Abstract
Magnetic resonance imaging (MRI) has been used for many years for anatomic evaluation of the kidney. Recently developed methods attempt to go beyond anatomy to give information about the health and function of the kidneys. Several methods, including diffusion-weighted MRI, renal blood oxygen level–dependent MRI, renal MR elastography, and renal susceptibility imaging, show promise for providing unique insight into kidney function and severity of fibrosis. However, substantial limitations in accuracy and practicality limit the immediate clinical application of each method. Further development and improvement are necessary to achieve the ideal of a noninvasive image-based measure of renal fibrosis. Our brief review provides a short explanation of these emerging MRI methods and outlines the promising initial results obtained with each as well as current limitations and barriers to clinical implementation.
Keywords: renal fibrosis, kidney imaging, MRI
CKD afflicts 14% of the population in the United States and over 10% of the world’s population.1 The annual cost of health care in the United States for patients with CKD covered by Medicare totals about $50.4 billion. CKD progresses to ESRD. Almost 600,000 people in the United States suffer from ESRD, and together with patients with CKD, they account for about one third of total health care expenditures in the United States.2
Renal fibrosis is the common pathway of progression of CKD, characterized by the abnormal accumulation of macrophages,3 the appearance of myofibroblasts recruited to the kidney or derived from cells resident in the kidney,4 and the deposition of a fibrotic interstitial matrix, including collagen.5–7 As fibrosis increases, tubular epithelial cells lose their resorptive function, and the kidney decreases in size.
Several drugs in various stages of clinical trial show encouraging potential to halt or reverse the progression of renal fibrosis.8 Assessment of renal fibrosis requires invasive kidney biopsy with nontrivial risk of serious complication.9 A noninvasive method of assessing renal fibrosis would be a valuable tool to diagnose CKD and monitor antifibrotic therapy. Toward this goal, several magnetic resonance imaging (MRI) methods are in development, which may be able to provide information about kidney fibrosis and renal function.
Diffusion-Weighted Imaging
Diffusion-weighted MRI gives information about the motion of water molecules in tissue. In diffusion-weighted imaging (DWI), a strong magnetic gradient field (called the positive diffusion gradient) causes water molecules to accumulate phase. A second gradient field, which is the negative of the first field (called the negative diffusion gradient), undoes this phase accumulation. For water molecules that have not moved in the time between the positive and negative diffusion gradients, the phase accumulation caused by the positive gradient is exactly undone by the negative gradient, and these molecules experience no net change in phase. However, water molecules that are in motion in the short time interval between the two gradients accumulate phase during the positive diffusion gradient, which is not completely undone by the negative gradient. Accumulation of phase causes signal loss. Therefore, moving water molecules give less signal (less intensity on the final image) than water molecules that are stationary. The amount of signal loss increases with increased distance traveled by water molecules in the time between the positive and negative diffusion gradients. Water molecules that diffuse freely experience the greatest signal loss, whereas molecules with motion that is constrained by surrounding structures experience less signal loss. This signal loss can be modeled to give a quantity called the apparent diffusion coefficient (ADC) for each pixel in an image that expresses how freely water is able to diffuse in the tissue. DWI can be performed rapidly with standard MRI equipment, and therefore, technical barriers to clinical implementation are low.
Tissue with higher cellularity generally shows lower ADC, because diffusion of water molecules is impeded by hydrophobic cell membranes. Accumulation of collagen and other matrix components in renal fibrosis would be expected to impair free diffusion of water and thus, lower ADC. Numerous studies in both animal models and humans have shown significant decrease in ADC in diseased kidneys compared with healthy kidneys.10–21 Some animal and human studies have directly compared ADC with degree of fibrosis in biopsy or postmortem histology. In a mouse model of renal fibrosis caused by unilateral ureter ligation, ADC decreased in the cortex of fibrotic kidneys, and the amount of decrease in ADC was correlated with cellular density and α-smooth muscle actin, markers of renal fibrosis.19 Human studies have shown significant correlation of ADC with percentage fibrosis determined on histology of core biopsy specimens in CKD.11,12,14 Several human studies have shown significant associations between eGFR or serum creatinine and ADC.10,13,14,16,20–22
An extension of DWI known as diffusion tensor imaging (DTI) gives additional information about the organization of tissue. In DTI, diffusion imaging is performed repeatedly with diffusion gradients oriented in different directions. This allows quantification of not only the freedom of water molecules to diffuse (ADC) but also, the preferential directions of diffusion. For water in free solution, there is no preferential direction for diffusion; motion is random in all directions. However, in tissues with an organized structure, water may diffuse more freely in one direction than another. For instance, in the tubules of the renal medulla, water may diffuse preferentially along the direction of the tubule. The degree to which diffusion shows a preference for direction is quantified as fractional anisotropy (FA). FA measured by DTI has a range of zero to one and represents the degree of spatial organization of tissue.
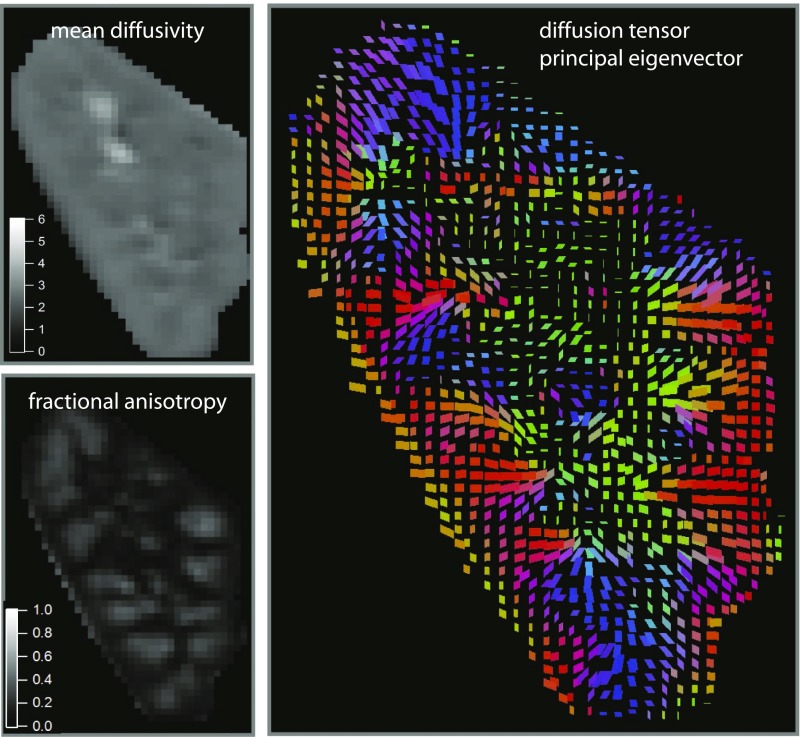
DTI and measurement of FA have been performed in the kidney (Figure 1). In a series of 40 renal transplant recipients, medullary FA was found to be correlated with eGFR, and FA was significantly lower in subjects with impaired transplant kidney function that did not subsequently recover.13 Another study found correlation between the severity of glomerulosclerosis on histology of biopsy specimens and both cortical and medullary FA in 75 subjects with glomerulosclerosis.11 Significantly decreased medullary FA was observed in another study in 47 subjects with renal disease of various etiologies compared with 17 normal controls.23 Correlation between medullary FA and eGFR has been observed in several studies.13–15
Figure 1.
Renal DTI shows preferential directions of diffusion corresponding to the anatomic organization of kidney tissue. A single coronal slice from the kidney of a normal volunteer is shown. Mean diffusivity (upper left panel) is equivalent to ADC in DWI. FA (lower left panel) quantifies the extent to which diffusion occurs with a preference for direction. FA is higher in the medulla than the cortex, indicating that diffusion in the medulla tends to have a preferential direction. This direction is illustrated in the plot of the principal eigenvector (preferred direction of perfusion) shown in the right panel. In this color-coded plot of direction, red is right-left, green is anterior-posterior, and blue is superior-inferior. The eigenvector plot shows that diffusion in the medulla occurs preferentially in a radial direction from the center of the kidney toward the periphery. Images courtesy of Eric Sigmund. Modified from ref. 26, with permission.
Although preclinical animal and human studies have shown associations of ADC and FA with severity of CKD, most studies show significant overlap in ADC and FA values between normal and diseased subjects. Statistically significant associations have been shown, but the power to diagnose CKD with DWI remains unproven. One study showed a sensitivity of 75% and a specificity of 70% for ADC measurement to detect CKD of stage 3 or worse.21 Most studies have not included receiver operating characteristic (ROC) analysis or other analysis of accuracy. The true meaning of ADC changes in fibrosis is also controversial. ADC values reflect not only the random motion of water molecules but also, motion of plasma water through blood vessels in capillary beds. Increased tissue perfusion causes increase in ADC. One experiment in a rat model of unilateral ureteral ligation suggests that the decrease in ADC seen in fibrotic kidneys in vivo may actually represent decreased renal perfusion rather than the effect of fibrosis and that ADC of fibrotic kidneys in postmortem analysis is actually higher than that of normal kidneys secondary to dilation of the tubules and expansion of the extracellular space in fibrosis.24 The evaluation of the clinical usefulness of DWI is hampered by marked variability in reported ADC values between institutions in normal and diseased subjects. The effect of tissue perfusion on ADC values depends in part on the choices of diffusion gradient strength and duration, which are often dictated by the capabilities of specific MRI machines. The perfusion component of ADC can be estimated with a method known as intravoxel incoherent motion DWI, which models the decay of signal caused by diffusion as two simultaneous processes, capillary perfusion and true diffusion.25–27 Some of the variability in ADC measurement represented in the literature can be reconciled with this more sophisticated model.28 ADC measurement accuracy can also be improved by optimal choice of diffusion parameters for the specific range of ADC expected in the kidney.29
Renal Blood Oxygen Level–Dependent Imaging
Renal hypoxia may be a functional rather than anatomic marker for fibrosis. As CKD worsens, the number of capillaries in the peritubular capillary bed within the renal medulla decreases,30 resulting in decreased medullary oxygen tension. Hypoxia contributes to the activation of a complex array of cytokines and other factors leading to tubulointerstitial fibrosis and impaired renal function31 and progression to ESRD requiring dialysis.32–34
Blood oxygenation level–dependent (BOLD) is an MRI method that obtains information about renal oxygenation through a tissue parameter T2*, which is mapped on a pixel by pixel basis within an image (Figure 2). Increased levels of deoxyhemoglobin cause decreased T2*, and numerous published studies have shown an inverse association of T2* with renal oxygen levels.35–39 Furosemide transiently decreases renal medullary hypoxia, and this effect has been seen in BOLD MRI in both animals and healthy human subjects.35,36,40,41
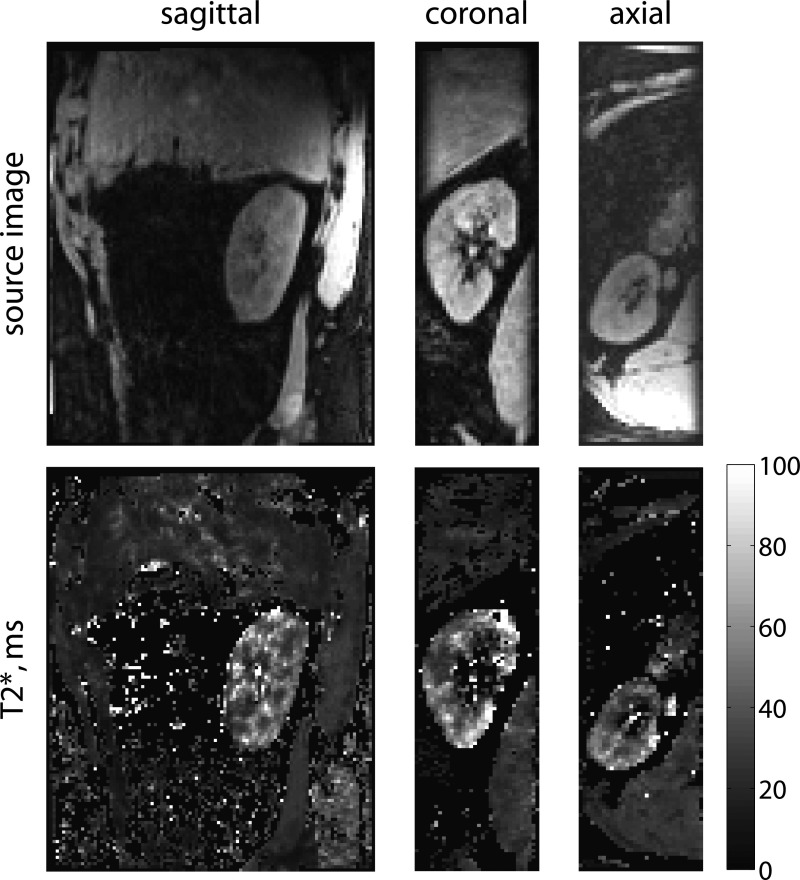
Figure 2.
Renal BOLD T2* map shows higher blood oxygenation levels in the cortex than medulla. T2* maps and source images from a normal volunteer in three planes from a full three-dimensional dataset are shown. The images were acquired with a free-breathing prospectively navigated method,58 with a total scan time of 5 minutes and 13 seconds. T2* is higher in the cortex than the medulla, reflecting higher levels of blood oxygenation in the cortex.
The important question of whether BOLD MRI, with or without diuretic challenge, reflects the severity of kidney disease remains open. Some studies have shown differences in the baseline T2* values in normal kidneys versus diabetic kidneys correlating with degree of kidney disease in humans42 and a rat model,43 whereas another study shows no correlation at all between severity of renal disease and measured T2*.44 Similarly, some data have suggested that the change in T2* after furosemide administration or water diuresis is attenuated in diabetic kidneys45,46 or elderly subjects,47 whereas a study in a rat model of diabetes showed no difference in the renal T2* response to furosemide between normal kidneys and diabetic kidneys.17
This variability may arise, because many factors affect renal T2* in addition to deoxyhemoglobin concentration. Experimental data consistently show changes in PO2 reflected in changes in T2*, but the absolute values of T2* have shown limited usefulness as a measure of tissue PO2. Models incorporating additional factors, such as tissue perfusion, vascular volume, and kidney water content, show some promise in teasing out tissue PO2 levels from the renal BOLD MRI signal.38,48
Magnetic Resonance Elastography
Magnetic resonance (MR) elastography is a method of quantifying tissue stiffness with MRI.49 In this method, a mechanical transducer is placed against the body surface and causes mechanical vibration that propagates through the tissue. MRI is performed in synchrony with the mechanical vibration. MRI phase images can detect small displacements of tissue throughout the imaging volume. These displacement maps can then be used to calculate the stiffness of tissue throughout the imaging volume (Figure 3).
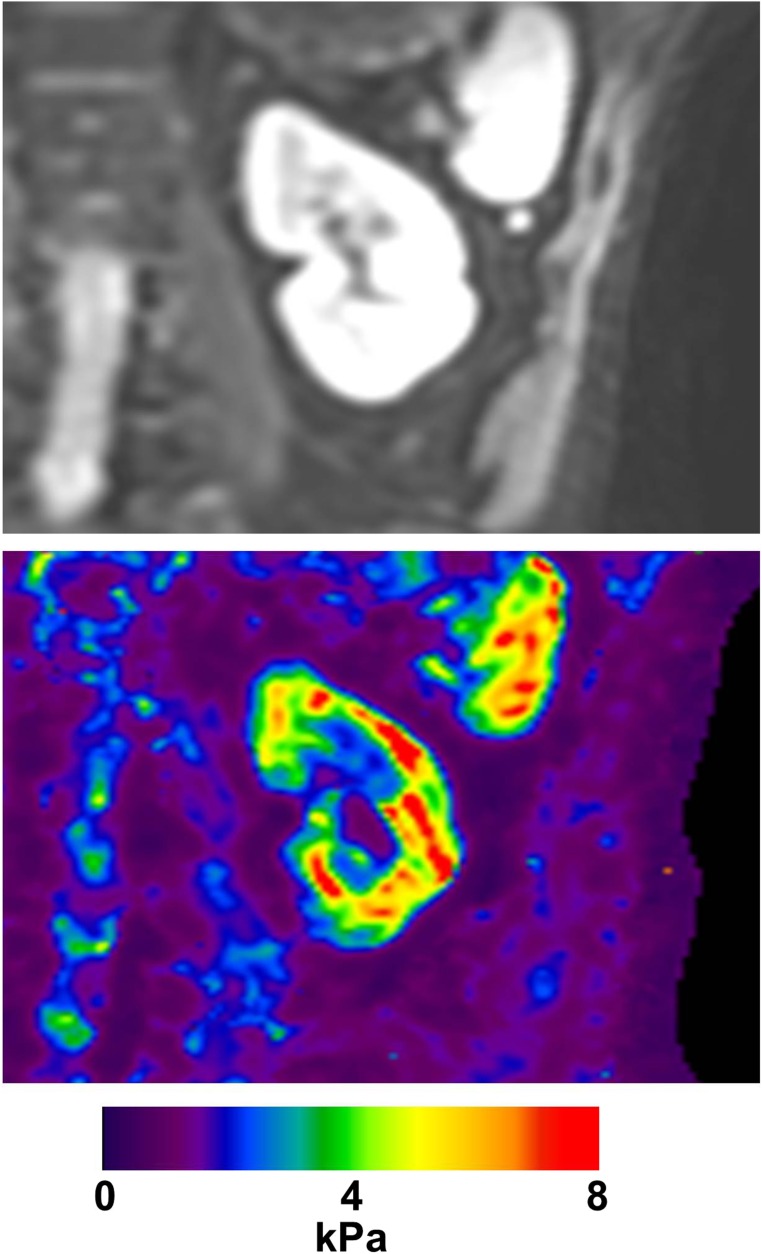
Figure 3.
Renal MRI elastography measures the stiffness of the kidney. A map of stiffness over a single coronal slice of the kidney of a normal volunteer is shown. Images courtesy of Richard Ehman.
Renal fibrosis increases the stiffness of the kidneys, which could be detected by MR elastography. However, several factors other than fibrosis affect kidney stiffness. A study of kidney stiffness using the related technique of ultrasound elastography in pigs showed that kidney stiffness varies with the filling pressure of the renal collecting system, the rate of arterial and venous blood flow, and the direction of the sound wave relative to the kidney.50 A renal MR elastography study in pig kidneys with surgical ligation of the renal artery with varying degrees of stenosis showed decrease in stiffness of the renal cortex with acute stenosis, likely due to the decrease in cortical turgor caused by decreased blood flow, but no significant change in the stiffness of the cortex or medulla of kidneys affected by chronic renal artery stenosis, despite considerable fibrosis in these kidneys on histology.51 It is hypothesized that the increased stiffness of the kidneys caused by fibrosis may be offset by decreased stiffness due to decreased renal blood flow reducing renal turgor. A subsequent study by the same group showed some increase in medullary stiffness on MR elastography of pig kidneys with a different model of renal artery stenosis, with elastography results significantly but weakly correlated with severity of fibrosis on histology. Published human kidney MR elastography work has so far been limited to proof of concept and reproducibility studies.52–54
Susceptibility-Weighted MRI
Magnetic susceptibility is a physical property of tissue that varies slightly between different tissue types and has a complex effect on MR images. For example, susceptibility-weighted imaging is useful for depicting small veins in and around the brain. The potential of susceptibility-weighted imaging in the kidney is unclear, but one study suggests that water loading causes changes in MRI phase images of the medulla of human kidneys.55 Sophisticated mathematical analysis of MRI phase images can go beyond susceptibility weighting to yield quantitative maps of estimated tissue susceptibility. In a study of mice with deficient angiotensin receptor type 1 (AT1), significant differences in susceptibility were seen between wild-type mice and mice with AT1 deficiency.56 Because AT1 deficiency leads to renal disease, including fibrosis, it was hypothesized that susceptibility changes in diseased kidneys represented the effect of accumulating collagen and other proteins. However, gadolinium contrast agent was used in this study to shorten imaging time, which itself strongly affects susceptibility, and it is possible that the observed differences in susceptibility could be related to changes in the kidney other than or in addition to fibrosis that affect gadolinium distribution. An extension of susceptibility imaging known as susceptibility tensor imaging has been applied to excised and fixed rat kidneys and has shown the intriguing potential to provide information about renal tissue microstructure on the scale of the renal tubule that may be relevant to renal fibrosis.57 However, this method is limited to ex vivo imaging and is not currently feasible for human imaging, because it requires extremely long imaging times and also requires that the tissue be placed in a variety of orientations relative to the main magnetic field of the MRI machine, which is not possible for human subjects in a conventional toroidal MRI system.
Summary
MRI has been used for many years to depict the anatomy of the kidney. Newer MRI methods aim to move beyond anatomic imaging to provide information about fibrosis and kidney function. DWI, renal BOLD, renal elastography, and renal susceptibility imaging are MRI methods that may be useful for evaluating renal fibrosis, but each still requires further development and validation. DWI, the most extensively studied method, has the advantages of being fast and simple to perform with existing MRI hardware. The limited accuracy of this technique for evaluation of renal fibrosis may be improved by better standardization of protocols and routine incorporation of more sophisticated models to differentiate tubular, vascular, and fibrosis contributions to changes in diffusion parameters. Many confounding factors have led to contradictory published renal BOLD MRI results so far. Efforts to advance BOLD MRI are motivated by the compelling promise of noninvasive measurement of renal oxygen levels. MR elastography directly measures the stiffness of the kidney, potentially a measure of fibrosis, but is also confounded by other variables, such as renal blood flow and water content. Modeling these factors to tease out the component of kidney stiffness due to fibrosis may improve the usefulness of elastography. Finally, susceptibility imaging offers intriguing information about the microscopic structure of the kidney but is currently only feasible ex vivo. Presently, no MRI method offers a clinically useful evaluation of renal fibrosis, but these methods are on the near-horizon. Experimental data so far show promising correlations between MR results and renal fibrosis and function, which encourage us to continue development and improvement of MRI kidney imaging methods for the evaluation of renal fibrosis.
Disclosures
None.
Acknowledgments
The authors acknowledge support from the National Institute of Health grant (R01DK063183-06).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Eckardt KU, Bernhardt WM, Weidemann A, Warnecke C, Rosenberger C, Wiesener MS, Willam C: Role of hypoxia in the pathogenesis of renal disease. Kidney Int Suppl 99: S46–S51, 2005 [DOI] [PubMed] [Google Scholar]
- 2.U.S. Renal Data System : USRDS 2015 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2015 [Google Scholar]
- 3.Wang Y, Harris DC: Macrophages in renal disease. J Am Soc Nephrol 22: 21–27, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Quaggin SE, Kapus A: Scar wars: Mapping the fate of epithelial-mesenchymal-myofibroblast transition. Kidney Int 80: 41–50, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Duffield JS: Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest 124: 2299–2306, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eddy AA: Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int Suppl (2011) 4: 2–8, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y: Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney Int 69: 213–217, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Tampe D, Zeisberg M: Potential approaches to reverse or repair renal fibrosis. Nat Rev Nephrol 10: 226–237, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Tøndel C, Vikse BE, Bostad L, Svarstad E: Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988-2010. Clin J Am Soc Nephrol 7: 1591–1597, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cakmak P, Yağcı AB, Dursun B, Herek D, Fenkçi SM: Renal diffusion-weighted imaging in diabetic nephropathy: Correlation with clinical stages of disease. Diagn Interv Radiol 20: 374–378, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Q, Ma Z, Wu J, Fang W: DTI for the assessment of disease stage in patients with glomerulonephritis–Correlation with renal histology. Eur Radiol 25: 92–98, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Inoue T, Kozawa E, Okada H, Inukai K, Watanabe S, Kikuta T, Watanabe Y, Takenaka T, Katayama S, Tanaka J, Suzuki H: Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol 22: 1429–1434, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanzman RS, Ljimani A, Pentang G, Zgoura P, Zenginli H, Kröpil P, Heusch P, Schek J, Miese FR, Blondin D, Antoch G, Wittsack HJ: Kidney transplant: Functional assessment with diffusion-tensor MR imaging at 3T. Radiology 266: 218–225, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Xu Y, Zhang J, Zhen J, Wang R, Cai S, Yuan X, Liu Q: Chronic kidney disease: Pathological and functional assessment with diffusion tensor imaging at 3T MR. Eur Radiol 25: 652–660, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Lu L, Sedor JR, Gulani V, Schelling JR, O’Brien A, Flask CA, MacRae Dell K: Use of diffusion tensor MRI to identify early changes in diabetic nephropathy. Am J Nephrol 34: 476–482, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Namimoto T, Yamashita Y, Mitsuzaki K, Nakayama Y, Tang Y, Takahashi M: Measurement of the apparent diffusion coefficient in diffuse renal disease by diffusion-weighted echo-planar MR imaging. J Magn Reson Imaging 9: 832–837, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Ries M, Basseau F, Tyndal B, Jones R, Deminière C, Catargi B, Combe C, Moonen CW, Grenier N: Renal diffusion and BOLD MRI in experimental diabetic nephropathy. Blood oxygen level-dependent. J Magn Reson Imaging 17: 104–113, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Thoeny HC, De Keyzer F, Oyen RH, Peeters RR: Diffusion-weighted MR imaging of kidneys in healthy volunteers and patients with parenchymal diseases: Initial experience. Radiology 235: 911–917, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Togao O, Doi S, Kuro-o M, Masaki T, Yorioka N, Takahashi M: Assessment of renal fibrosis with diffusion-weighted MR imaging: Study with murine model of unilateral ureteral obstruction. Radiology 255: 772–780, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X, Fang W, Ling H, Chai W, Chen K: Diffusion-weighted MR imaging of kidneys in patients with chronic kidney disease: Initial study. Eur Radiol 20: 978–983, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Yalçin-Şafak K, Ayyildiz M, Ünel SY, Umarusman-Tanju N, Akça A, Baysal T: The relationship of ADC values of renal parenchyma with CKD stage and serum creatinine levels. Eur J Radiol Open 3: 8–11, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toya R, Naganawa S, Kawai H, Ikeda M: Correlation between estimated glomerular filtration rate (eGFR) and apparent diffusion coefficient (ADC) values of the kidneys. Magn Reson Med Sci 9: 59–64, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Gaudiano C, Clementi V, Busato F, Corcioni B, Orrei MG, Ferramosca E, Fabbri E, Berardi P, Santoro A, Golfieri R: Diffusion tensor imaging and tractography of the kidneys: Assessment of chronic parenchymal diseases. Eur Radiol 23: 1678–1685, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Boor P, Perkuhn M, Weibrecht M, Zok S, Martin IV, Gieseke J, Schoth F, Ostendorf T, Kuhl C, Floege J: Diffusion-weighted MRI does not reflect kidney fibrosis in a rat model of fibrosis. J Magn Reson Imaging 42: 990–998, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M: Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168: 497–505, 1988 [DOI] [PubMed] [Google Scholar]
- 26.Notohamiprodjo M, Chandarana H, Mikheev A, Rusinek H, Grinstead J, Feiweier T, Raya JG, Lee VS, Sigmund EE: Combined intravoxel incoherent motion and diffusion tensor imaging of renal diffusion and flow anisotropy. Magn Reson Med 73: 1526–1532, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Sigmund EE, Vivier PH, Sui D, Lamparello NA, Tantillo K, Mikheev A, Rusinek H, Babb JS, Storey P, Lee VS, Chandarana H: Intravoxel incoherent motion and diffusion-tensor imaging in renal tissue under hydration and furosemide flow challenges. Radiology 263: 758–769, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Zhang JL, Sigmund EE, Chandarana H, Rusinek H, Chen Q, Vivier PH, Taouli B, Lee VS: Variability of renal apparent diffusion coefficients: Limitations of the monoexponential model for diffusion quantification. Radiology 254: 783–792, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang JL, Sigmund EE, Rusinek H, Chandarana H, Storey P, Chen Q, Lee VS: Optimization of b-value sampling for diffusion-weighted imaging of the kidney. Magn Reson Med 67: 89–97, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bohle A, Mackensen-Haen S, Wehrmann M: Significance of postglomerular capillaries in the pathogenesis of chronic renal failure. Kidney Blood Press Res 19: 191–195, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Norman JT, Fine LG: Intrarenal oxygenation in chronic renal failure. Clin Exp Pharmacol Physiol 33: 989–996, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Fine LG, Bandyopadhay D, Norman JT: Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia. Kidney Int Suppl 75: S22–S26, 2000 [PubMed] [Google Scholar]
- 33.Fine LG, Norman JT: Chronic hypoxia as a mechanism of progression of chronic kidney diseases: From hypothesis to novel therapeutics. Kidney Int 74: 867–872, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Nangaku M: Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Brezis M, Agmon Y, Epstein FH: Determinants of intrarenal oxygenation. I. Effects of diuretics. Am J Physiol 267: F1059–F1062, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Warner L, Glockner JF, Woollard J, Textor SC, Romero JC, Lerman LO: Determinations of renal cortical and medullary oxygenation using blood oxygen level-dependent magnetic resonance imaging and selective diuretics. Invest Radiol 46: 41–47, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedersen M, Dissing TH, Mørkenborg J, Stødkilde-Jørgensen H, Hansen LH, Pedersen LB, Grenier N, Frøkiaer J: Validation of quantitative BOLD MRI measurements in kidney: Application to unilateral ureteral obstruction. Kidney Int 67: 2305–2312, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Pohlmann A, Arakelyan K, Hentschel J, Cantow K, Flemming B, Ladwig M, Waiczies S, Seeliger E, Niendorf T: Detailing the relation between renal T2* and renal tissue pO2 using an integrated approach of parametric magnetic resonance imaging and invasive physiological measurements. Invest Radiol 49: 547–560, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Pohlmann A, Cantow K, Hentschel J, Arakelyan K, Ladwig M, Flemming B, Hoff U, Persson PB, Seeliger E, Niendorf T: Linking non-invasive parametric MRI with invasive physiological measurements (MR-PHYSIOL): Towards a hybrid and integrated approach for investigation of acute kidney injury in rats. Acta Physiol (Oxf) 207: 673–689, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Prasad PV, Edelman RR, Epstein FH: Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation 94: 3271–3275, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Priatna A, Epstein FH, Spokes K, Prasad PV: Evaluation of changes in intrarenal oxygenation in rats using multiple gradient-recalled echo (mGRE) sequence. J Magn Reson Imaging 9: 842–846, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yin WJ, Liu F, Li XM, Yang L, Zhao S, Huang ZX, Huang YQ, Liu RB: Noninvasive evaluation of renal oxygenation in diabetic nephropathy by BOLD-MRI. Eur J Radiol 81: 1426–1431, 2012 [DOI] [PubMed] [Google Scholar]
- 43.dos Santos EA, Li LP, Ji L, Prasad PV: Early changes with diabetes in renal medullary hemodynamics as evaluated by fiberoptic probes and BOLD magnetic resonance imaging. Invest Radiol 42: 157–162, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michaely HJ, Metzger L, Haneder S, Hansmann J, Schoenberg SO, Attenberger UI: Renal BOLD-MRI does not reflect renal function in chronic kidney disease. Kidney Int 81: 684–689, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Economides PA, Caselli A, Zuo CS, Sparks C, Khaodhiar L, Katsilambros N, Horton ES, Veves A: Kidney oxygenation during water diuresis and endothelial function in patients with type 2 diabetes and subjects at risk to develop diabetes. Metabolism 53: 222–227, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Epstein FH, Veves A, Prasad PV: Effect of diabetes on renal medullary oxygenation during water diuresis. Diabetes Care 25: 575–578, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Epstein FH, Prasad P: Effects of furosemide on medullary oxygenation in younger and older subjects. Kidney Int 57: 2080–2083, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Zhang JL, Morrell G, Rusinek H, Warner L, Vivier PH, Cheung AK, Lerman LO, Lee VS: Measurement of renal tissue oxygenation with blood oxygen level-dependent MRI and oxygen transit modeling. Am J Physiol Renal Physiol 306: F579–F587, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL: Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science 269: 1854–1857, 1995 [DOI] [PubMed] [Google Scholar]
- 50.Gennisson JL, Grenier N, Combe C, Tanter M: Supersonic shear wave elastography of in vivo pig kidney: Influence of blood pressure, urinary pressure and tissue anisotropy. Ultrasound Med Biol 38: 1559–1567, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Warner L, Yin M, Glaser KJ, Woollard JA, Carrascal CA, Korsmo MJ, Crane JA, Ehman RL, Lerman LO: Noninvasive In vivo assessment of renal tissue elasticity during graded renal ischemia using MR elastography. Invest Radiol 46: 509–514, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee CU, Glockner JF, Glaser KJ, Yin M, Chen J, Kawashima A, Kim B, Kremers WK, Ehman RL, Gloor JM: MR elastography in renal transplant patients and correlation with renal allograft biopsy: A feasibility study. Acad Radiol 19: 834–841, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Low G, Owen NE, Joubert I, Patterson AJ, Graves MJ, Glaser KJ, Alexander GJ, Lomas DJ: Reliability of magnetic resonance elastography using multislice two-dimensional spin-echo echo-planar imaging (SE-EPI) and three-dimensional inversion reconstruction for assessing renal stiffness. J Magn Reson Imaging 42: 844–850, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rouvière O, Souchon R, Pagnoux G, Ménager JM, Chapelon JY: Magnetic resonance elastography of the kidneys: Feasibility and reproducibility in young healthy adults. J Magn Reson Imaging 34: 880–886, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding J, Xing W, Wu D, Chen J, Pan L, Sun J, Xing S, Dai Y: Evaluation of renal oxygenation level changes after water loading using susceptibility-weighted imaging and T2* mapping. Korean J Radiol 16: 827–834, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie L, Sparks MA, Li W, Qi Y, Liu C, Coffman TM, Johnson GA: Quantitative susceptibility mapping of kidney inflammation and fibrosis in type 1 angiotensin receptor-deficient mice. NMR Biomed 26: 1853–1863, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie L, Dibb R, Cofer GP, Li W, Nicholls PJ, Johnson GA, Liu C: Susceptibility tensor imaging of the kidney and its microstructural underpinnings. Magn Reson Med 73: 1270–1281, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morrell G, Zhang JL, Kaggie J, Lee VS: 3D renal BOLD imaging with a prospectively navigated free breathing pulse sequence. Presented at the 22nd Meeting of the International Society of Magnetic Resonance in Medicine, Milan, Italy, May 10, 2014 [Google Scholar]