations by Samkurashvili and Luse, who proposed that pol II complexes pass through a structural transition around register 25 (31). They observed that complexes with 20, 23 and 25 nt RNAs have ExoIII footprint borders very close to the site of NMP addition, reminiscent of arrested complexes. In their paper transcription complexes were assembled in crude HeLa cell extracts, stalled by NTP omission, rinsed with sarkosyl and purified by gel filtration. A direct comparison with our results is complicated by differences in the stalling protocols (NTP depriviation versus 3′-OMeGTP chain termination), uncertainty in transcription complex composition and detection method (ExoIII footprint versus permanganate sensitivity). Despite these differences, similarities with our 26G and 27G complexes assembled in a minimal pol II system and in the absence of TFIIS are quite remarkable.
An important issue in the potassium permanganate sensitivity assay concerns the homogeneity in the stalled complexes (for a discussion see 43). In the interpretation of the data it is assumed that all individual transcription complexes have the same DNA–protein structure. Alternatively, the data are the average of the most prominent configurations. This would complicate the interpretation considerably. For several reasons we do not think that heterogeneity is a complicating factor in our study. First of all, the permanganate incubation was minimized to 20 s and the length of NTP incubation was optimized for the highest yield of RNA product. Secondly, with the inclusion of 3′-OMeGTP as a RNA chain terminator <10% of the RNA products have lengths other than those expected (Fig. 2). Thirdly, we never observed permanganate sensitivity of the thymidine residue 3 bp beyond the stall site (see for example Figs 5A and 7), which indicates that no significant portion of complexes transcribed beyond the stall site. Finally, only a very small portion of 3′-OMeGTP-stalled complexes can be chased by an excess of GTP in the absence of TFIIS (Fig. 7 and data not shown). This means that almost all of the RNA products formed are capped at their 3′-terminus by 3′-OMeGMP. All of these stalled RNAs can be chased to run-off products when TFIIS is added together with excess GTP (Fig. 7 and data not shown), implying that TFIIS efficiently removes the 3′-OMeGMP cap to allow chain elongation. Finally, the assumption that our complexes are homogeneous is compatible (see below) with the dimensions of the ‘grooves’ on pol II proposed to harbor nascent RNA, the RNA–DNA hybrid and the remaining lagging half of the bubble (5).
Addition of TFIIS to the 26G, 27G and 31G complexes reverses retraction of the transcription bubble. This is concomitant with an accumulation of short RNAs (Fig. 4), which are most likely cleavage products. These observations are fully explained by assuming that these complexes have become arrested. This is also supported by the chase experiment of Figure 6. Arrested complexes can be reactivated by TFIIS-induced cleavage releasing short oligonucleotides at the 3′-end of the nascent RNA, which becomes disengaged from the catalytic center of the polymerase upon arrest (reviewed in 38,39). The trimmed RNA becomes re-aligned with respect to the catalytic center. The nascent RNA can then be re-extended, which induces permanganate sensitivity of non-template residues up to the stall site. Our observations confirm the model of TFIIS-induced re-alignment with the active center. TFIIS reverses 26G and 27G retraction, suggesting that TFIIS can assist transcription complexes to attain the sliding clamp mode of elongation. TFIIS not only reverses back-tracking of the 26G, 27G and 31G complexes, but also seems to limit the size of the transcription bubble at its lagging edge (Figs 5 and 7). Several explanations are possible. First, a small portion of stalled transcription complexes may have a tendency to back-track. TFIIS reverses this process, resulting in a homogeneous population of complexes. In this scenario one would expect a TFIIS-induced accumulation of small RNAs in all cases, which is not observed (see Fig. 4, lanes 2, 4, 6 and 18). Secondly, TFIIS could bind directly to the non-template strand, thus protecting it from permanganate modification. It has been suggested that TFIIS can bind to single-stranded nucleic acids through its zinc ribbon domain, as discussed (44). Finally, a mere association of TFIIS to the transcription complex could alter the pol II–DNA contacts within the DNA exit channel, resulting in stabilization of double-stranded DNA at the lagging part of the transcription bubble. In this respect it is noteworthy that TFIIS has been shown to functionally interact with Rbp6 (45). This subunit lies at the base of an inverted funnel-shaped cavity of pol II, which is on the opposite side of the DNA to the entry and exit channels (5). This funnel was proposed to harbor the 3′-end of arrested RNAs and also to provide access to TFIIS (5). These structural considerations make it unlikely that TFIIS contacts the DNA directly. Rather, it seems that TFIIS association induces altered pol II–DNA contacts in the DNA exit channel, as suggested (39).
From biophysical and biochemical studies it has become clear that bacterial and eukaryotic RNA polymerases are quite similar. As mentioned, the size and position of the open region prior to transcription initiation are almost identical (see 4). Also, transcription shifts from abortive to productive mode at very similar registers (37,46). In addition, our present results strongly contribute to the view that pol II transcription complexes undergo an important structural transition around registers 25–27 (31), as was also noted previously for bacterial RNAP (32).
What could be the reason for this transition? In this respect it is interesting to review the structural models for T.aquaticus RNAP (6) and yeast pol II (5) and the proposed paths of the DNA template and RNA product. The DNA entry channel can harbor ∼9 bp (RNAP) or ∼20 bp (pol II) of double-stranded DNA, which it has been proposed is propelled towards the active center by a helical screw rotation (5). In pol II this downstream DNA would be retained by a flexible ‘clamp’ within the enzyme comprised of the N-terminal region of Rpb1 and the C-terminal region of Rpb2 (5). Compatible with the observed continuous template opening (this paper and 37), downstream DNA melts just before the active center. In both RNA polymerases it has been proposed that the incoming DNA duplex and the RNA–DNA hybrid make an angle of almost 90° at the active center. The RNA–DNA hybrid is placed at the upstream edge of the flexible clamp of pol II. An important feature of the RNA–DNA hybrid exit channel of T.aquaticus RNAP is a ‘rudder’-like structure formed by region C of β′ at a distance from the active center compatible with a RNA–DNA hybrid of 8–9 bp. A corresponding rudder-like structure has been detected in improved electron density maps of pol II (P.Cramer and R.Kornberg, personal communication). It was proposed that in RNAP this rudder redirects protruding RNA away from the DNA template under a flexible flap structure formed by βF,G,H (6). In the case of pol II the rudder probably directs the RNA towards groove 1 as it extends from the edge of the clamp structure (P.Cramer and R.Kornberg, personal communication). Groove 1 in pol II can accomodate nascent RNA 10–20 nt from the active center. This curved groove is located at the base of the flexible clamp and leads back to downstream DNA (5). Filling of this groove could tighten the grip of the flexible clamp on downstream DNA. Conceivably, the groove is filled to completion when the RNA is 26–27 nt long. This would obviously mark a structural transition in the transcription complex and could explain why complexes stalled at these positions are liable to back-tracking and transcriptional arrest.
The path of the non-template strand could also be involved in the instability of the 26/27 complexes. This strand has to be outside the RNA–DNA hybrid exit channel, because this cannot accommodate both the RNA–DNA hybrid and the non-template strand. What happens with the template strand when the 5′-end of a 9–10 nt RNA encounters the rudder? The template DNA strand may be threaded through groove 2 (5). The profound curvature of this groove (and of groove 1) is only compatible with binding single-stranded nucleic acids. The non-template strand could re-anneal only after the template strand has passed through the narrow part of groove 2. The narrow part of groove 2 (<20 Å in diameter) extends for 60 Å from the end of the proposed RNA–DNA hybrid before widening (P.Cramer and R.Kornberg, personal communication). This model has two attractive features. Localizing the non-template strand away from polymerase structures explains its sensitivity to modifying agents like permanganate (16). Secondly, combining the length of the RNA–DNA hybrid with the dimensions of the narrow part of groove 2 indicates a minimal length for the transcription bubble of ∼20 nt (with no base stacking interactions occuring within the bubble). Base stacking interactions would allow a maximal bubble size of 27 nt. These considerations are compatible with a model in which filling of grooves 1 and 2 is complete when the RNA is 25–27 nt long. This possibly constitutes the structural change observed around registers 25–27.
Thus, it is very possible that clamp movement induced by RNA binding in groove 1 and filling of groove 2 by the DNA template strand are coordinated and result in a stable configuration of the transcription complex. It is also possible that these structural changes are accompanied by TFIIH dissociation from the complex. However, it is unlikely that TFIIH action is a prerequisite for inducing the structural change required for promoter escape, because pol II can productively transcribe negatively supercoiled or premelted DNA templates in the absence of TFIIH (11,12,16,47). Resolving the details of the structural transition around registers 25–27 further requires a combination of biochemical experiments, such as precise DNA–protein crosslinking studies and three-dimensional structure determination of pol II transcription complexes at these stages of the transcription cycle.
Acknowledgments
ACKNOWLEDGEMENTS
We are grateful to Nell Shimazaki and Caroline Kane (University of California Berkeley) for supplying recombinant human TFIIS protein. We would like to thank Roger Kornberg, Patrick Cramer, Richard Ebright, Donal Luse, Peter van der Vliet and members of our laboratory for stimulating discussions. In addition, we thank Patrick Cramer, Robert Sijbrandi and Thomas Albert for critical review of this manuscript. U.F. was supported by a long-term fellowship from EMBO. In addition, U.F. and M.T. were supported by a Pionier grant from the Netherlands Organization for Scientific Research.
References
- 1.Eick D., Wedel,A. and Heumann,H. (1994) From initiation to elongation: comparison of transcription by prokaryotic and eukaryotic RNA polymerases. Trends Genet., 10, 292–296. [DOI] [PubMed] [Google Scholar]
- 2.Geiduschek E.P. and Kassavetis,G.A. (1995) Comparing transcriptional initiation by RNA polymerases I and III. Curr. Opin. Cell Biol., 7, 344–351. [DOI] [PubMed] [Google Scholar]
- 3.Zawel L. and Reinberg,D. (1995) Common themes in assembly and function of eukaryotic transcription complexes. Annu. Rev. Biochem., 64, 533–561. [DOI] [PubMed] [Google Scholar]
- 4.Fiedler U. and Timmers,H.T.M. (2000) Peeling by binding or twisting by cranking: models for promoter opening and transcription initiation by RNA polymerase II. Bioessays, 22, 316–326. [DOI] [PubMed] [Google Scholar]
- 5.Cramer P., Bushnell,D.A., Fu,J., Gnatt,A.L., Maier-Davis,B., Thompson,N.E., Burgess,R.R., Edwards,A.M., David,P.R. and Kornberg,R.D. (2000) Architecture of RNA polymerase II and implications for the transcription mechanism. Science, 288, 640–649. [DOI] [PubMed] [Google Scholar]
- 6.Zhang G., Campbell,E.A., Minakhin,L., Richter,C., Severinov,S. and Darst,S.A. (1999) Crystal structure of Thermus aquaticus core RNA polymerase at 3.3 angstrom resolution. Cell, 98, 811–824. [DOI] [PubMed] [Google Scholar]
- 7.Conaway R.C. and Conaway,J.W. (1993) General initiation factors for RNA polymerase II. Annu. Rev. Biochem., 62, 161–190. [DOI] [PubMed] [Google Scholar]
- 8.Orphanides G., Lagrange,T. and Reinberg,D. (1996) The general transcription factors of RNA polymerase II. Genes Dev., 10, 2657–2683. [DOI] [PubMed] [Google Scholar]
- 9.Roeder R.G. (1996) The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem. Sci., 21, 327–335. [PubMed] [Google Scholar]
- 10.Bunick D., Zandomeni,R., Ackerman,S. and Weinmann,R. (1982) Mechanism of RNA polymerase II-specific initiation of transcription in vitro: ATP requirement and uncapped runoff transcripts. Cell, 29, 877–886. [DOI] [PubMed] [Google Scholar]
- 11.Goodrich J.A. and Tjian,R. (1994) Transcription factors IIE and IIH and ATP hydrolysis direct promoter clearance by RNA polymerase II. Cell, 77, 145–156. [DOI] [PubMed] [Google Scholar]
- 12.Timmers H.T.M. (1994) Transcription initiation by RNA polymerase II does not require hydrolysis of the β–γ phosphoanhydride bond of ATP. EMBO J., 13, 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coin F. and Egly,J.-M. (1998) Ten years of TFIIH. Cold Spring Harbor Symp. Quant. Biol., 63, 105–110. [DOI] [PubMed] [Google Scholar]
- 14.Svejstrup J.Q., Vichi,P. and Egly,J.-M. (1996) The multiple roles of transcription/repair factor TFIIH. Trends Biochem. Sci., 21, 346–350. [PubMed] [Google Scholar]
- 15.Wang W., Carey,M. and Gralla,J.D. (1992) Polymerase II promoter activation: closed complex formation and ATP-driven start site opening. Science, 255, 450–453. [DOI] [PubMed] [Google Scholar]
- 16.Holstege F.C.P., van der Vliet,P.C. and Timmers,H.T.M. (1996) Opening of an RNA polymerase II promoter occurs in two distinct steps and requires the basal transcription factors IIE and IIH. EMBO J., 15, 1666–1677. [PMC free article] [PubMed] [Google Scholar]
- 17.Tirode F., Busso,D., Coin,F. and Egly,J.-M. (1999) Reconstitution of the transcription factor TFIIH: assignment of function for the three enzymatic subunits, XPB, XPD and cdk7. Mol. Cell, 3, 87–95. [DOI] [PubMed] [Google Scholar]
- 18.Winkler G.S., Araujo,S.J., Fiedler,U., Vermeulen,W., Coin,F., Egly,J.-M., Hoeijmakers,J.H.J., Wood,R.D., Timmers,H.T.M. and Weeda,G. (2000) TFIIH with inactive XPD helicase functions in transcription initiation but is defective in DNA repair. J. Biol. Chem., 275, 4258–4266. [DOI] [PubMed] [Google Scholar]
- 19.Kim T.K., Ebright,R.H. and Reinberg,D. (2000) Mechanism of ATP-dependent promoter melting by transcription factor IIH. Science, 288, 1418–1422. [DOI] [PubMed] [Google Scholar]
- 20.Dvir A., Conaway,R.C. and Conaway,J.W. (1997) A role for TFIIH in controlling the activity of early RNA polymerase II elongation complexes. Proc. Natl Acad. Sci. USA, 94, 9006–9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar K.P., Akoulitchev,S. and Reinberg,D. (1998) Promoter proximal stalling results from the inability to recruit transcription factor IIH to the transcription complex and is a regulated event. Proc. Natl Acad. Sci. USA, 95, 9767–9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradsher J., Coin,F. and Egly,J.-M. (2000) Distinct roles for helicases of TFIIH in transcript intiation and promoter escape. J. Biol. Chem., 275, 2532–2538. [DOI] [PubMed] [Google Scholar]
- 23.Akoulitchev S., Makela,T.P., Weinberg,R.A. and Reinberg,D. (1995) Requirement for TFIIH kinase activity in transcription by RNA polymerase II. Nature, 377, 557–560. [DOI] [PubMed] [Google Scholar]
- 24.Mäkelä T.P., Parvin,J.D., Kim,J., Huber,L.J., Sharp,P.A. and Weinberg,R.A. (1995) A kinase-deficient transcription factor TFIIH is functional in basal and activated transcription. Proc. Natl Acad. Sci. USA, 92, 5174–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serizawa H., Makela,T.P., Conaway,J.W., Conaway,R.C., Weinberg,R.A. and Young,R.A. (1995) Association of Cdk-activating kinase subunits with transcription factor TFIIH. Nature, 374, 280–282. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Y., Yan,M. and Gralla,J.D. (1996) A three-step pathway of transcription initiation leading to promoter clearance at an activated RNA polymerase II promoter. Mol. Cell. Biol., 16, 1614–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y. and Kornberg,R.D. (1994) Interplay of positive and negative effectors in function of the C-terminal repeat domain of RNA polymerase II. Proc. Natl Acad. Sci. USA, 91, 2362–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zawel L., Kumar,P. and Reinberg,D. (1995) Recycling of the general transcription factors during RNA polymerase II transcription. Genes Dev., 9, 1479–1490. [DOI] [PubMed] [Google Scholar]
- 29.Stelzer G., Goppelt,A., Lottspeich,F. and Meisterernst,M. (1994) Repression of basal transcription by HMG2 is counterparted by TFIIH-associated factors in an ATP-dependent process. Mol. Cell. Biol., 14, 4712–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holstege F.C.P. and Timmers,H.T.M. (1997) Analysis of open complex formation during RNA polymerase II transcription initiation using heteroduplex templates and potassium permanganate probing. Methods, 12, 203–211. [DOI] [PubMed] [Google Scholar]
- 31.Samkurashvili I. and Luse,D.S. (1998) Structural changes in the RNA polymerase II transcription complex during transition from initiation to elongation. Mol. Cell. Biol., 18, 5343–5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nudler E., Goldfarb,A. and Kashlev,M. (1994) Discontinuous mechanism of transcription elongation. Science, 265, 793–796. [DOI] [PubMed] [Google Scholar]
- 33.Lee D.N. and Landick,R. (1992) Structure of RNA and DNA chains in paused transcription complexes containing Escherichia coli RNA polymerase. J. Mol. Biol., 228, 759–777. [DOI] [PubMed] [Google Scholar]
- 34.Holstege F.C.P., Tantin,D., Carey,M., van der Vliet,P.C. and Timmers,H.T.M. (1995) The requirement for the basal transcription factor IIE is determined by the helical stability of promoter DNA. EMBO J., 14, 810–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 36.Timmers H.T.M. and Sharp,P.A. (1991) The mammalian TFIID protein is present in two functionally distinct complexes. Genes Dev., 5, 1946–1956. [DOI] [PubMed] [Google Scholar]
- 37.Holstege F.C.P., Fiedler,U. and Timmers,H.T.M. (1997) Three transitions in the RNA polymerase II-transcription complex during initiation. EMBO J., 16, 7468–7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uptain S.M., Kane,C.M. and Chamberlin,M.J. (1997) Basic mechanisms of transcript elongation and its regulation. Annu. Rev. Biochem., 66, 117–172. [DOI] [PubMed] [Google Scholar]
- 39.Wind M. and Reines,D. (2000) Transcription elongation factor SII. Bioessays, 22, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conaway J.W., Shilatifard,A., Dvir,A. and Conaway,R.C. (2000) Control of elongation by RNA polymerase II. Trends Biochem. Sci., 25, 375–380. [DOI] [PubMed] [Google Scholar]
- 41.Cipres-Palacin G. and Kane,C.M. (1994) Cleavage of the nascent transcript induced by TFIIS is insufficient to promote read-through of intrinsic blocks to elongation by RNA polymerase II. Proc. Natl Acad. Sci. USA, 91, 8097–8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas M.J., Platas,A.A. and Hawley,D.K. (1998) Transcriptional fidelity and proofreading by RNA polymerase II. Cell, 93, 627–637. [DOI] [PubMed] [Google Scholar]
- 43.Krummel B. and Chamberlin,M.J. (1992) Structural analysis of ternary complexes of Escherichia coli RNA polymerase. J. Mol. Biol., 225, 221–237. [DOI] [PubMed] [Google Scholar]
- 44.Awrey D.E., Shimasaki,N., Koth,C., Weilbaecher,R., Olmsted,V., Kazanis,S., Shan,X., Arello,J., Arrowsmith,C.H., Kane,C.M. and Edwards,A.M. (1998) Yeast transcription elongation factor (TFIIS), structure and function. J. Biol. Chem., 273, 22595–22605. [DOI] [PubMed] [Google Scholar]
- 45.Ishiguro A., Nogi,Y., Hisatake,K., Muramatsu,M. and Ishihama,A. (2000) The Rpb6 subunit of fission yeast RNA polymerase II is a contact target of the transcription elongation factor TFIIS. Mol. Cell. Biol., 2000, 1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaychikov E., Denissova,L. and Heumann,H. (1995) Translocation of the Escherichia coli transcription complex observed in the registers 11 to 20: “jumping” of RNA polymerase and assymmetric expansion and contraction of the “transcription bubble”. Proc. Natl Acad. Sci. USA, 92, 1739–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parvin J.D., Timmers,H.T.M. and Sharp,P.A. (1992) Promoter specificity of basal transcription factors. Cell, 68, 1135–1144. [DOI] [PubMed] [Google Scholar]