Abstract
The complement system, consisting of soluble and cell membrane–bound components of the innate immune system, has defined roles in the pathophysiology of renal allograft rejection. Notably, the unavoidable ischemia-reperfusion injury inherent to transplantation is mediated through the terminal complement activation products C5a and C5b-9. Furthermore, biologically active fragments C3a and C5a, produced during complement activation, can modulate both antigen presentation and T cell priming, ultimately leading to allograft rejection. Earlier work identified renal tubule cell synthesis of C3, rather than hepatic synthesis of C3, as the primary source of C3 driving these effects. Recent efforts have focused on identifying the local triggers of complement activation. Collectin-11, a soluble C-type lectin expressed in renal tissue, has been implicated as an important trigger of complement activation in renal tissue. In particular, collectin-11 has been shown to engage L-fucose at sites of ischemic stress, activating the lectin complement pathway and directing the innate immune response to the distressed renal tubule. The interface between collectin-11 and L-fucose, in both the recipient and the allograft, is an attractive target for therapies intended to curtail renal inflammation in the acute phase.
Keywords: complement, lectin pathway, collectin-11, renal transplantation
The innate immune system, composed of humoral, cellular, and physical barrier defenses, plays an essential role in the immediate and nonspecific response to invading pathogens. A vital subset of innate immunity is the complement system. Complement defends against invading organisms, clears immune complexes and cell debris, and provides an interface between innate and adaptive immunity.1 Specifically, complement consists of interactive soluble proteins, membrane-bound receptors, and regulatory proteins. For many years, the focus of immune modulation in organ transplantation has been on the adaptive immune system. However, it has been within the last 15 years that several new aspects of complement biology with respect to solid organ transplantation have emerged. We now know that complement helps direct the alloimmune response to solid organ transplants not only through contributing to damage associated with ischemia-reperfusion injury but also through a significant role in the augmentation of T cell– and B cell–mediated immunity.2,3 Indeed, not only has the role of complement as an effector of Antibody-Mediated Rejection (ABMR) been updated,4 but it is also required to prime effective antibody production against donor tissue.5 In addition, recent evidence points to complement activation as a significant factor in the progression of chronic native kidney disease, which is likely to have generic application relevant to the transplanted organ.6 How these observations will be translated to the clinical realm remains to be identified, as any intervention will need to weigh its effect on organ injury and rejection with dampening the antimicrobial functions of the complement system, such as opsonisation, cell lysis, and recruitment of neutrophils and other inflammatory cells.7 It is our belief that therapeutic strategies can be designed to specifically target the key initiators of complement activation at the relevant location, such as complement-binding anti-HLA antibodies in the vascular compartment or the initiator(s) of local complement activation in the extravascular compartment of the allograft itself. In this article, we will discuss the roles of various complement recognition pathways in ischemia-reperfusion injury and allograft rejection with particular focus on the role of carbohydrate both as a danger signal and a potential target underpinning complement activation after ischemic insult and likely downstream adaptive immune recognition.
Our review will cover (1) The Complement System, (2) Complement in the Development of Adaptive Immunity, (3) Role of Complement in ABMR, (4) The Lectin Pathway (LP) as a Mediator of Organ Injury, (5) Role of mannan-binding lectin (MBL) in Tissue Injury, (6) Role of Ficolins in Tissue Injury, and (7) Collectin-11 as a Mediator of Renal Epithelial Inflammation, followed by our concluding remarks.
The Complement System
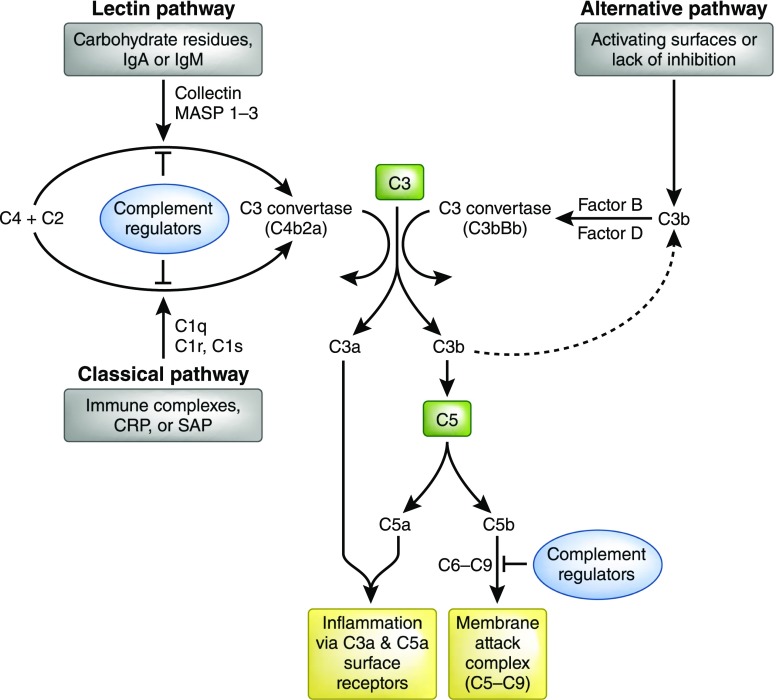
Complement is activated on the surface of pathogens as well as damaged or infected cells via one of three main pathways: the classical, alternative, or lectin pathway.8 These distinct pathways converge at the formation of C3, which is then cleaved to form C5 convertase, with the subsequent production of the terminal pathway complement components, comprising C5a and the membrane attack complex (C5b-9) (Figure 1). Classical pathway activation typically occurs through antibody-antigen complex binding of C1q to the Fc portion of IgM or IgG,1,9,10 which forms part of the C1 complex with classical pathway–specific serine proteases C1r and C1s. The alternative pathway, activated by hydrolysis of plasma C3 and enhanced in some circumstances by an absence of complement inhibitors on cell membranes, also functions as an amplification pathway after the generation of C3b by the classical or lectin pathways.11,12 In contrast, LP initiation takes place through pattern-recognition molecules such as MBL, ficolins, surfactant proteins, and the recently identified C-type lectin, Collectin-11 (CL-11; CL-K1), all of which bind to carbohydrate motifs.
Figure 1.
The complement cascade. The complement system is activated by one of three major pathways: classical, lectin, or alternative. The classical pathway is triggered by C1 binding to immune surveillance molecules such as IgG, IgM, C-reactive protein (CRP), or serum amyloid protein (SAP) which are attached to the target sequence. The LP is triggered by the binding of collectins, such as MBL and collectin-11, or ficolins to carbohydrate residues on a pathogenic surface or IgA and IgM molecules. The alternative pathway is initiated by direct binding of C3b to activating surfaces. All three pathways converge at the production of the central complement component C3. That is, all pathways form enzyme complexes (classical or alternative convertases) that cleave either C3 (into C3a and C3b) or C5 (into C5a and C5b). C5b triggers the terminal pathway by creating a pore in the target cell membrane via the formation of the membrane attack complex (C5b-C9). Soluble complement effectors C3a and C5a are detected by specific cell receptors thereby promoting inflammation. Complement inhibition occurs via a variety of molecules ultimately inhibiting C3 and C5 convertase or blocking the formation of the membrane attack complex (C5b-C9).
Under normal physiologic conditions, complement activation is controlled by surface-bound and soluble proteins that mediate the degradation of complement convertases, ultimately preventing the formation of complement effectors C3a, C3b, C5a, and C5b-9. Fluid phase complement-regulating plasma proteins include C1 esterase inhibitor (C1 INH), C4b binding protein, factor H, and factor I. Cell-membrane regulatory proteins include decay-accelerating factor (DAF; CD55), membrane cofactor protein (MCP; CD46), and CR1 (CD35). These proteins modulate the complement response and protect host cells and tissues from damage related to complement activation.13 During inflammation and cell stress this equilibrium shifts away from regulation and can lead to uncontrolled complement-mediated injury and rejection.14 Indeed, after renal ischemia-reperfusion injury, which is an unavoidable consequence of transplantation, postischemic renal dysfunction is dependent on the local conversion of tubule-derived C3 to its activated form,15 which growing evidence suggests is mediated through triggering of the LP,16,17 discussed in more detail below.
Complement in the Development of Adaptive Immunity
The role of complement in regulating T cell alloimmunity was discovered when it was observed that wild-type mice do not acutely reject renal allografts from C3-deficient donors.18 Further support, implicating a role in regulating B cell alloimmunity, came from a study of C3-deficient mice that were unable to produce high-affinity IgG responses against MHC in skin grafts.5 During interactions between antigen presenting cells and T cells, the alternative pathway complement components C3, factor B, and factor D are released along with the terminal pathway complement component C5.19–21 Additionally, complement receptors C3aR and C5aR are upregulated on T cells in response to the expression of these complement components.22,23 Increased production of complement effector products and corresponding receptor expression, in parallel with reduced cell surface DAF, favors complement activation. With increased local production of C3a and C5a and engagement of their respective receptors, T cells and antigen presenting cells are stimulated (Figure 2).22 Via intracellular AKT signaling, the antiapoptotic Bcl2 protein is upregulated whereas the proapoptotic Fas molecule is downregulated, thereby enhancing T cell proliferation and diminishing T cell apoptosis.22,24 Therefore, complement could indirectly play a role in antibody production through regulation of T cell immunity,18 as well as direct stimulation of B cell priming by opsonized antigen.25,26
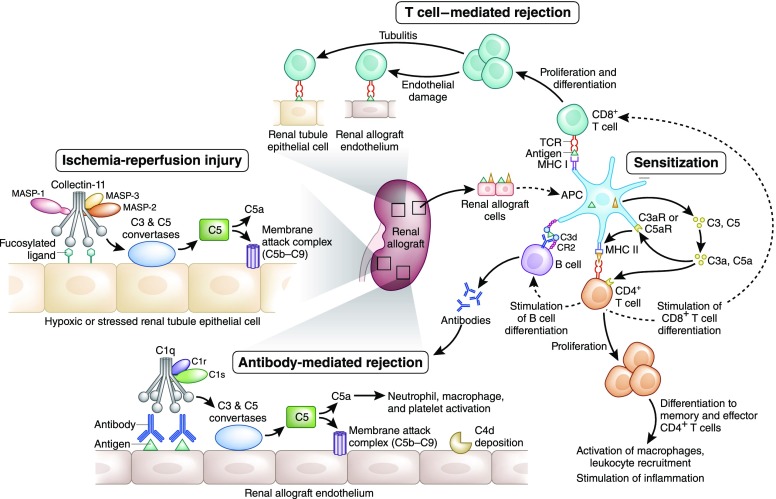
Figure 2.
Complement activation pathways and allograft immunity. Complement-mediated injury may occur in the intravascular or extravascular compartments. Ischemia-reperfusion injury increases extravascular cell surface expression of a fucosylated ligand that is thought to be recognized by Collectin-11 which associates with MASP-2, in conjunction with MASP-1 and MASP-3, to activate complement via the LP. After the cleavage of C3 and C5, the membrane attack complex (C5b-9) forms, resulting in inflammatory injury and cell death. Complement plays several roles in sensitization against donor alloantigen. Antigen presenting cells (APCs) express complement components C3 and C5 in addition to complement receptors C3aR (C3a Receptor) and C5aR1 (C5a Receptor 1). Generated by complement activation in the extracellular space, C3a and C5a enhance APC priming of T cells by increasing the presentation of alloantigens and the expression of costimulatory molecules. Additionally, C3a and C5a promote CD4+ T cell differentiation and cell longevity. Furthermore, APCs promote proliferation and differentiation of CD4+ and CD8+ T cells. CD8+ T cells mediate cellular rejection in both the intravascular and extravascular compartments which is identified pathologically as endothelitis and tubulitis, respectively. CD4+ T cells stimulate B cell proliferation and ultimately antibody production. In addition, the B cell response to alloantigen may be directly enhanced by complement,5 because it has been reported for nontransplant antigens25,30 that opsonisation by C3b and its metabolite C3d can enhance antigen presentation via the complement receptor CR2, which is present both on follicular dendritic cells and B cells in secondary lymphoid tissue.26 Binding of the B cell receptor with the opsonized antigen lowers the threshold for B cell activation and allows for class switching of the donor-specific antibody from IgM to IgG. ABMR occurs when donor-specific antibodies recognize antigens on renal allograft endothelial cells engaging with the C1q, C1r, and C1s complex to initiate complement activation via the classical pathway. Again, C3 and C5 convertases are created and the membrane attack complex subsequently formed. Clinical evidence of complement activation is generally ascribed to the identification of C4d on evaluation of a renal biopsy specimen. All rejection pathways whether complement or cellular based present clinically with evidence of graft dysfunction characterized by increasing serum creatinine and decreasing urine output.
Role of Complement in ABMR
ABMR is defined as the development of a donor-specific antibody in conjunction with histologic changes on graft biopsy and deterioration of graft function.27 As an effector of acute ABMR, the role of complement is well established, although this function has recently been extended in the context of chronic rejection. In 1969, Terasaki showed that patients with alloantibody capable of inducing complement-dependent cell lysis were more likely to suffer from hyperacute rejection.28 Donor-reactive anti-HLA antibodies have been described as mediators of acute and chronic transplant injury29 and it has been documented that complement depletion impairs antibody production.30
ABMR occurs via the classical complement pathway when complement component C1q binds to donor-specific anti-HLA antibody (DSA) at the site of antibody attachment to the endothelium (Figure 2). Loupy et al. have generated evidence that the capacity of serum DSA to interact with C1q could determine the cytotoxic potential of these antibodies and therefore be used to risk stratify and diagnose ABMR.4 They reported that the development of a C1q-binding DSA in the first year post-transplantation was an independent risk factor for allograft loss and was associated with a higher risk of ABMR. Furthermore, their results showed that patients who developed complement-binding DSAs had the lowest 5-year rate of graft survival as compared with patients with non–complement-binding DSAs and patients without DSAs.4 On the other hand, Sicard et al., evaluating the ability of DSAs to bind C1q and C3d, a terminal C3 cleavage product, found that C3d was associated with a higher risk of graft loss, whereas C1q did not achieve statistical significance.31 Further studies have since demonstrated the relevance of C1q-binding DSAs beyond the first year post-transplantation as a potential marker for both adult and pediatric patients at high risk for late ABMR and subsequent graft loss.32,33 Lefaucheur et al. extended these initial observations of complement-binding DSAs and concluded that acute ABMR was mainly driven by the IgG3 subtype DSA whereas subclinical ABMR was driven by IgG4 DSA.34 Furthermore, these antibody subtypes along with C1q-binding DSA were again independently associated with allograft failure. What remains unknown is how durable these correlations are, because the capacity of antibodies to bind complement may change over time.32 Regardless, it appears that the ability to correlate complement-binding DSAs with distinct patterns of ABMR could be extremely helpful in identifying patients who would benefit from additional immune modulation of the complement system, such as treatment with eculizumab.31 Initial work on complement system modulation showed that in presensitized mice bearing heart allografts, therapeutic inhibition of complement with anti-C5, in addition to treatment with cyclosporine and cyclophosphamide, prevented acute rejection and prolonged allograft survival.35 This therapeutic approach was extended to humans with the study of eculizumab, the anti–human C5 mAb. Clinical studies have since demonstrated reduced ABMR.36,37 However, the risk of downstream complement inhibition increases susceptibility to sepsis and encapsulated organisms. In an attempt to target upstream complement components, C1 INH is currently being tested in a clinical trial as a treatment of acute ABMR and is showing some promise.38 It should be noted, however, that C1 INH is a broad protease inhibitor with actions that extend to the alternative and lectin pathways, as well as noncomplement proteases.39
The LP as a Mediator of Organ Injury
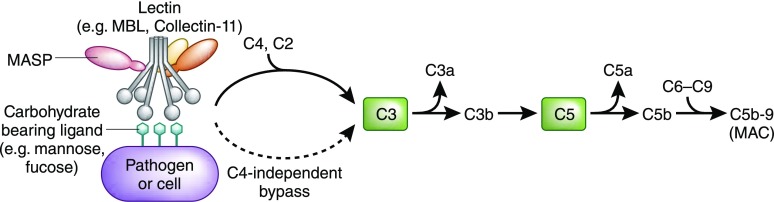
Renal ischemia-reperfusion insult leads to local, extravascular compartment complement activation through a C4-independent pathway with subsequent formation of C5a and C5b-9, suggesting a contribution to continued inflammation via the alternative pathway.15 Although a subsequent study in complement factor B–deficient mice confirmed a role for the alternative pathway, the complement recognition pathway that triggered injury was not conclusively identified at this stage.40 More recently, the LP was implicated as the primary mode of renal complement activation and subsequent tissue injury after ischemic insult.17,41 Complement activation via the LP begins when one of a number of pattern recognition receptors (PRRs) binds to a pathogen-associated molecular pattern (PAMP) that is displayed on the surface of an invading microorganism, or when a PRR binds to a damage-associated molecular pattern (DAMP) displayed on endogenous ligands during inflammation or cell stress (Figure 3).42 There are a variety of LP PRRs in the complement system, which include: collectins, which are soluble collagen-like lectins, such as MBL and collectin-11 (CL-11)43; surfactant proteins (SP-A and SP-D); and ficolins (ficolin-1, ficolin-2, and ficolin-3).44 A common and key feature of these PRRs is their interaction with MBL-associated serine proteases (MASPs), of which there are three, named MASP-1, MASP-2, and MASP-3.45,46 The LP was initially thought to be activated through the action of MASP-1 and MASP-2,44 whereas MASP-3 has been implicated in activation of the alternative pathway.47 The observation of complement activation when the collectin-MASP complex binds to ligands of invading pathogens or endogenous material demonstrated that LP activation was critically dependent on the action of MASP-2.48 In rodent models, MASP-2 has been shown to be an essential mediator of reperfusion injury in both native and transplanted organs.16,17,49 Traditionally, LP activation proceeds through MASP-2–mediated cleavage of C4 and C2 leading to formation of C3 convertase (C4b2a). Recent work, however, has shown the activity of a C4-independent bypass lectin activation pathway.16 In this study, MASP-2 was found to mediate injury in the absence of C4 in both cardiac and intestinal ischemia-reperfusion injury. Further studies in a renal isograft model comparing wild-type and MASP-2–deficient mice showed that renal function was preserved with MASP-2 deficiency and the effect of MASP-2 was independent of C4, implicating the C4 bypass route of activation as an important mediator of tissue injury.17 These observation help explain why C4-deficient mice are afforded no protection in both native renal ischemia15 and cell-mediated allograft rejection models.50 Studies on human sera from MASP-2–deficient patients support the existence of a C4-bypass pathway in man.16 Thus, there exists a novel mechanism of LP activation and a number of candidate pattern recognition molecules that could trigger this mechanism. We will now consider the evidence for the most relevant of these molecules in the context of allograft injury, especially of the kidney.
Figure 3.
The LP. The LP is triggered by collectins, ficolins, and surfactant proteins binding to a carbohydrate moiety such as mannose or fucose that is expressed on a pathogen or stressed cell. The pathway then progresses to formation of C3 convertase and subsequent C3 cleavage which results in terminal complement pathway activation and ultimately formation of C5b-9 (membrane attack complex, MAC). Note that the LP is known to proceed through MASP-2–mediated cleavage of C4 and C2 before formation of C3 convertase. However, recent work has shown the activity of a C4-independent lectin activation pathway leading to cleavage of C3.16
Role of MBL in Tissue Injury
Early efforts focused on well established PRRs as potential initiators of the LP, such as MBL. Models of renal and cardiac ischemia-reperfusion injury highlighted a potential role for MBL due to colocalization of MBL with complement.51 Although these associations suggested MBL mediated the observed injury, a classical pathway mediator of the injury could not be ruled out due to the ability of MBL to bind to carbohydrate moieties on IgA52 and IgM,53 in addition to carbohydrates on microorganisms. Additionally, ischemic heart,54,55 intestinal, and skeletal injury models identified a role for MBL and the LP in renal allograft rejection.56,57 However, the interaction between MBL and target ligands in the extravascular renal compartment after ischemia may be limited due to the large molecular mass of MBL (which is found in serum as a macromolecular complex) and the fact that it is synthesized almost solely in the liver.58 Furthermore, clinical evidence has been somewhat conflicting. In one study, as predicted, higher pretransplant levels of MBL were associated with more severe kidney rejection.59 In a separate study, patients with type I diabetes and renal failure receiving simultaneous kidney-pancreas transplants showed improved survival rates, associated with gene polymorphisms conferring lower MBL levels.60 However, a number of studies have identified an inverse correlation between MBL and graft outcome, where low levels of MBL lead to poor transplant outcome. For example, a recent study showed that low pretransplant levels of MBL in renal transplant recipients correlated with increased severity of renal inflammation and an increase in tubular cell necrosis.61 Similarly, a Swiss study showed that MBL-2 polymorphisms resulting in low serum MBL levels were associated with poor graft outcome.62 To add to the controversy, however, in a study comparing donor and recipient gene profiles for MBL-2 and MASP-2 it was shown that there was no association with graft outcome.63
Role of Ficolins in Tissue Injury
Three ficolins have been described in humans, namely ficolin-1, ficolin-2, and ficolin-3.42 There have been a handful of studies that have examined their role in human allograft rejection. One study of >1200 donor and recipient pairs investigating lectin gene profiles, specifically ficolin-2 gene haplotypes, showed no association with allograft survival.63 However, another study of common functional polymorphisms in the ficolin-2 gene revealed an association between this polymorphism and lower incidence of renal transplant rejection.64 Furthermore, high pretransplant levels of ficolin-3, the most abundant ficolin in serum, showed strong association with poor allograft survival after renal transplantation.65,66
Collectin-11 as a Mediator of Renal Epithelial Inflammation
Collectin-11 was first identified over a decade ago as a component of the innate immune system.67 However, it is only recently that the wider biologic significance of this molecule has begun to emerge. The biologically active structure of CL-11 is a trimer, approximately 300 kD.43 It is composed of monomers with a globular head containing a carbohydrate recognition domain and a collagenous tail that contains MASP binding motifs.68 Synthesis occurs in many tissues, including the liver, brain, heart, and the kidney.43,67,69 Previous work with CL-11 demonstrated an important role in host defense against bacteria and other pathogens via its carbohydrate recognition domain, which shows a preference for L-fucose in humans.43,67 In patients with disseminated intravascular coagulation, serum levels of CL-11 were typically double those of healthy control subjects.70 More recently, CL-11 was shown to have strong binding to nucleic acids, thus indicating a potential role in clearance of apoptotic cells after inflammation or injury.71
Renal tubule epithelial cells appear to be the main source of CL-11 in normal renal tissue, with increased expression after renal ischemia-reperfusion injury.69 Additionally, CL-11 was found to colocalize with carbohydrate ligand L-fucose on inflamed proximal tubule cells. A critical observation was that complement component C3d, a marker for complement activation, was present on the basolateral surface of the renal tubule cells in the hypoxia-sensitive zone only where CL-11 and L-fucose engage.69 Further support for the role of CL-11 in complement-mediated renal injury was the observation that CL-11–deficient mice were resistant to postischemic renal failure and associated complement-mediated damage, indicating the role of local complement activation in the extravascular compartment. The mechanism for this is believed to be through abnormal expression of a fucosylated ligand at the hypoxia-stressed cell surface, thereby allowing CL-11 to bind and subsequently initiate MASP-2–mediated complement activation.69 Understanding the nature of the fucosylated ligand(s) is critical to the design and application of relevant therapies to block the interaction of these molecules in the setting of renal inflammation.
Conclusion
As laboratory investigation into complement-binding DSAs as well as the triggering of CL-11–mediated complement activation continues, work is taking place in the clinical realm to further understand and modulate the role of complement-mediated injury in renal transplantation. Clinical trials will aid in determining if complement inhibition is a viable therapeutic option. There is no doubt that therapies such as eculizumab (anti-C5) have shown great promise for certain complement-mediated pathologies such as paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Indeed, clinical trials continue on the potential use of C1 INH and eculizumab in treating and preventing ABMR of solid organ transplants.13,36 Recent bench work has identified various complement components at play in both the vascular and extravascular compartments. It is likely that these components cause allograft injury via separate, although not mutually exclusive complement recognition pathways, which may have a subsequent effect on how future therapies are delivered in the clinic. Examples include specific DSA subtypes as well as the LP as potential earlier targets for intervention rather than the downstream complement components, C3 or C5, to reduce the risk of ABMR or the pathogenic response to ischemia-reperfusion, respectively. Local production of CL-11 at the site of tissue injury may be the distinguishing feature that sets it apart in functional terms from other pattern recognition molecules of the lectin complement pathway. Further understanding of complement-binding DSAs and the initiation of the LP and associated tissue ligands will allow for a clearer picture of the molecular and cellular mechanisms at play and hopefully lead to more precise therapeutic interventions.
Disclosures
None.
Acknowledgments
Some of the work described was supported by the Medical Research Council grants MR/J006742/1 and MR/L020254/1 and G1001141, and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St. Thomas’s NHS Foundation Trust and King’s College London.
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Beltrame MH, Catarino SJ, Goeldner I, Boldt AB, de Messias-Reason IJ: The lectin pathway of complement and rheumatic heart disease. Front Pediatr 2: 148, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacks SH, Zhou W: The role of complement in the early immune response to transplantation. Nat Rev Immunol 12: 431–442, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Pratt JR, Basheer SA, Sacks SH: Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med 8: 582–587, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, Suberbielle C, Frémeaux-Bacchi V, Méjean A, Desgrandchamps F, Anglicheau D, Nochy D, Charron D, Empana JP, Delahousse M, Legendre C, Glotz D, Hill GS, Zeevi A, Jouven X: Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369: 1215–1226, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Marsh JE, Farmer CK, Jurcevic S, Wang Y, Carroll MC, Sacks SH: The allogeneic T and B cell response is strongly dependent on complement components C3 and C4. Transplantation 72: 1310–1318, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Fearn A, Sheerin NS: Complement activation in progressive renal disease. World J Nephrol 4: 31–40, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricklin D, Hajishengallis G, Yang K, Lambris JD: Complement: A key system for immune surveillance and homeostasis. Nat Immunol 11: 785–797, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walport MJ: Complement. First of two parts. N Engl J Med 344: 1058–1066, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Jensenius JC: The mannan-binding lectin (MBL) pathway of complement activation: Biochemistry, biology and clinical implications. Adv Exp Med Biol 564: 21–22, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Ali YM, Lynch NJ, Haleem KS, Fujita T, Endo Y, Hansen S, Holmskov U, Takahashi K, Stahl GL, Dudler T, Girija UV, Wallis R, Kadioglu A, Stover CM, Andrew PW, Schwaeble WJ: The lectin pathway of complement activation is a critical component of the innate immune response to pneumococcal infection. PLoS Pathog 8: e1002793, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thurman JM: Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol 123: 7–13, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thurman JM, Holers VM: The central role of the alternative complement pathway in human disease. J Immunol 176: 1305–1310, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Frémeaux-Bacchi V, Legendre CM: The emerging role of complement inhibitors in transplantation. Kidney Int 88: 967–973, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Yamanaka K, Kakuta Y, Miyagawa S, Nakazawa S, Kato T, Abe T, Imamura R, Okumi M, Maeda A, Okuyama H, Mizuno M, Nonomura N: Depression of complement regulatory factors in rat and human renal grafts is associated with the progress of acute T-cell mediated rejection. PLoS One 11: e0148881, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, Stahl GL, Sacks SH: Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest 105: 1363–1371, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwaeble WJ, Lynch NJ, Clark JE, Marber M, Samani NJ, Ali YM, Dudler T, Parent B, Lhotta K, Wallis R, Farrar CA, Sacks S, Lee H, Zhang M, Iwaki D, Takahashi M, Fujita T, Tedford CE, Stover CM: Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc Natl Acad Sci USA 108: 7523–7528, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asgari E, Farrar CA, Lynch N, Ali YM, Roscher S, Stover C, Zhou W, Schwaeble WJ, Sacks SH: Mannan-binding lectin-associated serine protease 2 is critical for the development of renal ischemia reperfusion injury and mediates tissue injury in the absence of complement C4. FASEB J 28: 3996–4003, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cravedi P, Heeger PS: Complement as a multifaceted modulator of kidney transplant injury. J Clin Invest 124: 2348–2354, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heeger PS, Lalli PN, Lin F, Valujskikh A, Liu J, Muqim N, Xu Y, Medof ME: Decay-accelerating factor modulates induction of T cell immunity. J Exp Med 201: 1523–1530, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou W, Patel H, Li K, Peng Q, Villiers MB, Sacks SH: Macrophages from C3-deficient mice have impaired potency to stimulate alloreactive T cells. Blood 107: 2461–2469, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Peng Q, Li K, Patel H, Sacks SH, Zhou W: Dendritic cell synthesis of C3 is required for full T cell activation and development of a Th1 phenotype. J Immunol 176: 3330–3341, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, Shapiro VS, Dubyak GR, Heeger PS, Medof ME: Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity 28: 425–435, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng Q, Li K, Anderson K, Farrar CA, Lu B, Smith RA, Sacks SH, Zhou W: Local production and activation of complement up-regulates the allostimulatory function of dendritic cells through C3a-C3aR interaction. Blood 111: 2452–2461, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS: Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood 112: 1759–1766, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang Y, Xu C, Fu YX, Holers VM, Molina H: Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J Immunol 160: 5273–5279, 1998 [PubMed] [Google Scholar]
- 26.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT: C3d of complement as a molecular adjuvant: Bridging innate and acquired immunity. Science 271: 348–350, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Gosset C, Lefaucheur C, Glotz D: New insights in antibody-mediated rejection. Curr Opin Nephrol Hypertens 23: 597–604, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Patel R, Terasaki PI: Significance of the positive crossmatch test in kidney transplantation. N Engl J Med 280: 735–739, 1969 [DOI] [PubMed] [Google Scholar]
- 29.Lachmann N, Terasaki PI, Budde K, Liefeldt L, Kahl A, Reinke P, Pratschke J, Rudolph B, Schmidt D, Salama A, Schönemann C: Anti-human leukocyte antigen and donor-specific antibodies detected by luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation 87: 1505–1513, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Pepys MB: Role of complement in induction of antibody production in vivo. Effect of cobra factor and other C3-reactive agents on thymus-dependent and thymus-independent antibody responses. J Exp Med 140: 126–145, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sicard A, Ducreux S, Rabeyrin M, Couzi L, McGregor B, Badet L, Scoazec JY, Bachelet T, Lepreux S, Visentin J, Merville P, Fremeaux-Bacchi V, Morelon E, Taupin JL, Dubois V, Thaunat O: Detection of C3d-binding donor-specific anti-HLA antibodies at diagnosis of humoral rejection predicts renal graft loss. J Am Soc Nephrol 26: 457–467, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calp-Inal S, Ajaimy M, Melamed ML, Savchik C, Masiakos P, Colovai A, Akalin E: The prevalence and clinical significance of C1q-binding donor-specific anti-HLA antibodies early and late after kidney transplantation. Kidney Int 89: 209–216, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Fichtner A, Süsal C, Höcker B, Rieger S, Waldherr R, Westhoff JH, Sander A, Opelz G, Tönshoff B: Association of C1q-fixing DSA with late graft failure in pediatric renal transplant recipients. Pediatr Nephrol 31: 1157–1166, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Lefaucheur C, Viglietti D, Bentlejewski C, Duong van Huyen JP, Vernerey D, Aubert O, Verine J, Jouven X, Legendre C, Glotz D, Loupy A, Zeevi A: IgG donor-specific anti-human HLA antibody subclasses and kidney allograft antibody-mediated injury. J Am Soc Nephrol 27: 293–304, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Arp J, Liu W, Faas SJ, Jiang J, Gies DR, Ramcharran S, Garcia B, Zhong R, Rother RP: Inhibition of terminal complement components in presensitized transplant recipients prevents antibody-mediated rejection leading to long-term graft survival and accommodation. J Immunol 179: 4451–4463, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Stegall MD, Diwan T, Raghavaiah S, Cornell LD, Burns J, Dean PG, Cosio FG, Gandhi MJ, Kremers W, Gloor JM: Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant 11: 2405–2413, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Locke JE, Magro CM, Singer AL, Segev DL, Haas M, Hillel AT, King KE, Kraus E, Lees LM, Melancon JK, Stewart ZA, Warren DS, Zachary AA, Montgomery RA: The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am J Transplant 9: 231–235, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Montgomery RA, Orandi BJ, Racusen L, Jackson AM, Garonzik-Wang JM, Shah T, Woodle ES, Sommerer C, Fitts D, Rockich K, Zhang P, Uknis ME: Plasma-derived C1 esterase inhibitor for acute antibody-mediated rejection following kidney tTransplantation: Results of a randomized double-blind placebo-controlled pilot study. Am J Transplant 16: 3468–3478, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Davis AE 3rd, Mejia P, Lu F: Biological activities of C1 inhibitor. Mol Immunol 45: 4057–4063, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thurman JM, Ljubanovic D, Edelstein CL, Gilkeson GS, Holers VM: Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J Immunol 170: 1517–1523, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Farrar CA, Asgari E, Schwaeble WJ, Sacks SH: Which pathways trigger the role of complement in ischaemia/reperfusion injury? Front Immunol 3: 341, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Genster N, Takahashi M, Sekine H, Endo Y, Garred P, Fujita T: Lessons learned from mice deficient in lectin complement pathway molecules. Mol Immunol 61: 59–68, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Selman L, Hansen S: Structure and function of collectin liver 1 (CL-L1) and collectin 11 (CL-11, CL-K1). Immunobiology 217: 851–863, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Garred P, Genster N, Pilely K, Bayarri-Olmos R, Rosbjerg A, Ma YJ, Skjoedt MO: A journey through the lectin pathway of complement-MBL and beyond. Immunol Rev 274: 74–97, 2016 [DOI] [PubMed] [Google Scholar]
- 45.Dahl MR, Thiel S, Matsushita M, Fujita T, Willis AC, Christensen T, Vorup-Jensen T, Jensenius JC: MASP-3 and its association with distinct complexes of the mannan-binding lectin complement activation pathway. Immunity 15: 127–135, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Thiel S, Vorup-Jensen T, Stover CM, Schwaeble W, Laursen SB, Poulsen K, Willis AC, Eggleton P, Hansen S, Holmskov U, Reid KB, Jensenius JC: A second serine protease associated with mannan-binding lectin that activates complement. Nature 386: 506–510, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Iwaki D, Kanno K, Takahashi M, Endo Y, Matsushita M, Fujita T: The role of mannose-binding lectin-associated serine protease-3 in activation of the alternative complement pathway. J Immunol 187: 3751–3758, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Wallis R: Interactions between mannose-binding lectin and MASPs during complement activation by the lectin pathway. Immunobiology 212: 289–299, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farrar CA, Zhou W, Lin T, Sacks SH: Local extravascular pool of C3 is a determinant of postischemic acute renal failure. FASEB J 20: 217–226, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Lin T, Zhou W, Farrar CA, Hargreaves RE, Sheerin NS, Sacks SH: Deficiency of C4 from donor or recipient mouse fails to prevent renal allograft rejection. Am J Pathol 168: 1241–1248, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Vries B, Walter SJ, Peutz-Kootstra CJ, Wolfs TG, van Heurn LW, Buurman WA: The mannose-binding lectin-pathway is involved in complement activation in the course of renal ischemia-reperfusion injury. Am J Pathol 165: 1677–1688, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roos A, Rastaldi MP, Calvaresi N, Oortwijn BD, Schlagwein N, van Gijlswijk-Janssen DJ, Stahl GL, Matsushita M, Fujita T, van Kooten C, Daha MR: Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol 17: 1724–1734, 2006 [DOI] [PubMed] [Google Scholar]
- 53.McMullen ME, Hart ML, Walsh MC, Buras J, Takahashi K, Stahl GL: Mannose-binding lectin binds IgM to activate the lectin complement pathway in vitro and in vivo. Immunobiology 211: 759–766, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Busche MN, Pavlov V, Takahashi K, Stahl GL: Myocardial ischemia and reperfusion injury is dependent on both IgM and mannose-binding lectin. Am J Physiol Heart Circ Physiol 297: H1853–H1859, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walsh MC, Bourcier T, Takahashi K, Shi L, Busche MN, Rother RP, Solomon SD, Ezekowitz RA, Stahl GL: Mannose-binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J Immunol 175: 541–546, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Zhang M, Austen WG Jr, Chiu I, Alicot EM, Hung R, Ma M, Verna N, Xu M, Hechtman HB, Moore FD Jr, Carroll MC: Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc Natl Acad Sci USA 101: 3886–3891, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, Kessler B, Shimaoka M, Chan R, Friend D, Mahmood U, Weissleder R, Moore FD, Carroll MC: Identification of the target self-antigens in reperfusion injury. J Exp Med 203: 141–152, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farrar CA, Zhou W, Sacks SH: Role of the lectin complement pathway in kidney transplantation. Immunobiology 221: 1068–1072, 2016 [DOI] [PubMed] [Google Scholar]
- 59.Berger SP, Roos A, Mallat MJ, Fujita T, de Fijter JW, Daha MR: Association between mannose-binding lectin levels and graft survival in kidney transplantation. Am J Transplant 5: 1361–1366, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Berger SP, Roos A, Mallat MJ, Schaapherder AF, Doxiadis II, van Kooten C, Dekker FW, Daha MR, de Fijter JW: Low pretransplantation mannose-binding lectin levels predict superior patient and graft survival after simultaneous pancreas-kidney transplantation. J Am Soc Nephrol 18: 2416–2422, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Ibernon M, Moreso F, O’Valle F, Grinyo JM, Moral RG, Seron D: Low serum mannose-binding lectin levels are associated with inflammation and apoptosis in early surveillance allograft biopsies. Transpl Immunol 31: 152–156, 2014 [DOI] [PubMed] [Google Scholar]
- 62.Golshayan D, Wójtowicz A, Bibert S, Pyndiah N, Manuel O, Binet I, Buhler LH, Huynh-Do U, Mueller T, Steiger J, Pascual M, Meylan P, Bochud PY; Swiss Transplant Cohort Study : Polymorphisms in the lectin pathway of complement activation influence the incidence of acute rejection and graft outcome after kidney transplantation. Kidney Int 89: 927–938, 2016 [DOI] [PubMed] [Google Scholar]
- 63.Damman J, Kok JL, Snieder H, Leuvenink HG, van Goor H, Hillebrands JL, van Dijk MC, Hepkema BG, Reznichenko A, van den Born J, de Borst MH, Bakker SJ, Navis GJ, Ploeg RJ, Seelen MA: Lectin complement pathway gene profile of the donor and recipient does not influence graft outcome after kidney transplantation. Mol Immunol 50: 1–8, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Eikmans M, de Canck I, van der Pol P, Baan CC, Haasnoot GW, Mallat MJ, Vergunst M, de Meester E, Roodnat JI, Anholts JD, van Thielen M, Doxiadis II, de Fijter JW, van der Linden PJ, van Beelen E, van Kooten C, Kal-van Gestel JA, Peeters AM, Weimar W, Roelen DL, Rossau R, Claas FH: The functional polymorphism Ala258Ser in the innate receptor gene ficolin-2 in the donor predicts improved renal transplant outcome. Transplantation 94: 478–485, 2012 [DOI] [PubMed] [Google Scholar]
- 65.Bay JT, Hein E, Sørensen SS, Hansen JM, Garred P: Pre-transplant levels of ficolin-3 are associated with kidney graft survival. Clin Immunol 146: 240–247, 2013 [DOI] [PubMed] [Google Scholar]
- 66.Smedbråten YV, Sagedal S, Mjøen G, Hartmann A, Fagerland MW, Rollag H, Mollnes TE, Thiel S: High ficolin-3 level at the time of transplantation is an independent risk factor for graft loss in kidney transplant recipients. Transplantation 99: 791–796, 2015 [DOI] [PubMed] [Google Scholar]
- 67.Keshi H, Sakamoto T, Kawai T, Ohtani K, Katoh T, Jang SJ, Motomura W, Yoshizaki T, Fukuda M, Koyama S, Fukuzawa J, Fukuoh A, Yoshida I, Suzuki Y, Wakamiya N: Identification and characterization of a novel human collectin CL-K1. Microbiol Immunol 50: 1001–1013, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Ma YJ, Skjoedt MO, Garred P: Collectin-11/MASP complex formation triggers activation of the lectin complement pathway--the fifth lectin pathway initiation complex. J Innate Immun 5: 242–250, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farrar CA, Tran D, Li K, Wu W, Peng Q, Schwaeble W, Zhou W, Sacks SH: Collectin-11 detects stress-induced L-fucose pattern to trigger renal epithelial injury. J Clin Invest 126: 1911–1925, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takahashi K, Ohtani K, Larvie M, Moyo P, Chigweshe L, Van Cott EM, Wakamiya N: Elevated plasma CL-K1 level is associated with a risk of developing disseminated intravascular coagulation (DIC). J Thromb Thrombolysis 38: 331–338, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Henriksen ML, Brandt J, Iyer SS, Thielens NM, Hansen S: Characterization of the interaction between collectin 11 (CL-11, CL-K1) and nucleic acids. Mol Immunol 56: 757–767, 2013 [DOI] [PubMed] [Google Scholar]