In the presence of netrin, tripartite motif protein 9 (TRIM9) promotes deleted in colorectal cancer (DCC) clustering, but TRIM9-dependent ubiquitination of DCC is reduced. Loss of ubiquitination promotes an interaction between DCC and FAK and FAK activation. FAK activation is required for the progression from SNARE assembly to exocytic vesicle fusion, which supplies membrane material for axon branching.
Abstract
Extracellular netrin-1 and its receptor deleted in colorectal cancer (DCC) promote axon branching in developing cortical neurons. Netrin-dependent morphogenesis is preceded by multimerization of DCC, activation of FAK and Src family kinases, and increases in exocytic vesicle fusion, yet how these occurrences are linked is unknown. Here we demonstrate that tripartite motif protein 9 (TRIM9)-dependent ubiquitination of DCC blocks the interaction with and phosphorylation of FAK. Upon netrin-1 stimulation TRIM9 promotes DCC multimerization, but TRIM9-dependent ubiquitination of DCC is reduced, which promotes an interaction with FAK and subsequent FAK activation. We found that inhibition of FAK activity blocks elevated frequencies of exocytosis in vitro and elevated axon branching in vitro and in vivo. Although FAK inhibition decreased soluble N-ethylmaleimide attachment protein receptor (SNARE)-mediated exocytosis, assembled SNARE complexes and vesicles adjacent to the plasma membrane increased, suggesting a novel role for FAK in the progression from assembled SNARE complexes to vesicle fusion in developing murine neurons.
INTRODUCTION
During development, extracellular axon guidance cues direct the extension and branching of axons, essential for appropriate anatomy and function in the adult brain. Disruption of axon extension and branching may lead to the defective connectivity implicated in neurodevelopmental and neuropsychiatric disorders (Engle, 2010; Grant et al., 2012). In the mammalian neocortex, the secreted axon guidance cue netrin-1 promotes attractive guidance and axon branching through the receptor deleted in colorectal cancer (DCC) (Kennedy and Tessier-Lavigne, 1995; Keino-Masu et al., 1996). Gene trap–mediated disruption or deletion of either Dcc or the gene encoding netrin-1 (Ntn1) in mice leads to defects in fiber tracts in the forebrain, including the corpus callosum and hippocampal commissure, and perinatal or embryonic lethality (Serafini et al., 1996; Fazeli et al., 1997; Bin et al., 2015; Yung et al., 2015). Netrin-dependent neuronal morphogenesis is preceded by several subcellular events. In the presence of netrin-1, DCC is recruited to clusters within the plasma membrane, where it homomultimerizes (Mille et al., 2009; Matsumoto and Nagashima, 2010; Wang et al., 2014; Gopal et al., 2016). The noncatalytic cytoplasmic tail of DCC interacts with nonreceptor tyrosine kinases, leading to their activation. These targets include FAK and Src family kinases (SFKs), which are involved in cell adhesion, migration survival, neuritogenesis, and axon outgrowth (Li et al., 2004; Liu et al., 2004; Meriane et al., 2004; Ren et al., 2004). The molecular regulators that control and coordinate DCC relocalization and downstream kinase activation are not fully elucidated.
We identified vertebrate TRIM9, an evolutionarily conserved class I tripartite motif (TRIM) protein (Berti et al., 2002; Tanji et al., 2010), as a key regulator of netrin-dependent morphogenesis in cortical and hippocampal neurons (Winkle et al., 2014; Winkle, Olsen, et al., 2016; Menon, Boyer, et al., 2015). The single invertebrate orthologues of Ntn1 and of Dcc regulate axon development through the single class I TRIM orthologue (Hao et al., 2010; Morikawa et al., 2011). Mammalian TRIM9 directly interacts with the cytoplasmic tail of DCC, the neuronal exocytic target (t)-SNARE SNAP25, and the filopodial actin polymerase VASP (Li et al., 2001; Winkle et al., 2014; Menon, Boyer, et al., 2015). Genetic deletion of murine Trim9 in cortical neurons is associated with a loss of netrin-1 responsiveness, elevated exocytosis, enhanced growth cone filopodial stability in vitro, and defects in axon branching and axon projections in vitro and in vivo (Winkle et al., 2014; Winkle, Olsen, et al., 2016; Menon, Boyer, et al., 2015). However, whether TRIM9 plays a role in DCC localization or FAK-dependent intracellular signal transduction downstream of DCC/netrin-1 remains unknown.
Here we find that DCC is ubiquitinated in a TRIM9-dependent manner. Our data are consistent with the hypothesis that DCC ubiquitination blocks the activation of FAK and SFK in the absence of netrin-1. Following netrin-1 stimulation, DCC ubiquitination is reduced, and TRIM9-dependent clustering and multimerization of DCC occurs. FAK also becomes phosphorylated and activated, and SNARE-mediated exocytosis and axon branching increase. Inhibition of FAK activity blocks netrin-dependent exocytosis and axon branching but, surprisingly, increases the number of assembled SNARE complexes and the density of vesicles found immediately adjacent to the plasma membrane. This suggests a novel requirement for FAK activity in the progression from an assembled SNARE complex to SNARE-mediated fusion, which is necessary for plasma membrane expansion during axon branching.
RESULTS
Deletion of Trim9 disrupts netrin-dependent clustering and multimerization of DCC
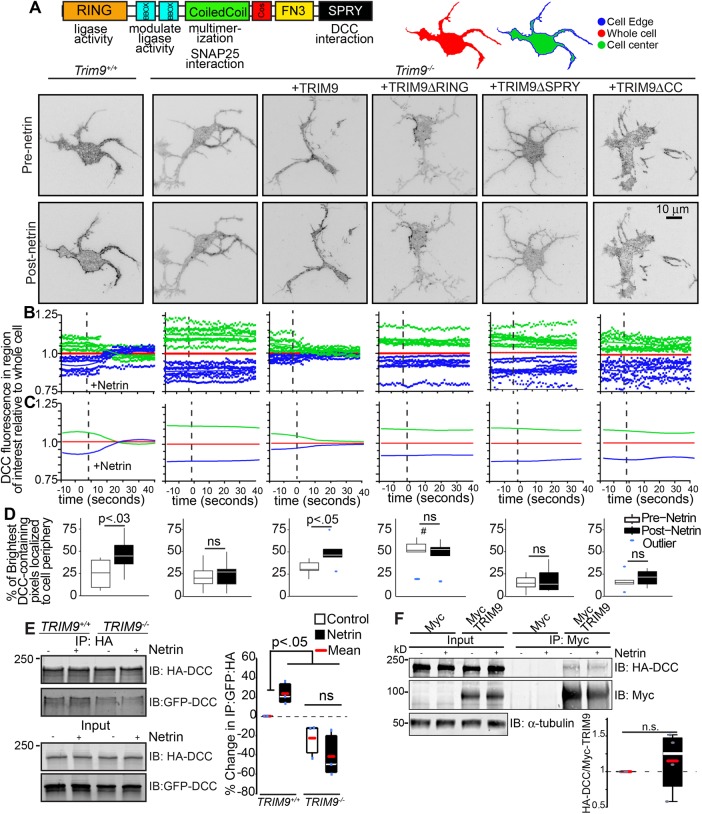
Application of netrin-1 induces clustering of its receptor DCC at the plasma membrane (Matsumoto and Nagashima, 2010; Gopal et al., 2016). Because we had observed colocalization of TRIM9 and DCC in punctae along the periphery of neurites (Winkle et al., 2014), we hypothesized that TRIM9 may play a role in netrin-dependent DCC clustering. Using total internal reflection fluorescence (TIRF) microscopy, we observed rapid mCherry-DCC cluster formation at the cell edge of Trim9+/+ neurons immediately following netrin-1 stimulation, which was absent in Trim9−/− neurons (Figure 1A and Supplemental Movie 1). To quantify the relocalization of DCC, we compared the change in mCherry-DCC fluorescence at the cell edge (Figure 1B, outer 0.64 μm, blue) and the cell center (green), normalized to the whole cell (Figure 1B, red). To reveal trends in fluorescence intensity changes, we fit these data to a local polynomial regression (Figure 1C). In Trim9+/+ neurons, DCC fluorescence increased at the cell edge and decreased in the cell center after netrin-1 stimulation, suggesting a rapid rearrangement of DCC. Clustered mCherry-DCC should exhibit higher fluorescence intensity than nonclustered DCC. To quantify changes in the localization of clustered DCC, we considered the localization of the brightest 10% of mCherry-DCC–containing pixels (Figure 1D). This revealed that the brightest DCC-containing pixels (likely DCC clusters) increasingly localized to the cell edge following netrin-1 stimulation in Trim9+/+ neurons. Netrin-dependent clustering of DCC was also observed on the apical plasma membrane by spinning-disk confocal microscopy (Supplemental Figure S1), indicating that DCC relocalization was not limited to the basal cell surface.
FIGURE 1:
Loss of Trim9 impairs netrin-dependent clustering and multimerization of DCC. (A) Inverted TIRF images of Trim9+/+ and Trim9−/− cortical neurons expressing fluorescently tagged DCC before and after netrin-1 application. Schematics of TRIM9 domain structure and functions (left), and of analysis regions of interest within cell mask, including whole cell (red), cell edge (blue, 0.64 μm) and cell center (green). (B) Graphs of average mean fluorescence of DCC in the denoted ROIs in individual cells over time. Values are normalized to the average intensity of the whole cell. (C) Local polynomial regression of data shown in B demonstrates trends in temporal changes in average DCC fluorescence intensity in each ROI. (D) The percent of the pixels containing the highest 10% of DCC fluorescence intensity in the TIRF illumination field that localize at the cell perimeter. The p values (assessed via two-way ANOVA with Tukey post hoc correction) show differences between each pre– and post–netrin-1 condition. #, p < 0.0005 difference from pre–netrin-1 Trim9+/+. (E) Immunoblot of inputs and immunoprecipitates (IPs) of HA-DCC. Coimmunoprecipitation of GFP-DCC increases following netrin-1 stimulation in TRIM9+/+ cells. The p values were assessed by Kruskal-Wallis ANOVA with LSD post hoc correction. (F) Immunoblot of Myc-TRIM9 and HA-DCC in inputs and IPs of Myc and Myc-TRIM9 in TRIM9+/+ HEK 293 cells. The p values were assessed by Mann-Whitney test.
This netrin-dependent redistribution and clustering of mCherry-DCC was absent in Trim9−/− neurons (Figure 1, A–D, and Supplemental Movie 1) and rescued by reintroduction of enhanced green fluorescent protein (eGFP)-TRIM9 (Figure 1, A–D, and Supplemental Movie 2, arrowheads), indicating that DCC clustering was dependent upon TRIM9. Expression of a TRIM9 mutant lacking the ubiquitin ligase RING domain (TRIM9ΔRING) induced clustering of DCC independent of netrin-1 (Figure 1, A–D, and Supplemental Movie 2). This gain of function suggests that the ligase domain of TRIM9 inhibits clustering of DCC in the absence of netrin-1. Expression of a TRIM9 variant lacking the DCC-binding SPRY domain (TRIM9ΔSPRY) or a mutant lacking the TRIM multimerization coiled-coil motif (TRIM9ΔCC) failed to rescue netrin-dependent clustering of DCC (Figure 1, A–D, and Supplemental Movie 2), suggesting that the interaction between TRIM9 and DCC and the multimerization of TRIM9 are both critical for TRIM9-dependent DCC clustering. These results reveal a netrin-stimulated, TRIM9-dependent spatial reorganization of DCC at the plasma membrane.
In addition to clustering in the plasma membrane, DCC homomultimerizes upon application of netrin-1, as revealed by the coimmunoprecipitation of differentially tagged DCC proteins (Mille et al., 2009; Bin et al., 2013). Because spatial clustering may involve multimerization (Figure 1, A–D), we hypothesized that TRIM9 may also be involved in DCC multimerization. Using previously reported TRIM9+/+ and TRIM9−/− HEK293 cells (Menon, Boyer, et al., 2015), we found that immunoprecipitation of HA-tagged DCC with antibodies against the HA epitope coprecipitated pHluorin-DCC (Figure 1E). Coimmunoprecipitation of pHluorin-DCC was enhanced by netrin-1 stimulation in TRIM9+/+ HEK293 cells and was reduced and netrin-insensitive in TRIM9−/− HEK293 cells. Thus multimerization of DCC was promoted by TRIM9. We did not observe netrin-dependent changes in the amount of DCC coimmunoprecipitated by TRIM9 (Figure 1F), indicating that netrin-dependent changes in DCC multimerization cannot be explained by an increased interaction between TRIM9 and DCC.
Loss of Trim9 leads to hyperactivation of FAK
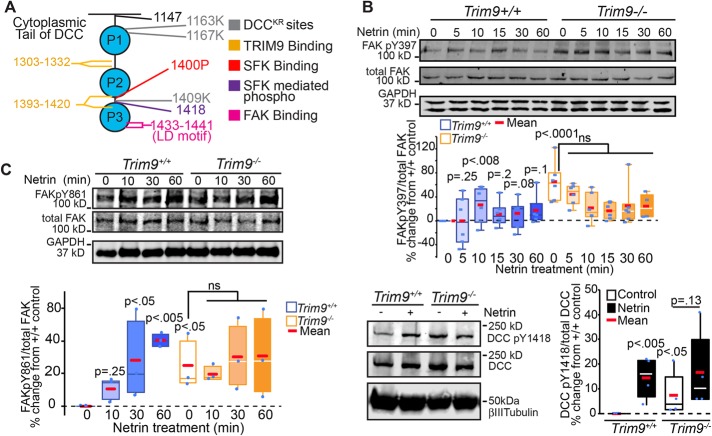
We hypothesized that the defects in netrin-dependent clustering and multimerization of DCC may be associated with defects in the netrin-triggered activation of FAK and SFK. Previous studies have shown that the nonreceptor tyrosine kinase FAK directly interacts with the COOH-terminal LD motif on the P3 domain of DCC (Figure 2A) and that FAK is autophosphorylated at Y397 (pY397) upon netrin stimulation, leading to FAK activation (Li et al., 2004; Liu et al., 2004; Ren et al., 2004). We similarly observed rapid netrin-dependent increases in FAK pY397 in Trim9+/+ neurons that returned to baseline and, surprisingly, found enhanced basal FAK pY397 in Trim9−/− neurons (Figure 2B). Although netrin-dependent clustering of DCC is associated with activation of FAK, these data suggest clustering is not necessary for FAK activation and, further, that TRIM9 constrains FAK activation in the absence of netrin.
FIGURE 2:
Loss of Trim9 leads to hyperactivation of FAK. (A) Schematic representation of the cytoplasmic tail of DCC showing key mutation and interaction sites. (B) Immunoblots of endogenous total FAK, FAKpY397, and GAPDH from cortical lysate. Quantification of percent change in each phosphorylation event, normalized to total FAK relative to Trim9+/+ neurons. The p values noted are for Kruskal-Wallis ANOVA with LSD post hoc correction to Trim9+/+ control, unless otherwise noted. (C) Immunoblots of endogenous total FAK, FAKpY861, and GAPDH from cortical lysate. Quantification of percent change in each phosphorylation event normalized to total FAK relative to Trim9+/+ neurons. The p values noted are for Kruskal-Wallis ANOVA with LSD post hoc correction to Trim9+/+ control, unless otherwise noted. (D) Immunoblots of endogenous DCC pY1418, DCC, and βIII tubulin in Trim9+/+ and Trim9−/− cortical neurons. Quantification of levels of pY1418/DCC relative to Trim9+/+ neurons. The p values noted are for Kruskal-Wallis ANOVA with LSD post hoc correction, compared with Trim9+/+ control, unless otherwise noted.
SFKs dock at pY397 on FAK and interact with the PXXP motif beginning at P1400 on the cytoplasmic tail of DCC (Li et al., 2004; Ren et al., 2004; Figure 2A). This interaction leads to a number of SFK-dependent phosphorylation events, including FAK pY861 and DCC pY1418. In Trim9+/+ neurons, these SFK-dependent phosphorylation events increased in response to netrin-1 stimulation (Figure 2, C and D), recapitulating previous results (Li et al., 2004; Liu et al., 2004; Meriane et al., 2004). However, these phosphorylation events were aberrantly elevated in Trim9−/− cortical neurons, suggesting that FAK hyperactivation promotes downstream phosphorylation events. TRIM9 interacts with two sites on the cytoplasmic tail of DCC (Winkle et al., 2014). These sites do not overlap with the established FAK binding site (Figure 2A), and we did not observe reduced interaction between TRIM9 and DCC upon netrin addition (Figure 1F), suggesting that a different TRIM9-dependent mechanism controls activation of FAK.
TRIM9-dependent ubiquitination of DCC does not alter protein stability
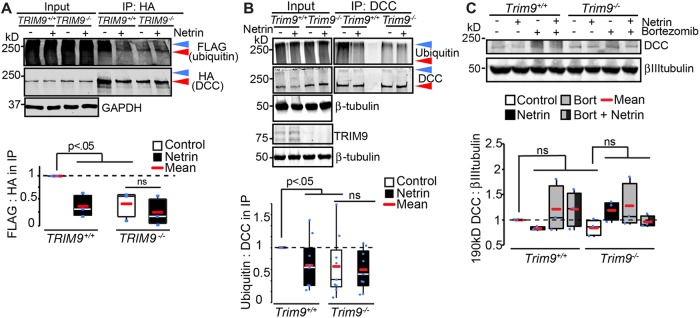
Because TRIM9 is a brain-enriched E3 ubiquitin ligase, and ubiquitination of DCC in embryonic cortical neurons and heterologous cells has been reported (Hu et al., 1997; Kim et al., 2005), we examined whether DCC ubiquitination was dependent on TRIM9. In TRIM9+/+ HEK293 cells, immunoprecipitation of HA-DCC under denaturing conditions revealed an HA+ band at the expected molecular weight (∼190 kDa; Figure 3A, red arrowhead) and a heavier HA+ band that migrated with coimmunoprecipitated FLAG-ubiquitin (Figure 3A, blue arrowhead). These higher molecular weight species are indicative of DCC ubiquitination. Either netrin-1 addition in the presence of TRIM9 or genetic loss of TRIM9 reduced FLAG-ubiquitin coimmunoprecipitated by HA-DCC (Figure 3A), suggesting that this modification is netrin-sensitive and TRIM9-dependent. To confirm the relevance of these results in neurons, we immunoprecipitated endogenous DCC from primary cultured Trim9+/+ or Trim9−/− embryonic cortical neurons under denaturing conditions and immunoblotted for DCC and ubiquitin (Figure 3B). This revealed that cortical neurons also exhibited TRIM9-dependent, netrin-sensitive DCC ubiquitination. SDS–PAGE and immunoblotting of cortical neuron lysate resolved a DCC immunoreactive band at the expected molecular weight of 190 kDa (Figure 3C). However, neither netrin addition, genetic loss of Trim9, nor treatment with the proteosome inhibitor bortezomib altered the level of this band, suggesting that TRIM9-dependent ubiquitination of DCC does not alter DCC stability.
FIGURE 3:
Netrin-sensitive, TRIM9-dependent ubiquitination of DCC. (A) SDS–PAGE and Western blots of inputs and HA-DCC IPs from TRIM9+/+ and TRIM9−/− HEK293 cells blotted for FLAG-ubiquitin and HA-DCC. High-molecular-weight ubiquitin in the IP is considered DCC ubiquitination (blue arrowhead). Red arrowhead demarcates DCC at expected molecular weight ∼190 kDa. The p values are from Kruskal-Wallis ANOVA with LSD post hoc correction. (B) SDS–PAGE and Western blots of inputs and DCC IPs from Trim9+/+ and Trim9−/− cortical neurons blotted for endogenous ubiquitin and DCC. Blue arrowhead demarcates ubiquitinated DCC, red arrowhead demarcates DCC at expected molecular weight ∼190 kDa. The p values are from Kruskal-Wallis ANOVA with LSD post hoc correction compared with Trim9+/+ control. (C) Immunoblot of cortical lysate shows DCC at expected molecular weight (∼190 kDa) is insensitive to inhibition of the proteasome (100 μM bortezomib) or deletion of Trim9.
A DCC mutant lacking ubiquitination sites shows increased interaction with FAK and increased phosphotyrosine levels
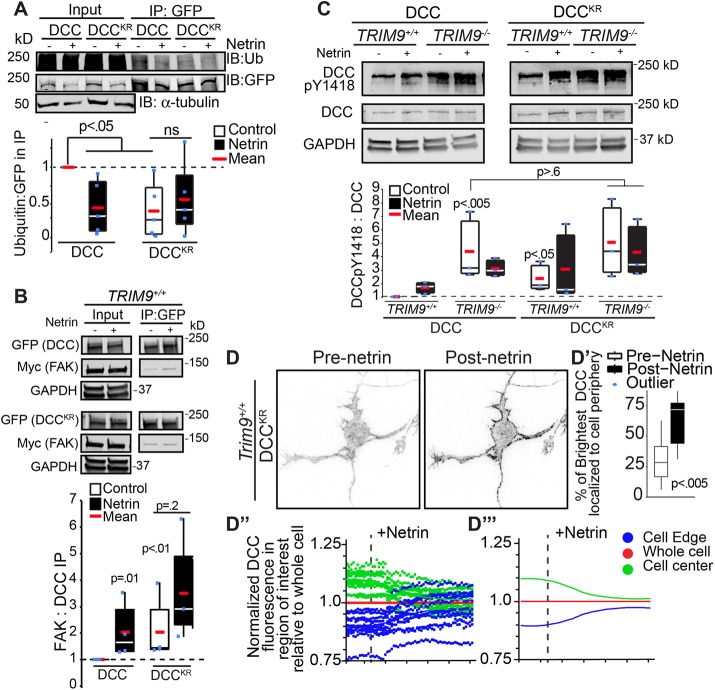
Bioinformatic analysis of the cytoplasmic tail of DCC identified three residues (K1163, K1167, and K1409) as putative ubiquitination sites, which we mutated to R (DCCKR). One of these K residues (K1409) is situated between the FAK and SFK binding sites, near Y1418 on DCC (Figure 2A). pHluorin-DCCKR exhibited reduced ubiquitination in HEK293 cells compared with pHluorin-DCC (Figure 4A). On the basis of the size of ubiquitin (∼8 kDa) relative to the P3 domain of DCC (∼3.7 kDa) and our observation that FAK is activated upon reduced DCC ubiquitination (Figures 2B and 3, A and B), we hypothesized that ubiquitination of DCC occludes access of FAK to the P3 domain and prevents the downstream activation of FAK and SFK. Consistent with this hypothesis, compared with pHluorin-DCC, pHluorin-DCCKR exhibited increased interaction with Myc-FAK in HEK293 cells in the absence of netrin, as measured by coimmunoprecipitation (Figure 4B), which was not further enhanced with netrin addition. Further, in TRIM9+/+ HEK293 cells, DCC pY1418 was enhanced on pHluorin-DCCKR as compared with pHlourin-DCC (Figure 4C). In TRIM9−/− cells, pY1418 DCC was enhanced compared with TRIM9+/+ HEK293 cells and insensitive to netrin-1 or the K-R mutations. These data suggest that TRIM9-dependent ubiquitination of DCC blocks access of FAK to DCC, which inhibits downstream FAK and SFK-dependent phosphorylation events. pHluorin-DCCKR did display rapid netrin-dependent clustering at the periphery of Trim9+/+ neurons (Figure 4D and Supplemental Movie 3), indicating that netrin-dependent clustering is independent of changes in the status of DCC ubiquitination.
FIGURE 4:
Ubiquitination of DCC regulates FAK interactions and activation, but not DCC clustering. (A) SDS–PAGE and Western blots of inputs and GFP-DCC IPs from TRIM9+/+ HEK293 cells blotted for FLAG-ubiquitin and GFP-DCC. High-molecular-weight ubiquitin in the IP is considered DCC ubiquitination. DCC ubiquitination in HEK293 cells is decreased upon addition of netrin-1 or mutation of K1163, K1167, and K1409 (DCCKR). The p values are from Kruskal-Wallis ANOVA with LSD post hoc correction. (B) Western blot of inputs and IPs of GFP-DCC or GFP-DCCKR and Myc-FAK from TRIM9+/+ and TRIM9−/− HEK293 cells. The p values noted are for Kruskal-Wallis ANOVA with LSD post hoc correction. (C) Immunoblots of DCC pY1418, DCC and GAPDH in HEK293 cells expressing GFP-DCC or GFP-DCCKR. The p values noted are for Kruskal-Wallis ANOVA with LSD post hoc compared with DCC in TRIM9+/+ control. (D) Inverted TIRF images of Trim9+/+ cortical neuron expressing fluorescently-tagged DCCKR before and after netrin-1 application. (D′) The percent of the pixels containing the highest 10% of DCC fluorescence intensity in the TIRF illumination field that localize at the cell perimeter. The p values assessed via t test show differences between each pre– and post–netrin-1 condition. (D″) Graphs of average mean fluorescence of DCC in the denoted ROIs in individual cells over time. (D‴) Local polynomial regression of data demonstrates trends in temporal changes in average DCC fluorescence intensity in each ROI.
Inhibition of FAK activity blocks axon branching
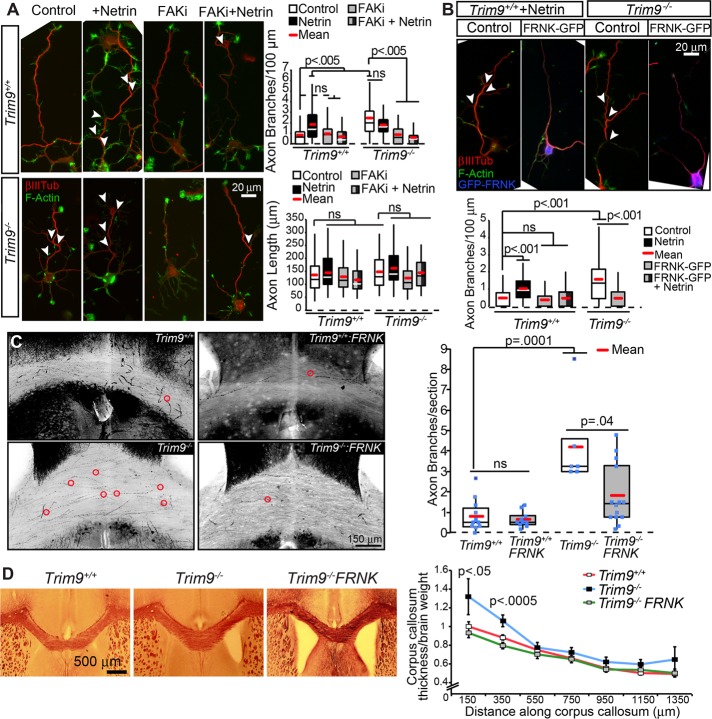
Netrin-1 promotes axon branching, whereas deletion of Trim9 aberrantly elevates branching in a netrin-independent manner (Dent et al., 2004; Winkle et al., 2014). FAK and SFK activity are required for netrin-dependent axon outgrowth and guidance (Liu et al., 2004; Ren et al., 2004; Mehlen and Rama, 2007) but have not been implicated in axon branching. To determine whether FAK activity was required for netrin-dependent axon branching and the elevated axon branching observed in Trim9−/− neurons, we used a pharmacological inhibitor, FAK inhibitor 14 (FAKi), which prevents autophosphorylation of FAK at Y397 (Golubovskaya et al., 2008) and, thus, FAK activity. FAKi treatment blocked netrin-dependent increases in axon-branching density in Trim9+/+ neurons and reduced the exuberant axon-branching phenotype in Trim9−/− neurons (Figure 5A) without significant alterations to axon length. Similar results were observed upon expression of FAK-related nonkinase (FRNK; Figure 5B), a noncatalytic variant of FAK that acts as a dominant negative (Richardson and Parsons, 1996). These data suggest that FAK activity is required downstream of DCC and TRIM9 to promote netrin-dependent axon branching and for the increased axon branching that occurs in the absence of Trim9.
FIGURE 5:
FAK activity is required for axon branching. (A) Phalloidin-stained F-actin (green) and βIII tubulin (red) in Trim9+/+ and Trim9−/− cortical neurons at 72 h in culture. Quantification of density of axon branches/100-μm axon length. The p values are from Kruskal-Wallis ANOVA with Bonferroni post hoc correction. (B) Phalloidin-stained F-actin (green) and βIII tubulin (red) ± GFP-FRNK (blue) in Trim9+/+ and Trim9−/− cortical neurons 72 h after plating. Quantification of density of axon branches/100-μm axon length. The p values are from Kruskal-Wallis ANOVA with Bonferroni post hoc correction. (C) Inverted maximal projections of Trim9+/+/Thy1-GFP, Trim9+/+/Thy1-GFP/NexCre/FRNKLoxSTOPLox, Trim9−/−/Thy1-GFP, and Trim9−/−/Thy1-GFP/NexCre/FRNKLoxSTOPLox corpus callosum sections. Red circles denote branch points. Quantification of average axonal branches per section per mouse (n = 7–15 mice/genotype). The p values are from Kruskal-Wallis ANOVA with Bonferroni post hoc correction. (D) Black Gold staining and quantification of position-matched coronal sections through Trim9+/+, Trim9−/−/Thy1-GFP, and Trim9−/−/Thy1-GFP/NexCre/FRNKLoxSTOPLox mice. Quantification of density of axon branches normalized to brain weight. The p values are from Kruskal-Wallis ANOVA.
Using a Thy1-GFP reporter mouse (Feng et al., 2000), we previously found that Trim9−/− mice exhibit aberrant axon branching within the corpus callosum (Winkle et al., 2014), which was associated with corpus callosum thickening. To test whether FAK activity was required for exuberant branching in vivo, we crossed Trim9−/−/Thy1-GFP mice with FRNKLoxSTOPLox (DiMichele et al., 2009) and Nex:CRE mice (Goebbels et al., 2006). The resultant mice express Cre in postmitotic cortical neurons, which promotes recombination and expression of FRNK specifically in these cells. The z-stacks of position-matched coronal sections through the corpus callosum were imaged by laser-scanning confocal microscopy, and GFP+ axons were inspected for branch points. FRNK expression did not affect axon branching in the corpus callosum of Trim9+/+ mice but did reduce the aberrant axon branching in the corpus callosum of Trim9−/− mice (Figure 5C and Supplemental Movie 4), suggesting that FAK activity is necessary for aberrant axon branching in the absence of Trim9 in vivo. Myelin staining of position-matched coronal sections revealed that FRNK expression also reduced the elevated thickness in the rostral portion of the Trim9−/− corpus callosum (Figure 5D), further supporting the hypothesis that this thickening is at least partially due to aberrant axon branching.
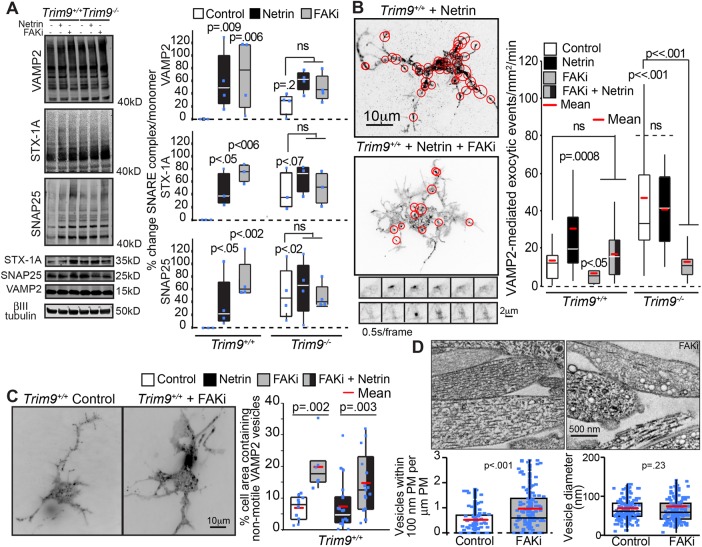
FAK inhibition blocks SNARE-dependent exocytosis but not SNARE-complex assembly
Axon branching increases the neuronal plasma membrane surface area, which necessitates insertion of new plasma membrane material (Winkle, Taylor, Dent, Gallo, Greif, and Gupton, 2016). We previously demonstrated that netrin-dependent axon branching and the exuberant axon branching in Trim9−/− neurons requires SNARE-mediated exocytosis and is associated with increased SNARE-complex assembly and an increased frequency of exocytosis (Winkle et al., 2014). Because FAK inhibition blocked axon branching (Figure 5), we hypothesized that FAK activation promotes the requisite SNARE-complex assembly and exocytosis, thus driving plasma membrane expansion during axon branching. We exploited FAKi treatment and the SDS-resistance of SNARE complexes (Söllner et al., 1993; Hayashi et al., 1995) to investigate this possibility (Figure 6A). Netrin-1 stimulation of Trim9+/+ neurons or deletion of Trim9 increased the amount of assembled SNARE complexes (Figure 6A) as previously observed. Unexpectedly, FAKi treatment increased basal levels of assembled SNARE complexes in Trim9+/+ neurons and failed to disrupt the elevated SNARE assembly in Trim9−/− neurons. These data suggested that FAK activity is not required for SNARE-complex formation. To determine whether the elevated SNARE complexes that were present following FAKi treatment were driving increased exocytosis, we tracked the frequency of vesicle-associated membrane protein 2 (VAMP2)-pHluorin vesicle fusion events via TIRF microscopy. Application of netrin-1 increased basal exocytic fusion frequency in Trim9+/+ cortical neurons as expected; however, this netrin-1–dependent response was blocked by FAKi treatment (Figure 6B and Supplemental Movie 5). Similarly, the aberrantly high exocytic vesicle fusion in Trim9−/− neurons was decreased by FAKi treatment (Figure 6B).
FIGURE 6:
Inhibition of FAK activity blocks SNARE-mediated exocytic vesicle fusion but not SNARE-complex formation. (A) Immunoblots and quantification of SNARE complexes (above 40 kDa) and monomers for VAMP2, syntaxin-1 (STX-1A), and SNAP25 shows that netrin-1 stimulation, genetic loss of Trim9, or FAKi treatment increases the accumulation of SNARE complexes in cortical neurons. The p values are from Kruskal-Wallis ANOVA with LSD post hoc correction compared with Trim9+/+ control, unless otherwise noted. (B) Quantification of VAMP2-pHluorin exocytic vesicle fusion events and example maximum projections of inverted time-lapse TIRF images of Trim9+/+ neurons expressing VAMP2-pHluorin treated with netrin-1 or netrin-1 and FAKi. Red circles indicate sites of vesicle fusion events. Montages represent examples of exocytic fusion events in a soma (top) and neurite (bottom). The p values are from Kruskal-Wallis ANOVA with Bonferroni post hoc correction to Trim9+/+ control, unless otherwise noted. (C) Average projection of TIRF time-lapse images of GFP-VAMP2 vesicles in Trim9+/+ control and FAKi-treated neurons. Quantification of percent of the total cell area containing nonmotile vesicles. The p values are from Kruskal-Wallis ANOVA with Tukey post hoc correction. (D) Representative transmission electron micrographs of Trim9+/+ control and FAKi-treated neurons reveals an increase in vesicle-like structures near the plasma membrane of FAKi-treated cells. Left, density of vesicles within 100 nm of the plasma membrane; p value from Mann-Whitney test. Right, vesicle diameter; p value from t test.
These data suggested the intriguing possibility that FAK activity functions in the progression from assembled trans-SNARE complexes to lipid bilayer fusion. We hypothesized that, during FAKi treatment, these nonproductive SNARE complexes might be tethering nonfusing vesicles to the plasma membrane. To test this, we imaged eGFP-VAMP2 within 100 nm of the basal plasma membrane of Trim9+/+ cortical neurons via TIRF microscopy to visualize vesicles at the basal neuronal surface (Figure 6C and Supplemental Movie 6). FAKi treatment was associated with an increased percentage of the basal neuronal membrane area containing nonmotile eGFP-VAMP2 in both the presence and absence of netrin-1 (Figure 6C and Supplemental Movie 6), supporting the hypothesis that FAK activity is required for the progression from assembled SNARE complexes to membrane fusion. To determine whether FAKi treatment increased the number of vesicles adjacent to the plasma membrane or altered vesicle size, we performed transmission electron microscopy of cultured neurons. This confirmed an increased density of vesicles within 100 nm of the plasma membrane in FAKi-treated neurons, with no change in vesicle diameter (Figure 6D). These data suggest FAK activity is required for the progression from trans-SNARE complex to vesicle fusion. Therefore, by modulating the activation of FAK, TRIM9 controls exocytosis and axon branching.
DISCUSSION
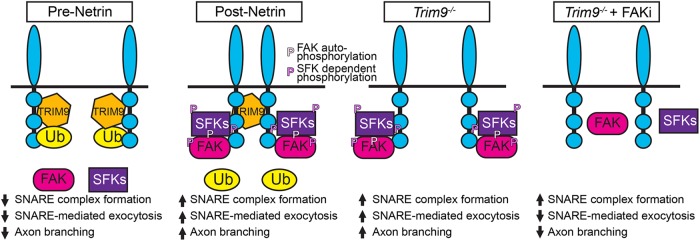
Here we demonstrate TRIM9-dependent mechanisms that control the localization and function of the netrin receptor DCC and the downstream signaling events and exocytic machinery that promote netrin-dependent axonal branching. Our data suggest that the TRIM9-dependent ubiquitination of DCC that occurs in the absence of netrin-1 precludes activation of FAK and SFKs (Figure 7), important regulators of netrin-dependent axonal morphogenesis. TRIM9 thus maintains the neuron in an immature morphology, yet poised for the rapid responses to netrin-1 required for axon branching. Upon addition of netrin-1, DCC ubiquitination is reduced, and TRIM9 mediates clustering and multimerization of DCC at the plasma membrane and promotes downstream interaction with and activation of FAK and SFKs, followed by elevated SNARE-mediated exocytosis and axon branching. Our studies suggest that this pathway is relevant in vivo as well. We identified an unexpected role for FAK activity in the progression from assembled SNAREs to exocytic vesicle fusion at the plasma membrane. Together with previous work demonstrating that TRIM9 constrains the function of the exocytic t-SNARE SNAP25 and the filopodial actin polymerase VASP in the absence of netrin-1 (Li et al., 2001; Mille et al., 2009; Bin et al., 2013; Winkle et al., 2014; Menon, Boyer, et al., 2015), these findings reveal an overarching function for TRIM9 in preparing the neuron for spatiotemporally controlled morphological responses to the axon guidance cue netrin-1. By moderating the local signaling necessary for axon branching, TRIM9 plays an integral role in establishing proper morphogenesis in vitro and in vivo, which is likely critical for behavior, as Trim9−/− mice exhibit severe deficits in spatial learning and memory (Winkle, Olsen, et al., 2016).
FIGURE 7:
Model of TRIM9-mediated regulation of signaling scaffolds. In the absence of netrin-1, DCC is ubiquitinated in the presence of TRIM9. The addition of ubiquitin inhibits FAK binding and downstream activation of FAK and SFK and constrains vesicle fusion and axon branching. The addition of netrin-1 results in loss of DCC ubiquitination and TRIM9-dependent clustering of DCC. FAK binds to the P3 domain of DCC and is autophosphorylated, leading to interactions with SFK and SFK-dependent phosphorylation events. This creates locally activated signaling complexes that promote vesicle fusion and axon branching. In the absence of TRIM9, the loss of DCC ubiquitination leads to aberrant interaction and activation of FAK and SFK, but a lack of signal complex clustering, leading to aberrant exocytosis and branching. Inhibition of FAK activity blocks subsequent SFK activity and SNARE-mediated exocytosis and branching.
Nondegradative roles for DCC ubiquitination
Classically, ubiquitination targets a substrate for degradation. Previous studies have demonstrated that DCC ubiquitination and proteasomal degradation of DCC are promoted by addition of netrin (Kim et al., 2005). After the acute netrin-1 treatments used here, ubiquitination of DCC was decreased in cortical neurons, but DCC levels were not. Our findings in both cortical neurons and HEK293 cells support TRIM9-dependent ubiquitination of DCC; however, whether TRIM9 directly ubiquitinates DCC remains unknown. Interestingly, the Siah family of ubiquitin ligases have been implicated in the ubiquitination of both DCC and TRIM9 in heterologous cells (Hu et al., 1997; Fanelli et al., 2004). Although loss of TRIM9 reduced DCC ubiquitination in both neurons and human cells, residual ubiquitination remained, suggesting that DCC may be a substrate of multiple ligases. Alternatively, TRIM9 may regulate the function of another ligase, such as Siah. How these ligases cooperate to regulate DCC remains to be determined.
In neurons, FAK and SFK are activated downstream of DCC binding to netrin-1, analogous to “outside-in” activation of integrin receptors triggered by binding their extracellular ligands. Integrins alternatively can be activated “inside-out” through cytoplasmic binding partners (Shattil et al., 2010; Ye and Petrich, 2011). Our data are consistent with a model in which TRIM9-dependent ubiquitination of the cytoplasmic tail of DCC prevents inside-out activation of this signaling pathway by occluding FAK binding to DCC (Figure 7). Inhibition of the interaction between FAK and DCC has previously been shown to prevent FAK- and SFK-dependent phosphorylation of FAK, Fyn, Src, and DCC (Li et al., 2004; Liu et al., 2004; Ren et al., 2004). Downstream of DCC pY1418, activation of guanine nucleotide exchange factors that activate Rac1 results in increased actin polymerization (Antoine-Bertrand et al., 2015). This actin polymerization may also participate in the formation of nascent axon branches. This nondegradative function of ubiquitination may represent a new mode of rapid protein and pathway regulation in the cell that prevents interactions and phosphorylation events.
Ligase-independent function of TRIM9
Here we discovered a ligase-independent function of TRIM9 in the spatial organization of DCC in response to netrin-1. The dramatic and rapid clustering of DCC at the cell periphery that occurred in response to netrin-1 was completely absent in Trim9−/− neurons. In the Caenorhabditis elegans anchor cell, the netrin orthologue unc-6 stabilizes oscillating clusters of the DCC orthologue unc-40, and the TRIM9 orthologue madd-2 facilitates ligand-independent clustering (Wang et al., 2014), similar to TRIM9ΔRING in mammalian cortical neurons, which suggests evolutionarily conserved functions for TRIM9 in DCC clustering. Structure–function experiments in neurons indicate that the DCC binding and multimerization domains of TRIM9 are necessary for this response, suggesting that the TRIM9 interaction with DCC is responsible for this spatial reorganization. However, that interaction alone cannot explain the netrin-dependent clustering as shown by coimmunoprecipitation experiments. Further, the observation that the TRIM9 mutant lacking the ligase domain could induce clustering in the absence of netrin-1 suggests that ligase domain or ligase activity of TRIM9 prevents DCC clustering. This is unlikely to occur through ubiquitination of DCC, as DCCKR did not exhibit aberrant clustering before netrin-1 application. Like most E3 ligases, TRIM9 is capable of autoubiquitination in vitro (Tanji et al., 2010). We speculate that netrin-dependent autoubiquitination of TRIM9 could permit TRIM9-dependent clustering of DCC, but this remains to be shown. DCC association with lipid rafts is required for netrin-1–dependent axonal outgrowth (Hérincs et al., 2005). An intriguing hypothesis is that these netrin-dependent DCC clusters represent regulated signaling microdomains possibly within lipid rafts near netrin-1 sources. The formation of these local domains may ensure the spatial and temporal specificity required to form collateral axon branches or may be required for axon turning during development.
TRIM9 employs a “double brake” on exocytosis and axon branching
Our previous work suggested that TRIM9 sequesters the exocytic t-SNARE SNAP25, preventing SNARE-complex assembly. Upon addition of netrin-1, TRIM9 releases SNAP25, which permits SNARE-complex formation, vesicle fusion, plasma membrane expansion, and axon branching (Winkle et al., 2014). This is consistent with our observations that loss of Trim9 increased the amount of SNAP25 assembled into SNARE complexes more than VAMP2 and syntaxin1a, suggesting alternative SNAREs such as VAMP7 and syntaxin 1b assemble with SNAP25. Here we identify a second, FAK-dependent mechanism that occurs after SNARE-complex assembly, yet before vesicle fusion, by which TRIM9 restrains membrane expansion. Our findings that FAK activity is necessary for vesicle fusion but not for SNARE-complex assembly suggest that FAK-dependent phosphorylation of an unknown substrate regulates the transition from assembled SNARE complexes to lipid bilayer fusion. Whether this is by promoting progression from a trans-SNARE to cis-SNARE complex, through promoting fusion pore opening, or by another mechanism is not known. Although no pY residues have been identified in the classical neuronal SNARE components, pY residues have been identified on a number of synaptic vesicle–associated proteins, such as the Ca2+-sensor synaptotagmin (Ballif et al., 2008; Wiśniewski et al., 2010; Trinidad et al., 2012). During synaptic vesicle release, calcium-bound synaptotagmin displaces complexin from the SNARE complex, allowing progression to the final stages of vesicle fusion (Giraudo et al., 2009). Whether a phosphorylation-dependent switch in synaptotagmin function or another exocytic component could be involved in developmental exocytosis in neurons is an exciting possibility. An alternative, intriguing possibility is that FAK-dependent modulation of the small Rho GTPase Cdc42, which functions in a late stage of exocytosis in yeast (Adamo et al., 2001; Myers et al., 2012), could be regulating vesicle fusion in neurons.
Taken together, our data indicate that FAK activity is necessary for netrin-dependent increases in exocytosis and axon branching and that the elevated FAK activity that occurs in the absence of Trim9 is required for the elevated exocytosis and axon branching in these neurons. Intriguingly, the relevance of this pathway in vivo is supported by our finding that expression of a noncatalytic version of FAK reduces the exuberant axon branching in the corpus callosum of Trim9−/− mice. Our previous studies indicate that TRIM9 is also required for netrin-dependent axon guidance (Menon, Boyer, et al., 2015). Application of the repulsive cue Sema3a decreases VAMP2-mediated exocytosis and Sema3a receptor trafficking, which regulates repulsive growth cone turning (Zylbersztejn et al., 2012). Analogously, we speculate that TRIM9 and FAK may regulate axon guidance via the modulation of VAMP2-mediated exocytosis.
MATERIALS AND METHODS
Animals
All mouse lines were on a C57BL/6J background and bred at the University of North Carolina (UNC) with approval from the Institutional Animal Care and Use Committee. Timed pregnant females were obtained by placing male and female mice together overnight; the following day was designated as embryonic day (E) 0.5 if the female had a vaginal plug. Trim9−/− and Thy1-GFP (Line M) mice were as previously described (Feng et al., 2000; Winkle et al., 2014). We crossed the Trim9−/−/Thy1GFP mice, with Nex-Cre mice (Goebbels et al., 2006) and FRNKloxp mice (DiMichele et al., 2009) (generous gift from Joan Taylor, UNC–Chapel Hill).
Antibodies, reagents, and plasmids
Antibodies used in this study include mouse anti-Myc (9E10; Santa Cruz Biotechnology and UNC Antibody Core Facility), rabbit anti-FLAG (Sigma), mouse anti-HA (Millipore), rabbit anti-GFP (Invitrogen), mouse anti-GFP (NeuroMab), rabbit anti-βIII tubulin (Covance), mouse anti-βIII tubulin (Covance), mouse anti-GAPDH (Santa Cruz Biotechnology), mouse anti-ubiquitin (Santa Cruz Biotechnology), mouse anti-Syntaxin 1A (Santa Cruz Biotechnology), rabbit anti-VAMP2 (Cell Signaling), goat anti-SNAP25 (Santa Cruz Biotechnology), rabbit anti-FAK-pY397 (Invitrogen), rabbit anti-FAK-pY861 (Novex), mouse anti-FAK (Invitrogen), goat anti-DCC (A-20, Santa Cruz Biotechnology), and rabbit anti-DCC pY1418 (generously provided by Nathalie LaMarche Vane, McGill University). Anti-Myc (9E10)-conjugated agarose beads (Santa Cruz Biotechnology), DCC-A20–conjugated agarose beads (Santa Cruz Biotechnology), or HA antibody–conjugated Sepharose beads (Cell Signaling) were used for immunoprecipitations. Alexa Fluor 488–, Alexa Fluor 568–, or Alexa Fluor 647–labeled secondary antibodies and phalloidin (Invitrogen) were used for immunocytochemistry. Far red–labeled secondary antibodies (Li-Cor) were used for Western blots.
Fluorescent and epitope-tagged TRIM9 mammalian expression plasmids have been previously described (Winkle et al., 2014). For generating pHluorin-DCC, pHluorin was subcloned into mCherry-DCC (Winkle et al., 2014). High-confidence ubiquitination sites were predicted using UbPred (Radivojac et al., 2010), and the DCCKR mutant was generated via GENEBlocks (Life Technologies). pHluorin-VAMP2, eGFP-VAMP2, and eGFP-VAMP7 were previously described (Miesenböck et al., 1998; Gupton and Gertler, 2010). HA-DCC and Myc-DCC were generous gifts from Tim Kennedy (McGill University). Myc-FAK was a generous gift from Joan Taylor (UNC–Chapel Hill). The rat FRNK construct was a kind gift of J.T. Parsons (University of Virginia, Charlottesville, VA). Recombinant chicken netrin-1 was concentrated from supernatant of HEK293 cells (Serafini et al., 1994; Lebrand et al., 2004). The FAK inhibitor 14 (Tocris), bortezomib (SCBT), and MG132 (American Peptide) were used at the indicated concentrations.
Cell culture and transfection
E15.5-dissociated cortical neuronal cultures were prepared as previously described (Viesselmann et al., 2011). Briefly, cortices were microdissected, and neurons were dissociated with trypsin and plated on poly-d-lysine (Sigma)-coated coverslips, glass-bottom movie dishes (MaTek), or tissue culture–treated plastic in Neurobasal medium (Invitrogen) supplemented with B27 (Invitrogen). For all plasmid transfections, dissociated neurons were resuspended in mouse neuron Lonza Nucleofector solution and electroporated in accordance with the manufacturer’s protocol before plating. TRIM9+/+ and CRISPR-generated, TRIM9−/− HEK293 cells were described previously (Menon, Boyer, et al., 2015). HEK293 cells were maintained in DMEM with glutamine (Invitrogen) supplemented with 10% fetal bovine serum (Hyclone). HEK293 cells were transfected using Lipofectamine 2000 (Invitrogen) as per the manufacturer’s protocol.
Immunoblotting and immunoprecipitation
For all immunoblotting and quantification, SDS–PAGE and immunoblot analyses were performed using standard procedures. All lysate concentrations were calculated via spectrophotometry using Bradford concentration reagent (Bio-Rad). Samples were diluted in 5X sample buffer, resolved via SDS–PAGE, and transferred to nitrocellulose, and the resulting Western blots were probed with primary and corresponding secondary antibodies as indicated. Signal was detected with Odyssey Imager (Li-Cor) and either analyzed via Li-Cor Image Studio Lite or ImageJ. All values were normalized to loading control and to wild-type control condition, unless otherwise noted.
Coimmunoprecipitations were performed using mouse immunoglobulin G–conjugated A/G beads (SCBT) to preclear lysates for 1 h at 4°C with agitation. For DCC multimerization studies, cells were cotransfected with HA-DCC and pHluorin-(GFP) DCC, treated, and lysed in lysis buffer (50 mM Tris, pH 7.5, 200 mM NaCl, 2 mM MgCl2, 10% glycerol, 1% NP-40, supplemented with protease and phosphatase inhibitors). HA-DCC and interacting proteins were immunoprecipitated from cell lysate overnight at 4°C. For FAK coimmunoprecipitation with pHluorin-DCC, cells were transfected with Myc-FAK and either pHluorin-DCC or pHluorin-DCCKR, treated, and lysed in lysis buffer. Lysates were incubated with DCC antibody overnight at 4°C. For DCC coimmunoprecipitation with Myc-TRIM9, cells were transfected with HA-DCC and Myc-TRIM9, treated, and lysed in lysis buffer. Lysates were incubated with Myc antibody overnight at 4°C. Beads were washed three times with lysis buffer, and bound proteins were prepared in sample buffer, resolved by SDS–PAGE, and analyzed by immunoblotting.
For measuring ubiquitination, HEK293 cells transfected with HA-DCC, pHluorin-DCC, or pHluorin-DCCKR and FLAG-Ub or cortical neurons at 48 h after plating were treated with 10 μM MG132 for 4 h ± 600 ng/ml netrin-1for 40 min. Cells were lysed in ubiquitin immunoprecipitation buffer (20 mM Tris-Cl, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 0.5% NP-40, 1% SDS, 2 mM dithiothreitol, 5 mM N-ethylmaleimide, 3 mM iodoacetamide, and protease and phosphatase inhibitors, pH = 7.3–7.4). For 5–6 million cells 270 μl of ubiquitin immunoprecipitation buffer was added, and the cells were incubated on ice for 10 min. Cells were removed from the dish and transferred into tubes. Thirty microliters of 1X PBS was added to each tube, and the tubes were gently vortexed. Samples were boiled immediately for 20 min until clear, then centrifuged at 14,000 rpm for 10 min. The boiled samples were diluted using IP buffer without SDS to reduce SDS concentration to 0.1%. DCC from HEK293 cells was immunoprecipitated using the appropriate tags. A similar protocol using antibodies against endogenous DCC and ubiquitin was used for untransfected cultured neurons. Immunoprecipitations were performed under conditions in which only covalent interactions were preserved with anti-GFP (mouse)/anti-HA (mouse) antibody or anti-DCC (goat) antibody coupled to protein A/G agarose beads.
For assessment of proteasomal degradation of DCC, Trim9+/+ and Trim9−/− E15.5 cortical neurons were treated with 100 nM bortezomib for 4 h before 40 min of 500 ng/ml netrin-1 stimulation and lysis. For assessment of FAK pY397 and pY861 in response to netrin-1 stimulation, 250 ng/ml netrin-1 was bath applied at the indicated times before lysis or left untreated for control. For assessment of total DCC and DCC pY1418 protein levels, 500 ng/ml netrin-1 was bath applied between at 72 h to dissociated Trim9+/+ and Trim9−/− E15.5 cortical neurons for 30 min before lysis or left untreated for control. Neurons were lysed in a lysis buffer and stabilized in SDS sample buffer and processed and quantified as described earlier. For assessment of SNARE complexes, assays were performed as extensively outlined in the literature (Winkle et al., 2014, 2016). In brief, cell lysates from untreated neurons and neurons that were netrin-1 (250 ng/ml) or FAKi (5 μM) treated for 1 h were stabilized in sample buffer and incubated at 37°C for complexes or 100°C for monomers. Resulting samples were processed and quantified as described earlier.
Live-cell imaging
Live-cell confocal z-stacks of mCherry-DCC were acquired at room temperature (RT) on an inverted Olympus IX81 zero-drift spinning-disk confocal microscope (Yokogawa CSU-X1) with Andor iQ acquisition software (Andor Revolution XD system), an electron-multiplying charge-coupled device (iXon), a 50-mW, 561-nm laser, band-pass emission filters (Semrock brightline) for 607–36 nm (TR-F607-036), and a 60× PlanSApo 1.2-W objective. Live-cell TIRF imaging was performed on an inverted microscope (IX81-ZDC2) with MetaMorph acquisition software, an electron-multiplying charge-coupled device (iXon), and an imaging chamber (Stage Top Incubator INUG2-FSX; Tokai Hit) maintained at 37°C and 5% CO2.
For measurement of DCC spatial clustering, neurons transfected with mCherry-DCC, pHluorin-DCC, or mCherry-DCC, and GFP-TRIM9 variants were imaged after being in culture for 48 h every 10 s for 8 min before and after stimulation with 500 ng/ml netrin-1, using a 100×, 1.49 NA TIRF objective and a solid-state 491-nm and 561-nm laser illumination at 120-nm penetration depth. Resulting image stacks were aligned using the ImageJ plug-in (TurboReg). For analysis of DCC clustering, cell masks were automatically generated using a cell-segmentation algorithm in MATLAB. The average of the pretreated image stacks was subtracted from each image followed by a sharpening mask and a Gaussian blur, and finally, a threshold was applied using Otsu’s method (Otsu, 1979) to create the cell mask. This cell mask was subdivided into an edge mask, defined as the outer pixels nearest the cell perimeter (0.64 μm in width), and the inner mask, defined as the remaining pixels that did not belong to the edge mask. DCC fluorescence intensity was corrected for photobleaching using the ImageJ CorrectBleach plug-in. Image stacks were temporally aligned to the “treatment frame,” at which time the cells were stimulated with netrin. The average pixel intensity for each region of interest (ROI; whole cell, perimeter, and cell center) was calculated for each time point and normalized to the whole-cell fluorescence. The temporal changes in average DCC fluorescence intensity for each ROI were fit using local polynomial regression fitting (LOESS). All LOESS curves were second-order polynomials fit with α = 0.75. For comparison of the brightest DCC-containing pixels (assumed to represent the most clustered DCC molecules) the pixel intensities of the pretreatment image stack and posttreatment stack were averaged. A threshold was applied to capture the top 10% of brightest pixels in the cell mask. The percentage of these pixels located within the cell edge mask was compared across each condition.
For exocytic vesicle fusion assays, neurons expressing VAMP2-pHluorin were imaged at 48 h after plating with a 100×, 1.49 NA TIRF objective and a solid-state 491-nm laser illumination at 100-nm penetration depth. Images were acquired every 0.5 s for 5 min. The frequency of exocytic events normalized per cell area and time is reported for the entire cell area. At 1 h before netrin-1–stimulated neurons were imaged, 500 ng/ml netrin-1 ± 1 μM FAKi was added to the dish. For imaging eGFP-VAMP2 containing vesicle motility at 48 h in vitro, Trim9+/+ neurons expressing eGFP-VAMP2 and cytoplasmic mCherry were imaged at 100× magnification every 2 s for 5 min with a TIRF illumination penetration depth of 100 nm. FAKi treatment (5 μM) occurred 30 min before imaging. Images were analyzed using ImageJ (Schindelin et al., 2012); a threshold of the mCherry images was used to measure the total area of the footprint of the cell, and an average projection of the eGFP-VAMP2 time lapse was used to identify nonmotile vesicles. The percentage of the neuronal footprint area containing nonmotile vesicles is reported.
Axon-branching assays
For in vitro axon-branching assays, 250 ng/ml netrin-1 was bath applied after 48 h in culture, and FAK-inhibited conditions were treated with 1 μM FAKi. Cells were fixed in 4% paraformaldehyde for 20 min at 72 h in culture, permeabilized for 10 min in 0.1% Triton X-100, blocked for 30 min in 10% bovine serum albumin, and incubated with indicated primary antibodies for 1 h at RT. Following three washes, neurons were incubated in spectrally distinct, species-appropriate, fluorescent secondary antibodies for 1 h at RT. Following three washes, cells were mounted in a TRIS/glycerol/n-propyl-gallate–based mounting media for imaging. Widefield epifluorescence images of pyramidal-shaped neurons were collected at RT on an inverted microscope with 40×, 1.4 NA Plan-Apochromat objective lens (Olympus), MetaMorph acquisition software, and an electron-multiplying charge-coupled device (iXon). Axon branches were defined as extensions off the primary axon that were ≥ 20 μm in length.
For analysis of axon branching in vivo, 200 μm coronal sections of 3-wk-old Thy1-GFP/Trim9+/+, Thy1-GFP/Trim9+/+/Nex-CRE/FRNKLoxSTOPLox, Thy1-GFP/Trim9−/−, and Thy1-GFP/Trim9−/−/Nex-CRE/FRNKLoxSTOPLox littermates mounted in DPX mountant (Fisher) were imaged on a confocal inverted microscope (Fluoview FV1200, Olympus) equipped with a 20×/0.75 NA Plan-Apochromat objective lens with a 488-nm argon laser. Multiarea z-stacks were stitched; ROIs containing potential axon branch points were identified in maximum projections. Candidate branches were confirmed by inspection of stitched z-stacks by the intersection and coalescence of two resolved axons. Candidate branches were rejected if axons were observed to intersect but not coalesce or if one axon left the focal plane without having reached the potential branch point.
Transmission electron microscopy
For electron microscopy, neurons were plated on ACLAR film in 35-mm dishes and prepared as previously described (Heckman, 2008). Briefly, after 48 h in vitro, neurons were left untreated or underwent a 30-min treatment with 5 μM FAKi. Subsequently they were rinsed with 1X phosphate-buffered saline (PBS; pH 7.5); fixed for 20 min in 0.1 M NaCl and 0.05 M cacodylate (pH 7.5) containing 2.5% wt/vol glutaraldehyde; washed two times PBS; and postfixed in a solution containing 1% wt/vol osmium tetroxide and 1.5% wt/vol potassium ferricyanide. After being dehydrated in graded hexylene glycol, cells were embedded in plastic (Spurr’s resin with ERL-4221). Thin sections (70 nm) were cut and collected on nickel mesh grids and contrasted with 4% wt/vol uranyl acetate followed by 0.4% wt/vol lead citrate. Sections were examined under a transmission electron microscope (Philips Tecnai 12). Circular structures with a membrane were considered vesicles. All vesicle diameters were measured. Vesicles within 100 nm of the plasma membrane were reported. All analysis was performed blinded.
Statistics
At least three independent experiments were performed for each assay. Data distribution normality was determined using the Shapiro-Wilks test. Normally distributed data were compared by unpaired t test for two independent samples. For more than two samples with normal distribution, statistical comparisons were made by analysis of variance (ANOVA) with Bonferroni (three to four comparisons) or Tukey (more than four comparisons) post hoc correction. For nonnormally distributed data, the Mann-Whitney test was used to compare two samples. For more than two samples, Kruskal-Wallis nonparametric ANOVA with the post hoc corrections described above was used. For analysis of Western blots, Kruskal-Wallis nonparametric ANOVAs with least significant difference (LSD) correction were made. Data are presented in Figures 1–6 as box-and-whisker plots indicating the 25th percentile (bottom boundary), median (middle line), 75th percentile (top boundary), and nearest observations within 1.5 times the interquartile range (whiskers). Outliers are defined as data points outside 1.5 times the interquartile range. Red lines indicate arithmetic means, individual blue data points are included when experimental observations per condition are less than 10, such as Western blots. Outliers are included in statistical analyses.
Supplementary Material
Acknowledgments
We thank Patrick Brennwald for thoughtful critique of the article. We thank Gerald Gordon, Robert Currin, Jennifer Marks, Lora Hartman, and the UNC Olympus Center for their expertise and generosity. We thank Carey Hanlin for technical assistance. This work was supported by National Institutes of Health GM108970 (S.L.G.), MH10965301 (S.L.G.), and F31 NS087837 (C.C.W.) and by American Heart Association 14POST20450085 (S.M.).
Abbreviations used:
- DCC
deleted in colorectal cancer
- DCCKR
non-ubiquitinatable DCC mutant
- FAKi
pharmacological FAK inhibitor 14
- FRNK
FAK-related nonkinase
- IP
immunoprecipitate
- Py
phosphotyrosine
- ROI
region of interest
- RT
room temperature
- SFK
Src family kinase
- STX-1A
syntaxin 1A
- TIRF
total internal reflection fluorescence
- TRIM
tripartite motif
- TRIM9ΔRING
TRIM9 lacking the ubiquitin ligase RING domain
- TRIM9ΔSPRY
TRIM9 variant lacking the DCC-binding SPRY domain
- VAMP
vesicle-associated membrane protein.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E16-08-0594) on July 12, 2017.
REFERENCES
Boldface names denote co–first authors.
- Adamo JE, Moskow JJ, Gladfelter AS, Viterbo D, Lew DJ, Brennwald PJ. Yeast Cdc42 functions at a late step in exocytosis, specifically during polarized growth of the emerging bud. J Cell Biol. 2001;155:581–592. doi: 10.1083/jcb.200106065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine-Bertrand J, Duquette PM, Alchini R, Kennedy TE, Fournier AE, Lamarche-Vane N. p120RasGAP mediates netrin-1-induced cortical axon outgrowth and guidance. J Biol Chem. 2015;291:4589–4602. doi: 10.1074/jbc.M115.674846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballif BA, Carey GR, Sunyaev SR, Gygi SP. Large-scale identification and evolution indexing of tyrosine phosphorylation sites from murine brain. J Proteome Res. 2008;7:311–318. doi: 10.1021/pr0701254. [DOI] [PubMed] [Google Scholar]
- Berti C, Messali S, Ballabio A, Reymond A, Meroni G. TRIM9 is specifically expressed in the embryonic and adult nervous system. Mech Dev. 2002;113:159–162. doi: 10.1016/s0925-4773(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Bin JM, Han D, Sun KLW, Croteau L-P, Dumontier E, Cloutier J-F, Kania A, Kennedy TE. Complete loss of netrin-1 results in embryonic lethality and severe axon guidance defects without increased neural cell death. Cell Rep. 2015;12:1099–1106. doi: 10.1016/j.celrep.2015.07.028. [DOI] [PubMed] [Google Scholar]
- Bin JM, Rajasekharan S, Kuhlmann T, Hanes I, Marcal N, Han D, Rodrigues SP, Leong SY, Newcombe J, Antel JP, et al. Full-length and fragmented netrin-1 in multiple sclerosis plaques are inhibitors of oligodendrocyte precursor cell migration. Am J Pathol. 2013;183:673–680. doi: 10.1016/j.ajpath.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Dent EW, Barnes AM, Tang F, Kalil K. Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J Neurosci. 2004;24:3002–3012. doi: 10.1523/JNEUROSCI.4963-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMichele LA, Hakim ZS, Sayers RL, Rojas M, Schwartz RJ, Mack CP, Taylor JM. Transient expression of FRNK reveals stage-specific requirement for focal adhesion kinase activity in cardiac growth. Circ Res. 2009;104:1201–1208. doi: 10.1161/CIRCRESAHA.109.195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle EC. Human genetic disorders of axon guidance. Cold Spring Harb Perspect Biol. 2010;2:a001784. doi: 10.1101/cshperspect.a001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanelli M, Fantozzi A, De Luca P, Caprodossi S, Matsuzawa S-I, Lazar MA, Pelicci PG, Minucci S. The coiled-coil domain is the structural determinant for mammalian homologues of Drosophila Sina-mediated degradation of promyelocytic leukemia protein and other tripartite motif proteins by the proteasome. J Biol Chem. 2004;279:5374–5379. doi: 10.1074/jbc.M306407200. [DOI] [PubMed] [Google Scholar]
- Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Giraudo CG, Garcia-Diaz A, Eng WS, Chen Y, Hendrickson WA, Melia TJ, Rothman JE. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323:516–521. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S, Bormuth I, Bode U, Hermanson O, Schwab MH, Nave K-A. Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis. 2006;44:611–621. doi: 10.1002/dvg.20256. [DOI] [PubMed] [Google Scholar]
- Golubovskaya VM, Nyberg C, Zheng M, Kweh F, Magis A, Ostrov D, Cance WG. A small molecule inhibitor, 1,2,4,5-benzenetetraamine tetrahydrochloride, targeting the Y397 site of focal adhesion kinase decreases tumor growth. J Med Chem. 2008;51:7405–7416. doi: 10.1021/jm800483v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal AA, Rappaz B, Rouger V, Martyn IB, Dahlberg PD, Meland RJ, Beamish IV, Kennedy TE, Wiseman PW. Netrin-1-regulated distribution of UNC5B and DCC in live cells revealed by TICCS. Biophys J. 2016;110:623–634. doi: 10.1016/j.bpj.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A, Fathalli F, Rouleau G, Joober R, Flores C. Association between schizophrenia and genetic variation in DCC: a case-control study. Schizophr Res. 2012;137:26–31. doi: 10.1016/j.schres.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Gertler FB. Integrin signaling switches the cytoskeletal and exocytic machinery that drives neuritogenesis. Dev Cell. 2010;18:725–736. doi: 10.1016/j.devcel.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao JC, Adler CE, Mebane L, Gertler FB, Bargmann CI, Tessier-Lavigne M. The tripartite motif protein MADD-2 functions with the receptor UNC-40 (DCC) in netrin-mediated axon attraction and branching. Dev Cell. 2010;18:950–960. doi: 10.1016/j.devcel.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Yamasaki S, Nauenburg S, Binz T, Niemann H. Disassembly of the reconstituted synaptic vesicle membrane fusion complex in vitro. EMBO J. 1995;14:2317–2325. doi: 10.1002/j.1460-2075.1995.tb07226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman C. Preparation of cultured cells for transmission electron microscope. Protoc Exchange. 2008 doi: 10.1038/nprot.2008.251. [Google Scholar]
- Hérincs Z, Corset V, Cahuzac N, Furne C, Castellani V, Hueber A-O, Mehlen P. DCC association with lipid rafts is required for netrin-1-mediated axon guidance. J Cell Sci. 2005;118:1687–1692. doi: 10.1242/jcs.02296. [DOI] [PubMed] [Google Scholar]
- Hu G, Zhang S, Vidal M, Baer JL, Xu T, Fearon ER. Mammalian homologs of seven in absentia regulate DCC via the ubiquitin-proteasome pathway. Genes Dev. 1997;11:2701–2714. doi: 10.1101/gad.11.20.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M. Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Tessier-Lavigne M. Guidance and induction of branch formation in developing axons by target-derived diffusible factors. Curr Opin Neurobiol. 1995;5:83–90. doi: 10.1016/0959-4388(95)80091-3. [DOI] [PubMed] [Google Scholar]
- Kim T-H, Lee HK, Seo IA, Bae HR, Suh DJ, Wu J, Rao Y, Hwang K-G, Park HT. Netrin induces down-regulation of its receptor, Deleted in Colorectal Cancer, through the ubiquitin-proteasome pathway in the embryonic cortical neuron. J Neurochem. 2005;95:1–8. doi: 10.1111/j.1471-4159.2005.03314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrand C, Dent EW, Strasser GA, Lanier LM, Krause M, Svitkina TM, Borisy GG, Gertler FB. Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron. 2004;42:37–49. doi: 10.1016/s0896-6273(04)00108-4. [DOI] [PubMed] [Google Scholar]
- Li W, Lee J, Vikis HG, Lee SH, Liu G, Aurandt J, Shen TL, Fearon ER, Guan JL, Han M, et al. Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat Neurosci. 2004;7:1213–1221. doi: 10.1038/nn1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chin LS, Weigel C, Li L. Spring, a novel RING finger protein that regulates synaptic vesicle exocytosis. J Biol Chem. 2001;276:40824–40833. doi: 10.1074/jbc.M106141200. [DOI] [PubMed] [Google Scholar]
- Liu G, Beggs H, Jürgensen C, Park HT, Tang H, Gorski J, Jones KR, Reichardt LF, Wu J, Rao Y. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci. 2004;7:1222–1232. doi: 10.1038/nn1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Nagashima M. Netrin-1 elevates the level and induces cluster formation of its receptor DCC at the surface of cortical axon shafts in an exocytosis-dependent manner. Cell. 2010;67:99–107. doi: 10.1016/j.neures.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Rama N. [Netrin-1 and axonal guidance: signaling and asymmetrical translation] Med Sci (Paris) 2007;23:311–315. doi: 10.1051/medsci/2007233311. [DOI] [PubMed] [Google Scholar]
- Menon S, Boyer NP, Winkle CC, McClain LM, Hanlin CC, Pandey D, Rothenfußer S, Taylor AM, Gupton SL. The E3 ubiquitin ligase TRIM9 is a filopodia off switch required for netrin-dependent axon guidance. Dev Cell. 2015;35:698–712. doi: 10.1016/j.devcel.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriane M, Tcherkezian J, Webber CA, Danek EI, Triki I, McFarlane S, Bloch-Gallego E, Lamarche-Vane N. Phosphorylation of DCC by Fyn mediates netrin-1 signaling in growth cone guidance. J Cell Biol. 2004;167:687–698. doi: 10.1083/jcb.200405053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Mille F, Llambi F, Guix C, Delloye-Bourgeois C, Guenebeaud C, Castro-Obregon S, Bredesen DE, Thibert C, Mehlen P. Interfering with multimerization of netrin-1 receptors triggers tumor cell death. Cell Death Differ. 2009;16:1344–1351. doi: 10.1038/cdd.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa RK, Kanamori T, Yasunaga K-I, Emoto K. Different levels of the Tripartite motif protein, Anomalies in sensory axon patterning (Asap), regulate distinct axonal projections of Drosophila sensory neurons. Proc Natl Acad Sci USA. 2011;108:19389–19394. doi: 10.1073/pnas.1109843108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JP, Robles E, Ducharme-Smith A, Gomez TM. Focal adhesion kinase modulates Cdc42 activity downstream of positive and negative axon guidance cues. J Cell Sci. 2012;125:2918–2929. doi: 10.1242/jcs.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern. 1979;smc-9:62–66. [Google Scholar]
- Radivojac P, Vacic V, Haynes C, Cocklin RR, Mohan A, Heyen JW, Goebl MG, Iakoucheva LM. Identification, analysis, and prediction of protein ubiquitination sites. Proteins. 2010;78:365–380. doi: 10.1002/prot.22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XR, Ming GL, Xie Y, Hong Y, Sun DM, Zhao ZQ, Feng Z, Wang Q, Shim S, Chen ZF, et al. Focal adhesion kinase in netrin-1 signaling. Nat Neurosci. 2004;7:1204–1212. doi: 10.1038/nn1330. [DOI] [PubMed] [Google Scholar]
- Richardson A, Parsons JT. A mechanism for regulation of the adhesion-associated protein tyrosine kinase pp125FAK. Nature. 1996;380:538–540. doi: 10.1038/380538a0. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Tanji K, Kamitani T, Mori F, Kakita A, Takahashi H, Wakabayashi K. TRIM9, a novel brain-specific E3 ubiquitin ligase, is repressed in the brain of Parkinson’s disease and dementia with Lewy bodies. Neurobiol Dis. 2010;38:210–218. doi: 10.1016/j.nbd.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinidad JC, Barkan DT, Gulledge BF, Thalhammer A, Sali A, Schoepfer R, Burlingame AL. Global identification and characterization of both O-GlcNAcylation and phosphorylation at the murine synapse. Mol Cell Proteomics. 2012;11:215–229. doi: 10.1074/mcp.O112.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viesselmann C, Ballweg J, Lumbard D, Dent EW. Nucleofection and primary culture of embryonic mouse hippocampal and cortical neurons. J Vis Exp 2011. 2011:2373. doi: 10.3791/2373. doi:10.3791/2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Linden LM, Naegeli KM, Ziel JW, Chi Q, Hagedorn EJ, Savage NS, Sherwood DR. UNC-6 (netrin) stabilizes oscillatory clustering of the UNC-40 (DCC) receptor to orient polarity. J Cell Biol. 2014;206:619–633. doi: 10.1083/jcb.201405026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkle CC, Hanlin CC, Gupton SL. Utilizing combined methodologies to define the role of plasma membrane delivery during axon branching and neuronal morphogenesis. J Vis Exp 2016. 2016 doi: 10.3791/53743. doi:10.3791/53743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkle CC, McClain LM, Valtschanoff JG, Park CS, Maglione C, Gupton SL. A novel netrin-1-sensitive mechanism promotes local SNARE-mediated exocytosis during axon branching. J Cell Biol. 2014;205:217–232. doi: 10.1083/jcb.201311003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkle CC, Olsen RHJ, Kim H, Moy SS, Song J, Gupton SL. Trim9 deletion alters the morphogenesis of developing and adult-born hippocampal neurons and impairs spatial learning and memory. J Neurosci. 2016;36:4940–4958. doi: 10.1523/JNEUROSCI.3876-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkle CC, Taylor KL, Dent EW, Gallo G, Greif KF, Gupton SL. Beyond the cytoskeleton: the emerging role of organelles and membrane remodeling in the regulation of axon collateral branches. Dev Neurobiol. 2016;76:1293–1307. doi: 10.1002/dneu.22398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wis´niewski JR, Nagaraj N, Zougman A, Gnad F, Mann M. Brain phosphoproteome obtained by a FASP-based method reveals plasma membrane protein topology. J Proteome Res. 2010;9:3280–3289. doi: 10.1021/pr1002214. [DOI] [PubMed] [Google Scholar]
- Ye F, Petrich BG. Kindlin: helper, co-activator, or booster of talin in integrin activation. Curr Opin Hematol. 2011;18:356–360. doi: 10.1097/MOH.0b013e3283497f09. [DOI] [PubMed] [Google Scholar]
- Yung AR, Nishitani AM, Goodrich LV. Phenotypic analysis of mice completely lacking netrin 1. Development. 2015;142:3686–3691. doi: 10.1242/dev.128942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylbersztejn K, Petkovic M, Burgo A, Deck M, Garel S, Marcos S, Bloch-Gallego E, Nothias F, Serini G, Bagnard D, et al. The vesicular SNARE synaptobrevin is required for semaphorin 3A axonal repulsion. J Cell Biol. 2012;196:37–46. doi: 10.1083/jcb.201106113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.