Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by innumerous fluid-filled cysts and progressive deterioration of renal function. Previously, we showed that periostin, a matricellular protein involved in tissue repair, is markedly overexpressed by cyst epithelial cells. Periostin promotes cell proliferation, cyst growth, interstitial fibrosis, and the decline in renal function in PKD mice. Here, we investigated the regulation of these processes by the integrin-linked kinase (ILK), a scaffold protein that links the extracellular matrix to the actin cytoskeleton and is stimulated by periostin. Pharmacologic inhibition or shRNA knockdown of ILK prevented periostin-induced Akt/mammalian target of rapamycin (mTOR) signaling and ADPKD cell proliferation in vitro. Homozygous deletion of ILK in renal collecting ducts (CD) of Ilkfl/fl;Pkhd1-Cre mice caused tubule dilations, apoptosis, fibrosis, and organ failure by 10 weeks of age. By contrast, Ilkfl/+;Pkhd1-Cre mice had normal renal morphology and function and survived >1 year. Reduced expression of ILK in Pkd1fl/fl;Pkhd1-Cre mice, a rapidly progressive model of ADPKD, decreased renal Akt/mTOR activity, cell proliferation, cyst growth, and interstitial fibrosis, and significantly improved renal function and animal survival. Additionally, CD-specific knockdown of ILK strikingly reduced renal cystic disease and fibrosis and extended the life of pcy/pcy mice, a slowly progressive PKD model. We conclude that ILK is critical for maintaining the CD epithelium and renal function and is a key intermediate for periostin activation of signaling pathways involved in cyst growth and fibrosis in PKD.
Keywords: ILK, matricellular proteins, periostin, PKD
Autosomal dominant polycystic kidney disease (ADPKD) is caused by mutations in PKD1 or PKD2, which encode for polycystin-1 and -2, respectively.1 The disorder is characterized by the formation of fluid-filled cysts, leading to loss of functional parenchyma, interstitial fibrosis, and enlarged kidneys. Several signaling pathways have been implicated in polycystic kidney disease (PKD)2; however, the mechanisms involved in disease progression remain unclear. Cyst-lining cells are characterized as being incompletely differentiated and express factors involved in tissue repair.3–5 As cysts expand, compression of neighboring nephrons also induces an injury response that activates repair mechanisms, including the Akt/mammalian target of rapamycin (mTOR) pathway.4,6,7 This perpetual renal injury-repair response contributes importantly to aberrant cell proliferation5,8 and relentless cyst growth.
Extracellular matrix (ECM) remodeling is an important part of wound healing9; however, excessive matrix deposition leads to fibrosis, a common feature of CKD.10 Matricellular proteins represent a family of soluble ECM molecules that regulate collagen fibrillogenesis and bind to integrins for communication between the cell and ECM.11,12 Persistent expression of these proteins is associated with inflammation, tissue fibrosis, and disease progression.13–18
We found that periostin, a matricellular protein involved in tissue repair, was highly overexpressed in ADPKD, autosomal recessive PKD, and rodent models of PKD, suggesting that periostin expression is a common feature of renal cystic disease.19 Knockout of periostin reduced mTOR activity, cyst growth, and interstitial fibrosis, and significantly improved the survival of pcy/pcy mice.20
αVβ3-integrins, the receptors for periostin, promote proliferation of cancer cells21 by activating integrin-linked kinase (ILK)22 and stimulating downstream Akt/mTOR and GSK-3β/β-catenin signaling pathways.23,24 ILK interacts with β1- integrin and was originally characterized as a kinase.23,25–30 However, it remains unclear if ILK is a bona fide kinase because the kinase domain lacks conserved motifs found in conventional kinases, and mutations that should render the kinase inactive failed to alter mouse development.28,31–33 Nevertheless, it is generally agreed that ILK serves as a scaffolding protein critical for the formation of a multiprotein complex with adaptor proteins PINCH and α-parvin.34,35 ILK-PINCH-Parvin (IPP) signaling affects phosphorylation of downstream molecules such as Akt and the mitogen-activated protein kinases36–39 and plays a central role in ECM-integrin signaling.40,41 The IPP ternary complex is responsible for binding β-integrins to the actin cytoskeleton and recruiting focal adhesion components that mediate cell proliferation, survival, and migration during tissue repair.32,42 ILK activation of Akt and mTOR mediates proliferation of cancer cells.43,44 In addition, PINCH interacts with adaptor protein NCK2, leading to crosstalk between the ILK and receptor tyrosine kinase (RTK) pathways.35,40,45
We determined if ILK mediates periostin-induced ADPKD cell proliferation and if knocking down ILK in renal collecting ducts (CDs) reduces cystic disease in two models of PKD. The results indicate that ILK is a key intermediate in the activation of cellular pathways involved in cyst growth. We propose that targeting the periostin-integrin-ILK axis may be a potential therapeutic approach to slow cyst growth and fibrosis in PKD.
Results
Effect of Periostin on ILK-mTOR Signaling and Proliferation of ADPKD Cells
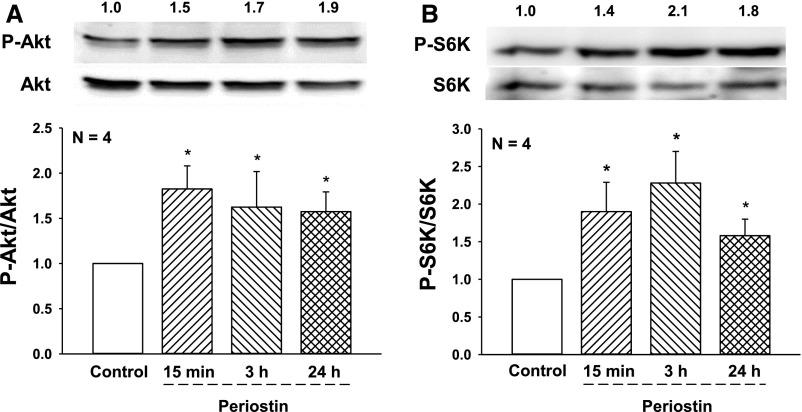
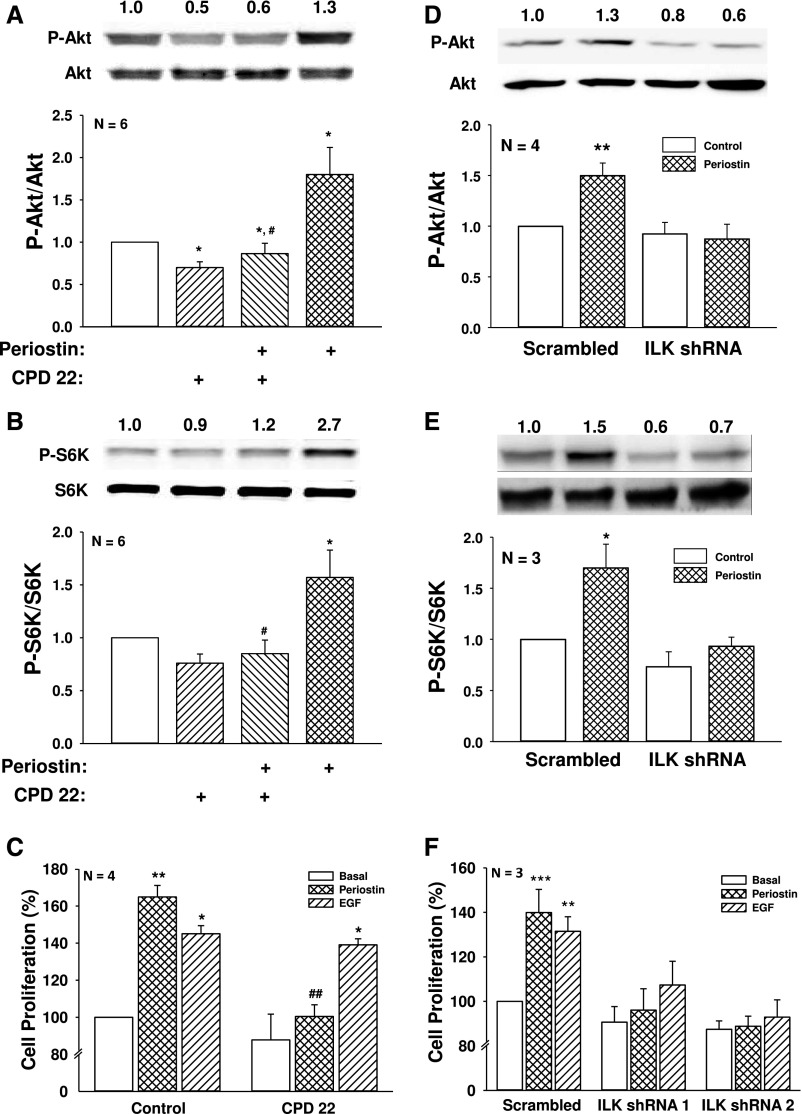
ILK was immunoprecipitated from primary ADPKD and normal human kidney (NHK) cells and incubated with GSKα/β protein or myelin basic protein in a kinase assay.25,46 Consistent with previous reports, ILK appeared to be capable of phosphorylating these substrates (Supplemental Figure 1); however, it is unclear if other components of the complex were immunoprecipitated with ILK.29,33,36 Basal ILK activity was higher in ADPKD than NHK cells and periostin caused a further increase in ILK activity in ADPKD cells. Previously, we found that periostin stimulated the proliferation of ADPKD cells, but not NHK cells, a difference that may be related to increased expression of αV-integrin in the cystic cells.19 Periostin increased phosphorylated Akt (P-Akt/Akt) and S6K (P-S6K/S6K), a target of mTOR, in as little as 15 minutes and levels remained elevated for 24 hours (Figure 1). Incubation with CPD 22, an ILK inhibitor,47 blocked periostin-induced phosphorylation of Akt and S6K (Figure 2, A and B). Periostin and EGF increased ADPKD cell proliferation to similar levels. Incubation with CPD 22 blocked periostin-induced cell proliferation (Figure 2C) but did not affect the EGF response.
Figure 1.
Periostin stimulates the Akt/mTOR pathway in human ADPKD cells. Primary cyst epithelial cells from human ADPKD kidneys (n=4) were incubated in media containing 250 ng/ml periostin for 15 minutes, or 3 or 24 hours. Levels of (A) phosphorylated Akt (P-Akt) and (B) phosphorylated S6K (P-S6K), a downstream target of mTOR, were determined by immunoblot analysis. Numbers above representative immunoblots are (A) P-Akt/Akt and (B) P-S6K/S6K, normalized to control (set to 1.0). Bar graphs represent the mean and SEM for (A) P-Akt/Akt and (B) P-S6K/S6K from cell preparations from four different ADPKD kidneys. *P<0.05, versus control treatment.
Figure 2.
ILK inhibition or knockdown prevents periostin-induced Akt/mTOR signaling and ADPKD cell proliferation. ADPKD cells were treated with or without 2.5 µM CPD 22 for 1 hour and then periostin was added for an additional 15 minutes. Immunoblot analysis was used to measure (A) P-Akt/Akt and (B) P-S6K/S6K. (C) The effect of ILK inhibition on ADPKD cell proliferation was determined by treating cells with 250 ng/ml periostin or 25 ng/ml EGF for 48 hours in the presence or absence of 1 µM CPD 22. Cell numbers were counted using a BioRad TC20 cell counter. To confirm the role of ILK, ADPKD cells were infected with lentivirus containing shRNA against ILK or a nonspecific (scrambled) sequence. Cells were treated with 250 ng/ml periostin for 15 minutes and levels of (D) P-Akt and (E) P-S6K were measured by immunoblot analysis. Summary data for (D) P-Akt/Akt and (E) P-S6K/S6K were normalized to basal conditions of scrambled shRNA cells (set to 1.0). (F) ADPKD cells were infected with an ILK (shRNA 1 or shRNA 2) or a scrambled shRNA lentivirus, and then treated with periostin or 100 ng/ml EGF for 24 hours. Cell proliferation was measured by Promega MTT assay and normalized to basal proliferation of cells infected with the scrambled shRNA (set to 100%). Cell proliferation was also confirmed by counting cell numbers (Supplemental Figure 2, B and C). *P<0.05, **P<0.01, and ***P<0.001, compared with control or basal conditions of scrambled shRNA infected cells, and #P<0.05, ##P<0.01, compared with periostin alone.
To confirm the results, we used a lentiviral shRNA approach to knock down ILK expression in ADPKD cells. In three separate experiments, two ILK shRNA constructs were used, achieving 46%–54% reduction in ILK expression (Supplemental Figure 2A). Periostin increased P-Akt/Akt and P-S6K/S6K to the same level in the absence and presence of scrambled shRNA (data not shown). By contrast, ILK knockdown prevented periostin stimulation of the Akt/mTOR pathway (Figure 2, D and E) and blocked periostin-induced cell proliferation (Figure 2F). In contrast to ILK inhibition with CPD 22, disruption of the IPP complex by ILK knockdown decreased EGF-induced ADPKD cell proliferation (Figure 2F), supporting a role for the IPP complex in RTK signaling.40,48,49
Generation of Mice with CD-Specific Deletion of ILK
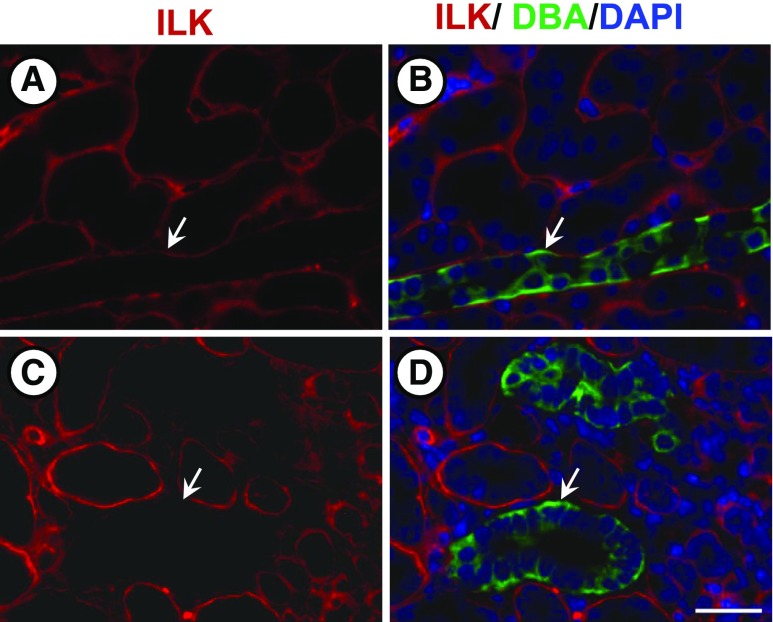
Ilkfl/fl mice50 were crossed with Pkhd1-Cre mice51 to generate wild-type (WT: Ilk+/+ CD), Ilkfl/+;Pkhd1-Cre (Ilk+/− CD), and Ilkfl/fl;Pkhd1-Cre (Ilk−/− CD) mice. ILK deletion in CDs was confirmed by coimmunofluorescence using an ILK antibody and Dolichos biflorus agglutinin (DBA, green) (Figure 3). Ilk−/− CD mice had lower body weight and developed a urine concentrating defect (Supplemental Table 1), consistent with a previous report.52 Kidneys of Ilk−/− CD mice had caspase-3–mediated anoikis53 with apoptotic cells in the lumen and dilated cortical tubules (Supplemental Figure 3). There was a significant increase in BUN as early as 25 days. By 10 weeks of age, Ilk−/− CD mice had reduced kidney size (Supplemental Table 1), massive levels of apoptosis, and renal fibrosis (data not shown), and the mice died by 10.4±0.34 weeks (n=14). By contrast, Ilk+/− CD mice had normal renal morphology and function, urine osmolality, and body weight, and survived beyond 1 year.
Figure 3.
CD-specific ILK knockout in mice. At PN day 25, Ilk+/+;Pkhd1-Cre (Ilk+/+ CD) and Ilkfl/fl;Pkhd1-Cre (Ilk−/− CD) mice were euthanized, and kidney sections were stained for ILK (red), DBA (green), and DAPI nuclear stain (blue). Left panels (A and C) are images of kidney sections that were stained for ILK expression (red). Right panels (B and D) are merged images in which ILK, DBA, and DAPI staining was superimposed. Top panels (A and B) show an Ilk+/+ CD mouse kidney section with normal ILK expression in a CD (arrow). Bottom panels (C and D) show that Ilk−/− CD mouse kidney sections lack ILK expression in the CDs. Scale bar=5 µm.
Effect of ILK on Cystic Disease Progression in ADPKD Mice
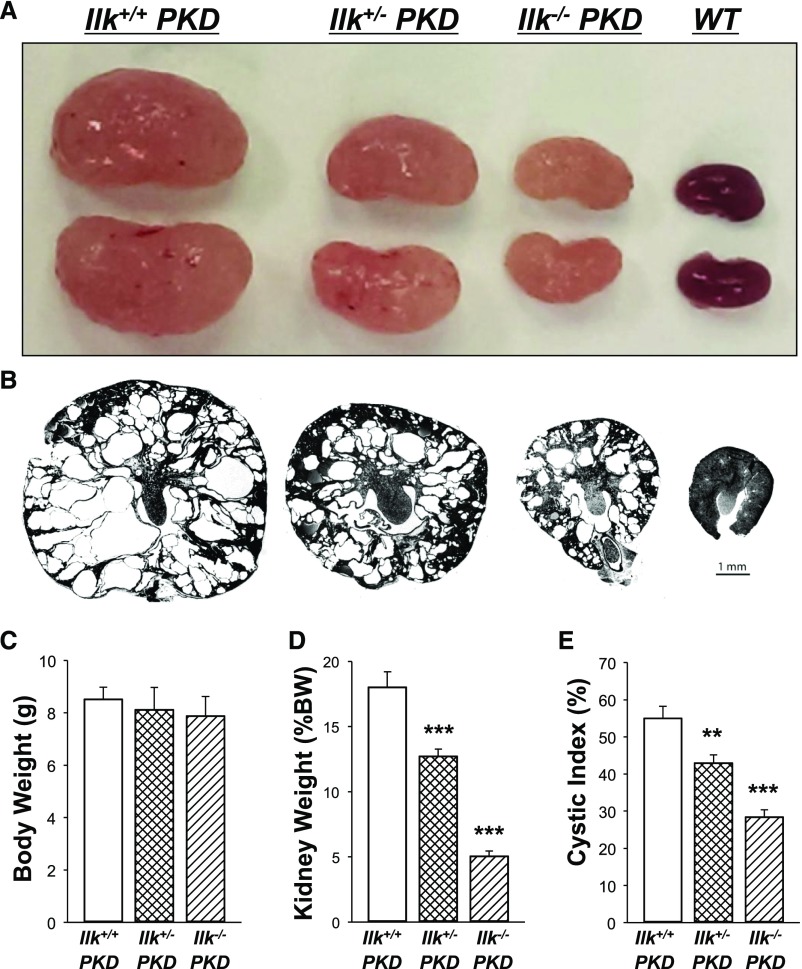
ILK expression was knocked down in CDs of Pkd1fl/fl;Pkhd1-Cre (PKD) mice, an orthologous ADPKD model with aggressive disease.51 These mice typically die by 33 days with massively enlarged kidneys. We generated Ilk+/+;Pkd1fl/fl;Pkhd1-Cre (Ilk+/+ PKD), Ilkfl/+;Pkd1fl/fl;Pkhd1-Cre (Ilk+/−PKD), and Ilkfl/fl;Pkd1fl/fl;Pkhd1-Cre (Ilk−/− PKD) mice (Figure 4). By 25 days, the PKD mice had massively enlarged kidneys (Figure 4, A and B) with percent kidney weight to body weight equaling 18.0% (Figure 4D), compared with 1.3% for WT mice (Supplemental Table 1). Loss of one or both alleles of ILK reduced kidney weight (% body weight) to 12.7% or 5.1%, respectively (Figure 4D). There was no difference in body weight among the three genotypes (Figure 4C). Cystic area of the kidneys was reduced from 55% in Ilk+/+ PKD mice to 43% and 28% in Ilk+/− PKD and Ilk−/− PKD mice, respectively (Figure 4, B and E). There were also fewer cysts in Ilk+/− PKD and Ilk−/− PKD kidneys; however, this difference was NS (data not shown).
Figure 4.
ILK knockdown decreases cyst growth and kidney weight in PKD mice. Representative images of (A) kidneys and (B) kidney sections from Ilk+/+;Pkd1fl/fl;Pkhd1-Cre (Ilk+/+ PKD), Ilkfl/+;Pkd1fl/fl;Pkhd1-Cre (Ilk+/−PKD), Ilkfl/fl;Pkd1fl/fl;Pkhd1-Cre (Ilk−/− PKD), and WT mice demonstrate that the loss of ILK expression in CD cells reduced cyst growth in PKD mice. All images were taken at the same magnification. Scale bar=1 mm. Bar graphs are mean±SEM for (C) Body weight and (D) kidney weight as a percentage of body weight (% body weight) for n=10 mice per group. (E) Cystic index is represented as the percentage of cystic area per total kidney cross-sectional surface area for n=7 mice per group. **P<0.01 and ***P<0.001, compared with Ilk+/+ PKD mice.
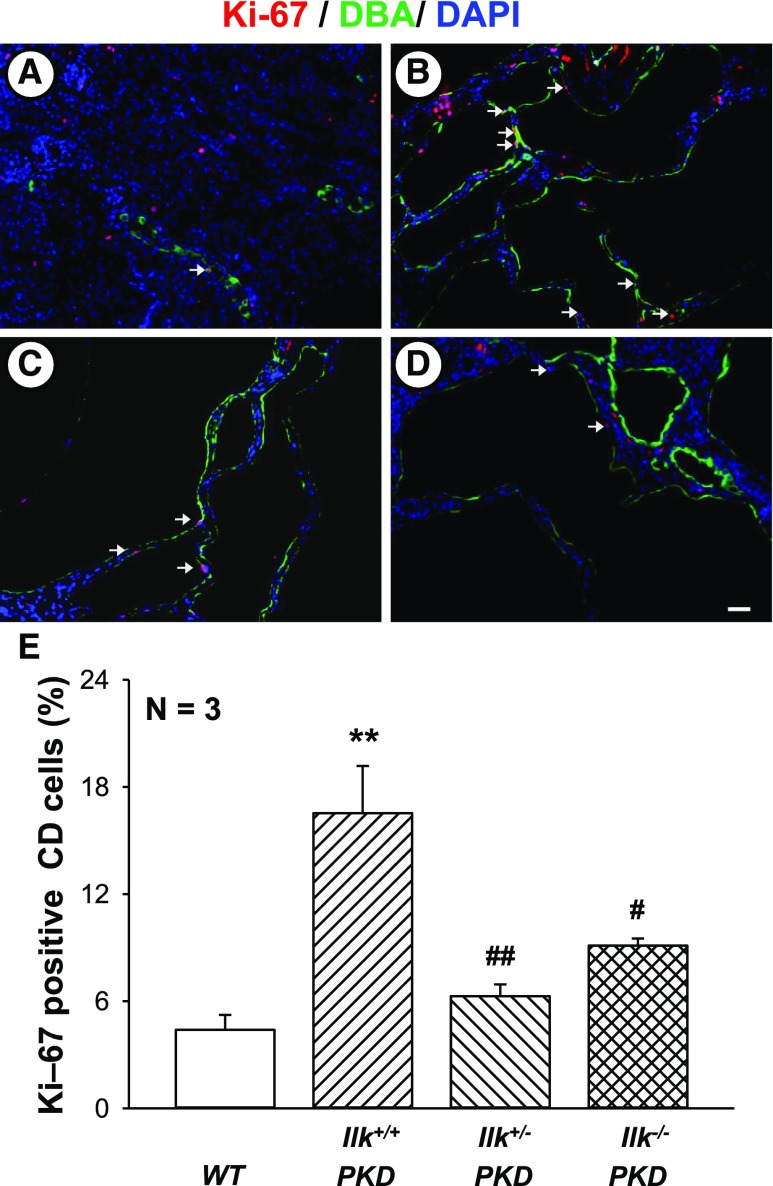
To determine if CD-specific knockdown of ILK decreased cell proliferation, the number of Ki-67–positive cells in DBA-positive tubules was determined using immunofluorescence (Figure 5). We found that Ki-67–positive cells were dramatically decreased with the loss of one or both alleles of ILK in CD cells (Figure 5E).
Figure 5.
ILK knockdown reduces renal cell proliferation in PKD mice. Representative kidney sections from (A) WT, (B) Ilk+/+;Pkd1fl/fl;Pkhd1-Cre (Ilk+/+ PKD), (C) Ilkfl/+;Pkd1fl/fl;Pkhd1-Cre (Ilk+/−PKD), and (D) Ilkfl/fl;Pkd1fl/fl;Pkhd1-Cre (Ilk−/− PKD) mice were stained with an antibody to Ki-67, a marker for cell proliferation (red). Tissues were also stained for DBA (green) to detect CDs and the DAPI nuclear stain (blue). Arrows indicate Ki-67–positive CD cells. All images were taken at the same magnification. Scale bar=5 µm. (E) Summary data for the number of cells positive for nuclear Ki-67, normalized to the total nuclei in DBA-positive CDs. Data are mean±SEM for kidneys from three mice per group. **P<0.01 compared with WT, #P<0.05 and ##P<0.01 compared with Ilk+/+ PKD.
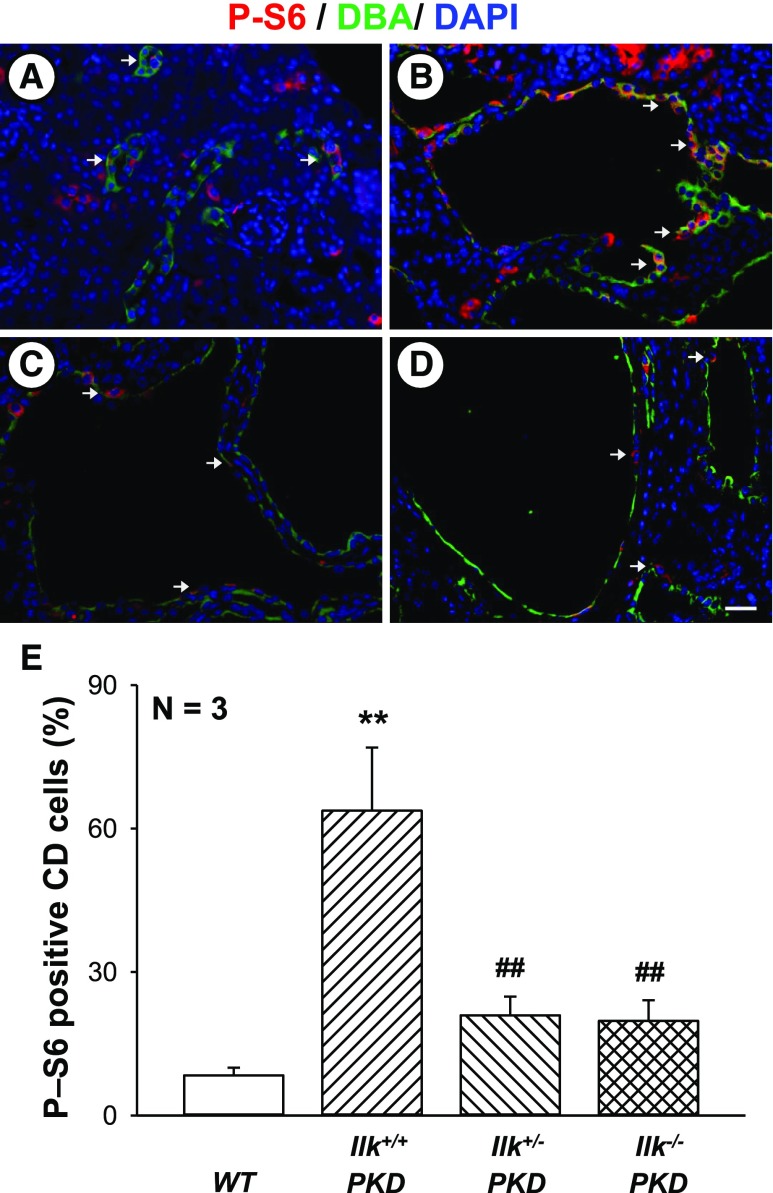
Pkd1fl/fl;Pkhd1-Cre kidneys had higher P-Akt/Akt levels than WT kidneys6,54 and partial loss or complete ablation of ILK reduced P-Akt/Akt (Supplemental Figure 4, A and B). Percentage of CD cells with phosphorylated S6 (P-S6) was higher in Ilk+/+ PKD compared with WT mice, and partial or complete loss of ILK significantly diminished P-S6 in CD-derived cysts (Figure 6). These data support the hypothesis that ILK is a key regulator of Akt/mTOR signaling in PKD.
Figure 6.
ILK knockdown decreases mTOR signaling in PKD kidneys. Representative sections from (A) WT, (B) Ilk+/+;Pkd1fl/fl;Pkhd1-Cre (Ilk+/+ PKD), (C) Ilkfl/+;Pkd1fl/fl;Pkhd1-Cre (Ilk+/−PKD), and (D) Ilkfl/fl;Pkd1fl/fl;Pkhd1-Cre (Ilk−/− PKD) mice were stained with an antibody to P-S6 (red). Tissues were also stained for DBA (green) to detect CD and the DAPI nuclear stain (blue). Arrows indicate cytoplasmic P-S6 in CD cells. All images were taken at the same magnification. Scale bar=5 µm. (E) Percentage of cytosolic P-S6–positive cells, normalized to the total nuclei in DBA-positive CDs. Data are mean±SEM for kidneys from three mice per group. **P<0.01 compared with WT, ##P<0.01 compared with Ilk+/+ PKD.
To determine the effect of ILK on RTK signaling, we examined levels of ERK activity (P-ERK/ERK) (Supplemental Figure 4, A and C). Ilk+/+ PKD kidneys showed a significant increase in ERK activity compared with WT.55 Loss of one allele of ILK decreased P-ERK; however, this was NS. By contrast, ILK ablation in Ilk−/− PKD kidneys significantly reduced P-ERK. We think that disruption of IPP complex due to loss of ILK also affects RTK signaling.40,48,49
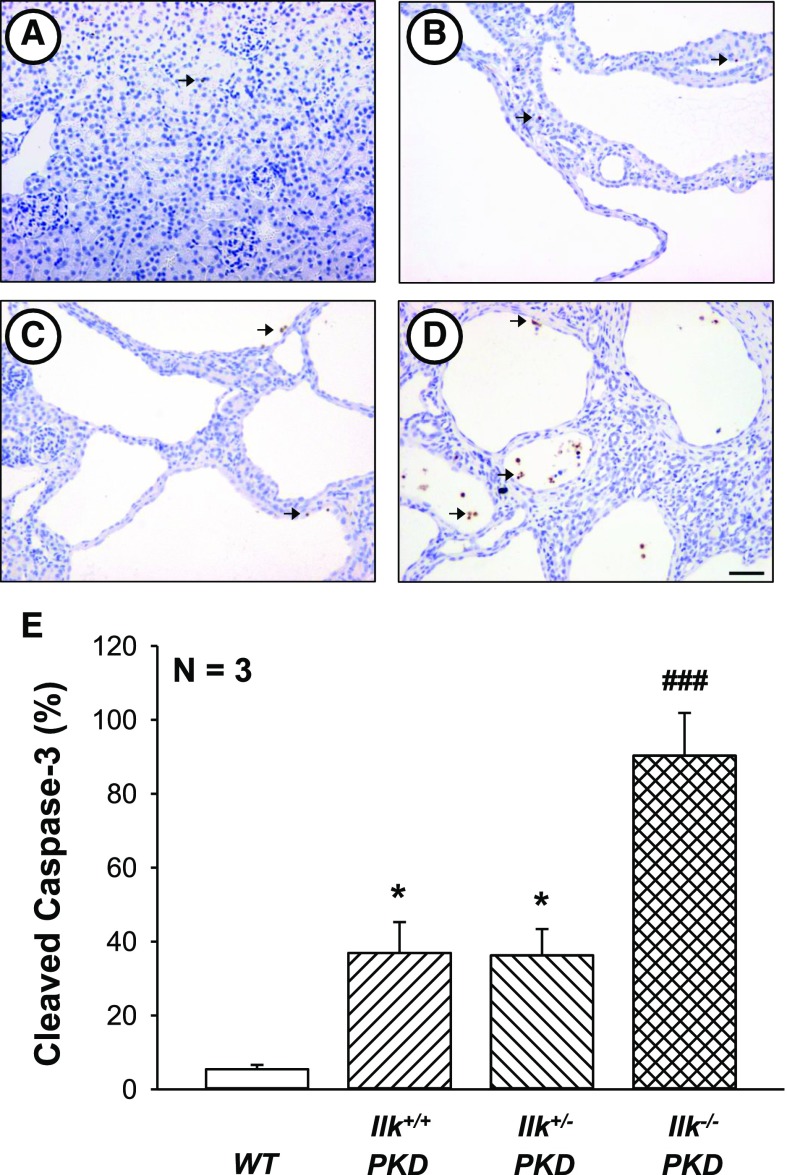
There was an increase in cleaved caspase-3–positive nuclei in PKD kidneys (Figure 7). Complete loss of ILK further increased the number of apoptotic cells and caused CD cells to be shed into the lumen (Figure 7). Previously, loss of cystic cells due to apoptosis was shown to reduce PKD progression.56,57
Figure 7.
ILK ablation induces caspase-3-mediated apoptosis in PKD kidneys. Representative kidney sections from (A) WT, (B) Ilk+/+;Pkd1fl/fl;Pkhd1-Cre (Ilk+/+ PKD), (C) Ilkfl/+;Pkd1fl/fl;Pkhd1-Cre (Ilk+/−PKD), and (D) Ilkfl/fl;Pkd1fl/fl;Pkhd1-Cre (Ilk−/− PKD) mice were stained with an antibody to cleaved caspase-3 to visualize apoptotic cells (brown staining). All images were taken at the same magnification. Scale bar=50 µm. (E) Total number of cleaved caspase-3–positive cells in the entire section was counted and normalized to the noncystic surface area of the kidney (represented as percentage cleaved caspase). Data are means±SEM for kidneys from three mice per group. *P<0.05 compared with WT, ###P<0.001 compared with Ilk+/+ PKD.
Effect of ILK on Interstitial Fibrosis, Renal Function, and Survival in PKD Kidneys
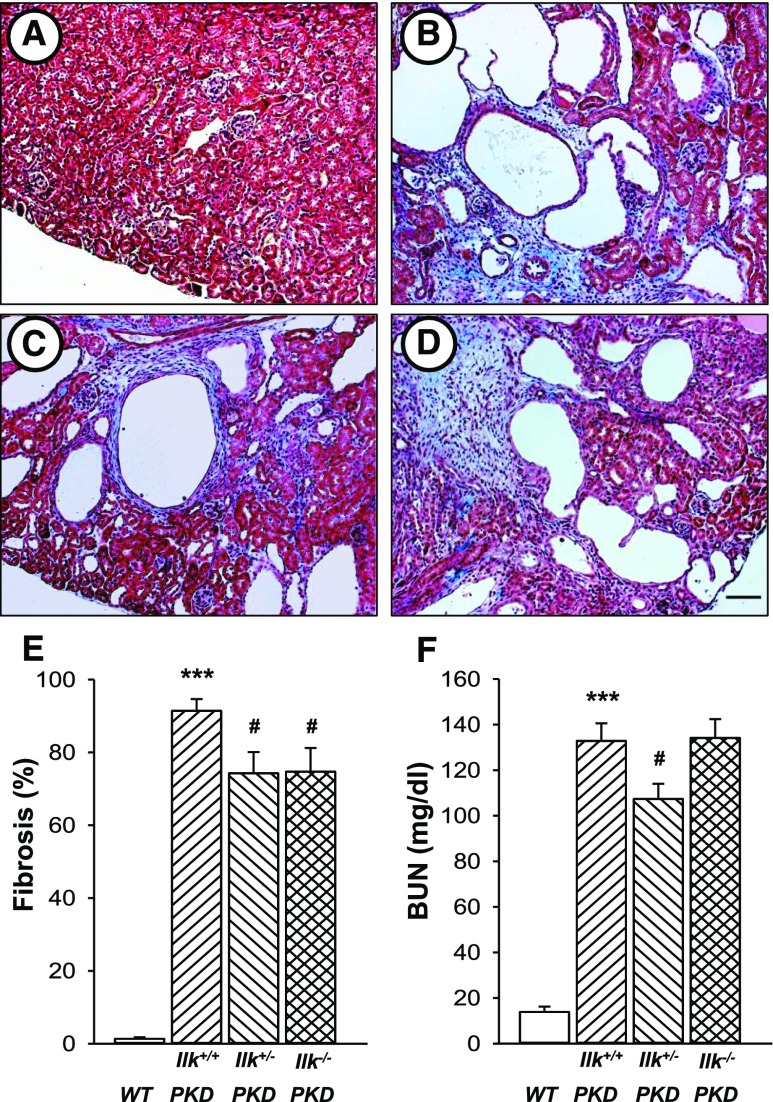
Pkd1fl/fl;Pkhd1-Cre mice develop extensive interstitial damage involving fibrosis and edema, a precursor for collagen deposition and fibrosis. At postnatal (PN) day 25, there was massive fibrosis/edema in Ilk+/+ PKD kidneys with 91% of the interstitial space showing pathologic damage (Figure 8). Ilk+/+ PKD mice showed a marked increase in BUN (132.8±7.7 mg/dl; normal range is 8–33 mg/dl). Loss of one or both alleles of ILK in CD cells caused a reduction in fibrosis. Decreased expression of ILK in Ilk+/−PKD mice reduced BUN levels, compared with Ilk+/+ PKD mice. By contrast, BUN levels of Ilk−/− PKD mice were similar to Ilk+/+ PKD mice, suggesting that renal injury due to loss of ILK confounds the improved cystic phenotype in these mice.
Figure 8.
ILK knockdown reduces renal interstitial fibrosis and improves kidney function in PKD mice. Representative kidney sections from (A) WT, (B) Ilk+/+;Pkd1fl/fl;Pkhd1-Cre (Ilk+/+ PKD), (C) Ilkfl/+;Pkd1fl/fl;Pkhd1-Cre (Ilk+/−PKD), and (D) Ilkfl/fl;Pkd1fl/fl;Pkhd1-Cre (Ilk−/− PKD) mice were stained with Masson trichrome to visualize pathogenic collagen deposition (stained blue). All images were taken at the same magnification. Scale bar=50 µm. (E) Tissue sections were coded to conceal the group assignment before being visually scored for percentage of interstitial fibrosis and edema per total area of the tissue section. Data are means±SEM for kidneys from seven mice per group. (F) Serum was isolated from WT (n=5), Ilk+/+ PKD (n=10), Ilk+/− PKD (n=10), and Ilk−/− PKD (n=7) and BUN was determined to assess renal function. ***P<0.001 compared with WT, #P<0.05 compared with Ilk+/+ PKD.
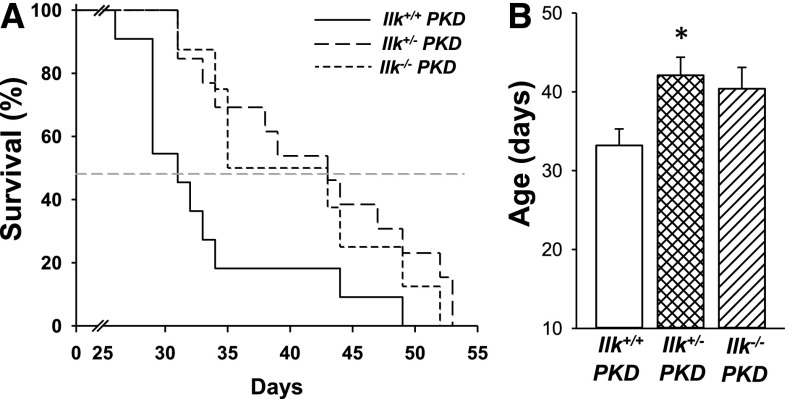
Next, we determined if knockdown of ILK alters the lifespan of the PKD mice. Pkd1fl/fl;Pkhd1-Cre mice expressing normal ILK lived to approximately 33 days (Figure 9). PKD mice heterozygous at the ILK locus (Ilk+/− PKD) survived to 42 days, displaying a 27% increase in lifespan. Complete ILK knockout increased survival to 40 days; however, this was NS. These data indicate that a reduction in ILK decreased cyst growth by inhibiting mitogenic pathways, leading to improved renal function and survival. By contrast, complete loss of ILK induced cell death, contributing, in part, to the decrease in cyst burden.
Figure 9.
ILK knockdown increases the survival of PKD mice. (A) Kaplan–Meier survival curve for Ilk+/+;Pkd1fl/fl;Pkhd1-Cre (Ilk+/+ PKD, n=11), Ilkfl/+;Pkd1fl/fl;Pkhd1-Cre (Ilk+/−PKD, n=13), and Ilkfl/fl;Pkd1fl/fl;Pkhd1-Cre (Ilk−/− PKD, n=8) mice. The dotted line represents the time point at which 50% of the mice had died. ILK knockdown in CD cells extended the survival of PKD mice. (B) Heterozygous loss of ILK significantly increased mean survival age from 33±2 for the Ilk+/+ PKD mice to 42±2 days for the Ilk+/− PKD mice, *P<0.05. Complete loss of ILK (Ilk−/− PKD) appeared to extend survival; however, the difference did not reach statistical significance.
Effect of ILK on PKD Progression in pcy/pcy Mice
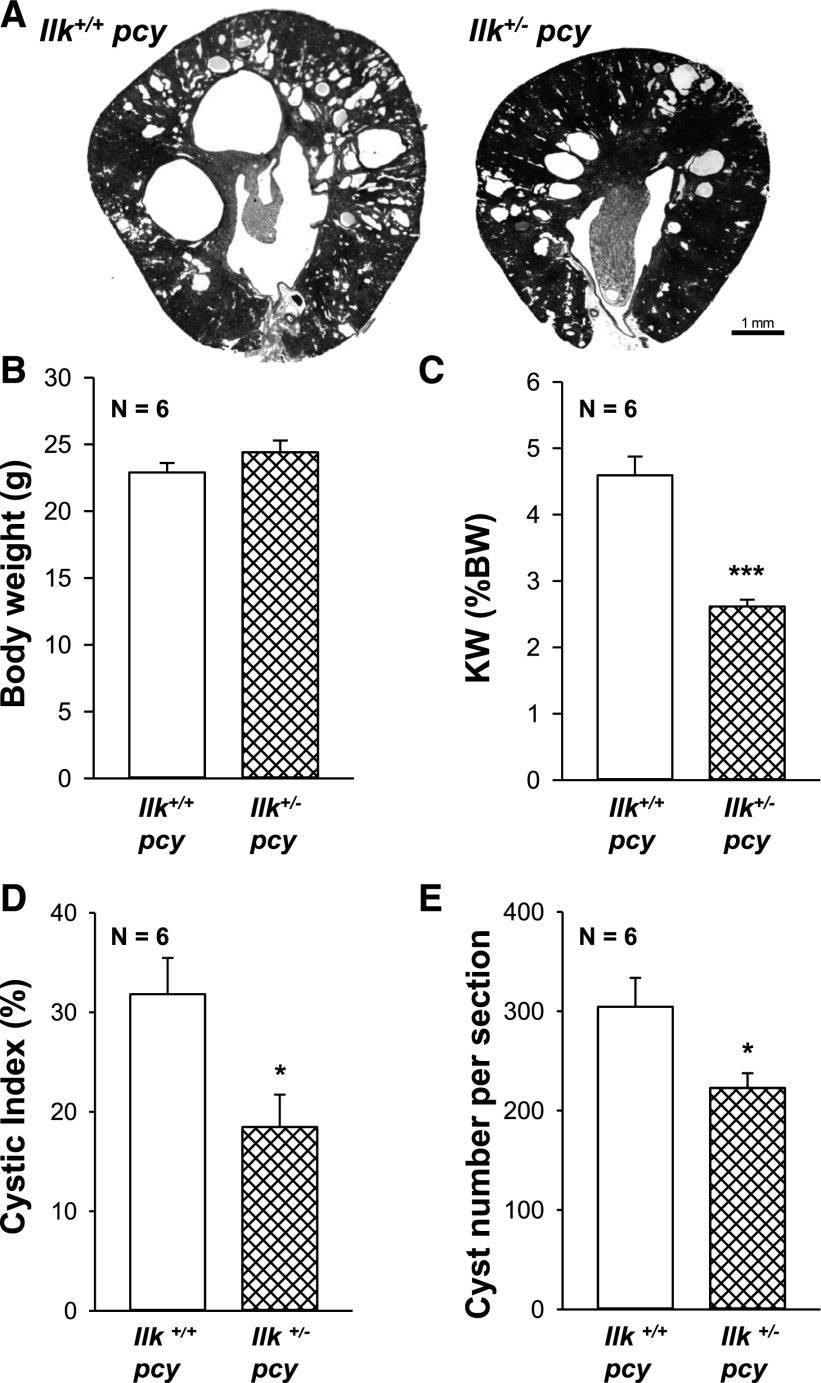
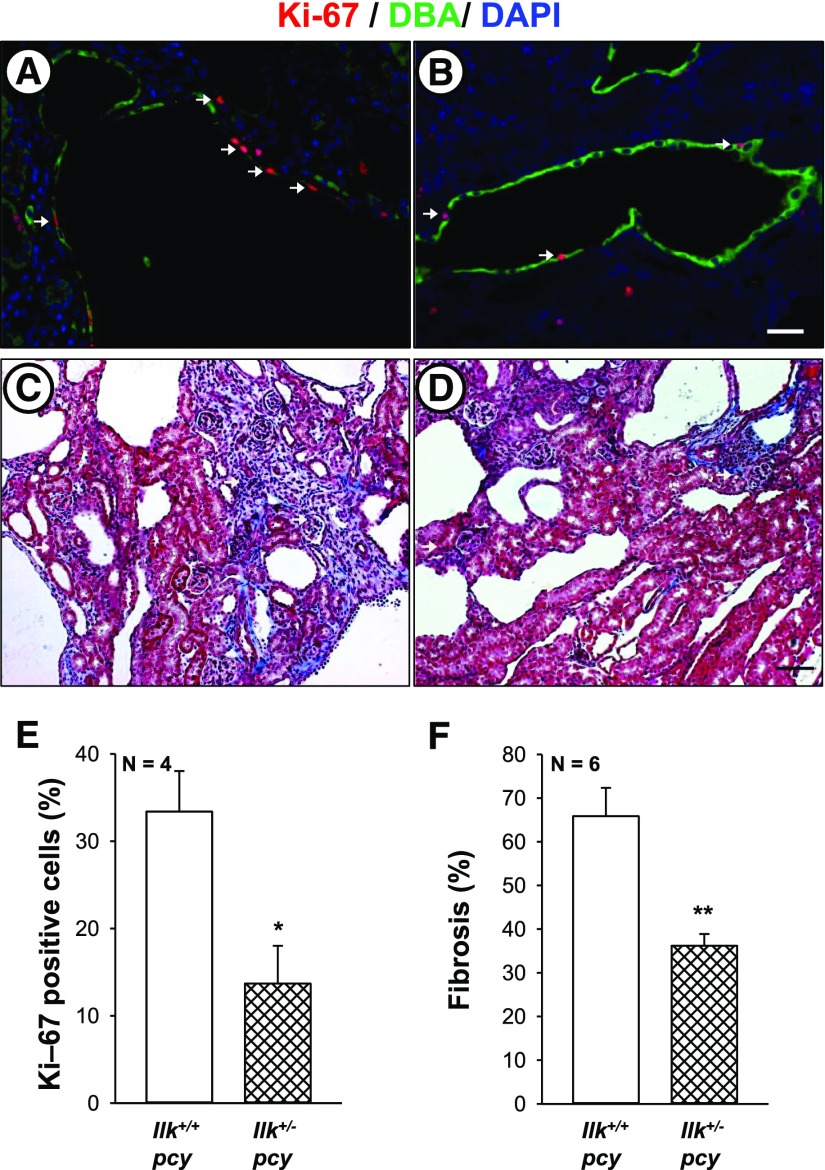
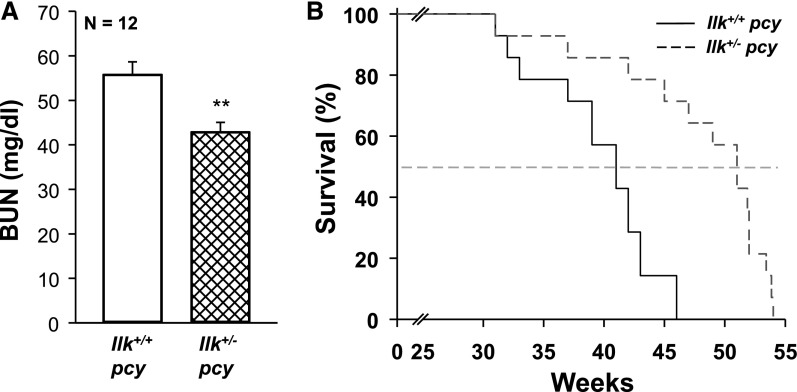
Next, we examined the role of ILK in pcy/pcy (pcy) mice, a well characterized slowly progressive model of PKD.58,59 We crossed Ilk fl/+ and Pkhd1-Cre mice to pcy mice to generate Ilk+/+;pcy/pcy (Ilk+/+ pcy) and Ilkfl/+;Pkhd1-Cre;pcy/pcy (Ilk+/− pcy) mice. Male Ilk+/+ pcy and Ilk+/− pcy mice were euthanized at 10 weeks, and body weight, kidney weight (% body weight), cystic index, and fibrosis were measured (Figure 10). We found that Ilk+/− pcy mice had a pronounced reduction in kidney weight (% body weight), with no effect on body weight. There was a pronounced reduction in cystic area and cyst number compared with Ilk+/+ pcy mice. mTOR signaling is elevated in pcy kidneys60 and reduced ILK expression downregulated both P-S6K and total S6K (Supplemental Figure 5). Measurement of Ki-67–positive cells showed a 59% reduction in proliferating cells in the Ilk+/− pcy kidneys (Figure 11, A–E). There was prominent interstitial fibrosis in pcy mice, and reduced ILK decreased fibrotic area by >50% (Figure 11F). We found that the average BUN for Ilk+/+ pcy mice at 25 weeks was 57.4 mg/dl, demonstrating a decline in renal function (Figure 12A). These mice lived to an average age of 40 weeks (Figure 12B).20 By comparison, BUN for Ilk+/− pcy littermates was significantly lower (42.0 mg/dl) and the mice survived to 48 weeks, indicating a 20% increase in lifespan. Taken together, these data demonstrate that ILK plays an important role in renal cyst growth and fibrosis in PKD.
Figure 10.
ILK knockdown attenuates renal cyst development in pcy mice. (A) Representative images of kidney cross-sections of Ilk+/+;pcy/pcy (Ilk+/+ pcy) and Ilkfl/+;Pkhd1-Cre;pcy/pcy (Ilk+/− pcy) mice at 10 weeks of age. Images were taken at the same magnification. Scale bar=1 mm. (B) Heterozygous loss of ILK in CD cells had no effect on body weight. However, there was a dramatic reduction in (C) kidney weight (% body weight), (D) cystic index, and (E) cyst number per section in Ilk+/−pcy mice compared with Ilk+/+ pcy mice. *P<0.05, ***P<0.001 compared with Ilk+/+ pcy mice.
Figure 11.
ILK knockdown reduces renal cell proliferation and fibrosis in pcy mice. Proliferating cells were determined in kidney sections from (A) Ilk+/+;pcy/pcy (Ilk+/+ pcy) and (B) Ilkfl/+;Pkhd1-Cre;pcy/pcy (Ilk+/− pcy) mice that were stained for Ki-67 (red), DBA (green), and DAPI (blue). Arrows indicate Ki-67–positive cells. Images (A and B) were taken at the same magnification. Scale bar=5 µm. Representative kidney sections from (C) Ilk+/+ pcy and (D) Ilk+/− pcy mice were stained with Masson trichrome to visualize interstitial fibrosis. Scale bar=50 µm. (E) Comparison of nuclear Ki-67 staining in Ilk+/+ pcy and Ilk+/− pcy sections, normalized to total nuclei. (F) Tissue sections stained with Masson trichrome were coded to conceal the group assignment and visually scored for percentage of interstitial edema and fibrosis. *P<0.05 and **P<0.01, compared with Ilk+/+ pcy.
Figure 12.
ILK knockdown improves renal function and increases the survival of pcy mice. Ilk+/+;pcy/pcy (Ilk+/+ pcy) and Ilkfl/+;Pkhd1-Cre;pcy/pcy (Ilk+/− pcy) mice were given standard chow and water ad libitum and monitored regularly until moribund. (A) At 25 weeks, blood was collected from the tail vein and BUN was measured to assess renal function (n=12 mice per group). **P<0.01, compared with Ilk+/+ pcy. (B) Kaplan–Meier survival curve indicates that heterozygous loss of ILK significantly increased the average lifespan of the pcy mice (n=14 mice per group). Average survival age increased from 40±2 weeks for the Ilk+/+ pcy mice to 48±2 weeks for the Ilk+/− pcy mice, P<0.001.
Discussion
Our results demonstrate that (1) ILK is a central intermediate for periostin activation of the Akt/mTOR pathway and proliferation of human ADPKD cells; (2) pharmacologic inhibition of ILK blocked periostin-induced Akt/mTOR signaling and cell proliferation; (3) heterozygous knockdown of ILK in CDs of WT mice had no effect on renal morphology or function, whereas complete ILK knockout caused caspase-3–mediated anoikis, dilated cortical tubules with apoptotic cells in the lumens, interstitial fibrosis, and death of the mice by 10 weeks of age; (4) reduced ILK expression in Pkd1fl/fl;Pkhd1-Cre and pcy/pcy mice decreased renal Akt/mTOR activity, cell proliferation, cyst growth, and interstitial fibrosis, and significantly extended the survival of PKD mice; and (5) homozygous deletion of ILK in the CDs of Pkd1fl/fl;Pkhd1-Cre mice decreased cystic index, in part, due to apoptosis of the cystic cells.
In PKD, aberrant cell proliferation is responsible for the formation and growth of renal cysts; however, the mechanisms remain unclear. One hypothesis is that mutations in the PKD genes incite a maladaptive repair mechanism, involving pathways that activate cell proliferation.4 Abnormal and extensive expression of ECM molecules, including laminins, collagens, and matricellular proteins, accelerate cyst growth through activation of integrin signaling.18,19,61–63 Laminin-332 (α3, β3, γ2), also called laminin-5, has been shown to be abnormally expressed in PKD and contributes to cyst epithelial cell proliferation and cyst growth.61,62 Recently, we discovered that periostin, a tissue repair molecule, was highly overexpressed in ADPKD, autosomal recessive PKD, and animal models of cystic disease.19 Periostin binding to αV-integrins activates mTOR and promotes the proliferation of human ADPKD cells. Global knockout of periostin in pcy mice led to a striking reduction in renal mTOR activity, cell proliferation, cystic area, and tubulointerstitial fibrosis.20
Integrins transmit signals from the external environment into the cell. Several integrins, including αV, α8, β1, and β4, are aberrantly expressed by cyst-lining cells in PKD19,54,61,64,65 and deletion of β1 integrin prevented cystogenesis in a Pkd1 mutant mouse model.54 ILK directly binds to β integrins,23,25,26 linking the actin cytoskeleton to the ECM, and plays a role in cytoskeleton reorganization by recruiting regulatory proteins, such as parvin, PINCH, paxillin, and kindlin-2.39,40,66,67
ECM and matricellular proteins regulate gene expression by integrin signaling through ILK, which has been shown to be necessary for embryonic development of the kidneys. Deletion of ILK in the ureteric bud of Ilkfl/fl;HoxB7-Cre mice caused tubule obstruction, and the mice died at 8 weeks of age.39 Conditional knockout of ILK using a tamoxifen-inducible Cre caused a urinary concentrating defect which the authors attribute to a disruption of AQP2 expression and localization; however, morphologic changes in the kidneys were not reported.52 Here, we show that PN ablation of ILK in CDs caused a urinary concentrating defect and a decline in body weight and the mice died by 10 weeks of age. Loss of ILK caused anoikis and tubule dilations in the kidneys. Thus, complete knockout of ILK appeared to disrupt the connection between the ECM and cell cytoskeleton, leading to detachment of CD cells into the lumen and tubule obstruction.
Pkd1fl/fl;Pkhd1-Cre mice die at approximately 33 days of age due to renal failure. Complete knockout of ILK caused apoptosis of the cystic epithelial cells, contributing to a reduction in the cyst area and size of the cystic kidneys. Mice with heterozygous knockout of ILK in CDs had normal kidneys, urinary concentrating ability, and BUN, and lived beyond 1 year. Importantly, reduction in ILK expression in the Pkd1fl/fl;Pkhd1-Cre mice and pcy mice decreased Akt/mTOR signaling, cell proliferation, cyst number, cystic index, and fibrosis. There was improved renal function and increased lifespan of the PKD mice. Previously, a small-molecule ILK inhibitor was shown to reduce renal fibrosis in a model of obstructive nephropathy,68 suggesting that ILK inhibition may be an effective approach for the treatment of ADPKD. Because complete knockout of ILK in CD cells caused renal injury, long-term use of an ILK inhibitor would need to be considered with caution.
In summary, aberrant secretion of periostin by cystic epithelial cells contributes to a maladaptive repair process that activates the ILK-Akt-mTOR pathway, promoting cyst growth and fibrosis. We propose that targeting the periostin-integrin-ILK axis may be a potential therapeutic approach to slow cyst growth and fibrosis in PKD.
Concise Methods
Human ADPKD and NHK Cells
Primary cultures of ADPKD and NHK cells were generated by the PKD Biomaterials and Biomarkers Core in the Kansas PKD Center at the Kansas University Medical Center (KUMC). ADPKD kidneys were obtained from the surgery department at KU hospital and other hospitals participating in the Polycystic Kidney Research Retrieval Program through the assistance of the PKD Foundation. NHKs, unsuitable for transplantation, were obtained from the Midwest Transplant Network (Kansas City, KS), an organ retrieval agency. The protocol for the use of discarded human tissues for research complies with federal regulations and was approved by the Institutional Review Board at KUMC.
Primary cell cultures were prepared as described previously.69,70 Cells were propagated in DMEM/F12 supplemented with 5% FBS, 5 µg/ml insulin, 5 µg/ml transferrin, and 5 ng/ml sodium selenite (ITS) and penicillin G and streptomycin (P/S). After cells had reached 70%–80% confluence in the flasks, they were lifted from the plastic with a trypsin-EDTA solution (Sigma Chemical, Saint Louis, MO) and counted using a hemocytometer.
Cell Proliferation Measurements
ADPKD cells (7.5 × 104) were seeded into individual wells of six-well plates containing DMEM/F12, ITS, 1% FBS, and P/S. After cells reached approximately 50% confluence, the serum was reduced to 0.002%. After 24 hours, cells were treated with 250 ng/ml human recombinant periostin (75 kD; Biovendor, Asheville, NC) or 100 ng/ml EGF (Sigma Chemical) alone or in the presence of 1 μM CPD 22 (EMD Millipore, Billerica, MA), an ILK inhibitor,47 for an additional 24 hours. Each treatment was performed in triplicate. Cells were harvested by trypsinization, counted using an automated cell counter (Bio-Rad, Hercules, CA), and normalized to the control-treated group. In some experiments, cell proliferation was determined by an MTT assay.71 Briefly, ADPKD cells (4 × 103) were seeded into the wells of a 96-well plate (six wells per experimental condition) and grown under similar conditions as described above. After the 24-hour treatment, cell proliferation was measured using CellTiter 96 nonradioactive cell proliferation (MTT) assay (Promega, Madison, WI).
ILK Knockdown in Human ADPKD Cells
ILK was knocked down in human ADPKD cells using an shRNA lentiviral approach. Six unique ILK shRNA constructs (RHS4531-EG3611), each with a high specificity for the ILK mRNA, and a scrambled nonsilencing shRNA construct (RHS4346) were purchased from GE Healthcare (Waukesha, WI). HEK293T cells were transfected with 4.5 µg ILK or scrambled shRNA plasmid DNA and the packaging vectors (4.5 µg psPAX2 and 1.8 µg pMD2.G) using Lipofectamine 200 (Invitrogen, Carlsbad, CA). The cells were incubated for 18 hours for lentivirus production. Fresh media was added, and the conditioned media containing the lentivirus was harvested at 24 and 48 hours. Human ADPKD cells were seeded into 100-mm petri dishes for 24 hours. Lentiviral harvest media from the two time points was added successively to the ADPKD cells. After viral infection, media bathing the ADPKD cells was switched to DME/F12+1% FBS for 2 days. Using immunoblot analysis, ILK knockdown efficiency was determined by band densitometry of ILK protein from ADPKD cells infected with ILK shRNA compared with the scrambled shRNA. We selected two shRNAs with the best knockdown efficiencies (mature antisense sequences for ILK shRNA1: 5′-ACACTACGGCTATTGAGTG-3′, and ILK shRNA2: 5′-ATACGGTTGAGATTCTGGC-3′). Alignment of the reverse complement of the antisense sequences targeted only ILK and no other mRNAs. To determine if ILK knockdown inhibits periostin-induced cell proliferation, ADPKD cells were infected with individual ILK shRNA or the scramble shRNA and then treated with 250 ng/ml periostin or EGF (positive control) for 24 hours. Cell proliferation was determined by either cell count (n=1) or MTT assay (n=3).
CD-Specific ILK Knockdown and Knockout in Mice
Ilkfl/fl mice50 were crossed to mice expressing Pkhd1-Cre, which maximally express Cre recombinase in the CDs at PN day 751 to generate Ilkfl/+;Pkhd1-Cre mice. These mice were crossed to produce Ilk+/+;Pkhd1-Cre (WT: Ilk+/+ CD), Ilkfl/+;Pkhd1-Cre (Ilk+/− CD), and Ilkfl/fl;Pkhd1-Cre (Ilk−/− CD) mice. We observed normal Mendelian inheritance. Mice were provided food and water ad libitum and monitored daily. At 5 and 10 weeks of age, mice were water-restricted for 3 hours and urine was collected for measurement of osmolality using a vapor pressure osmometer. Afterward, the mice were euthanized in an isoflurane chamber, and blood was collected by cardiac puncture for measurement of BUN followed by exsanguination and removal of the kidneys, as described previously.20 The protocol for use of these mice was approved by the Institutional Animal Care and Use Committee at KUMC.
CD-Specific Knockout of ILK in Pkd1 Mutant Mice and pcy Mice
To study the effect of ILK knockdown in PKD, we bred Pkd1fl/fl mice72 with Ilkfl/fl mice. Ilk fl/+;Pkd1fl/fl mice were crossed with Ilkfl/+;Pkd1fl/+;Pkhd1-Cre mice to generate litters of PKD mice (Pkd1fl/fl;Pkhd1-Cre) with Ilk+/+;Pkd1fl/fl;Pkhd1-Cre (Ilk+/+ PKD), Ilkfl/+;Pkd1fl/fl;Pkhd1-Cre (Ilk+/−PKD), and Ilkfl/fl;Pkd1fl/fl;Pkhd1-Cre (Ilk−/− PKD) ILK alleles in CD cells. Noncystic littermate controls (Ilk+/+ CD, Ilk+/− CD, Ilk−/− CD) were also generated using this breeding strategy. One group of mice was set aside for survival study where food and water were provided ad libitum and mice were monitored every day until death or euthanized when there were signs of imminent death, including labored breathing and inability to move.73 A second group of mice was assessed at PN day 25. These mice were anesthetized, and serum and tissues were harvested as described above, and euthanized by exsanguination. All mice were F3–F5 generations on the C57BL/6 background.
To confirm the results in a slowly progressive model of PKD, we knocked down ILK expression in CDs of pcy/pcy (pcy) mice, a model orthologous to human nephronophthisis type 3 (NPHP3). Disease progression in pcy mice is similar to ADPKD, with kidney enlargement to several times normal size and progressive interstitial fibrosis.74 Previously, we showed that kidney volume increased exponentially up to 20 weeks of age, after which there was a plateau as renal parenchyma was replaced with fibrosis.74 In general, these mice survive to approximately 40 weeks of age.20 Ilkfl/+ mice and Pkhd1-Cre mice were bred to pcy mice and backcrossed for eight generations. These mice were then bred to obtain Ilk+/+;pcy/pcy (Ilk+/+ pcy) and Ilkfl/+;Pkhd1-Cre;pcy/pcy (Ilk+/− pcy) mice. Because mice with complete loss of ILK died by 10 weeks, Ilk−/− pcy mice were not used. In one group, the mice were euthanized for tissue analysis at 10 weeks of age, as described above. Mice in the survival study had free access to food and water ad libitum. At 25 weeks, 50–100 μl of blood was collected from the tail vein for BUN analysis. These mice were monitored for survival as described above.
Genotyping
We used a PCR method for genotyping ILK floxed mice. The forward primer sequence was 5′-GTTTCGCGGTGCTTCTGTG-3′, and the reverse sequence was 5′-AAAAGCCTTTGATCCTTGAATGG-3′. For genotyping WT and floxed Pkd1 alleles, the forward primer was 5′-CCTGCCTTGCTCTACTTTCC-3′, and the reverse primer was 5′-AGGGCTTTTCTTGCTGGTCT-3′. For genotyping for Cre alleles, the forward sequence was 5′-GCGGTCTGGCAGTAAAAACTATC-3′, and the reverse primer was 5′-GTGAAACAGCATTGCTGTCACTT-3′.
Western Blot Analysis
ADPKD and NHK cells were seeded onto plastic petri dishes (100 mm diameter) containing DMEM/F12 medium with 1% FBS. Serum was reduced to 0.002% at 75% confluency. Cells were then treated as indicated and lysates were prepared in triton lysis buffer at specific time points. Lysates were also prepared from renal tissues as described previously.75 Total protein content was determined using BCA protein assay (Pierce, Rockford, IL) and 50 μg of protein was used for immunoblot analysis.71 The following antibodies were used: P-Akt (9271; Cell Signaling Technology, Beverly, MA [CS]), Akt (CS 9272), GAPDH (CS 2118), ILK (EP-1593Y; Origene, Rockville, MD), sc13075 (Santa Cruz, CA), P-S6K (CS 9234), and S6K (CS 9292). After incubation with an HRP-linked secondary antibody against rabbit or mouse (NA934, NA931; GE Healthcare), the blots were detected with ECL (RPN2232; GE Healthcare), imaged in AI 600 imager, and quantified with Biorad Quantity One program.
Histology, Immunohistochemistry, and Immunofluorescence
Kidney cross-sections were fixed in 4% paraformaldehyde overnight, embedded into paraffin blocks, and 5 μm sections were placed on glass slides. Immunohistochemical staining for cleaved caspase-3 (CS 4858) was performed by the Department of Pathology and Laboratory Medicine core facility at KUMC. For quantification of cleaved caspase-3 staining, sections were blinded for the experimental condition, and the total number of cleaved caspase-3–stained cells was manually counted and normalized to the total noncystic surface area of the kidney (% cleaved caspase-3). For immunofluorescence, tissue slices were deparaffinized, rehydrated, and incubated with the following primary antibodies: Ki-67 (ab15880; Abcam [ab] Inc., Cambridge, MA) and Ki-67 blocking peptide (ab15881), ILK (EP-1593Y; Origene), and P-S6 (CS 4858), followed by incubation with fluorescent-conjugated secondary antibody (ab 150084). Sections were costained with FITC-conjugated DBA (FL-1031; Vector Laboratories, Burlingame, CA) to label CDs and mounted with DAPI (P36931; ThermoFisher Scientific). Slides were coded to conceal the group assignment and three nonoverlapping fields per section were imaged using a Nikon Eclipse Ti microscope (Nikon Instruments, Melville, NY). Quantification of nuclear Ki-67 and cytoplasmic P-S6 staining was performed with a multiwavelength cell-scoring application in Metamorph Image Analysis software (Molecular Devices, Sunnyvale, CA).
Measurement of Renal Cystic Area
Tissue sections were stained with hematoxylin and eosin and imaged using a dissecting microscope connected to a digital camera (Leica Microsystems, Buffalo Grove, IL). Slides were coded, and total number of cysts, cystic area, and total area of the kidney were measured using Image Pro Premiere (Media Cybernetics Inc., Silverspring, MD) as described previously.20
Measurement of Tissue Fibrosis
Tissue sections were stained with Masson trichrome, causing collagen fibers to appear blue.20 Pkd1fl/fl;Pkhd1-Cre mice have aggressive disease, and die of massively enlarged kidneys before excessive collagen/ECM deposition. Accordingly, we observed only mild collagen deposition within the cortex, whereas there were dramatic prefibrotic edematous areas in the cortex and detectable fibrosis within the medulla. Tissues were scored by the naïve observer assigning a percentage of cortical area with prefibrotic edema and collagen deposition per total area of the cortex within the section. We also measured renal fibrosis in pcy mice which develop significant renal fibrosis by 10 weeks. Percentage fibrosis was scored on the basis of both prefibrotic and fibrotic areas.
Measurement of BUN
Serum was isolated from blood collected at the time of euthanasia by centrifugation at 10,000 rpm for 10 minutes. Urea concentration was estimated using a colorimetric QuantiChrom Urea assay kit (DIUR 500; Bioassay Systems, Hayward, CA) and BUN was calculated using the formula BUN=(Urea/2.14).
Statistical Analyses
Data are expressed as mean±SEM. Unless noted otherwise, statistical significance was determined by one-way ANOVA and Student–Newman–Keuls post-test for multiple comparisons or unpaired t test for comparison between control and treated groups. For survival studies, the Gehan–Breslow analysis was performed to determine statistical significance of the survival curves, and pairwise analysis was performed using the Bonferroni Method.
Disclosures
None.
Supplementary Material
Acknowledgments
Ilkfl/fl mice were provided by Dr. Udayan Apte at Kansas University Medical Center (KUMC), Pkd1fl/fl mice were purchased from Jackson Laboratory, and Pkhd1-Cre mice were received from the University of Texas Southwestern O’Brien Center Core (National Institutes of Health P30DK079328). Dr. Timothy Fields at KUMC provided advice on the pathologic examination of the tissue sections. We thank Marsha Danley for technical assistance with immunohistochemistry.
This work was supported by a grant from the National Institutes of Diabetes and Digestive and Kidney Disease (R01DK081579 to D.P.W.) and a fellowship through the KUMC Biomedical Research Training Program (A.R.). Generation of primary cultures of autosomal dominant polycystic kidney disease and normal human kidney cells was supported by the PKD Biomaterials and Biomarkers Repository Core in the Kansas PKD Research and Translational Core Center (DK106912 to J.P.C.).
Portions of this work were published previously in abstract form (J Am Soc Nephrol 25: 413A, 2014; J Am Soc Nephrol 26: 131A, 2015; and J Am Soc Nephrol 27: 773A, 2016).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016111235/-/DCSupplemental.
References
- 1.Ong AC, Harris PC: A polycystin-centric view of cyst formation and disease: The polycystins revisited. Kidney Int 88: 699–710, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres VE, Harris PC: Autosomal dominant polycystic kidney disease: The last 3 years. Kidney Int 76: 149–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calvet JP: Polycystic kidney disease: Primary extracellular matrix abnormality or defective cellular differentiation? Kidney Int 43: 101–108, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Weimbs T: Regulation of mTOR by polycystin-1: Is polycystic kidney disease a case of futile repair? Cell Cycle 5: 2425–2429, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Weimbs T: Polycystic kidney disease and renal injury repair: Common pathways, fluid flow, and the function of polycystin-1. Am J Physiol Renal Physiol 293: F1423–F1432, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Wahl PR, Le Hir M, Vogetseder A, Arcaro A, Starke A, Waeckerle-Men Y, Serra AL, Wuthrich RP: Mitotic activation of Akt signalling pathway in Han:SPRD rats with polycystic kidney disease. Nephrology (Carlton) 12: 357–363, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Torres VE, Boletta A, Chapman A, Gattone V, Pei Y, Qian Q, Wallace DP, Weimbs T, Wüthrich RP: Prospects for mTOR inhibitor use in patients with polycystic kidney disease and hamartomatous diseases. Clin J Am Soc Nephrol 5: 1312–1329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weimbs T, Olsan EE, Talbot JJ: Regulation of STATs by polycystin-1 and their role in polycystic kidney disease. JAK-STAT 2: e23650, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker JT, McLeod K, Kim S, Conway SJ, Hamilton DW: Periostin as a multifunctional modulator of the wound healing response. Cell Tissue Res 365: 453–465, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eddy AA: Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int Suppl (2011) 4: 2–8, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bornstein P, Sage EH: Matricellular proteins: Extracellular modulators of cell function. Curr Opin Cell Biol 14: 608–616, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Norris RA, Moreno-Rodriguez R, Hoffman S, Markwald RR: The many facets of the matricelluar protein periostin during cardiac development, remodeling, and pathophysiology. J Cell Commun Signal 3: 275–286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kudo A: Periostin in fibrillogenesis for tissue regeneration: Periostin actions inside and outside the cell. Cell Mol Life Sci 68: 3201–3207, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conway SJ, Molkentin JD: Periostin as a heterofunctional regulator of cardiac development and disease. Curr Genomics 9: 548–555, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sen K, Lindenmeyer MT, Gaspert A, Eichinger F, Neusser MA, Kretzler M, Segerer S, Cohen CD: Periostin is induced in glomerular injury and expressed de novo in interstitial renal fibrosis. Am J Pathol 179: 1756–1767, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, Muller SJ, Fahy JV: Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA 107: 14170–14175, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiodoni C, Colombo MP, Sangaletti S: Matricellular proteins: From homeostasis to inflammation, cancer, and metastasis. Cancer Metastasis Rev 29: 295–307, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Wilson PD, Hreniuk D, Gabow PA: Abnormal extracellular matrix and excessive growth of human adult polycystic kidney disease epithelia. J Cell Physiol 150: 360–369, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Wallace DP, Quante MT, Reif GA, Nivens E, Ahmed F, Hempson SJ, Blanco G, Yamaguchi T: Periostin induces proliferation of human autosomal dominant polycystic kidney cells through alphaV-integrin receptor. Am J Physiol Renal Physiol 295: F1463–F1471, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace DP, White C, Savinkova L, Nivens E, Reif GA, Pinto CS, Raman A, Parnell SC, Conway SJ, Fields TA: Periostin promotes renal cyst growth and interstitial fibrosis in polycystic kidney disease. Kidney Int 85: 845–854, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD: Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res 62: 5358–5364, 2002 [PubMed] [Google Scholar]
- 22.Cruet-Hennequart S, Maubant S, Luis J, Gauduchon P, Staedel C, Dedhar S: alpha(v) integrins regulate cell proliferation through integrin-linked kinase (ILK) in ovarian cancer cells. Oncogene 22: 1688–1702, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Hannigan G, Troussard AA, Dedhar S: Integrin-linked kinase: A cancer therapeutic target unique among its ILK. Nat Rev Cancer 5: 51–63, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Grashoff C, Thievessen I, Lorenz K, Ussar S, Fässler R: Integrin-linked kinase: Integrin’s mysterious partner. Curr Opin Cell Biol 16: 565–571, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S: Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature 379: 91–96, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Wu C, Dedhar S: Integrin-linked kinase (ILK) and its interactors: A new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J Cell Biol 155: 505–510, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piao Z, Hong CS, Jung MR, Choi C, Park YK: Thymosin β4 induces invasion and migration of human colorectal cancer cells through the ILK/AKT/β-catenin signaling pathway. Biochem Biophys Res Commun 452: 858–864, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Hannigan GE, McDonald PC, Walsh MP, Dedhar S: Integrin-linked kinase: Not so ‘pseudo’ after all. Oncogene 30: 4375–4385, 2011 [DOI] [PubMed] [Google Scholar]
- 29.McDonald PC, Oloumi A, Mills J, Dobreva I, Maidan M, Gray V, Wederell ED, Bally MB, Foster LJ, Dedhar S: Rictor and integrin-linked kinase interact and regulate Akt phosphorylation and cancer cell survival. Cancer Res 68: 1618–1624, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Persad S, Attwell S, Gray V, Mawji N, Deng JT, Leung D, Yan J, Sanghera J, Walsh MP, Dedhar S: Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: Critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem 276: 27462–27469, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Lange A, Wickström SA, Jakobson M, Zent R, Sainio K, Fässler R: Integrin-linked kinase is an adaptor with essential functions during mouse development. Nature 461: 1002–1006, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Wickström SA, Lange A, Montanez E, Fässler R: The ILK/PINCH/parvin complex: The kinase is dead, long live the pseudokinase! EMBO J 29: 281–291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukuda K, Knight JD, Piszczek G, Kothary R, Qin J: Biochemical, proteomic, structural, and thermodynamic characterizations of integrin-linked kinase (ILK): Cross-validation of the pseudokinase. J Biol Chem 286: 21886–21895, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tu Y, Huang Y, Zhang Y, Hua Y, Wu C: A new focal adhesion protein that interacts with integrin-linked kinase and regulates cell adhesion and spreading. J Cell Biol 153: 585–598, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tu Y, Li F, Goicoechea S, Wu C: The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol Cell Biol 19: 2425–2434, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eke I, Koch U, Hehlgans S, Sandfort V, Stanchi F, Zips D, Baumann M, Shevchenko A, Pilarsky C, Haase M, Baretton GB, Calleja V, Larijani B, Fässler R, Cordes N: PINCH1 regulates Akt1 activation and enhances radioresistance by inhibiting PP1alpha. J Clin Invest 120: 2516–2527, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Persad S, Attwell S, Gray V, Delcommenne M, Troussard A, Sanghera J, Dedhar S: Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc Natl Acad Sci USA 97: 3207–3212, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White DE, Coutu P, Shi YF, Tardif JC, Nattel S, St Arnaud R, Dedhar S, Muller WJ: Targeted ablation of ILK from the murine heart results in dilated cardiomyopathy and spontaneous heart failure. Genes Dev 20: 2355–2360, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smeeton J, Zhang X, Bulus N, Mernaugh G, Lange A, Karner CM, Carroll TJ, Fässler R, Pozzi A, Rosenblum ND, Zent R: Integrin-linked kinase regulates p38 MAPK-dependent cell cycle arrest in ureteric bud development. Development 137: 3233–3243, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Legate KR, Montañez E, Kudlacek O, Fässler R: ILK, PINCH and parvin: The tIPP of integrin signalling. Nat Rev Mol Cell Biol 7: 20–31, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Qin J, Wu C: ILK: A pseudokinase in the center stage of cell-matrix adhesion and signaling. Curr Opin Cell Biol 24: 607–613, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuda K, Gupta S, Chen K, Wu C, Qin J: The pseudoactive site of ILK is essential for its binding to alpha-Parvin and localization to focal adhesions. Mol Cell 36: 819–830, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boppart MD, Burkin DJ, Kaufman SJ: Activation of AKT signaling promotes cell growth and survival in α7β1 integrin-mediated alleviation of muscular dystrophy. Biochim Biophys Acta 1812: 439–446, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koul D, Shen R, Bergh S, Lu Y, de Groot JF, Liu TJ, Mills GB, Yung WK: Targeting integrin-linked kinase inhibits Akt signaling pathways and decreases tumor progression of human glioblastoma. Mol Cancer Ther 4: 1681–1688, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Tu Y, Li F, Wu C: Nck-2, a novel Src homology2/3-containing adaptor protein that interacts with the LIM-only protein PINCH and components of growth factor receptor kinase-signaling pathways. Mol Biol Cell 9: 3367–3382, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maydan M, McDonald PC, Sanghera J, Yan J, Rallis C, Pinchin S, Hannigan GE, Foster LJ, Ish-Horowicz D, Walsh MP, Dedhar S: Integrin-linked kinase is a functional Mn2+-dependent protein kinase that regulates glycogen synthase kinase-3β (GSK-3beta) phosphorylation. PLoS One 5: e12356, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee SL, Hsu EC, Chou CC, Chuang HC, Bai LY, Kulp SK, Chen CS: Identification and characterization of a novel integrin-linked kinase inhibitor. J Med Chem 54: 6364–6374, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malan D, Elischer A, Hesse M, Wickström SA, Fleischmann BK, Bloch W: Deletion of integrin linked kinase in endothelial cells results in defective RTK signaling caused by caveolin 1 mislocalization. Development 140: 987–995, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Azimifar SB, Böttcher RT, Zanivan S, Grashoff C, Krüger M, Legate KR, Mann M, Fässler R: Induction of membrane circular dorsal ruffles requires co-signalling of integrin-ILK-complex and EGF receptor. J Cell Sci 125: 435–448, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Terpstra L, Prud’homme J, Arabian A, Takeda S, Karsenty G, Dedhar S, St-Arnaud R: Reduced chondrocyte proliferation and chondrodysplasia in mice lacking the integrin-linked kinase in chondrocytes. J Cell Biol 162: 139–148, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma M, Tian X, Igarashi P, Pazour GJ, Somlo S: Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat Genet 45: 1004–1012, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cano-Peñalver JL, Griera M, Serrano I, Rodríguez-Puyol D, Dedhar S, de Frutos S, Rodríguez-Puyol M: Integrin-linked kinase regulates tubular aquaporin-2 content and intracellular location: A link between the extracellular matrix and water reabsorption. FASEB J 28: 3645–3659, 2014 [DOI] [PubMed] [Google Scholar]
- 53.Attwell S, Roskelley C, Dedhar S: The integrin-linked kinase (ILK) suppresses anoikis. Oncogene 19: 3811–3815, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Lee K, Boctor S, Barisoni LM, Gusella GL: Inactivation of integrin-β1 prevents the development of polycystic kidney disease after the loss of polycystin-1. J Am Soc Nephrol 26: 888–895, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tao S, Kakade VR, Woodgett JR, Pandey P, Suderman ED, Rajagopal M, Rao R: Glycogen synthase kinase-3β promotes cyst expansion in polycystic kidney disease. Kidney Int 87: 1164–1175, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou JX, Li X: Apoptosis in polycystic kidney disease: From pathogenesis to treatment. In: Polycystic Kidney Disease, edited by LI X, Brisbane, Australia, Codon Publications, 2015 [PubMed] [Google Scholar]
- 57.Zhou X, Fan LX, Sweeney WE Jr, Denu JM, Avner ED, Li X: Sirtuin 1 inhibition delays cyst formation in autosomal-dominant polycystic kidney disease. J Clin Invest 123: 3084–3098, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi H, Calvet JP, Dittemore-Hoover D, Yoshida K, Grantham JJ, Gattone VH 2nd : A hereditary model of slowly progressive polycystic kidney disease in the mouse. J Am Soc Nephrol 1: 980–989, 1991 [DOI] [PubMed] [Google Scholar]
- 59.Olbrich H, Fliegauf M, Hoefele J, Kispert A, Otto E, Volz A, Wolf MT, Sasmaz G, Trauer U, Reinhardt R, Sudbrak R, Antignac C, Gretz N, Walz G, Schermer B, Benzing T, Hildebrandt F, Omran H: Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat Genet 34: 455–459, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Gattone VH 2nd , Sinders RM, Hornberger TA, Robling AG: Late progression of renal pathology and cyst enlargement is reduced by rapamycin in a mouse model of nephronophthisis. Kidney Int 76: 178–182, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Joly D, Morel V, Hummel A, Ruello A, Nusbaum P, Patey N, Noël L-H, Rousselle P, Knebelmann B: β4 Integrin and Laminin 5 are aberrantly expressed in polycystic kidney disease. Am J Pathol 163: 1791–1800, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vijayakumar S, Dang S, Marinkovich MP, Lazarova Z, Yoder B, Torres VE, Wallace DP: Aberrant expression of laminin-332 promotes cell proliferation and cyst growth in ARPKD. Am J Physiol Renal Physiol 306: F640–F654, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song X, Di Giovanni V, He N, Wang K, Ingram A, Rosenblum ND, Pei Y: Systems biology of autosomal dominant polycystic kidney disease (ADPKD): Computational identification of gene expression pathways and integrated regulatory networks. Hum Mol Genet 18: 2328–2343, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Daïkha-Dahmane F, Narcy F, Dommergues M, Lacoste M, Beziau A, Gubler MC: Distribution of alpha-integrin subunits in fetal polycystic kidney diseases. Pediatr Nephrol 11: 267–273, 1997 [DOI] [PubMed] [Google Scholar]
- 65.Zeltner R, Hilgers KF, Schmieder RE, Porst M, Schulze BD, Hartner A: A promoter polymorphism of the alpha 8 integrin gene and the progression of autosomal-dominant polycystic kidney disease. Nephron Clin Pract 108: c169–c175, 2008 [DOI] [PubMed] [Google Scholar]
- 66.Fukuda K, Bledzka K, Yang J, Perera HD, Plow EF, Qin J: Molecular basis of kindlin-2 binding to integrin-linked kinase pseudokinase for regulating cell adhesion. J Biol Chem 289: 28363–28375, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ussar S, Wang HV, Linder S, Fässler R, Moser M: The Kindlins: Subcellular localization and expression during murine development. Exp Cell Res 312: 3142–3151, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Li Y, Tan X, Dai C, Stolz DB, Wang D, Liu Y: Inhibition of integrin-linked kinase attenuates renal interstitial fibrosis. J Am Soc Nephrol 20: 1907–1918, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pinto CS, Raman A, Reif GA, Magenheimer BS, White C, Calvet JP, Wallace DP: Phosphodiesterase isoform regulation of cell proliferation and fluid secretion in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 27: 1124–1134, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wallace DP, Grantham JJ, Sullivan LP: Chloride and fluid secretion by cultured human polycystic kidney cells. Kidney Int 50: 1327–1336, 1996 [DOI] [PubMed] [Google Scholar]
- 71.Reif GA, Yamaguchi T, Nivens E, Fujiki H, Pinto CS, Wallace DP: Tolvaptan inhibits ERK-dependent cell proliferation, Cl− secretion, and in vitro cyst growth of human ADPKD cells stimulated by vasopressin. Am J Physiol Renal Physiol 301: F1005–F1013, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG: A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med 13: 1490–1495, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ray MA, Johnston NA, Verhulst S, Trammell RA, Toth LA: Identification of markers for imminent death in mice used in longevity and aging research. J Am Assoc Lab Anim Sci 49: 282–288, 2010 [PMC free article] [PubMed] [Google Scholar]
- 74.Wallace DP, Hou YP, Huang ZL, Nivens E, Savinkova L, Yamaguchi T, Bilgen M: Tracking kidney volume in mice with polycystic kidney disease by magnetic resonance imaging. Kidney Int 73: 778–781, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Nagao S, Nishii K, Yoshihara D, Kurahashi H, Nagaoka K, Yamashita T, Takahashi H, Yamaguchi T, Calvet JP, Wallace DP: Calcium channel inhibition accelerates polycystic kidney disease progression in the Cy/+ rat. Kidney Int 73: 269–277, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.