Abstract
Atherosclerotic renovascular disease (RVD) reduces renal blood flow (RBF) and GFR and accelerates poststenotic kidney (STK) tissue injury. Preclinical studies indicate that mesenchymal stem cells (MSCs) can stimulate angiogenesis and modify immune function in experimental RVD. We assessed the safety and efficacy of adding intra-arterial autologous adipose-derived MSCs into STK to standardized medical treatment in human subjects without revascularization. The intervention group (n=14) received a single infusion of MSC (1.0 × 105 or 2.5 × 105 cells/kg; n=7 each) plus standardized medical treatment; the medical treatment only group (n=14) included subjects matched for age, kidney function, and stenosis severity. We measured cortical and medullary volumes, perfusion, and RBF using multidetector computed tomography. We assessed tissue oxygenation by blood oxygen level–dependent MRI and GFR by iothalamate clearance. MSC infusions were well tolerated. Three months after infusion, cortical perfusion and RBF rose in the STK (151.8–185.5 ml/min, P=0.01); contralateral kidney RBF increased (212.7–271.8 ml/min, P=0.01); and STK renal hypoxia (percentage of the whole kidney with R2*>30/s) decreased (12.1% [interquartile range, 3.3%–17.8%] to 6.8% [interquartile range, 1.8%–12.9%], P=0.04). No changes in RBF occurred in medical treatment only subjects. Single-kidney GFR remained stable after MSC but fell in the medical treatment only group (−3% versus −24%, P=0.04). This first-in-man dose-escalation study provides evidence of safety of intra-arterial infusion of autologous MSCs in patients with RVD. MSC infusion without main renal artery revascularization associated with increased renal tissue oxygenation and cortical blood flow.
Keywords: renal artery stenosis, ischemia, stem cell, Renovascular disease
Atherosclerotic renovascular disease (RVD) reduces renal blood flow (RBF) and ultimately accentuates tissue hypoxia.1 Severe reductions in RBF eventually lead to vascular rarefication, inflammatory injury oxidative stress, and loss of renal function,2–4 which has been designated “ischemic nephropathy.”5 Many of these changes fail to reverse after restoring vessel patency alone,4,6, thereby reinforcing the need for more effective alternative or adjunctive therapy.
Experimental studies in swine and rodent RVD models show that infusion of mesenchymal stem cells (MSC) is associated with increased microvascular density and GFR beyond that attainable with restoring blood flow alone.7,8 Moreover, MSC administration leads to reductions in oxidative stress and renal inflammation that were not observed with revascularization alone.7 Whether these preclinical observations with MSC therapy extend to humans with atherosclerotic RVD is unknown.
The purpose of this initial study was to examine the safety and efficacy of intra-arterial injection of autologous adipose-derived MSC alone in a dose-escalation phase 1/2A study for patients with atherosclerotic RVD. We sought to evaluate RBF, cortical and medullary perfusion, GFR, and renal tissue oxygenation in poststenotic kidneys (STKs) and contralateral kidneys (CLKs) of patients with atherosclerotic RVD treated with intrarenal MSC and compared with a matched group of patients treated with standardized medical therapy alone.
Results
Study Cohort
Demographic and clinical features of the 28 patients with RVD studied are summarized in Table 1. There were no differences between groups in age, severity of stenosis, and pretreatment kidney function. Measured BP, weight, body mass index, and most biochemical values were also not different between groups.
Table 1.
Clinical, laboratory, and demographic data of medically treated atherosclerotic RVD patients with and without MSC
| Characteristic | Medical (n=14) | Medical plus MSCs (n=14) | P Value |
|---|---|---|---|
| Men/womena | 6:8 | 9:5 | 0.45 |
| Age, yr | 71.1±6.9 | 74.4±3.4 | 0.13 |
| Creatinine, mg/dl | 1.32±0.31 | 1.5±0.3 | 0.11 |
| Iothalamate clearance GFR, ml/min | 54.9±20.4 | 51.2±18.6 | 0.6 |
| Stenotic artery U/S Doppler “peak systolic velocity,” cm/s | 331±119.4 | 303.7±64.5 | 0.3 |
| SBP, mmHg | 138.6±17 | 145.2±14 | 0.26 |
| DBP, mmHg | 66.1±6.9 | 67.8±12.4 | 0.64 |
| Weight, kg | 77.2±16.7 | 86.2±17.8 | 0.17 |
| BMI, kg/m2 | 27.4±4.5 | 30.8±4.9 | 0.07 |
| Hemoglobin, g/dl | 12.4±1.3 | 13.2±1.3 | 0.1 |
| Hematocrit, % | 37.3±3.3 | 39.9±3.9 | 0.07 |
Mean±SD. N, number of patients; U/S, ultrasound; SBP, systolic BP; DBP, diastolic BP; BMI, body mass index.
Fisher exact test.
Safety Monitoring: Sequential Measurements after MSC Infusion
All patients tolerated the single MSC infusion without identified adverse clinical effects (specifically: fever, headache, vomiting, weakness, hematuria, or allergic reactions). On the day after intra-arterial infusion of MSC, serum creatinine rose slightly (1.52±0.35–1.72±0.45 mg/dl), persisting for 1 week and returning to baseline by 1 month (Table 2). Additionally, C-reactive protein (CRP) rose for 1 day (3.1±1.2–6.1±2.3), whereas lactate dehydrogenase was unchanged. White blood cell count and platelets rose slightly and remained above baseline for 1 month. These transient alterations in laboratory values resolved by the 3-month inpatient return visit. None of these were associated with evident pathology, discomfort, or adverse events. No changes occurred in neutrophil gelatinase-associated lipocalin (NGAL) or urine cytology during follow-up.
Table 2.
Safety data as reflected by sequential measurements after MSC infusion
| Variable | Baseline | 1 D | 1 Wk | 1 Mo | 2 Mo | 3 Mo |
|---|---|---|---|---|---|---|
| Creatinine mg/dl | 1.52±0.35 | 1.72±0.45a | 1.66±0.3a | 1.55±0.37 | 1.55±0.33 | 1.53±0.3 |
| CRP mg/L | 3.1±1.2 | 6.1±2.3 | 4.1±5.1 | 3.3±4.4 | 2.3±1.7 | 3.4±2.3 |
| LDH U/L | 214±51 | 202±41 | 196±44 | 188±50.7a | 185.2±53a | 206.5±48 |
| ESR mm/h | 19.8±12.9 | 14.6±10.5 | ||||
| Leukocytes × 109/L | 6.5±1.6 | 7.3±1.5a | 7.2±1.4a | 6.9±1.2a | 6.7±1.2 | 6.6±1.3 |
| Neutrophils × 109/L | 3.8±1.3 | 4.65±1.2a | 4.47±1.1a | 4.17±1.1a | 3.9±0.86 | 4.08±1.2 |
| Platelets × 103/L | 194±51 | 200±67a | 245±67a | 218.6±45a | 212.6±60 | 196±44 |
| NGAL ng/ml | 204±64 | 208±45 |
Data are expressed as mean±SD. LDH, lactate dehydrogenase; ESR, erythrocyte sedimentation rate; “Normal reference range ≤8 mg/L.”
P<0.05 versus baseline.
Cortical Perfusion and RBF Rose in MSC-Treated Kidneys
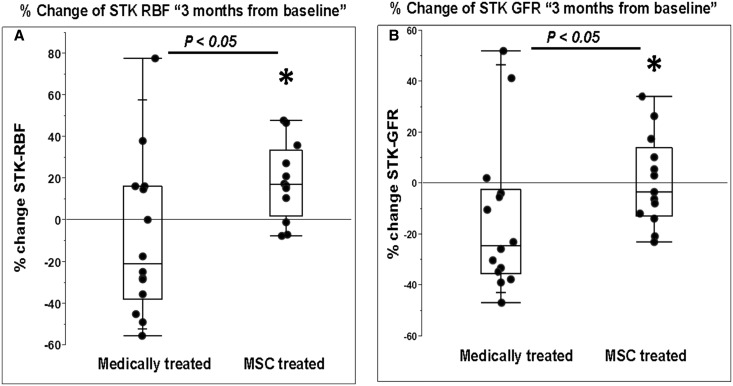
Results from quantitative multidetector computed tomography (MDCT) measurements of hemodynamics for individual STKs at baseline and after three months are summarized in Table 3. The total STK volume did not change in either medically-treated or MSC-treated groups. Both total RBF (Figure 1A) and cortical blood flow increased in the STKs, but medullary blood flow did not. Perfusion in the STKs rose in the MSC group from 2.02±0.69 to 2.4±1 ml/min per milliliter tissue (P=0.04) but remained unchanged in the medical treatment only group. Despite no direct intervention, changes of a similar magnitude were seen in cortical blood flow, total RBF, and cortical perfusion in the untreated CLKs in the MSC-treated group (Table 4) (Supplemental Figure 1 depicts individual changes in RBF). Whereas STK-iothalamate GFR (milliliter per minute per kidney) was unchanged in the MSC group (Table 3), the medical treatment group had a fall in STK-GFR at the 3-month follow-up (19 [13.8, 27.4] to 15.9 [9.7, 27.6] ml/min; P=0.05). When expressed as percent (%) change from baseline, the STK-GFR in the MSC group was unchanged (−3.3%) but fell in the medically treated group (−24.4%, P=0.04) (Figure 1B).
Table 3.
MDCT measurements of individual STK volume, tissue perfusion, blood flow, and iothalamate filtration
| Single Kidney | Medically Treated STK (n=14) | MSC-Treated STK (n=14) | ||
|---|---|---|---|---|
| Baseline | 3 Mo | Baseline | 3 Mo | |
| Total kidney volume (CT), ml | 90±42.9 | 86.6±41.3 | 98.9±41.2 | 98.9±41.3 |
| Cortical volume, ml | 59.7±27 | 56.3±28 | 61.2±31.8 | 61.8±29.6 |
| Medullary volume, ml | 30.3±17 | 30.3±16.2 | 37.8±12.7 | 37.2±13.3 |
| Cortical perfusion, ml/min per milliliter of tissue | 2.1±0.76 | 2.0±0.53 | 2.02±0.69 | 2.4±1a |
| Medullary perfusion, ml/min per milliliter of tissue | 0.94±0.5 | 0.86±0.27 | 0.7±0.24 | 0.8±0.37 |
| Total RBF, ml/minb | 132.5 (96, 255) | 128.1 (77.2, 233) | 151.8 (63.7, 222) | 185.5 (72.3, 259)a |
| Cortical blood flow, ml/minb | 114 (75.7, 221) | 106 (54, 186.3) | 125.6 (54.7, 192) | 138.3 (60.3, 226)a |
| Medullary blood flow, ml/minb | 26.3 (11.5, 42.3) | 20.3 (17.8, 36.4) | 31 (14.8, 38.9) | 29.4 (14.5, 36.9) |
| Single-kidney GFR, ml/min per kidneyb | 19 (13.8, 27.4) | 15.9 (9.7, 27.6) | 20.4 (12, 25) | 19.1 (14.3, 24.5) |
CT, computed tomography.
P<0.05 versus baseline.
Median (interquartile range) reported due to skewed data (both paired t test and Wilcoxon signed-rank test).
Figure 1.
MSC infusion increased STK-RBF and preserved GFR. Percent (%) change from baseline in STK-RBF and STK-GFR. (A) Differences in RBF (expressed as percent [%] change after 3 months) indicated a modest fall in RBF in the medically treated only group, whereas the MSC-treated group rose (P<0.05). (B) Relative change in measured STK-GFR decreased in medically treated group (−24.4%) during the 3-month follow-up but was preserved in the MSC group (−3.3%) (P<0.05). All patients received ACE/ARB treatment during these studies. ACE, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; IQR, interquartile range.
Table 4.
MDCT measurements of individual CLK or untreated kidney volume, tissue perfusion, blood flow, and iothalamate filtration
| Single Kidney | Medically Treated CLK (n=14) | MSC-Treated CLK (n=14) | ||
|---|---|---|---|---|
| Baseline | 3 Mo | Baseline | 3 Mo | |
| Total kidney volume (CT), ml | 136.2±40.6 | 130.2±36.3a | 124.9±35.4 | 125.9±38.2 |
| Cortical volume, ml | 92±29.8 | 85.3±29.8a | 76.8±23.8 | 78.9±24.8 |
| Medullary volume, ml | 44.2±13.8 | 44.9±11.8 | 48.1±15.4 | 46.9±15.4 |
| Cortical perfusion, ml/min per milliliter tissue | 2.5±0.66 | 2.6±0.64 | 2.3±0.58 | 2.8±0.7a |
| Medullary perfusion, ml/min per milliliter tissue | 0.95±0.26 | 1.15±0.39 | 0.74±0.19 | 0.9±0.26 |
| Total RBF, ml/min | 280.8±143 | 290.3±131.4 | 212.7±80.2 | 271.8±99.3a |
| Cortical blood flow, ml/min | 238.5±129.9 | 237.4±116.7 | 177.3±72.3 | 229.2±89a |
| Medullary blood flow, ml/min | 40.8±20.9 | 52.9±24a | 35.3±12.5 | 42.6±16.1a |
| Single-kidney GFR, ml/min per kidney | 34.3±13.1 | 34.4±13.9 | 29.5±13.2 | 30.9±12.4 |
Data are expressed as mean±SD. CT, computed tomography.
P<0.05 versus baseline (both paired t test and Wilcoxon signed-rank test).
Fractional Kidney Hypoxia Fell in MSC-Treated Kidneys
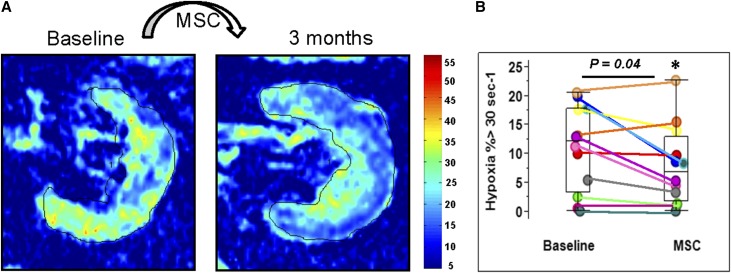
Tissue oxygenation levels defined both by cortical R2* values and fractional hypoxia (percent of whole kidney with R2*>30/s) did not differ between groups at baseline. Fractional hypoxia in the STK fell after 3 months in MSC-treated subjects (from 12.1% [3.3, 17.8] to 6.8% [1.8, 12.9]; P<0.04) Figure 2B, whereas no change was observed in the medically-treated group, from 10 (6.3, 25.1) to 9.6 (2.4, 37.7) (P=0.3) (Supplemental Table 1). Representative blood oxygen level–dependent (BOLD) images (R2* parametric maps) illustrating the change in hypoxia in an STK before and after MSC injection are illustrated in Figure 2A.
Figure 2.
MSC infusion decreased the levels of hypoxia in STKs. (A) Individual example of BOLD images (R2* parametric maps depicting effects of deoxyhemoglobin) illustrating the change in hypoxia in STK before and after MSC injection (left and right). There were lower R2* levels associated with hypoxia after MSC injection in both the cortical and medullary regions. (B) Fractional hypoxia levels in STK fell significantly after MSC treatment (P=0.04) (Supplemental Table 1). IQR, interquartile range.
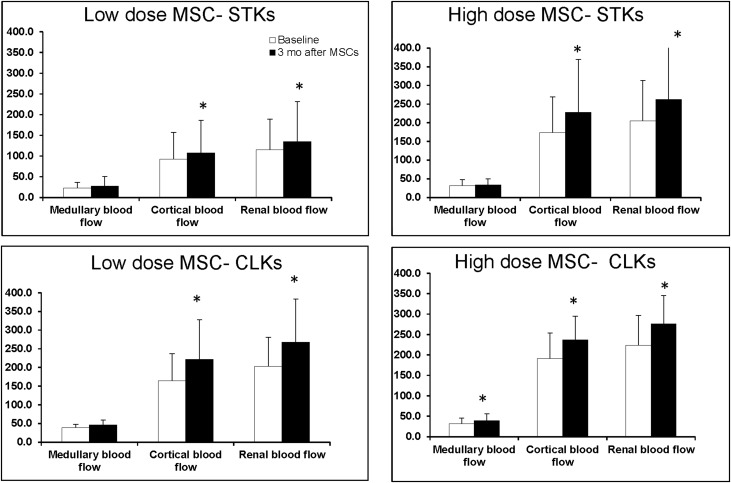
The rise in cortical and total tissue perfusion and RBF were of similar magnitude for both doses of MSC infused (Figure 3).
Figure 3.
Similar magnitude for both doses of MSC on RBF and perfusion. Medullary, cortical, and total RBF measurements before and 3 months after intra-arterial infusion of autologous adipose-derived MSC. Both low dose (1.0 × 105 cells/kg) and higher dose (2.5 × 105 cells/kg) of MSCs were associated with a rise in cortical and total perfusion and blood flow of similar magnitude. These changes were evident in both STK and CLK. No such changes were evident in patients undergoing medical therapy only.
Renal Vein Levels of Vascular Endothelial Growth Factor Decreased after MSC Treatment
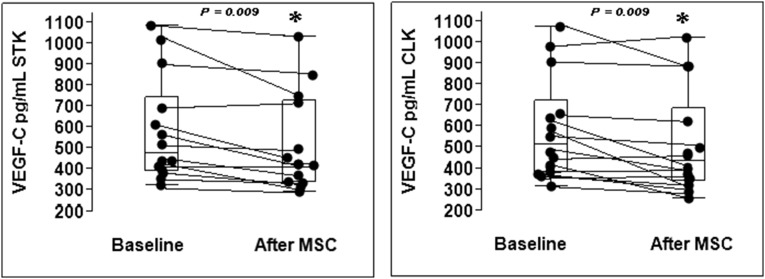
Renal vein levels of vascular endothelial growth factor (VEGF-C) decreased 3 months after MSC treatment compared with baseline (P=0.01) both at the STK and CLK (Figure 4). There were no such changes in the medically treated group. Renal vein levels of VEGF-A and inflammatory cytokines including IL-6, IFN-γ, TNF-α, monocyte chemoattractant protein-1 (MCP-1), and TGF-α tended to decrease in both kidneys after MSC treatment but did not reach statistical significance.
Figure 4.
Renal vein levels of VEGF-C decreased after MSC treatment. Renal vein levels of VEGF-C decreased 3 months after MSC treatment both in the STK and CLK (P<0.05). IQR, interquartile range.
Discussion
The results of this “first-in-man” phase 1/2A study indicate that intra-arterial infusion of autologous adipose tissue–derived MSCs in patients with atherosclerotic RVD increased cortical perfusion and RBF after 3 months, despite unchanged patency of the renal artery. Increased kidney perfusion was associated with measurable reduction in tissue fractional hypoxia and stabilization of GFR. MSC infusion was safe and well tolerated over the study follow-up period, with no identified adverse events related to MSC treatment. Serial safety laboratory tests demonstrated transient, minor changes in leukocyte counts, platelets, and CRP, all of which resolved by the return study visit at 3 months. Although our study was designed primarily to assess safety, these data also identified cortical hemodynamic effects associated with MSC treatment. These included the rise in renal cortical blood flow and perfusion in both the STK and nontreated kidneys at 3 months after MSC treatment. The reduction in hypoxia observed in the STK provided an independent measurement consistent with increased blood flow and tissue oxygenation. Whether these changes reflect new vessel formation (angiogenesis), redistribution of intrarenal flows, and/or altered intrarenal arterial shunting cannot be established with these data. Patients treated with intrarenal MSC and medical therapy had stable filtration, as reflected by the 3-month percent change in STK-GFR (−3%) compared with the medical treatment alone (−24%). To our knowledge, this is the first human experience with intra-arterial administration of cell-based therapy into ischemic kidneys.
Renal revascularization alone often fails to restore kidney function in atherosclerotic RVD, as reported in multiple recent clinical trials.9,10 Studies of single-kidney GFR beyond stenotic lesions demonstrate a loss of filtration during medical therapy, which may be obscured by increased filtration in the CLK.11–13 Our experimental studies in swine models of RVD demonstrate loss of microvascular structures14 that is magnified in the atherosclerotic environment2,15,16 and correlates with loss of GFR.6,17 Loss of cortical structural integrity appears in part related to oxidative stress and inflammation,14 which do not reverse after revascularization alone.4,6, This is consistent with data from human STKs that release higher levels of inflammatory cytokines (including NGAL and TNF-α) and “homing” signals that attract reparative progenitor cells under some conditions.4,18,19 Despite these signals, reparative mechanisms in human RVD usually are insufficient to restore vascular and tubular integrity after restoring vessel patency alone. In these studies we sought to evaluate the safety of infusing autologous MSC into the renal artery of human subjects with an ischemic environment. Results of our study in which inflammatory cytokines tended to decline after MSC treatment suggest that reparative and immunomodulatory properties of MSCs may partially abrogate vascular insufficiency and inflammatory injury pathways in the kidney that lead to progressive decline in kidney function. These results are consistent with data obtained in experimental large animal models.20
The rise in RBF and perfusion after MSC treatment might be related to MSC angiogenic properties and the active secretion of the proangiogenic factors by MSC.21,22 Improvements in the renal microcirculation after MSC treatment in experimental studies have been attributed to increased renal expression of the proangiogenic factor VEGF.7 Angiogenesis and restoration of flow to the ischemic tissue are particularly important to mitigate hypoxia and potentially improve the clinical course of RVD. In addition, MSC administration is thought to decrease oxidative stress7 and thereby increase the efficiency of oxygen utilization in the kidney.23 Remarkably, and consistent with animal experiments,24 our study showed that MSC treatment was also associated with increased cortical perfusion and RBF in the untreated CLKs. There are no methods currently for tracking the exact location of MSCs in human subjects. Using fluorescent markers in a pig model, labeled MSCs have been identified within both the STK and to a lesser extent in the CLK 4 weeks after injection.7 We and others have shown that CLKs express high levels of inflammatory cytokines (IL-6, TNF-α) and signs of fibrosis,4,24–26 which were attenuated by MSC treatment.8 These observations are consistent with “cross-talk” signaling between kidneys and/or wider systemic effects of signaling and homing signals for MSCs, in addition to systemic effects of hypertension and atherosclerosis per se.25,27 An additional effect observed after MSC treatment in this study was the reduction in fractional tissue hypoxia in the STK. Studies in patients with atherosclerotic RVD show that the STKs eventually develop higher levels of hypoxia compared with nonstenotic kidneys.1,4, Chronic hypoxia triggers inflammation and tubulointerstitial fibrosis, stimulates an increase in the expression of VEGF,28 and leads to chronic renal failure.29,30 Our results underscore the potential for MSC treatment to reduce hypoxia and improve tissue oxygenation in the chronically ischemic kidney.
Importantly, the increase in RBF, perfusion, and renal oxygenation was not associated with improvement in glomerular filtration in these patients. GFR was preserved as compared with the medically treated group which declined slightly during follow-up, as previously observed.12 It should be emphasized that angiotensin converting enzyme inhibitor/angiotensin receptor blocker therapy was continued throughout these protocol studies, in part because activation of the renin-angiotensin system affects tissue oxygenation31,32 and BOLD magnetic-resonance imaging (MRI) measurements. Because no changes were made to the main renal arterial lesions, poststenotic arterial pressures likely remained low, rendering trans-capillary filtration pressures dependent upon the renin-angiotensin system. Hence, filtration was likely limited partly by removal of efferent arteriolar vasomotor tone by renin-angiotensin system blockade.
Expression of VEGF-C is upregulated by proinflammatory cytokines such as IL-1B and TNF.33 Elevated levels of plasma VEGF-C are found in patients with vascular diseases associated with lymphangioleiomyomatosis,34,35 and inflammatory diseases where the levels of VEGFs have been correlated to measures of endothelial dysfunction.36 Our results after MSC treatment indicated decreased renal vein levels of VEGF-C, angiopoietin, and inflammatory cytokines both from the STK and CLK, consistent with an anti-inflammatory effect of MSCs associated with angiogenesis and increased RBF and perfusion.
Although advanced RVD can lead to renal hypoxia, previous studies showed that restoring vessel patency by revascularization alone fails to alter local inflammatory signals suggesting ongoing processes of tissue injury and inflammation.4 This study suggests that additional measures, such as intra-arterial MSCs, may partially abrogate those pathways. Recent experimental studies combining intrarenal infusion of MSCs and revascularization indicate that recovery of renal microvessels, blood flow, and glomerular filtration is possible in the STK.7
This study has limitations. It was an open-label, nonrandomized study, with a relatively limited number of patients. The medical treatment only group did not undergo angiography or arterial cannulation. For ethical reasons, we did not have a control group of healthy individuals treated with MSC. Although unlikely, there may have been effects related to the angiography or injection of material that caused effects attributed to the MSCs. Diabetic subjects were specifically excluded in this phase I/2A study. A longer follow-up period is needed to detect long-term effects.
Our results indicate that intra-arterial infusion of autologous MSC in patients with RVD is safe and well tolerated. MSC infusion at these doses increased cortical perfusion and RBF and reduced renal tissue hypoxia within STKs. These changes were associated with preserved STK-GFR as compared with a medically treated group in which STK-GFR declined over the same interval. These data underscore a potential adjunctive role for MSC in the management of ischemic renal disease. Further studies combining MSC with revascularization of the STK offer the potential to both restore large vessel patency and blood flow and to improve renal microcirculation in ischemic renal disease.
Concise Methods
Study Design and Patient Selection
In this first-in-man, open-label, single center, dose-escalation trial, we enrolled adult patients with significant RVD estimated by renal artery Doppler ultrasound velocity acceleration (average peak systolic velocity >300 cm/s) and/or magnetic resonance/computed tomography angiography with evident atherosclerotic stenosis (>60% lumen occlusion), and hypertension (systolic hypertension >155 mmHg and/or the use of at least two BP drugs) and/or with declining kidney function. Exclusion criteria included serum creatinine >2.5 mg/dl, diabetes mellitus, allergy to furosemide or iodinated contrast, pregnancy, kidney transplant, malignancy, or recent history of a major cardiovascular event within 6 months. Enrolled patients were admitted into the Clinical Research Unit at St. Mary’s Hospital, Rochester, Minnesota between June of 2013 and April of 2016. All patients received medical therapy consisting of agents blocking the renin-angiotensin system during these studies (angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers). Twenty-eight patients with RVD were included in the analysis: 14 were treated with standardized medical therapy plus a single intra-arterial infusion of autologous MSCs in the renal artery (MSC-treated group). The first seven were assigned to a low dose (1.0 × 105 cells/kg) and the next seven patients to a higher dose (2.5 × 105 cells/kg), on the basis of animal studies and investigational new drug (IND) #15082 with the Food and Drug Administration (FDA).7 When bilateral stenoses were identified (n=6), only the kidney with the most severe stenosis was infused with MSC. The other 14 patients who participated in the identical protocol without MSC were treated with standardized medical therapy alone (medically treated group) and were matched for age, GFR, sex, and stenosis severity. Informed, written consent was obtained as approved by the institutional review board of the Mayo Clinic in compliance with the Declaration of Helsinki. MSC preparation and administration were performed under IND# 15082 approved by the FDA. The study was registered with ClinicalTrials.gov (NCT02266394); https://clinicaltrials.gov/ct2/show/NCT02266394.
MSC Isolation, Preparation, and Safety Evaluation
Six weeks before planned administration, a subcutaneous abdominal fat biopsy sample (approximately 0.5–2 g) was obtained under sterile conditions. Enzymatic digestion of the fat was followed by selection and expansion of plastic adherent cells. Cells were cultured in Advanced MEM (Gibco Cat #12492021) supplemented with human platelet lysate (PLTMax; Mill Creek Life Sciences) over 2 weeks using good manufacturing practices as previously described until a sufficient number of cells were obtained for each treatment protocol.37 Release criteria for administration after final passage included sterility testing using anaerobic and aerobic culture, endotoxin, mycoplasma, karyotype, and FACS for surface markers characteristic of MSC.37,38
Interventions and Inpatient Study Protocol
Patients participated in this study during a 3-day inpatient protocol on two occasions (3 months apart) in the clinical research unit as previously described.4 Dietary intake was regulated at 150 mEq of sodium with an isocaloric diet prepared on site. The first study day included measurement of urinary sodium excretion and GFR by iothalamate clearance (iothalamate meglumine, Conray, Mallinckrodt) over three 30-minute timed collection periods after oral hydration (20 ml/kg).39,40 Single kidney–GFR was determined by apportioning the measured iothalamate clearance by percentage of blood flow for each kidney. BP was measured by automated oscillometric recordings including three values taken three times daily.
Renal Oxygenation Measured by BOLD MRI
On the second day, BOLD MRI examinations were performed on a (GE Twin Speed Signa EXCITE) 3.0T system (GE Medical Systems, Waukesha, WI) using a 12-channel torso phased array coil.1,41,42 The BOLD MRI is a measure of deoxyhemoglobin in the blood—because the oxygen tension (pO2) of capillary blood generally is thought to be in equilibrium with the surrounding tissue, changes estimated by BOLD MRI are interpreted as changes in tissue pO243
Cortical and Medullary Tissue Perfusion and Blood Flow Measured by MDCT
On the third study day, the common femoral vein was cannulated with a 6F sheath and blood samples drawn from the right and left renal veins with a 5F pigtail Cobra catheter (Cook, Inc., Bloomington, IN). The catheter was then advanced into the right atrium for central venous injection of contrast for flow studies using MDCT. For assessment of perfusion MDCT imaging was obtained using a dual-source 64-slice helical MDCT scanner (SOMATOM Definition, Siemens Medical Solutions) after a bolus injection of iopamidol 370 (0.5 ml/kg). Kidney volume study was performed in the helical mode to determine both cortical and medullary regional volumes.44
MSC Infusion, Laboratory Testing, and Safety Monitoring
After completing CT imaging, patients were returned to the angiography suite where standard aortic cannulation was performed through the femoral artery. Heparin (4000 U) was administered intravenously and the stenotic renal artery then selectively cannulated and imaged, after which MSCs suspended in 10 ml of Lactated Ringer’s solution were manually infused distal to stenosis over 5 minutes. Follow-up angiography was obtained once the infusion was complete, and the catheter removed. Patients remained hospitalized for observation for 24 hours after MSC administration. Blood samples were obtained the following day, day 7, and monthly until the 3-month return visit peripheral for complete blood count, CRP, urine cytology, lactate dehydrogenase, and markers of renal injury (e.g., NGAL). Renal vein blood samples for VEGF-C, angiopoietin, and inflammatory cytokine levels (including IL-6, IFN-γ, TNF-α, and MCP-1) were obtained from the STK and CLK renal vein of all patients, as previously described.4,26
Statistical Analyses
Results were expressed using mean values and SD or median values (interquartile range), as appropriate. Qualitative variables were expressed as number (percentage). Comparisons between the two groups of patients with RVD were performed using two-sample t tests with unequal variance (or the Wilcoxon rank-sum test for skewed data) for continuous variables, and a chi-squared test or Fisher exact test for categoric variables as appropriate. Comparisons between two kidneys within the same individuals and repeated measurements for specific kidneys within individuals before and after treatment were performed using paired t tests. Percent (%) change in STK-GFR was calculated as: ([(3 months STK-GFR−baseline STK-GFR)/baseline STK-GFR] × 100%). Statistical significance was accepted for P≤0.05. Statistical analysis was performed using JMP software package version 8.0 (SAS Institute Inc., Cary, NC). Complete and detailed methods are provided as Supplemental Material.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors would like to thank Beverly Tietje, for her role as study coordinator throughout the duration of this study. We also thank Darcie Radel, Greg Butler, Pranathi Madde, Kathy Allen, Kathleen Soiney, and Karin Zachau for product manufacture.
The Human Cell Therapy Lab receives support from the Department of Laboratory Medicine and Pathology, the Center for Translational Research, and the Center of Regenerative Medicine. Furthermore, we appreciate the generous philanthropic support of William and Karen Eby, as well as the charitable foundation in their names. This project was partly supported by National Institutes of Health (NIH) grants, including P01 HL85307 from the National Heart, Lung and Blood Institute (NHLBI); R01 DK100081, DK102325, K23 DK109134 and R01 DK73608 from the National Institute of Digestive, Diabetic and Kidney Diseases (NIDDK); as well as Clinical and Translational Science Award Grant UL1 RR024150 from NIH/The National Center for Research Resources. Our studies were also supported by funds from the Center of Regenerative Medicine at Mayo Clinic.
The content is solely the responsibility of the authors and does not represent the official views of the NHLBI, NIDDK, or NIH.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017020151/-/DCSupplemental.
References
- 1.Saad A, Crane J, Glockner JF, Herrmann SM, Friedman H, Ebrahimi B, Lerman LO, Textor SC: Human renovascular disease: Estimating fractional tissue hypoxia to analyze blood oxygen level-dependent MR. Radiology 268: 770–778, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO: Distinct renal injury in early atherosclerosis and renovascular disease. Circulation 106: 1165–1171, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Chade AR, Krier JD, Rodriguez-Porcel M, Breen JF, McKusick MA, Lerman A, Lerman LO: Comparison of acute and chronic antioxidant interventions in experimental renovascular disease. Am J Physiol Renal Physiol 286: F1079–F1086, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Saad A, Herrmann SM, Crane J, Glockner JF, McKusick MA, Misra S, Eirin A, Ebrahimi B, Lerman LO, Textor SC: Stent revascularization restores cortical blood flow and reverses tissue hypoxia in atherosclerotic renal artery stenosis but fails to reverse inflammatory pathways or glomerular filtration rate. Circ Cardiovasc Interv 6: 428–435, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garovic VD, Textor SC: Renovascular hypertension and ischemic nephropathy. Circulation 112: 1362–1374, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Eirin A, Ebrahimi B, Zhang X, Zhu XY, Tang H, Crane JA, Lerman A, Textor SC, Lerman LO: Changes in glomerular filtration rate after renal revascularization correlate with microvascular hemodynamics and inflammation in swine renal artery stenosis. Circ Cardiovasc Interv 5: 720–728, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eirin A, Zhu XY, Krier JD, Tang H, Jordan KL, Grande JP, Lerman A, Textor SC, Lerman LO: Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells 30: 1030–1041, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliveira-Sales EB, Boim MA: Mesenchymal stem cells and chronic renal artery stenosis. Am J Physiol Renal Physiol 310: F6–F9, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, Lipkin G, Nicholson A, Scoble J; ASTRAL Investigators : Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 361: 1953–1962, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D’Agostino RB Sr, Dworkin LD; CORAL Investigators : Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 370: 13–22, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung CM, Chrysochou C, Shurrab AE, Buckley DL, Cowie A, Kalra PA: Effects of renal volume and single-kidney glomerular filtration rate on renal functional outcome in atherosclerotic renal artery stenosis. Nephrol Dial Transplant 25: 1133–1140, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Herrmann SM, Saad A, Eirin A, Woollard J, Tang H, McKusick MA, Misra S, Glockner JF, Lerman LO, Textor SC: Differences in gfr and tissue oxygenation, and interactions between stenotic and contralateral kidneys in unilateral atherosclerotic renovascular disease. Clin J Am Soc Nephrol 11: 458–469, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura G, London GM, Safar ME, Kuramochi M, Omae T: Split intrarenal hemodynamics in renovascular hypertension. Clin Invest Med 14: 559–565, 1991 [PubMed] [Google Scholar]
- 14.Zhu XY, Chade AR, Rodriguez-Porcel M, Bentley MD, Ritman EL, Lerman A, Lerman LO: Cortical microvascular remodeling in the stenotic kidney: Role of increased oxidative stress. Arterioscler Thromb Vasc Biol 24: 1854–1859, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, Napoli C, Sawamura T, Textor SC, Lerman A, Lerman LO: Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol 23: 1295–1301, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Urbieta-Caceres VH, Lavi R, Zhu XY, Crane JA, Textor SC, Lerman A, Lerman LO: Early atherosclerosis aggravates the effect of renal artery stenosis on the swine kidney. Am J Physiol Renal Physiol 299: F135–F140, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eirin A, Zhu XY, Urbieta-Caceres VH, Grande JP, Lerman A, Textor SC, Lerman LO: Persistent kidney dysfunction in swine renal artery stenosis correlates with outer cortical microvascular remodeling. Am J Physiol Renal Physiol 300: F1394–F1401, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chade AR, Zhu XY, Krier JD, Jordan KL, Textor SC, Grande JP, Lerman A, Lerman LO: Endothelial progenitor cells homing and renal repair in experimental renovascular disease. Stem Cells 28: 1039–1047, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eirin A, Gloviczki ML, Tang H, Rule AD, Woollard JR, Lerman A, Textor SC, Lerman LO: Chronic renovascular hypertension is associated with elevated levels of neutrophil gelatinase-associated lipocalin. Nephrol Dial Transplant 27: 4153–4161, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu XY, Urbieta-Caceres V, Krier JD, Textor SC, Lerman A, Lerman LO: Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem Cells 31: 117–125, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo K, Shintani S, Shibata R, Murakami H, Murakami R, Imaizumi M, Kitagawa Y, Murohara T: Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol 29: 61–66, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Rubina K, Kalinina N, Efimenko A, Lopatina T, Melikhova V, Tsokolaeva Z, Sysoeva V, Tkachuk V, Parfyonova Y: Adipose stromal cells stimulate angiogenesis via promoting progenitor cell differentiation, secretion of angiogenic factors, and enhancing vessel maturation. Tissue Eng Part A 15: 2039–2050, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Welch WJ, Blau J, Xie H, Chabrashvili T, Wilcox CS: Angiotensin-induced defects in renal oxygenation: Role of oxidative stress. Am J Physiol Heart Circ Physiol 288: H22–H28, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Oliveira-Sales EB, Varela VA, Maquigussa E, Borges FT, Shimoura CG, Gomes G, Campos RR, Boim MA: Renovascular hypertension: Effects of mesenchymal stem cells in the contralateral hypertensive kidney in rats. Clin Exp Hypertens 38: 586–593, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Wright JR, Duggal A, Thomas R, Reeve R, Roberts IS, Kalra PA: Clinicopathological correlation in biopsy-proven atherosclerotic nephropathy: Implications for renal functional outcome in atherosclerotic renovascular disease. Nephrol Dial Transplant 16: 765–770, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Eirin A, Gloviczki ML, Tang H, Gossl M, Jordan KL, Woollard JR, Lerman A, Grande JP, Textor SC, Lerman LO: Inflammatory and injury signals released from the post-stenotic human kidney. Eur Heart J 34: 540–548a, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saad A, Herrmann SM, Textor SC: Chronic renal ischemia in humans: Can cell therapy repair the kidney in occlusive renovascular disease? Physiology (Bethesda) 30: 175–182, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka T, Nangaku M: Angiogenesis and hypoxia in the kidney. Nat Rev Nephrol 9: 211–222, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Fine LG, Norman JT: Chronic hypoxia as a mechanism of progression of chronic kidney diseases: From hypothesis to novel therapeutics. Kidney Int 74: 867–872, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Veron D, Reidy KJ, Bertuccio C, Teichman J, Villegas G, Jimenez J, Shen W, Kopp JB, Thomas DB, Tufro A: Overexpression of VEGF-A in podocytes of adult mice causes glomerular disease. Kidney Int 77: 989–999, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Norman JT, Fine LG: Intrarenal oxygenation in chronic renal failure. Clin Exp Pharmacol Physiol 33: 989–996, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Deng A, Miracle CM, Suarez JM, Lortie M, Satriano J, Thomson SC, Munger KA, Blantz RC: Oxygen consumption in the kidney: Effects of nitric oxide synthase isoforms and angiotensin II. Kidney Int 68: 723–730, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Ristimäki A, Narko K, Enholm B, Joukov V, Alitalo K: Proinflammatory cytokines regulate expression of the lymphatic endothelial mitogen vascular endothelial growth factor-C. J Biol Chem 273: 8413–8418, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Seeger H, Bonani M, Segerer S: The role of lymphatics in renal inflammation. Nephrol Dial Transplant 27: 2634–2641, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Lee AS, Lee JE, Jung YJ, Kim DH, Kang KP, Lee S, Park SK, Lee SY, Kang MJ, Moon WS, Kim HJ, Jeong YB, Sung MJ, Kim W: Vascular endothelial growth factor-C and -D are involved in lymphangiogenesis in mouse unilateral ureteral obstruction. Kidney Int 83: 50–62, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Karpanen T, Alitalo K: Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol 3: 367–397, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Crespo-Diaz R, Behfar A, Butler GW, Padley DJ, Sarr MG, Bartunek J, Dietz AB, Terzic A: Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant 20: 797–811, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E: Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 8: 315–317, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Textor SC, Turner ST: Renal vascular response to sodium loading in sons of hypertensive parents. Hypertension 17: 982–988, 1991 [DOI] [PubMed] [Google Scholar]
- 40.Wilson DM, Bergert JH, Larson TS, Liedtke RR: GFR determined by nonradiolabeled iothalamate using capillary electrophoresis. Am J Kidney Dis 30: 646–652, 1997 [DOI] [PubMed] [Google Scholar]
- 41.Gloviczki ML, Glockner J, Gomez SI, Romero JC, Lerman LO, McKusick M, Textor SC: Comparison of 1.5 and 3 T BOLD MR to study oxygenation of kidney cortex and medulla in human renovascular disease. Invest Radiol 44: 566–571, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Textor SC, Glockner JF, Lerman LO, Misra S, McKusick MA, Riederer SJ, Grande JP, Gomez SI, Romero JC: The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol 19: 780–788, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prasad PV, Edelman RR, Epstein FH: Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation 94: 3271–3275, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Daghini E, Primak AN, Chade AR, Krier JD, Zhu XY, Ritman EL, McCollough CH, Lerman LO: Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: Comparison with electron-beam CT. Radiology 243: 405–412, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.