Abstract
The absence of a positive family history (PFH) in 10%–25% of patients poses a diagnostic challenge for autosomal dominant polycystic kidney disease (ADPKD). In the Toronto Genetic Epidemiology Study of Polycystic Kidney Disease, 210 affected probands underwent renal function testing, abdominal imaging, and comprehensive PKD1 and PKD2 mutation screening. From this cohort, we reviewed all patients with and without an apparent family history, examined their parental medical records, and performed renal imaging in all available parents of unknown disease status. Subsequent reclassification of 209 analyzed patients revealed 72.2% (151 of 209) with a PFH, 15.3% (32 of 209) with de novo disease, 10.5% (22 of 209) with an indeterminate family history, and 1.9% (four of 209) with PFH in retrospect. Among the patients with de novo cases, we found two families with germline mosaicism and one family with somatic mosaicism. Additionally, analysis of renal imaging revealed that 16.3% (34 of 209) of patients displayed atypical PKD, most of which followed one of three patterns: asymmetric or focal PKD with PFH and an identified PKD1 or PKD2 mutation (15 of 34), asymmetric and de novo PKD with proven or suspected somatic mosaicism (seven of 34), or focal PKD without any identifiable PKD1 or PKD2 mutation (eight of 34). In conclusion, PKD without an apparent family history may be due to de novo disease, missing parental medical records, germline or somatic mosaicism, or mild disease from hypomorphic PKD1 and PKD2 mutations. Furthermore, mutations of a newly identified gene for ADPKD, GANAB, and somatic mosaicism need to be considered in the mutation-negative patients with focal disease.
Keywords: polycystic kidney disease, family history, human genetics
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disease worldwide, with a prevalence affecting approximately 1 in 500–1000 births.1 It is characterized by focal development and enlargement of cysts with increasing age, leading to the distortion of kidney architecture and ultimately, ESRD in a majority of patients.2 Mutations of two genes (i.e., polycystic kidney disease 1 [PKD1] and PKD2) account for 80%–85% and 15%–20%, respectively, of patient cohorts enriched with patients at high risk for progression to ESRD.3,4 In these studies, 7%–10% of the families were genetically unresolved (i.e., no mutations were detected in both PKD1 and PKD2). By contrast, in the Toronto Genetic Epidemiology Study of Polycystic Kidney Disease (TGESP), in which probands were ascertained with normal or mildly impaired renal function, 15.5% of the families were genetically unresolved.5 More recently, mutations of the gene GANAB have been identified as a rare cause for a mild form of ADPKD in genetically unresolved families.6
The diagnosis of ADPKD is most commonly established by ultrasonography, which is readily available and inexpensive.7 In equivocal patients or young at-risk subjects, magnetic resonance imaging (MRI) may also be used.8 For these imaging modalities, validated age-dependent diagnostic criteria have been established for diagnosis and disease exclusion; however, both required a positive family history of ADPKD. This is because the pretest probability of an at-risk subject (who has a positive family history) at birth is 50%. By contrast, in the absence of a positive family history, the pretest probability of ADPKD is that of the population risk (i.e., one in 500–1000).7,8 Thus, the absence of a positive family history poses a diagnostic challenge in 10%–25% of patients suspected of ADPKD. In a recent series of 24 patients suspected to have ADPKD without an apparent family history, six (25%) were found to have de novo pathogenic PKD1 mutations, three (12.5%) were found to have a positive family history in retrospect with pathogenic PKD1 mutations, and the remaining (15% or 62.5%) had no mutations detected in both PKD1 and PKD2.9 In the TGESP,5 210 affected probands underwent renal function testing, abdominal imaging, and comprehensive PKD1 and PKD2 mutation screening. From this cohort, we reviewed all patients with and without apparent family history, examined their parental medical records, and performed renal imaging in all available parents of unknown disease status. Here, we report a systematic study on the prevalence and causes as well as the renal imaging correlates of patients without an apparent family history of ADPKD.
Results
Clinical Characteristics of Patients with PKD without an Apparent Family History
We found that 27.8% (58 of 209) of the affected TGESP probands did not have an apparent family history of ADPKD. Among them, 15.3% (32 of 209) had de novo disease, 10.5% (22 of 209) had an indeterminate family history, and 1.9% (four of 209) had a positive family history in retrospect; their clinical characteristics are detailed in Table 1. Compared with those with a positive family history or de novo disease, our patients with either an indeterminate family history or a positive family history in retrospect were older at presentation. Not surprising, the latter patients also had a high prevalence of hypertension at presentation. Nonetheless, they exhibited milder disease compared with those with a positive family history, with comparable levels of creatinine clearance and smaller heighted-aged total kidney volume. Because of the study design, all our patients were ascertained to have mild disease as reflected by their kidney function and volume.5
Table 1.
Characteristics of study patients by family history classification
| Patient Characteristics | Positive Family History, n=151 | De Novo Disease, n=32 | Indeterminate Family History, n=22 | Positive Family History in Retrospect, n=4 |
|---|---|---|---|---|
| Age at presentation, yr | 36 [34 to 39] | 35 [30 to 39] | 42 [36 to 48] | 44 [35 to 52] |
| Women, % | 55 | 47 | 41 | 75 |
| Parental age of initial presentation, yr | 63 [61 to 64] | 63 [59 to 67] | 69 [63 to 74] | 68 [55 to 82] |
| Missing parental information, % | 0 | 0 | 100 | 0 |
| Hypertension at presentation, % | 35 | 28 | 64 | 100 |
| Creatinine clearance, ml/min per 1.73 m2 | 109 [104 to 114] | 117 [104 to 129] | 102 [91 to 114] | 108 [90 to 126] |
| Serum creatinine, mg/dl | 0.90 [0.87 to 0.93] | 0.88 [0.82 to 0.94] | 0.95 [0.85 to 1.1] | 0.78 [0.67 to 0.89] |
| Height-adjusted total kidney volume, ml/m | 707 [623 to 791] (108) | 583 [458 to 708] (23) | 418 [281 to 555] (14) | 383a (4) |
One patient with congenital unilateral kidney was excluded from analysis. Values are reported as mean [95% CI] or mean [95% CI] (n) for height-adjusted total kidney volume.
Median value.
Genetic Correlates of Patients with PKD without an Apparent Family History
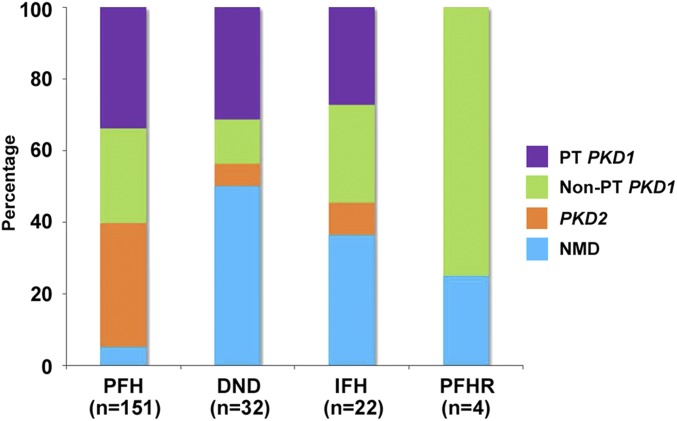
Figure 1 shows the distribution of PKD1 and PKD2 mutation classes in patients with different family history classification. Compared with those with a positive family history, in whom 95.3% (144 of 151, including one patient identified on rescreening; see below) had an identifiable PKD1 or PKD2 mutation, the mutation detection rate in patients with de novo disease and indeterminate family history was significantly lower at 55.5% (30 of 54; odds ratio, 0.06; 95% confidence interval [95% CI], 0.02 to 0.15; P<0.001 by Fisher exact test) (Figure 1, Supplemental Table 1). However, compared with those with a positive family history (i.e., 63.8% or 92 of 144), patients with de novo disease and an indeterminate family history (i.e., 86.6% or 26 of 30) had a higher proportion of PKD1 versus PKD2 mutations (odds ratio, 3.7; 95% CI, 1.2 to 11.1; P=0.02 by Fisher exact test). Although patients with a positive family history in retrospect also exhibited a high proportion of PKD1 mutations (75% or three of four and all nontruncating), the number is very small.
Figure 1.
The distribution of mutation classes in study patients differs according to their family history classification. DND, de novo disease; IFH, indeterminate family history; NMD, no mutations detected; non-PT PKD1, nontruncating PKD1 mutations; PFH, positive family history; PFHR, positive family history in retrospect; PKD2, PKD2 mutations; PT PKD1, protein-truncating PKD1 mutations.
Atypical PKD in Patients without an Apparent Family History
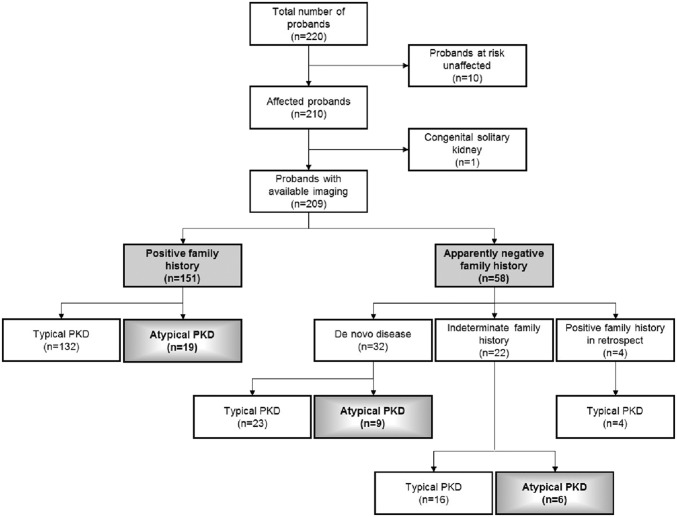
Figure 2 shows a flow diagram of our renal imaging study in the TGESP cohort. To delineate the relationship of atypical (i.e., asymmetric, unilateral, or focal) (Concise Methods) PKD with the different family history classification, an experienced abdominal radiologist (K.K.) reviewed the renal imaging (160 on the basis of computed tomography [CT] or MRI scans and 50 by ultrasound) patterns of all study patients without any knowledge of their clinical or genetic status. The renal images of one patient with congenital single kidney were not analyzed. Of 160 patients with CT or MRI scans reviewed, 30 met the criteria for atypical PKD: 22 with asymmetric disease, seven with focal disease, and one with unilateral disease. Of 49 patients with renal ultrasound reviewed, four met the criteria for atypical PKD: one with asymmetric disease and three with focal disease. In total, 16.3% (34 of 209) of our study patients had atypical PKD by renal imaging, and their clinical and genetic correlates are shown in Table 2. Most of these patients with atypical cases followed one of three patterns: asymmetric or focal PKD with positive family history and an identified PKD1 or PKD2 mutation (15 of 34), asymmetric and de novo PKD with proven or suspected somatic mosaicism (seven of 34), or focal PKD without any identifiable PKD1 or PKD2 mutation (eight of 34). Of note, the percentage of patients with an identifiable PKD1 or PKD2 mutation was significantly higher among those with typical compared with atypical PKD (i.e., 91.4% or 160 of 175 versus 58.8% or 20 of 34; P value <0.001 by Fisher exact test). Among those with atypical PKD, 20 had a positive family history, in whom 84.2% (16 of 20) were mutation positive; eight had de novo disease, in whom 37.5% (three of eight) were mutation positive; and six had an indeterminate family history, in whom 16.6% (one of six) were mutation positive. Thus, the presence of a positive family history or typical renal imaging pattern is associated with a high mutation detection rate for ADPKD. Examples of asymmetric and focal PKD are shown in Figures 3 and 4, respectively. The clinical characteristics of patients with asymmetric PKD are detailed in Supplemental Table 2. One mutation-negative patient with a highly unusual pattern of unilateral PKD is shown in Figure 4, C and D.
Figure 2.
Flowchart of systematic imaging review of the TGESP cohort. The study subjects were classified into subgroups with a positive or apparently negative family history and typical or atypical PKD.
Table 2.
Characteristics of patients with atypical PKD
| Patient Characteristics | Typical PKD | Atypical PKD | ||
|---|---|---|---|---|
| Asymmetrica | Focalb | Unilateralc | ||
| N | 175 | 23 | 10 | 1 |
| Age,d yr | 38 [31–47]e | 45 [38–52] | 49 [40–58] | 52 |
| Sex | ||||
| Men | 85 (48.6) | 11 (47.8) | 3 (30.0) | 1 (100) |
| Women | 90 (51.4) | 12 (52.2) | 7 (70.0) | 0 |
| Family history | ||||
| Positive | 131 (74.9) | 12 (52.3) | 7 (70.0) | 1 (100) |
| De novo PKD | 24 (13.7) | 6 (26) | 2 (20.0) | 0 |
| Indeterminate | 16 (9.1) | 5 (21.7) | 1 (10.0) | 0 |
| Positive in retrospect | 4 (2.3) | 0 | 0 | 0 |
| Mutation | ||||
| PKD1 | 115 (65.7)f | 7 (30.4) | 3 (30.0) | 0 |
| Truncating | 63 (36.0) | 6 (26.0) | 1 (10.0) | 0 |
| Nontruncating | 53 (30.3) | 1 (4.3) | 2 (20.0) | 0 |
| PKD2 | 46 (26.3)f | 7 (30.4) | 3 (30.0) | 0 |
| NMD | 14 (8.0) | 9 (39.2) | 4 (40.0) | 1 (100) |
Column percentages are shown in parentheses. NMD, no mutation detected.
Asymmetric PKD is defined as diffuse cystic involvement of one or both kidneys with >50% difference in volume between the two kidneys by CT/MRI or >50% difference in length between the two kidneys by ultrasound.
Focal PKD denotes mild disease with ample normal-appearing renal parenchyma and focal distribution of renal cysts (total number <30 per kidney), often with less than or equal to five cysts accounting for >50% of the individual kidney volume.
Unilateral PKD is defined as diffuse cystic involvement and marked enlargement of one kidney, with a normal contralateral kidney having, at most, two cysts.
At MRI or ultrasound.
Median value [interquartile range].
Includes one patient with bilineal (PKD1 and PKD2) disease.
Figure 3.
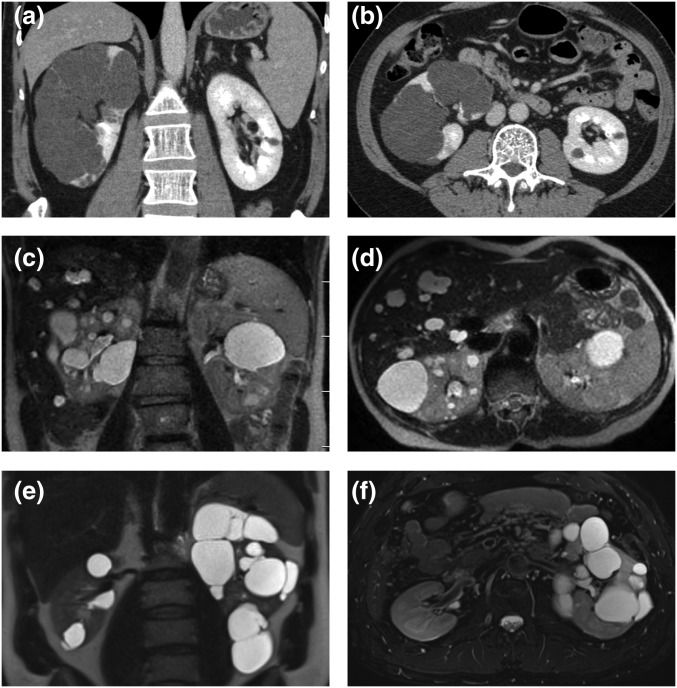
Representative images of three patients with asymmetric PKD. (A and B) Coronal and axial enhanced CT images depicting almost complete replacement of an enlarged right kidney by numerous cysts. (C and D) Coronal and axial T2-weighted MRI images show normal-sized kidneys. Cystic disease is predominantly within the right kidney and liver. (E and F) Coronal and axial T2-weighted MRI images reveal an enlarged left kidney with numerous cysts. In each patient, the contralateral kidney contained significantly fewer cysts and was of normal size.
Figure 4.
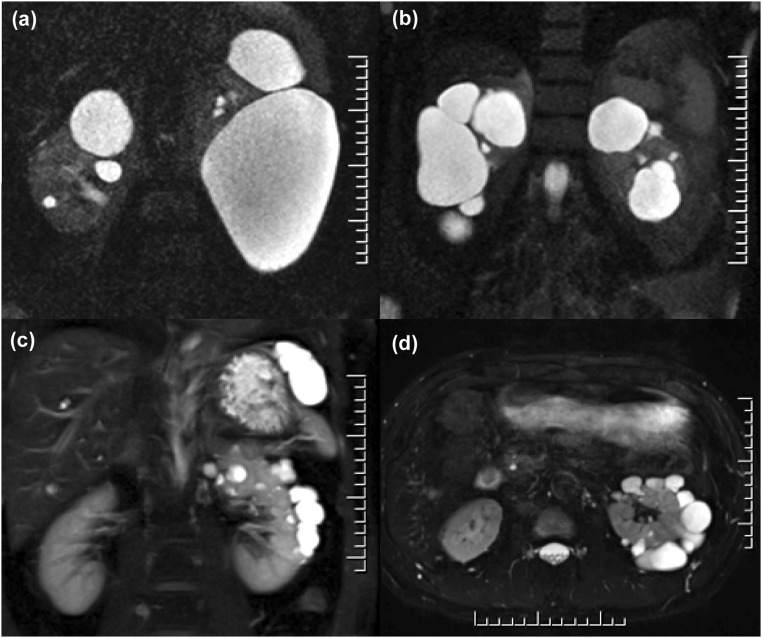
Examples of atypical PKD identified by renal imaging. Coronal MR images of three patients with (A and B) focal and (C) asymmetric PKD. (A) A 58-year-old man with a positive family history of kidney cysts in two brothers. He had multiple cysts (more than ten per kidney) bilaterally and enlarged kidneys from several large to very large cysts. His total kidney volume measured 3.63 L. He was screened negative for PKD1 and PKD2 mutations. (B) A 57-year-old man with negative family history of PKD presented with incidental findings of focal bilateral renal cysts (>20 cysts in each kidney) and normal kidney function. He was screened negative for PKD1 and PKD2 mutations. (C and D) Coronal and axial MR images of a 55 year old man with incidental findings of unilateral PKD (>50 cysts in his left kidney). He was mutation negative with an unconfirmed possible family history of renal cysts in his 83-year-old father.
Causes for Apparent Absence of Family History in ADPKD
Patients with De Novo Disease
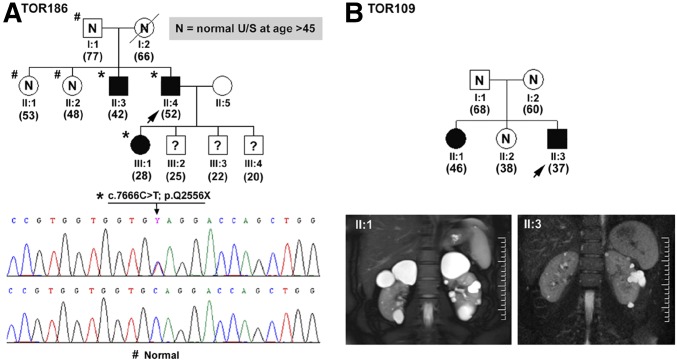
One half (16 of 32) of our patients with putative de novo PKD had an identifiable PKD1 or PKD2 mutation, and most (24 of 32) displayed a typical imaging pattern of ADPKD (Figure 1, Supplemental Tables 1 and 2). In eight of 16 mutation-positive patients, we confirmed their disease as de novo by paternity and mutation testing of their parents (data not shown); however, we were unable to confirm the de novo disease status of the remaining patients due to death or unavailability of one or both of their patients. Two mutation-negative patients with de novo disease presented with focal PKD (examples are in Figure 4, A and B, Table 2). Of the other mutation-negative patients, we found two families highly suspicious of germline mosaicism. In TOR186 (Figure 5A), we identified two affected siblings with unaffected parents. We detected a pathogenic PKD1 (c.7666C>T; p.Q2556X) mutation in both of them (II:3 and II:4) as well as the affected daughter of II:4 but not in their unaffected father (I:1). Their mother died of breast cancer at age 66 years old, with multiple negative renal ultrasound scans since 45 years old. Haplotype analysis (Supplemental Figure 1) shows that only one PKD1 haplotype (1-1-2-1-1) cosegregates with all three affected family members (II:3, II:4, and III:1). This haplotype originated from the unaffected father (I:1) and is also present in one unaffected (II:2) sibling. Taken together, these data strongly support the diagnosis of germline mosaicism in I:1. Similarly, our findings of two mildly affected siblings (II:1 and II:3) with unaffected parents (I:1 and I:2) in TOR109 (Figure 5B) also suggest germline mosaicism, although we did not detect a pathogenic PKD1 or PKD2 mutation in this family.
Figure 5.
Examples of germline mosaicism. (A) TOR186 is a pedigree with proven germline mosaicism. Two members (II:3 and II:4) inherited the same pathogenic PKD1 mutation (c.7666C>T; p.Q2556X) from their apparently unaffected parents. (B) TOR109 is a pedigree with suspected germline mosaicism with no detectable PKD1 or PKD2 mutation. U/S, ultrasound scan.
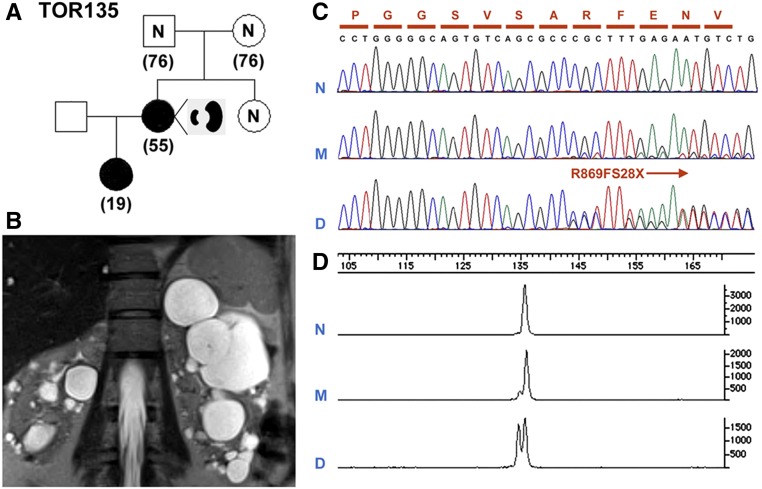
We found that 18.8% (six of 32) of our patients with de novo disease have asymmetric PKD (Figure 3, Supplemental Table 2, Table 2). In one family (TOR135), the proband presented with asymmetric PKD with a measured creatinine clearance of 78 ml/min at age 53 years old; she also has an affected daughter with typical imaging pattern of ADPKD (Figure 6, A and B). At ages 53 and 18 years old, their height-adjusted total kidney volumes were 533 and 563 ml/m, respectively. Sanger sequencing identified an unambiguous PKD1 frameshift (c.2605delC; p.R869fs28X) mutation in the affected daughter, but the sequence at this position in our proband was suggestive of nonspecific background. However, quantitative PCR revealed that there were low levels of mutant alleles (i.e., approximately one mutant allele compared to every 10 normal alleles) in her leukocyte DNA (Figure 6, C and D). We further screened randomly selected clones of individual PCR amplicons encompassing the mutation site in the proband and her affected daughter. We found that 13.5% (42 of 311) of the PCR clones screened in the proband carried the PKD1 mutation. By contrast, 53.4% (174 of 326) of the clones screened in her daughter carried the PKD1 mutation (data not shown). Taken together, these data indicate that the proband is a PKD1 somatic mosaic with germline disease transmission to her daughter. Two of five remaining patients with de novo and asymmetric PKD had a PKD1 mutation, whereas the other three were mutation negative (Supplemental Table 2).
Figure 6.
An example of PKD1 somatic mosaicism. (A) A pedigree (TOR135) with somatic mosaicism and germline disease transmission. (B) MRI shows asymmetric PKD in the affected mother with somatic mosaicism. (C) Sanger sequencing showing a 1-bp PKD1 frameshift deletion (c.2605delC; p.R869FS28X) unequivocally in the daughter (D) but not in the mother (M). (D) Quantitative analysis by capillary electrophoresis of the PCR product encompassing the PKD1 mutation site shows that the ratio of mutant to normal alleles is approximately 1:1 in the daughter (D) but only approximately 1:10 the mother (M).
Patients with Indeterminate Family History
Twenty-two patients had an indeterminate family history due to inaccessibility of the medical record of one or both of the parents as a result of parental death (n=17), adoption (n=3), or estrangement (n=2). Most of them (72.7% or 16 of 22) had an identifiable PKD1 or PKD2 mutation with a typical imaging pattern of ADPKD (Supplemental Table 1). Of the eight mutation-negative patients, five had asymmetric PKD (Table 2) suspicious of somatic mosaicism; none have any affected children. One other mutation-negative patient had focal PKD.
Patients with a Positive Family History in Retrospective
Four patients had a positive family history in retrospect; all had very mild disease with typical imaging pattern of ADPKD (Table 1). Three of them had a nontruncating PKD1 mutation (Table 1, Supplemental Table 1), whereas in one, no mutation was detected. The PKD in their older relatives and affected parents was also very mild; all had normal or near-normal renal function (data not shown).
Patients with No Identifiable PKD1 and PKD2 Mutations
We rescreened 32 mutation-negative patients by targeted resequencing using a gene panel that contains PKD1, PKD2, GANAB, and HNF1B. We identified a frameshift PKD1 mutation (c.5014_5015delAG; p.R1672fs97X; confirmed by Sanger sequencing on a locus-specific PKD1 template) in a patient with a positive family history previously screened by Athena Diagnostic. We also identified three missense GANAB variants (i.e., p.R331C, p.H872Y, and p.R54W) with pathogenic potential predicted by bioinformatics algorithms (Supplemental Table 3). R331C was considered likely neutral by a previous study on the basis of a functional assay, in which the expression of this mutant in GANAB-null cells restored surface localization of polycysin-1.6 H872Y was identified in the Exome Aggregation Consortium (ExAC) database 398 times, including four homozygotes, and is unlikely to be pathogenic. R54W has been reported in the ExAC database seven times and is not very well conserved; however, in the absence of functional analysis, we are unable to determine its pathogenicity at this time. No mutations (including large gene rearrangements) were identified in our HNF1B screen.
Discussion
The diagnosis of ADPKD is commonly established by ultrasonography7 and in equivocal patients or young at-risk subjects, MRI.8 For these imaging modalities, validated age-dependent diagnostic criteria have been established; however, both required a positive family history.1 The absence of a positive family history, therefore, poses a diagnostic challenge, because these imaging tests can no longer be applied. Furthermore, in this setting, the differential diagnosis will need to broaden to include other genetic and nongenetic causes of renal cystic diseases, especially in those patients with atypical features.1 In a recent series of 24 patients suspected to have ADPKD without an apparent family history, nine patients were found to have pathogenic PKD1 mutations due to either de novo disease or a positive family history in retrospect. However, the majority (15 of 24) of the patients remained genetically unresolved.9
The study cohort from the TGESP, which comprised 209 affected probands and their families from a single geographic region, provided us a unique opportunity to define the prevalence and causes as well as the renal imaging correlates of patients without an apparent family history of ADPKD. We found that 27.8% of probands did not have an apparent family history of ADPKD as a result of putative de novo disease (15.3%), indeterminate family history (10.5%), or positive family history in retrospect (1.9%). A limitation of our study is that we were only able to definitively prove de novo disease by paternity and mutation testing in one half of our mutation-positive patients. A recent study has highlighted the importance of differentiating patients with PKD and atypical renal imaging patterns, because they are associated with mild disease and may have different etiologies.10 We found that 16.3% of study patients had an atypical imaging pattern, which manifested as asymmetric (11.0%), focal (4.8%), or unilateral (0.5%) PKD; they were less likely to have an identifiable PKD1 or PKD2 mutation than those with a typical imaging pattern. We also found that patients with a positive family history (including four in retrospect) had a very high rate of identifiable PKD1 or PKD2 mutations compared with those without. However, patients with de novo disease displayed a high ratio of PKD1 versus PKD2 mutations (i.e., 7:1, respectively), suggesting that PKD1 is more prone to somatic mutagenesis than PKD2, likely due to its large size and complex structure.11,12
By integrating the renal imaging and genetic results with the family history information, we have identified suspected or proven (i.e., two germline, nine somatic, and one with both) mosaicism in 5.7% of our probands. Mosaicism refers to the occurrence of two genetically distinct cell populations within an individual resulting from a somatic mutation during embryogenesis.13–15 Depending on the cell type and developmental stage when the mutation occurs, three clinical syndromes can arise: (1) germline mosaicism involving the germ cells only, (2) somatic mosaicism involving the body cells only, and (3) gonadal and somatic mosaicism involving both the germline and somatic cells.14 Thus, the presence of two affected siblings without affected parents provides a clue to identify two families with germline mosaicism (Figure 5). However, focal and asymmetric disease is a key feature of somatic mosaicism due to dilution and variable involvement of the affected cells.15 Thus, the presence of de novo and asymmetric or unilateral PKD is a clinical finding highly suspicious of somatic mosaicism. In the case of the proband from TOR135, she was proven to be a PKD1 somatic and germline mosaic (Figure 6). At age 53 years old, her disease was mild due to asymmetric PKD, and her height-adjusted kidney volume was comparable with that of her 18-year-old affected daughter. By contrast, through germline transmission, the disease in her daughter is severe (Mayo Clinic classification 1E) and typical of that associated with a truncating PKD1 mutation. The diagnosis of mosaicism is challenging, because Sanger sequencing will typically miss the pathogenic mutation due to the low signal-to-noise ratio resulting from dilution of the mutant cells and the variable degree of tissue involvement in an affected individual as illustrated in the proband from TOR135. The key here is to identify the mutation by screening an affected offspring of the suspected mosaic. However, six of our suspected PKD somatic mosaics did not have any children, and three others with children did not have any affected children. The application of targeted PKD1 and PKD2 resequencing with very high read depths to detect variant allele fraction to approximately 10% coupled with screening of multiple (i.e., blood, buccal mucosal, and urine epithelial) cell types may provide a promising approach in diagnosing PKD mosaicism.16
PKD without an apparent family history can be due to very mild disease that is not initially recognized in the affected parent, which is exemplified by our patients with a positive family history in retrospect. Interestingly, three of four patients with a positive family history in retrospect were found to have nontruncating PKD1 mutations, many of which have been recently shown to be associated with mild disease.3–5,17,18 Similarly, patients with a positive family history in retrospect are affected due to very mild disease associated with PKD2 mutations as well.3–5 We also found that 13.4% (28 of 209) of our study cohort did not have an identifiable PKD1 or PKD2 mutation (Table 1). Excluding ten with asymmetric PKD suspected or proven to harbor somatic mosaicism, 8.6% (18 of 209) of our patients remain genetically unresolved. Most of the latter patients displayed mild or focal disease with a positive family history, suggestive of GANAB mutations.6 However, we did not identify any definitively pathogenic GANAB mutations. Thus, the genetic causes in these patients remain unsolved but could be due to missed PKD1/PKD2 mutations (e.g., intronic or synonymous exonic mutations that cause atypical splicing) and/or additional GANAB-like gene(s) that modify intracellular polycystin-1 trafficking.
In conclusion, our study of 209 families revealed that 27.8% of the probands did not have an apparent family history of ADPKD as a result of de novo disease (15.3%), indeterminate family history (10.5%), and positive family history in retrospect (1.9%). Moreover, 16.3% of them had an atypical imaging pattern manifested as asymmetric (11.0%), focal (4.8%), or unilateral (0.5%) disease. By integrating the renal imaging and genetic results with their family history information, we found that PKD without an apparent family history might be due to de novo mutations, missing parental medical records, germline or somatic mosaicism, and mild disease from hypomorphic PKD1 mutations. Of our patients with genetically unresolved cases, those suspected of somatic mosaicism are amenable to improved detection by targeted resequencing, whereas the remaining patients with mild or focal disease should be screened for atypical splice mutations in PKD1 and PKD2 as well as GANAB mutations.
Concise Methods
Study Population
We conducted a systematic study to delineate the causes behind the apparent lack of positive family history in patients with suspected ADPKD from the TGESP cohort5 according to a prespecified protocol approved by the institutional review board at the University Health Network in Toronto, Ontario, Canada. All study patients (n=210) previously underwent renal function testing, abdominal imaging, and comprehensive PKD1 and PKD2 mutation screen at a single site in Toronto. From this cohort, we identified all patients with and without an apparent family history, examined their parental medical records, and performed renal imaging in all available parents of unknown disease status. On the basis of the updated data, we classified our families as (1) de novo disease if the patient had ten or more cysts per kidney, but both parents did not each have a total of three kidney cysts, and/or if we identified a mutation in the patient but did not identify it in both of their parents; (2) indeterminate family history if parental medical records or genetic information were unavailable; or (3) positive family history in retrospect if at least one parent had an age-dependent minimal number of cysts for ADPKD according to the unified ultrasound diagnostic criteria or if a pathogenic PKD1 or PKD2 mutation was identified in both the patient and the affected parent.
Genetic Studies
DNA samples from all study patients were screened by bidirectional sequencing of the coding regions and splice junctions of both PKD1 and PKD2.5 For PKD1 screening, we used a validated PCR protocol to generate locus-specific amplicons.19 Nonsense, frameshift, and canonical splice site mutations are grouped as protein-truncating mutations, whereas nonsynonymous missense or atypical splice site mutations are grouped as nontruncating mutations. Small in-frame insertions/deletions affecting fewer than five amino acids are grouped as a separate mutation class. All nontruncating mutations and in-frame insertions/deletions were evaluated for their potential pathogenicity using prediction algorithms (Align GVGD,19 PolyPhen-2,20 SIFT,21 PROVEAN,22 and Human Splicing Finder23), by review of the PKD mutation database (http://pkdb.mayo.edu), and by segregation analysis with additional affected family members whenever possible. All mutation-negative patients were rescreened by multiplex ligation–dependent probe amplification for detection of large gene rearrangements.24
We genotyped ten family members of TOR186 with five polymorphic simple sequence repeat markers at the PKD1 locus. The locations of these markers relative to PKD1 are as follows (the number between markers denotes intermarker distance in centimorgans): HBAP1–2.0-KG8/PKD1-0.8-D16S2618–1.2-D16S423–1.3-D16S2622. KG8 is an intragenic marker located within the 3′ end of PKD1. Genotyping was performed using a published protocol and analyzed by PAGE.25 All genotyping was performed and scored by K.W., who had no knowledge of the clinical status of the study subjects. Paternity testing was performed on DNA samples of the test patients and their parents by genotyping 15 polymorphic tetranucleotide simple sequence repeat markers from the AmpFLSTR Identifiler Kit (Applied Biosystem, Foster City, CA) according to the manufacturer’s instructions and analyzed on capillary electrophoresis.
Cloning of PCR amplicons encompassing the PKD1 (c.2605delC; p.R869FS28X) mutation in TOR135 was done using the TOPO TA Cloning Kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.26 Four microliters PCR products from the mosaic mother and her affected daughter (Figure 4A) were used for the ligation reaction, and the mixture was incubated at room temperature for 10 minutes. Then, 2 μl TOPO Cloning reaction mix was added to One Shot Chemically Competent Escherichia coli and mixed gently. After incubation on ice for 10 minutes, the mixture was heat shocked at 42°C for 30 seconds and transferred to ice immediately; 250 μl SOC medium was added into the vial containing the reaction mix and shaken horizontally at 37°C at 200 rpm for 1 hour. Fifty microliters transformation mix was spread on two prewarmed selective LB plates (containing 100 μg/ml ampicillin with 40 μl 40 mg/ml X-gal) and incubated at 37°C overnight. All white colonies were inoculated on a plate, numbered, and resuspended individually in 50 μl water. After 5 minutes of heating at 95°C and centrifugation, 1 μl supernatant from each colony was added into a final 10-μl PCR reaction using the same PCR conditions, except that the cycle number was reduced to 30. At least 300 randomly chosen clones (with 335-bp DNA inserts) each were screened in the mosaic mother and her affected daughter by SmuI restriction digestion, which yielded four (i.e., 35, 51, 111, and 138 bp) fragments from the wild-type allele and three (i.e., 35, 51, and 249 bp) from the mutant allele, because the latter abolished one restriction site.
In patients with no mutations detected, targeted resequencing was performed to screen all of the exons and flanking intronic sequences of PKD1, PKD2, GANAB, and HNF1B for mutation. Libraries were prepared using an NEBNext Ultra DNA Preparation Kit and hybridized to custom capture baits (SureSelect; Agilent Technologies). The enriched libraries were sequenced with 150-bp paired end reads on an Illumina HiSeq 4000. FASTQ files were aligned to the hg19 reference genome (UCSC Genome Browser), and realignment and recalibration were performed with the Genome Analysis Toolkit (GATK). Multisample variant calling was performed with the GATK Haplotype Caller, and variants were filtered with Variant Quality Score Recalibration for both SNVs and indels. Variant mining was performed with Golden Helix SNP & Variation Suite v.8. Large deletions in HNF1B were searched by the copy number variations analysis. In addition, multiplex-dependent probe ligation amplification was performed to detect HNF1B deletions in patients with equivocal CNV analysis results.
Renal Imaging Studies
The diagnosis of ADPKD in the study subjects was confirmed by ultrasound- or MRI-based criteria.7,8 For patients without a positive family history, the diagnosis of ADPKD required the presence of at least ten cysts in each kidney, with both kidneys >13 cm in length. An experienced abdominal radiologist (K.K.) reviewed the renal imaging patterns of all study patients (160 on the basis of MRI or CT scans and 50 by ultrasound) without any knowledge of their clinical and genetic status. The renal image of one patient with congenital single kidney was not analyzed. From this review, the renal imaging pattern of all study patients was classified as typical or atypical for ADPKD. On the basis of cyst distribution and the ratio of right kidney volume (RKV) to left kidney volume (LKV), atypical PKD was reported as (1) asymmetric, (2) unilateral, or (3) focal, similar to the class 2 patterns reported by Irazabal et al.10 Asymmetric PKD was defined using a set of conservative criteria (i.e., diffuse cystic involvement of one or both kidneys with 50% or more difference between RKV and LKV).27 Unilateral disease was defined as unilateral diffuse cystic involvement causing marked enlargement of one kidney with a normal contralateral kidney volume (normal single kidney volume <138 ml in men and <113 ml in women) having, at most, two cysts by MRI. Focal disease was defined as unilateral or bilateral distribution of renal cysts mainly affecting one pole or where five cysts or fewer accounted for 50% or more of TKV (with a difference between RKV and LKV of <50%).23 Ultrasound was used for evaluation of 50 patients without MRI or CT. We defined asymmetric PKD on ultrasound by 50% or larger length difference between the two kidneys. Unilateral PKD was defined as above and was not seen in any of the patients reviewed. Focal disease was defined by the presence of cysts affecting one pole of the kidney with relative sparing of other parts of the same kidney.
Statistical Analyses
Continuous variables were reported as mean (95% CI) if normally distributed and median (interquartile range) if not, and discrete variables were reported as percentages. Two-tailed t test for continuous trait and Fisher exact test for discrete traits were used for statistical testing using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank all of the study subjects for their participation.
This work was supported by Canadian Institutes of Health Research grant MOP 123429 (to A.D.P. and Y.P.) and National Institutes of Health for the Molecular Genetics and Proteomics Core of the Mayo Translational PKD Center grant DK090728 (to P.C.H.).
Part of this study was presented at the American Society of Nephrology Kidney Week November 4, 2013 in Atlanta, Georgia.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016090938/-/DCSupplemental.
References
- 1.Barua M, Pei Y: Diagnosis of autosomal-dominant polycystic kidney disease: An integrated approach. Semin Nephrol 30: 356–365, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Grantham JJ, Mulamalla S, Swenson-Fields KI: Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol 7: 556–566, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Cornec-Le Gall E, Audrézet M-P, Chen J-M, Hourmant M, Morin M-P, Perrichot R, Charasse C, Whebe B, Renaudineau E, Jousset P, Guillodo M-P, Grall-Jezequel A, Saliou P, Férec C, Le Meur Y: Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol 24: 1006–1013, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heyer CM, Sundsbak JL, Abebe KZ, Chapman AB, Torres VE, Grantham JJ, Bae KT, Schrier RW, Perrone RD, Braun WE, Steinman TI, Mrug M, Yu AS, Brosnahan G, Hopp K, Irazabal MV, Bennett WM, Flessner MF, Moore CG, Landsittel D, Harris PC; HALT PKD and CRISP Investigators : Predicted mutation strength of non-truncating PKD1 mutations aids genotype-phenotype correlation in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 27: 2872–2884, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang Y-H, Conklin J, Chan W, Roslin NM, Liu J, He N, Wang K, Sundsbak JL, Heyer CM, Haider M, Paterson AD, Harris PC, Pei Y: Refining genotype-phenotype correlation in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 27: 1861–1868, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porath B, Gainullin VG, Cornec-Le Gall E, Dillinger EK, Heyer CM, Hopp K, Edwards ME, Madsen CD, Mauritz SR, Banks CJ, Baheti S, Reddy B, Herrero JI, Bañales JM, Hogan MC, Tasic V, Watnick TJ, Chapman AB, Vigneau C, Lavainne F, Audrézet M-P, Ferec C, Le Meur Y, Torres VE, Harris PC; Genkyst Study Group, HALT Progression of Polycystic Kidney Disease Group; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease : Mutations in GANAB, encoding the glucosidase IIα subunit, cause autosomal dominant polycystic kidney and liver disease. Am J Hum Genet 98: 1193–1207, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, Parfrey P, Cramer B, Coto E, Torra R, San Millan JL, Gibson R, Breuning M, Peters D, Ravine D: Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 20: 205–212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pei Y, Hwang YH, Conklin J, Sundsbak JL, Heyer CM, Chan W, Wang K, He N, Rattansingh A, Atri M, Harris PC, Haider MA: Imaging-based diagnosis of autosomal dominant polycystic kidney disease. J Am Soc Nephrol 26: 746–753, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reed B, McFann K, Kimberling WJ, Pei Y, Gabow PA, Christopher K, Petersen E, Kelleher C, Fain PR, Johnson A, Schrier RW: Presence of de novo mutations in autosomal dominant polycystic kidney disease patients without family history. Am J Kidney Dis 52: 1042–1050, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, Bae KT, Chapman AB, Grantham JJ, Mrug M, Hogan MC, El-Zoghby ZM, Harris PC, Erickson BJ, King BF, Torres VE; CRISP Investigators : Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J Am Soc Nephrol 26: 160–172, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossetti S, Strmecki L, Gamble V, Burton S, Sneddon V, Peral B, Roy S, Bakkaloglu A, Komel R, Winearls CG, Harris PC: Mutation analysis of the entire PKD1 gene: Genetic and diagnostic implications. Am J Hum Genet 68: 46–63, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris PC, Bae KT, Rossetti S, Torres VE, Grantham JJ, Chapman AB, Guay-Woodford LM, King BF, Wetzel LH, Baumgarten DA, Kenney PJ, Consugar M, Klahr S, Bennett WM, Meyers CM, Zhang QJ, Thompson PA, Zhu F, Miller JP: Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 17: 3013–3019, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Gottlieb B, Beitel LK, Trifiro MA: Somatic mosaicism and variable expressivity. Trends Genet 17: 79–82, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Freed D, Stevens EL, Pevsner J: Somatic mosaicism in the human genome. Genes (Basel) 5: 1064–1094, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell IM, Shaw CA, Stankiewicz P, Lupski JR: Somatic mosaicism: Implications for disease and transmission genetics. Trends Genet 31: 382–392, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossetti S, Hopp K, Sikkink R, Sundsbak JL, Lee YK, Kubly V, Eckloff BW, Ward CJ, Winearls CG, Torres VE, Harris PC: Identification of gene mutation in autosomal dominant polycystic kidney disease through targeted resequencing. J Am Soc Nephrol 23: 915–933, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossetti S, Kubly VJ, Consugar MB, Hopp K, Roy S, Horsley SW, Chauveau D, Rees L, Barratt TM, van’t Hoff WG, Niaudet P, Torres VE, Harris PC: Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int 75: 848–855, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pei Y, Lan Z, Wang KR, Garcia-Gonzalez M, He N, Dicks E, Parfrey P, Germino G, Watnick T: Attenuated renal disease severity associated with a missense PKD1 mutation. Kidney Int 81: 412–417, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossetti S, Consugar MB, Chapman AB, Torres VE, Guay-Woodford LM, Grantham JJ, Bennett WM, Meyers CM, Walker DL, Bae K, Zhang QJ, Thompson PA, Miller JP, Harris PC; CRISP Consortium : Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 18: 2143–2160, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR: A method and server for predicting damaging missense mutations. Nat Methods 7: 248–249, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng PC, Henikoff S: Predicting deleterious amino acid substitutions. Genome Res 11: 863–874, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi Y, Sims GE, Murphy S, Miller JR, Chan AP: Predicting the functional effect of amino acid substitutions and indels. PLoS One 7: e46688, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desmet FO, Hamroun D, Lalande M, Collod-Béroud G, Claustres M, Béroud C: Human splicing finder: An online bioinformatics tool to predict splicing signals. Nucleic Acids Res 37: e67, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Consugar MB, Wong WC, Lundquist PA, Rossetti S, Kubly VJ, Walker DL, Rangel LJ, Aspinwall R, Niaudet WP, Ozen S, David A, Velinov M, Bergstralh EJ, Bae KT, Chapman AB, Guay-Woodford LM, Grantham JJ, Torres VE, Sampson JR, Dawson BD, Harris PC; CRISP Consortium : Characterization of large rearrangements in autosomal dominant polycystic kidney disease and the PKD1/TSC2 contiguous gene syndrome. Kidney Int 74: 1468–1479, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pei Y, Paterson AD, Wang KR, He N, Hefferton D, Watnick T, Germino GG, Parfrey P, Somlo S, St George-Hyslop P: Bilineal disease and trans-heterozygotes in autosomal dominant polycystic kidney disease. Am J Hum Genet 68: 355–363, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang K, Zhao X, Chan S, Cil O, He N, Song X, Paterson AD, Pei Y: Evidence for pathogenicity of atypical splice mutations in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 4: 442–449, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheong B, Muthupillai R, Rubin MF, Flamm SD: Normal values for renal length and volume as measured by magnetic resonance imaging. Clin J Am Soc Nephrol 2: 38–45, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.