Abstract
IMPORTANCE
The Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) Trial showed that aggressive medical therapy was more effective than stenting for preventing stroke in patients with symptomatic intracranial stenosis. However, 15% of patients in the medical group still experienced a primary end point during a median follow-up of 32.7 months.
OBJECTIVE
To determine baseline features that were associated with a high rate of a primary end point in the medical arm of the SAMMPRIS Trial.
DESIGN, SETTING, AND PARTICIPANTS
A post hoc analysis of patients in the medical arm only of the SAMMPRIS trial. Enrollment occurred between October 2008 and April 2013 and included 227 patients randomized to medical management alone. Baseline demographic features, vascular risk factors, qualifying event, brain imaging, and angiographic features were analyzed. Bivariate and multivariable proportional hazard regression modeling was performed to relate baseline features to the time until a primary end point. The post hoc analysis was conducted from November 2014 to June 2015.
INTERVENTIONS
The SAMMPRIS Trial compared stenting with aggressive medical management in patients with a stroke or transient ischemic attack attributed to 70% to 99% stenosis of a major intracranial artery.
MAIN OUTCOMES AND MEASURES
The primary outcome was any of the following: stroke or death within 30 days of enrollment, ischemic stroke in the territory of the symptomatic intracranial artery beyond 30 days after enrollment, or any stroke or death within 30 days after stenting a patient in the medical group during follow-up
RESULTS
A total of 227 patients were included in the study, 82 of whom were female, and the mean (SD) age was 59.5 (11.8) years. Being female (hazard ratio [HR], 1.9; 95% CI, 0.96-3.7), having diabetes mellitus (HR, 1.8; 95% CI, 0.9-3.5), not taking a statin at enrollment (HR, 2.6; 95% CI, 1.2-5.7), stroke as the qualifying event (HR, 2.5; 95% CI, 1.03-6.0), Rankin grade of 1 or greater (HR, 2.3; 95% CI, 0.9-5.5), old infarct in the territory of the stenotic artery (HR, 2.6; 95% CI, 1.3-5.1), and greater than 80% stenosis (HR, 1.9; 95% CI, 0.9-3.7) were associated (P < .10) with higher risk on bivariate analysis. Factors that were significantly associated with a primary end point on multivariable analyses were old infarct in the territory (HR, 2.6; 95% CI, 1.3-5.3; P = .006), stroke as the qualifying event (HR, 3.0; 95% CI, 1.1-7.7; P = .03), and no statin use at enrollment (HR, 2.4; 95% CI, 1.1-5.2; P = .03).
CONCLUSIONS AND RELEVANCE
Old infarct in the territory of the stenosis, new stroke presentation, and absence of statin use at enrollment were independently associated with high rates of the primary end point in the medical group in the SAMMPRIS Trial. These features may be useful for selecting high-risk patients for future clinical trials evaluating alternative therapies for intracranial stenosis.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00576693
Intracranial atherosclerotic stenosis is a common cause of stroke worldwide that is associated with a particularly high risk for recurrent stroke, especially in patients with severe (70%-99%) arterial stenosis.1-7 The Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) Trial was performed to determine whether stenting in combination with aggressive medical management was superior to aggressive medical management alone for preventing the primary end point, which largely consisted of stroke in the territory of the stenotic artery.8-11 The final results of the SAMMPRIS Trial showed that aggressive medical management was of greater benefit in lowering the risk for the primary end point compared with stenting at 30 days, 1 year, 2 years, and 3 years of follow-up.10,11
Efforts to improve medical and endovascular treatments for intracranial stenosis depend in part on identifying risk factors associated with a high risk for recurrent stroke with each treatment. A previous article by our group studied baseline features associated with a high risk for periprocedural stroke, which constituted most of the primary end points in the stenting arm of the SAMMPRIS Trial.12 We now report the results of an analysis to identify baseline features associated with a high risk for the primary end point in the medical group in the SAMMPRIS Trial.
Methods
Study Design and Patient Eligibility
Details of the SAMMPRIS Trial design and results have been published previously.8-11 Briefly, the SAMMPRIS Trial was a randomized, superiority, multicenter clinical trial funded by the National Institute of Neurological Disorders and Stroke (NINDS). The US Food and Drug Administration issued an Investigational Device Exemption (G050157) to perform the study with the Wingspan stent system (Stryker Neurovascular; formerly Boston Scientific Neuro-vascular),13 which had been approved under a Humanitarian Device Exemption in 2005 for patients with 50% to 99% intracranial stenosis refractory to antithrombotic treatment. Eligible patients were 30 to 80 years old and had nondisabling stroke or transient ischemic attack (TIA) within 30 days prior to enrollment attributable to angio-graphically verified 70% to 99% atherosclerotic stenosis of a major intracranial artery.14 All patients provided written informed consent to participate. Institutional review boards at all 50 participating sites in the United States approved the study protocol. Approval for the coordination of the SAMMPRIS Trial, which included this post hoc analysis (conducted from November 2014 to June 2015), was provided by the institutional review board at the Medical University of South Carolina.
Aggressive Medical Management Treatment
The details and rationale of aggressive medical management have been published.9 Briefly, medical treatment consisted of aspirin (325 mg daily) for the duration of follow-up and clopidogrel (75 mg daily) for 90 days after enrollment and risk-factor management, primarily targeting systolic blood pressure less than 140 mm Hg (<130 mm Hg, if diabetic) and low-density lipoprotein cholesterol level of less than 70 mg/dL (to convert to millimoles per liter, multiply by 0.0259). Additionally, diabetes mellitus and lifestyle behaviors, including smoking cessation, ideal body mass index, and exercise, were managed by the local study team working with a lifestyle modification program15 and the primary care physician.
Duration of Follow-up and Assessment of End Points
Patients were assessed at study enrollment, 4 days, 30 days, and subsequently every 4 months until a primary end point occurred or a final closeout visit was done, which occurred during February 2013 to April 2013, 2 years after the last patient was enrolled. Patients with a suspected stroke typically underwent brain magnetic resonance imaging or computed tomography and were assessed by the primary site neurologist. A second site neurologist, blinded to treatment, assessed patients with minor events that were more difficult to classify and might have been subject to observer bias by the unblinded neurologist (ie, prolonged TIA or minor stroke associated with an increase in the National Institutes of Health Stroke Scale score of ≤3). Both neurologists’ assessments were sent for central adjudication. The primary end point was any of the following: any stroke or death within 30 days after enrollment, ischemic stroke in the territory of the qualifying artery beyond 30 days of enrollment, or any stroke or death within 30 days after stenting a patient in the medical group. We defined ischemic stroke as a new focal neurological deficit of sudden onset, lasting for at least 24 hours, that was not associated with a hemorrhage on brain computed tomography or magnetic resonance imaging. Strokes were categorized as either in or out of the territory of the qualifying stenotic artery.
Statistical Methods
For the patients randomized to medical therapy only, the association between the time to the primary end point and selected baseline characteristics, including demographic features, vascular risk factors, qualifying event, brain imaging, and angiographic features, was assessed using proportional hazards regression in both bivariate and multivariable analyses. Candidate factors for the multivariable analysis were selected based on P ≤ .20 in the bivariate analyses or based on factors identified in the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Trial.16 In the multivariable analyses, significant factors related to the primary end point were identified from among the candidate factors using backward elimination with P < .05 set for retention in the model. A Kaplan-Meier plot was constructed for each of the significant factors showing the cumulative probability of the primary end point vs time since randomization according to the levels of the factor. Patients who either withdrew from the study or were lost to follow-up before experiencing a primary end point were censored at the last study contact. Two-sided P < .05 values were considered statistically significant. All analyses were done using SAS version 9.3 (SAS Institute).
Results
There were 227 patients (mean [SD] age, 59.5 [11.8] years) randomized to aggressive medical management alone. The baseline characteristics for this treatment group as a whole have been published previously.11 The median duration of follow-up was 32.7 months (interquartile range, 24.2-40.5; range, 0.03-52.6). There were 24 patients (11%) who either withdrew (13 patients) or were lost to follow-up (11 patients). All 11 lost patients and 7 of 13 withdrawn patients were followed up for at least 1 year. Of the 227 patients in the medical group, 34 (15%) had a primary end point during follow-up, of which 31 (91%) were ischemic strokes in the territory of the stenotic artery, 2 were ischemic strokes in another territory within 30 days of enrollment, and 1 was a nonstroke death within 30 days of enrollment.11
Bivariate Analyses
The results of the bivariate analyses are shown in Table 1 and Table 2. These analyses identified the following factors that were associated (P < .10) with a higher risk for a primary end point: female, diabetes, no statin use at enrollment, stroke as the qualifying event, Rankin grade of 1 or greater, old infarct in the territory of the stenotic artery, and greater percentage stenosis. Only 3 of these factors were significantly associated (P < .05) with an increased risk for the primary end point: old infarct in the territory of the stenotic artery on baseline imaging (hazard ratio [HR], 2.6; 95% CI, 1.3-5.1; P = .007), absence of statin use at enrollment (HR, 2.6; 95% CI, 1.2-5.7; P = .01), and stroke rather than TIA as the qualifying event (HR, 2.5; 95% CI, 1.03-6.0; P = .04). Only 6 of 75 patients (8.0%) with TIA as the qualifying event had a primary end point, whereas 28 of 152 patients (18.4%) whose qualifying event was a stroke had a primary end point during follow-up (Table 1).
Table 1.
Primary End Point in the SAMMPRIS Trial Medical Group vs Categorical Baseline Factors
| Factor | No. | Primary End Point, No. (%) | Probability of the Primary End Point, % (95% CI)a | Hazard Ratio (95% CI)b | P Valueb | ||
|---|---|---|---|---|---|---|---|
| By 1 y | By 2 y | ||||||
| Age, y | |||||||
| <60 | 119 | 16 (13.5) | 11.0 (6.5-18.2) | 11.9 (7.2-19.2) | 1.4 (0.7-2.7) | .37 | |
| ≥60 | 108 | 18 (16.7) | 14.4 (8.9-22.7) | 16.5 (10.6-25.2) | |||
| Sex | |||||||
| Male | 145 | 17 (11.7) | 9.2 (5.4-15.3) | 10.7 (6.6-17.1) | 1.9 (0.96-3.7) | .06 | |
| Female | 82 | 17 (20.7) | 18.8 (11.8-29.3) | 20.1 (12.8-30.8) | |||
| Race/ethnicity | |||||||
| White | 162 | 23 (14.2) | 12.6 (8.3-18.9) | 13.3 (8.8-19.6) | 1.2 (0.6-2.5) | .61 | |
| Nonwhite | 65 | 11 (16.9) | 12.8 (6.6-23.9) | 16.2 (9.0-28.0) | |||
| Diabetes mellitusc | |||||||
| No | 124 | 14 (11.3) | 10.6 (6.3-17.6) | 10.6 (6.3-17.6) | 1.8 (0.9-3.5) | .10 | |
| Yes | 103 | 20 (19.4) | 15.1 (9.4-23.8) | 18.3 (11.9-27.4) | |||
| Hypertension | |||||||
| No | 24 | 5 (20.8) | 17.4 (6.9-39.9) | 17.4 (6.9-39.9) | 0.7 (0.3-1.8) | .43 | |
| Yes | 203 | 29 (14.3) | 12.1 (8.3-17.5) | 13.7 (9.6-19.3) | |||
| Absence of statin use at enrollment | |||||||
| No | 196 | 25 (12.8) | 10.4 (6.8-15.7) | 12.0 (8.2-17.6) | 2.6 (1.2-5.7) | .01 | |
| Yes | 31 | 9 (29.0) | 27.1 (14.6-47.1) | 27.1 (14.6-47.1) | |||
| BMI | |||||||
| <29 | 106 | 13 (12.3) | 10.7 (6.1-18.6) | 10.7 (6.1-18.6) | 1.4 (0.7-2.7) | .37 | |
| ≥29 | 121 | 21 (17.4) | 14.2 (9.1-21.9) | 16.8 (11.2-24.8) | |||
| Smoking | |||||||
| Smoker, current or former | 149 | 24 (16.1) | 13.2 (8.6-19.9) | 14.7 (9.8-21.6) | 0.8 (0.4-1.7) | .54 | |
| Nonsmoker | 78 | 10 (12.8) | 11.7 (6.2-21.2) | 13.0 (7.2-22.8) | |||
| Physical activity | |||||||
| In target | 66 | 6 (9.1) | 7.8 (3.3-17.7) | 7.8 (3.3-17.7) | 2.0 (0.8-4.9) | .11 | |
| Out of target | 161 | 28 (17.4) | 14.6 (10.0-21.2) | 16.6 (11.6-23.5) | |||
| Systolic BP, mm Hg, median | |||||||
| <144 | 107 | 20 (18.7) | 15.1 (9.6-23.5) | 17.1 (11.1-25.7) | 0.6 (0.3-1.2) | .18 | |
| ≥144 | 120 | 14 (11.7) | 10.3 (6.0-17.5) | 11.2 (6.7-18.6) | |||
| LDL cholesterol, core, median, mg/dL | |||||||
| <90 | 107 | 15 (14.0) | 14.2 (8.8-22.4) | 14.2 (8.8-22.4) | 1.1 (0.6-2.2) | .72 | |
| ≥90 | 119 | 19 (16.0) | 11.3 (6.7-18.6) | 14.0 (8.8-21.8) | |||
| HDL cholesterol, core, median, mg/dL | |||||||
| <36.3 | 104 | 14 (13.5) | 9.7 (5.3-17.2) | 11.7 (6.8-19.7) | 1.3 (0.6-2.5) | .50 | |
| ≥36.3 | 122 | 20 (16.4) | 15.3 (9.9-23.1) | 16.2 (10.6-24.2) | |||
| Glucose level, mg/dL | |||||||
| <100 | 77 | 9 (11.7) | 7.9 (3.6-16.8) | 10.6 (5.5-20.2) | [Reference] | .27 | |
| 100-200 | 129 | 20 (15.5) | 15.1 (9.9-22.6) | 15.1 (9.9-22.6) | 1.4 (0.7-3.2) | ||
| >200 | 18 | 5 (27.8) | 16.7 (5.7-43.2) | 22.2 (9.0-48.9) | 2.5 (0.8-7.3) | ||
| QE | |||||||
| TIA | 75 | 6 (8.0) | 5.6 (2.1-14.1) | 7.0 (3.0-15.9) | 2.5 (1.03-6.0) | .04 | |
| Stroke | 152 | 28 (18.4) | 16.1 (11.1-23.1) | 17.5 (12.3-24.7) | |||
| QE to enrollment, median, d | |||||||
| ≤7 | 115 | 17 (14.8) | 13.4 (8.3-21.2) | 13.4 (8.3-21.2) | 0.98 (0.5-1.9) | .96 | |
| >7 | 112 | 17 (15.2) | 11.9 (7.1-19.6) | 14.7 (9.3-22.9) | |||
| Antithrombotic at QE | |||||||
| No | 87 | 13 (14.9) | 11.6 (6.4-20.6) | 11.6 (6.4-20.6) | 0.98 (0.5-2.0) | .96 | |
| Yes | 140 | 21 (15.0) | 13.3 (8.6-20.2) | 15.6 (10.5-22.9) | |||
| Old infarct in the territory | |||||||
| No | 147 | 15 (10.2) | 7.6 (4.3-13.4) | 9.1 (5.4-15.2) | 2.6 (1.3-5.1) | .007 | |
| Yes | 75 | 18 (24.0) | 21.7 (13.9-33.0) | 23.1 (15.0-34.5) | |||
| Rankin grade | |||||||
| <1 | 68 | 6 (8.8) | 7.4 (3.2-17.0) | 7.4 (3.2-17.0) | 2.3 (0.9-5.5) | .07 | |
| ≥1 | 159 | 28 (17.6) | 14.9 (10.2-21.6) | 17.0 (11.9-23.9) | |||
| NIH scale score | |||||||
| ≤1 | 139 | 19 (13.7) | 11.7 (7.3-18.4) | 12.4 (7.9-19.2) | 1.4 (0.7-2.7) | .38 | |
| <1 | 88 | 15 (17.1) | 14.2 (8.3-23.6) | 16.7 (10.2-26.6) | |||
| % Stenosis, central reader | |||||||
| <80 | 154 | 19 (12.3) | 10.6 (6.6-16.7) | 12.0 (7.7-18.3) | 1.9 (0.9-3.7) | .07 | |
| ≥80 | 71 | 15 (21.1) | 17.7 (10.4-29.1) | 19.2 (11.6-30.7) | |||
| Symptomatic artery | |||||||
| Basilar | 51 | 6 (11.8) | 7.9 (3.0-19.7) | 9.9 (4.3-22.2) | [Reference] | .23 | |
| Vertebral | 22 | 3 (13.6) | 9.5 (2.5-33.0) | 9.5 (2.5-33.0) | 1.2 (0.3-4.6) | ||
| MCA | 105 | 13 (12.4) | 11.8 (6.9-19.8) | 12.8 (7.6-21.1) | 1.1 (0.4-2.8) | ||
| ICA | 49 | 12 (24.5) | 21.0 (11.9-35.5) | 23.2 (13.6-38.0) | 2.2 (0.8-5.9) | ||
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; HDL, high-density lipoprotein; ICA, internal carotid artery; LDL, low-density lipoprotein; MCA, middle cerebral artery; NIH, National Institutes of Health; QE, qualifying event; TIA, transient ischemic attack.
SI conversion factors: To convert cholesterol to millimoles per liter, multiply by 0.0259; glucose to millimoles per liter, multiply by 0.0555.
Kaplan-Meier estimates of the cumulative probability of a primary end point by 1 and 2 years after enrollment
Hazard ratio and P value from a Cox proportional hazard regression model relating time until a primary end point to the indicated factor with that factor as the only variable in the model. The hazard ratio is calculated for the second category relative to the first category or to the indicated reference category.
Considered diabetic at baseline if there was a history of diabetes or the baseline hemoglobin A1c level of 6.5 mmol/mol or greater.
Table 2.
Primary End Point in the SAMMPRIS Trial Medical Group vs Continuous Factors
| Factor | Hazard Ratio (95% CI)a | P Valuea |
|---|---|---|
| Age, y | 1.1 (0.8-1.4) | .59 |
| BMI | 1.1 (0.7-1.8) | .73 |
| Systolic BP, mm Hg | 0.9 (0.8-1.1) | .39 |
| LDL cholesterol, mg/dL | 1.0 (0.9-1.1) | .93 |
| HDL cholesterol, mg/dL | 1.1 (0.8-1.6) | .39 |
| Glucose, mg/dL | 1.03 (0.98-1.08) | .24 |
| Time from QE to enrollment, d | 0.9 (0.6-1.3) | .20 |
| % Stenosis | 1.5 (0.98-2.2) | .06 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; QE, qualifying event.
SI conversion factors: To convert cholesterol tomillimoles per liter, multiply by 0.0259; glucose to millimoles per liter, multiply by 0.0555.
Hazard ratio and P value from a Cox proportional hazard regression model relating time until a primary end point to the indicated factor with that factor as the only variable in the model. The hazard ratio is based on a 10-unit increase in the factor.
Multivariable Analyses
The following factors with bivariate P ≤ .15 for the association with the primary end point were included as candidate factors for the multivariable analysis: qualifying event, old infarct in the territory, absence of statin use at enrollment, sex, diabetes, physical activity, Rankin grade, percentage stenosis, and time from qualifying event to enrollment. Age and race/ethnicity were also included based on outcome results from the WASID Trial, as was symptomatic artery given its clinical relevance. Because there were 7 patients missing data for 1 or more of these factors, the multivariable analysis included 220 medically treated patients. The statistically significant factors identified for the final model using backward elimination with effect size provided by HRs were: old infarct in the territory (patients with old infarct at higher risk: HR, 2.6; 95% CI, 1.3-5.3; P = .006), qualifying event (stroke patients at higher risk: HR, 3.0; 95% CI, 1.1-7.7; P = .03), and absence of statin use at enrollment (HR, 2.4; 95% CI, 1.1-5.2; P = .03) (Table 3) (Figure). Among the 48 patients with stroke as the qualifying event and an old infarct in the territory (21% of whom were not taking a statin), their 1-year and 2-year rates of a primary end point were 32.1% (95% CI, 20.7%-47.5%) and 34.3% (95% CI, 22.6%-49.8%), respectively.
Table 3.
Baseline Factors Related to the Primary End Point in the SAMMPRIS Trial Medical Group in Multivariable Analysis
| Factor | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Old infarct in the territory, yes vs no | 2.6 (1.3-5.3) | .006 |
| Absence of statin use at enrollment, yes vs no | 2.4 (1.1-5.2) | .03 |
| Qualifying event, stroke vs transient ischemic attack | 3.0 (1.1-7.7) | .03 |
Figure.
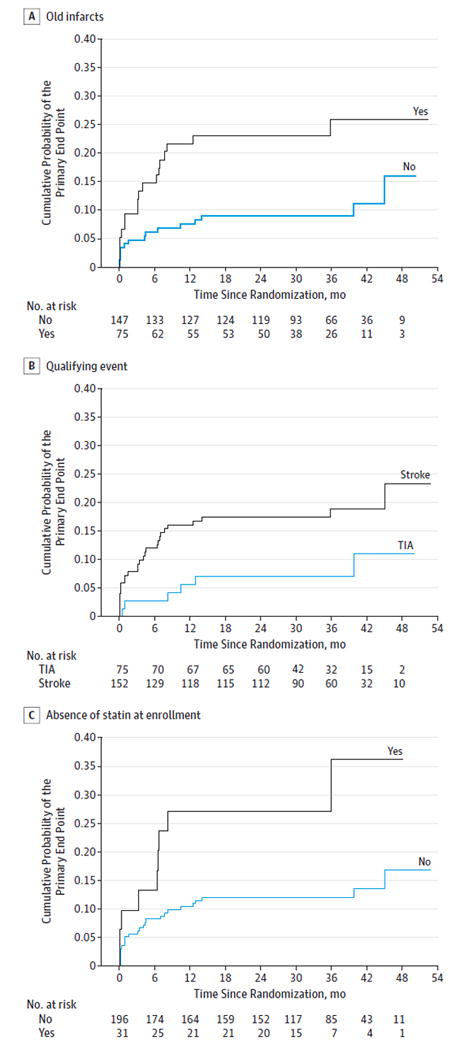
Cumulative Probability of Primary End Point vs Time Since Randomization
For the variables not included in the final model, Table 4 shows the P values for each factor when that factor is added to a model that included old infarct in the territory, absence of statin use at enrollment, and qualifying event. Two of the factors (percentage stenosis and diabetes) had P values between .05 and ≥.10.
Table 4.
Nonsignificant Baseline Factors in the SAMMPRIS Trial Medical Group in Multivariable Analysis
| Factor | P Valuea |
|---|---|
| % Stenosis, continuous | .07 |
| Diabetes | .10 |
| Symptomatic artery | .11 |
| Physical activity, pace score in/out of target | .15 |
| Rankin score, <1 vs ≥1 | .21 |
| Time from qualifying event to enrollment | .44 |
| Sex | .46 |
| Age, continuous | .46 |
| Race/ethnicity | .81 |
P value for adding each factor separately to a model containing old infarcts in the territory, statin use at enrollment, and qualifying event.
Discussion
Although the risk for a primary end point in the medical arm of the SAMMPRIS Trial was half of the predicted rate,8-11 this analysis shows that there are still subgroups of patients at high risk for stroke despite aggressive medical therapy. The high-risk features are the presence of an old infarct in the territory of the stenotic artery, stroke as the qualifying event, and absence of statin use at enrollment. The highest-risk patients are those who present with a new ischemic stroke and have an old infarct in the territory on brain imaging. The patients with 2 strokes in the territory before enrollment probably had unstable stenotic plaques or impaired collateral blood flow or both that put them at high risk for a third stroke in the territory.
The association between the absence of statin use at enrollment and high risk for recurrent stroke is somewhat surprising given that virtually all patients in the SAMMPRIS Trial were prescribed statins at enrollment. One possible explanation for this finding is that the use of statins before enrollment may have led to earlier stabilization of the symptomatic atherosclerotic plaque in these patients, which could have lowered their early and longer-term risk for stroke.
Two other baseline features were associated with P values between .05 and ≤.10 when combined individually with old infarcts in the territory, statin use at enrollment, and stroke as the qualifying event in the multivariable analysis: percentage stenosis and diabetes. In the WASID Trial in which patients with 50% to 99% stenosis were enrolled, severe stenosis was most significantly associated with a poor outcome in medically treated patients.7 Although the association between the severity of stenosis and outcome in the WASID Trial primarily compared patients with 70% to 99% stenosis vs less than 70% stenosis, the WASID Trial showed that the 2-year rates of stroke were 31% in patients with 80% to 89% stenosis compared with 18% in patients with 70% to 79% stenosis.7 In the SAMMPRIS Trial, only patients with 70% to 99% stenosis were enrolled and the 2-year rates of a primary endpoint were 19.2% in patients with greater than 80% stenosis compared with 12.0% in patients with 70% to 79% stenosis (Table 1).
Both the bivariate and multivariable analyses showed P values for the association between diabetes and a primary end point in the range of .05 to .10. For medically managed patients, the 2-year rate of the primary end point in the SAMMPRIS Trial was 18.3% for patients with diabetes compared with 10.6% for those without diabetes (Table 1). In the WASID Trial, diabetes was significantly associated with an increased risk for stroke in the territory on bivariate analysis but was not significant on multivariable analysis in the WASID Trial.7 Because both the SAMMPRIS and WASID trials had limited numbers of patients compared with most other stroke prevention trials, it is possible that there was insufficient statistical power in either the SAMMPRIS or WASID trial to establish diabetes as a statistically significant risk factor for stroke in medically treated patients.
The location of stenosis that was associated with the highest primary end point rate in the medical group in the SAMMPRIS Trial was the intracranial internal carotid artery (2-year rate, 23.2%; Table 1). Of note, the vertebral (2-year rate, 9.5%) and basilar (2-year rate, 9.9%) arteries were associated with the lowest primary end point rate. However, the location of stenosis was not significantly associated with outcome on bivariate analysis in the medical arm and the P value on multivariable analysis for location was .11. The WASID Trial also did not show an association between the location of stenosis and outcome.7
In the SAMMPRIS Trial, the 2-year rate for the primary end point in the medical group was 20.1% for women and 10.7% for men (HR, 1.9; P = .06) (Table 1). The association between sex and increased risk for a primary end point was weaker on the multivariable analysis, but it is of interest that female sex was associated with an increased risk for stroke in the territory on bivariate analysis (P = .047) and multivariable analysis (P = .051) in the WASID Trial.7
The results of this study and a previous study by our group that evaluated risk factors for periprocedural stroke in the stenting arm of the SAMMPRIS Trial12 showed that the high-risk features for recurrent ischemic stroke in both treatment arms have minimal overlap. The high risk features for periprocedural ischemic stroke in the stenting arm on multivariable analyses were older age, nonsmoking status, diabetes, and basilar artery stenosis.12 Old infarct in the territory of the stenotic artery and nonperforator territory stroke as the qualifying event were significantly associated with periprocedural ischemic stroke in the SAMMPRIS Trial on bivariate analysis but not on multivariable analysis.12 Despite the discordance between risk factors for recurrent stroke in both treatment groups, it is important to note that subgroup analyses in the SAMMPRIS Trial failed to show that stenting was superior to medical therapy alone in any subgroup in the SAMMPRIS Trial.17
This analysis also identified a few subgroups at particularly low risk for a primary end point while undergoing aggressive medical treatment. In particular, patients presenting with TIA alone had 1- and 2-year rates of a primary end point of only 5.6% and 7.0%, respectively (Table 1). Patients who were already in target for exercise at study entry also had low rates of the primary end point at 1 and 2 years (7.8% at both points; Table 1). These results supplement the risk factor analyses from the SAMMPRIS Trial showing that exercise during follow-up was the most important determinant of a good outcome in the medical arm.18
This study had some limitations, including the post hoc nature, multiple comparisons, and the limited sample size, which was a result of enrollment being stopped early because of the clear superiority of medical treatment. Thus, it is possible that both type I and type II errors were made. Nevertheless, the consistency between many of the predictors of higher risk among SAMMPRIS and WASID patients strongly supports the validity of our findings, despite the improvements in medical management since WASID was conducted.
Conclusions
We have identified that old infarct in the territory of a stenotic artery, stroke presentation, and absence of statin use at enrollment were associated with a high risk for recurrent stroke with aggressive medical therapy in patients with recently symptomatic 70% to 99% intracranial arterial stenosis. Other possible high-risk features include the severity of stenosis and diabetes. Although the primary results of the SAMMPRIS Trial showed aggressive medical therapy is superior to stenting, the risk for the primary end point was still high (15% during a median follow-up of 32.7 months) in the medical group. This implies that there is still an urgent need to develop better treatments for this disease, especially in high-risk subgroups that fared particularly poorly with medical therapy alone. The features identified in this analysis will be useful for choosing eligibility criteria for future trials focused on novel therapies for improving the outcome of high-risk patients with intracranial stenosis.
Key Points.
Question
In patients with recent transient ischemic attack or stroke attributed to 70% to 99% intracranial arterial stenosis, which patient characteristics are associated with a higher risk for recurrent stroke despite aggressive medical management?
Findings
In the medical arm of the SAMMPRIS Trial (that showed aggressive medical management alone was superior to stenting), the statistically significant baseline characteristics in multivariable analyses were old infarct in the territory of the stenotic artery on brain imaging, stroke as the qualifying event for the trial, and no statin use at enrollment.
Meaning
Although the SAMMPRIS Trial showed aggressive medical management is superior to stenting, there are subgroups at higher risk for recurrent stroke despite aggressive medical management for whom it is urgent to develop additional therapies.
Acknowledgments
Funding/Support: This study was funded by a research grant (U01 NS058728) from the US Public Health Service National Institute of Neurological Disorders and Stroke (NINDS). In addition, the following Clinical and Translational Science Awards, funded by the National Institutes of Health (NIH), provided local support for the evaluation of patients in the trial: Medical University of South Carolina (UL1RR029882), University of Florida (UL1RR029889), University of Cincinnati (UL1RR029890), and University of California, San Francisco (UL1RR024131). Stryker Neurovascular (formerly Boston Scientific Neurovascular) provided study devices and supplemental funding for third-party device distribution, site monitoring, and study auditing. This research was also supported by the Investigator-Sponsored Study Program of AstraZeneca, which donated rosuvastatin (Crestor) to study patients. INTERVENT provided the lifestyle modification program to the study at a discounted rate. Walgreens pharmacies provided study medications, except rosuvastatin, to patients at a discounted price (paid for by the study). The PACE self-assessment forms for physical activity and smoking cessation were provided by the San Diego Center for Health Interventions LLC. DrsWaters, Turan, and Chimowitz and Mr Lynn reported receiving grant funding from the NIH/NINDS. Dr Turan also received a K23 grant from NIH/NINDS unrelated to this project.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Group Information: The SAMMPRIS Trial members include the following (in order of the number of participants enrolled): Oregon Health & Science University: S. Barnwell, H. Lutsep, B. Petersen, G. Nesbit, W. Clark, J. Fields, D. Larsen, S. Enochs, K. Feest, B. Dugan, and S. Jamieson. University of Florida: B. Hoh, M. Waters, B. Miller, A. Khanna, T. Sheehan, L. Carlton, R. Boyette, and N. Davis. State University of New York at Buffalo: E. Levy, A. Siddiqui, M. Hourihane, R. Sawyer, C. Ionita, M. Donovan, A. Crumlish, M. Hartney, J. McAdoo, and D. Holler. University of Alabama: M. Harrigan, A. Alexandrov, J. Rothrock, I. Lopez, D. Patterson, L. Cava, T. Taylor, L. Nelson, B. Casey, K. Albright, and H. Walker. Houston Methodist Hospital: D. Chiu, R. Klucznik, J. Volpi, O. Diaz, J. Ling, D. McCane, and L. Katz. Barrow Neurological Institute: C. McDougall, F. Albuquerque, D. Fiorella, J. Clark, J. Frey, G. Moguel-Cobos, S. Ladha, B. Kasel, J. Cacciola, S. Porter, C. Kelly, N. Honea, and H. Jahnke. The University of Texas Southwestern Medical Center at Dallas: L. Pride, B. Welch, M. Johnson, J. Lee, R. Novakovic, C. Hall, and K. Johnson. Medical College of Wisconsin: O. Zaidat, M. Torbey, D. Book, J. Lynch, B. Fitzsimmons, A. Helms, C. Pierzchalski, J. Delap, C. Miller, E. Brandenburg, E. Das, and A. Farrow-Schmidt. Riverside Methodist Hospital, Columbus: R. Budzik, P. Pema, G. Eubank, K. Johnson, N. Sample, M. Taylor, W. Metz, L. Allison, T. Campbell, and C. Crabtree. Wayne State University: S. Chaturvedi, A. Xavier, K. Rajamani, P. Bhattacharya, R. Madhaven, F. Mada, K. Sawaya, L. Salowich-Palm, D. Murray, and D. Vaphiadis. Medical University of South Carolina: A. Turk, M. Chimowitz, T. Turan, I. Chaudry, R. Turner, R. Adams, N. Papamitsakis, A.Walker, G. Starr, E. Debenham, J. Garry, J. Peterson, and S. Helwig. MultiCare/Tacoma General Hospital: B. Kott, J. Huddlestone, A. Nohara, L. Lynam, Y. Zhu, M. Barnhart, C. Holsey, M. Wall-Tweten, S. Warner, and D. Sherif. Moses Cone Health System: S. Deveshwar, P. Sethi, C. Dohmeier, Y. Yan, M. Reynolds, K. Willis, C. Weymann, D. Champey, W. Hickling,W. Harbison, D. Parker, A. Howard, S. Hammonds, A. Overton, T. Goodpasture, P. Henderson, C. Martin, and M. Johnson. State University of New York at Stony Brook: H. Woo, D. Fiorella, C. Perkins, L. Donarummo, M. Guido, L. Krupp, P. Coyle, O. Gerber, S. Fiore, D. Madigan, M. Baumeister, V. Geronimo, and D. McHugh. Forsyth Medical Center: D. Heck, C. Chase, A. Runheim, H. Kraft, J. Dodds, D. Greene-Chandos, D. Norwood, Y. Whitley, T. Browning, and M. Burdette. West Virginia University: A. Rai, C. Brooks, C. Nance, L. Gutmann, P. Altemus, J. Domico, M. Power, T. Weimer, and R. Whitescarver. Cleveland Clinic: P. Rasmussen, T. Masaryk, R. Gupta, I. Katzan, K. Uchino, J. Gebel, M. Hussain, M. Lu, C. Bae, J. Provencio, D. Andrews-Hinders, T. Wheeler, L. Strozniak, N. Tighe, A. Richmond, G. Toth, and J. Turczyk. Henry Ford Hospital: P. Mitsias, M. Kole, A. Katramados, A. Russman, B. Silver, N. Sripathi, and J. Jones. Florida Hospital, Orlando: F. Hellinger, D. Honeycutt, A. Isa, L. Bilanovic, B. Brutsch, K. Beaudry, C. Caplan, C. Basignani, and K. Donaldson. University of Pittsburgh: T. Jovin, V. Reddy, L. Weschler, B. Jankowitz, S. Zaidi, M. Horowitz, R. Lin, M. Hammer, M. Jumaa, S. DeCesare, D. Crowley-Lisowski, A. Adams, J. Oakley, and J. Billigen. St Luke’s Episcopal Hospital, Houston, Texas: M. Mawad, H. Morsi, E. Bershad, J. Suarez, G. Lopez, S. Moore, M. Pierce, and G. DeFreitas. Columbia University Medical Center: M. Elkind, P. Meyers, J. Willey, J. Roberts, T. Corporan, and R. Aragon Garcia. University of Mississippi Medical Center: R. Buciuc, H. Uschmann, A. Auchus, R. Leacock, E. Brumfield, M. Bankston, and D. Gordy. Emory University: F. Tong, F. Nahab, J. Dion, C. Cawley, M. Frankel, R. Gupta, R. Nogueira, A. Webb, L. Ayala, K. Hanson, and E. Holland. University of Miami: J. Romano, D. Yavagal, S. Koch, M. Aziz-Sultan, M. Katsnelson, I. Campo-Bustillo, and M. Lichtenberger. Weill Cornell Medical College: Y. Gobin, D. Leifer, H. Riina, M. Fink, D. Jamieson, A. Segal, H. White, J. Caronna, B. Nikolov, K. Salvaggio, M. Velcheva, and M. Ogg. Rush University Medical Center: J. Conners, D. Lopes, S. Prabhakaran, V. Lee, M. Shanks, M. Catalano, G. Griffin, T. Cole, and C. Woods. University of Pennsylvania: R. Hurst, S. Kasner, J. Weigele, B. Cucchiara, S. Messe, N. Shafi, M. Mullen, D. Rose, I. Rybinnik, L. Sansing, M. McGarvey, E. Augelli, M. DeSanto, N. Gallatti, L. Ventura, and J. Luciano. University of Cincinnati: T. Abruzzo, A. Ringer, P. Khatri, D. Kanter,M. Flaherty, D. Kleindorfer, S. Kempisty, K. Sullivan, C. Moore, L. Sprafka, C. Koenig, B. Reinert. Central DuPage Hospital: H. Shownkeen, A. Mazumdar, H. Echiverri, R. Bajwa, K. Dzamashvili, R. Sucholeiki,M. Iacob, J. Smith, S. Lidtke, P. Pastore, and K. Woodson. Case Western Reserve University: R. Tarr, J. Sunshine, C. Sila, E. Westbrook, M. DeGeorgia, S. Pundik, S. Sundararajan, A. Furlan, D. Korosec, and D. Diorio. Cedars Sinai Medical Center: M. Alexander, D. Palestrant, P. Lyden, C. Miller, R. Singh, C. Serrano, E. Ferguson, F. Lin, P. Kornbluth, T. Zorge, S. Song, and T. Babcock. Duke University Medical Center: T. Smith, C. Graffagnino, V. Chilukuri, J. Stoner, D. Bombard, and L. Gauger. Erlanger Medical Center: B. Baxter, D. Rankine, T. Devlin, D. Jones, J. Sparks, and D Gaddis. Baylor Medical Center, Dallas, Texas: I. Thacker, J. Hise, D. Graybeal, R. Choudry, A. Okai, E. Broyles, M. Johnson, R. Smith, M. Sams, and M. Castillo. Sacred Heart Medical Center: C. Zylak, M. Geraghty, T. Powell, W. Bender, E. Rawner, R. Franks, B. Aaron, T. Cantrell, and K. Worrell. Thomas Jefferson University: R. Rosenwasser, P. Jabbour, C. Pineda, R. Bell, D. Brock, D. August, E. Simons, A. Salvatore, M. Wakefield, M. Sheridan, K. Salgado, and J. Furlong. Scott and White Memorial Hospital: W. Lesley, R. Lenehan, J. Clark, R. Wardlow, B. Wulbrecht, M. Carney, S. Taggart, and R. Castillo. Glendale Adventist Medical Center: G. Rappard, D. Thompson, L. Lee, C. Galicia, J. Cann, I. Zoltzman, A. Ghosalkar, and L. Oliveria Berndt. Providence Medical Center, Southfield,Michigan: R. Fessler, J. Mick, B. Kole, B. Silverman, P. Cullis, M. Holtzman, N. Warra, Y. Will-Murphy, M. Silverman, K. Telck, V. Gordon, J. Kelly, M. Lahey, N. Zakhem, and C. Rieck. Carolinas Medical Center: J. Bernard, S. Dibert, J. Story, J. Martin, and M. Price. Sentara Medical Center: J. Agola, R. Zweifler, J. Rathbun, S. Cummings, P. Hollsten, and S. Bright. Massachusetts General Hospital: J. Pryor, S. Silverman, R. Nogueira, F. Buonanno, M. Ning, A. Singhal, N. Rost, B. Rista, A. Chutinet, and T. Thankachan. Inova Fairfax Medical Center: R. Pergolizzi, C. Putman, J. Cochran, R. Lipsky, J. Rosecan, M. Greenberg, K. John, C. Restak, and K. Boucher. University of California, San Francisco: R. Higashida, M. Nguyen- Huynh, J. Tatum, S. Poisson, J. Scanlon, C. Yancey, and S. Stason. Good Samaritan Hospital, San Jose: R. Malek, H. Sachdev, N. Sachdev, M. Emami, and U. Kelly-Tolley. Mayo Clinic Arizona: B. Chong, B. Demaerschalk, M. Aguilar, D. Dodick, T. Ingall, B. Vargas, K. Noe, J. Sirven, J. Drazkowski, E. Boyd, and J. Dunshee. Johns Hopkins University: P. Gailloud, R. Wityk, P. Nyquist, B. Kohler, and A. Jones.Washington University, St Louis: C. Moran, C. Derdeyn, D. Cross, D. Carpenter, A. Ford, J. Lee, A. Zazulia, N. Hantler, J. Newgent, J. Rickmann, J. Serna, and L. Carpenter. MedstarWashington Hospital Center,Washington, DC: W. Bank, A. Liu, A. Hsia, M. Lin, M. Schlosberg, J. Martin, M. Cota, and I. Fleming.
Footnotes
Author Contributions: Dr Chimowitz had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design:Waters, Hoh, Lynn, Turan, Derdeyn, Fiorella, Khanna, Janis, Chimowitz.
Acquisition, analysis, or interpretation of data: Waters, Hoh, Lynn, Kwon, Turan, Derdeyn, Khanna, Sheehan, Lane, Janis, Montgomery, Chimowitz.
Drafting of the manuscript:Waters, Lynn, Chimowitz.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Lynn.
Obtained funding: Lynn, Turan, Chimowitz.
Administrative, technical, or material support:Waters, Lynn, Kwon, Derdeyn, Fiorella, Khanna, Montgomery.
Study supervision:Waters, Hoh, Lynn, Turan, Janis, Chimowitz.
Additional Contributions: The Regulatory and Clinical Research Institute (Minneapolis,Minnesota) provided assistance in designing the site-monitoring processes and performing the site-monitoring visits. The VA Cooperative Studies Program Clinical Research Pharmacy Coordinating Center (Albuquerque, New Mexico) handled the procurement, labeling, distribution, and inventory management of the study devices and rosuvastatin.
Conflict of Interest Disclosures: DrWaters has received fees as a medical expert in medical legal cases unrelated to this research. Dr Turan has received support from AstraZeneca and Stryker Neurovascular related to this study, other support from CardioNet (consultant), personal fees from Gore and Boehringer Ingelheim for participating as a stroke adjudicator in clinical trials unrelated to this work, and personal fees as an expert witness in medical legal cases unrelated to this research. Dr Derdeyn has relationships with companies that manufacture medical devices for the treatment of cerebrovascular disease in general, although none directly involved in this study. These includeW. L. Gore and Associates (scientific advisory board and consultant), MicroVention Inc (Angiographic Core Lab for clinical trial), Penumbra Inc (data and safety monitoring board member for clinical trial), and Pulse Therapeutics (chair, scientific advisory board). Dr Fiorella has received payment for research/salary support from Siemens, MicroVention, and Sequent Medical; consulting fees from Covidien/Ev3, Codman and Shurtleff, and Penumbra; royalties from Codman and Shurtleff (REVIVE); and ownership and stock interests in Vascular Simulators LLC, TDC Technologies, and CVSL.MsMontgomery has received fees from MicroVention Inc as a consultant. Dr Chimowitz has received support from AstraZeneca and Stryker Neurovascular (formerly Boston Scientific Neurovascular) related to this study. He also reports personal fees from Gore Associates, Merck /Parexel, and Medtronic for participating as a stroke adjudicator or data safety monitoring board member on clinical trials unrelated to the submittedwork. He also reports personal fees as an expertwitness in medical legal cases related to stroke. No other disclosures were reported.
Contributor Information
Michael F. Waters, Department of Neurology, McKnight Brain Institute, University of Florida College of Medicine, Gainesville; Department of Neuroscience, McKnight Brain Institute, University of Florida College of Medicine, Gainesville.
Brian L. Hoh, Department of Neurosurgery, McKnight Brain Institute, University of Florida College of Medicine, Gainesville.
Michael J. Lynn, Department of Biostatistics and Bioinformatics, Emory University Rollins School of Public Health, Atlanta, Georgia.
Hyung-Min Kwon, Department of Neurosciences, Medical University of South Carolina, Charleston; Department of Neurology, Seoul Metropolitan Government–Seoul National University, Boramae Medical Center, Seoul, South Korea.
Tanya N. Turan, Department of Neurosciences, Medical University of South Carolina, Charleston.
Colin P. Derdeyn, Mallinckrodt Institute of Radiology and the Departments of Neurology and Neurosurgery, Washington University School of Medicine, St Louis, Missouri.
David Fiorella, Department of Neurosurgery, State University of New York, Stony Brook.
Anna Khanna, Department of Neurology, McKnight Brain Institute, University of Florida College of Medicine, Gainesville.
Tiffany O. Sheehan, Department of Neurology, McKnight Brain Institute, University of Florida College of Medicine, Gainesville.
Bethany F. Lane, Department of Biostatistics and Bioinformatics, Emory University Rollins School of Public Health, Atlanta, Georgia.
Scott Janis, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda.
Jean Montgomery, Department of Biostatistics and Bioinformatics, Emory University Rollins School of Public Health, Atlanta, Georgia.
Marc I. Chimowitz, Department of Neurosciences, Medical University of South Carolina, Charleston.
References
- 1.Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39(8):2396–2399. doi: 10.1161/STROKEAHA.107.505776. [DOI] [PubMed] [Google Scholar]
- 2.Arenillas JF. Intracranial atherosclerosis: current concepts. Stroke. 2011;42(1 suppl):S20–S23. doi: 10.1161/STROKEAHA.110.597278. [DOI] [PubMed] [Google Scholar]
- 3.Wong KS, Li H. Long-term mortality and recurrent stroke risk among Chinese stroke patients with predominant intracranial atherosclerosis. Stroke. 2003;34(10):2361–2366. doi: 10.1161/01.STR.0000089017.90037.7A. [DOI] [PubMed] [Google Scholar]
- 4.Kasner SE, Chimowitz MI, Lynn MJ, et al. Warfarin Aspirin Symptomatic Intracranial Disease Trial Investigators. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113(4):555–563. doi: 10.1161/CIRCULATIONAHA.105.578229. [DOI] [PubMed] [Google Scholar]
- 5.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction: the Northern Manhattan Stroke Study. Stroke. 1995;26(1):14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 6.Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Trial Investigators. Design, progress and challenges of a double-blind trial of warfarin versus aspirin for symptomatic intracranial arterial stenosis. Neuroepidemiology. 2003;22(2):106–117. doi: 10.1159/000068744. [DOI] [PubMed] [Google Scholar]
- 7.Chimowitz MI, Kokkinos J, Strong J, et al. The warfarin-aspirin symptomatic intracranial disease study. Neurology. 1995;45(8):1488–1493. doi: 10.1212/wnl.45.8.1488. [DOI] [PubMed] [Google Scholar]
- 8.Chimowitz MI, Lynn MJ, Turan TN, et al. SAMMPRIS Investigators. Design of the stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis trial. J Stroke Cerebrovasc Dis. 2011;20(4):357–368. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turan TN, Lynn MJ, Nizam A, et al. SAMMPRIS Investigators. Rationale, design, and implementation of aggressive risk factor management in the Stenting and Aggressive Medical Management for Prevention of Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) Trial. Circ Cardiovasc Qual Outcomes. 2012;5(5):e51–e60. doi: 10.1161/CIRCOUTCOMES.112.966911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chimowitz MI, Lynn MJ, Derdeyn CP, et al. SAMMPRIS Trial Investigators. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365(11):993–1003. doi: 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis Trial Investigators. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. 2014;383(9914):333–341. doi: 10.1016/S0140-6736(13)62038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiorella D, Derdeyn CP, Lynn MJ, et al. SAMMPRIS Trial Investigators. Detailed analysis of periprocedural strokes in patients undergoing intracranial stenting in Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) Stroke. 2012;43(10):2682–2688. doi: 10.1161/STROKEAHA.112.661173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bose A, Hartmann M, Henkes H, et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: theWingspan Study. Stroke. 2007;38(5):1531–1537. doi: 10.1161/STROKEAHA.106.477711. [DOI] [PubMed] [Google Scholar]
- 14.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardizedmethod for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21(4):643–646. [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon NF, Salmon RD, Franklin BA, et al. Effectiveness of therapeutic lifestyle changes in patients with hypertension, hyperlipidemia, and/or hyperglycemia. Am J Cardiol. 2004;94(12):1558–1561. doi: 10.1016/j.amjcard.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 16.Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Warfarin-Aspirin Symptomatic Intracranial Disease Trial Investigators. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352(13):1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 17.Lutsep HL, Lynn MJ, Cotsonis GA, et al. SAMMPRIS Investigators. Does the Stenting Versus Aggressive Medical Therapy Trial support stenting for subgroups with intracranial stenosis? Stroke. 2015;46(11):3282–3284. doi: 10.1161/STROKEAHA.115.009846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turan TN, Nizam A, Lynn MJ, et al. Relationship between risk factor control and vascular events in the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) Trial. Stroke. 2014;45 AWP130. [Google Scholar]


