Abstract
Objective
To determine pharmacy availability of ulipristal acetate (UPA) and compare to availability of levonorgestrel-containing emergency contraceptive pills (LNG-ECPs).
Methods
We conducted an observational population-based study utilizing a telephone-based secret shopper methodology. Researchers called all 198 unique retail pharmacies in Hawaii on December 2013–June 2014, representing themselves as patients and physicians.
Results
Only 2.6% of pharmacies had UPA immediately available, though 22.8% reported ability to order UPA. In contrast, 82.4% reported immediate availability of LNG-ECPs. No significant difference in availability was reported to patients and physicians.
Conclusions
Availability of UPA is limited and significantly lower compared to LNG-ECPs. The study period did overlap with a change in distributor for UPA, likely capturing some disruption of the supply chain.
Implications
Systems-based interventions are needed to address barriers to obtaining UPA.
Keywords: Ulipristal acetate, Emergency contraception, Pharmacies, Availability, United States, Hawaii
1. Introduction
Ulipristal acetate (UPA) is a second-generation selective progesterone receptor modulator US Food and Drug Administration approved in 2010 as a prescription emergency contraceptive to be taken within 120 h of unprotected intercourse [1,2]. Compared to levonorgestrel emergency contraceptive pills (LNG ECPs), UPA prevents more pregnancies when taken 1, 3 and 5 days after unprotected sex [3]. In addition, UPA may be more effective than LNG ECPs in overweight and obese women [4]. Given its higher efficacy, it is estimated that use of UPA would result in 37,589 fewer unintended pregnancies and savings of more than US$116.3 million in direct health care expenditures annually in the US compared to LNG ECPs [3,5]. Despite the potential for UPA to reduce the risk of unintended pregnancies after unprotected intercourse, little information is available on its availability or information provided by pharmacy staff.
2. Materials and methods
To determine the availability of UPA in Hawaii’s pharmacies, we conducted a two-arm cross-sectional telephone secret shopper study of all unique retail pharmacies in the state. Pharmacies were excluded if they did not serve the general public or were unable to be reached after three attempts.
In the first arm, callers represented themselves as uninsured, unemployed 18-year-old females attempting to fill a prescription for UPA. In the second arm, physician researchers placed calls stating that they had written a UPA prescription for a patient and asking whether it could be filled that day. In both arms, callers followed a semistructured questionnaire to obtain information on same-day UPA availability, ability to order UPA, availability of LNG ECPs and out-of-pocket ECP prices. Calls were made between December 2013 and July 2014, Monday through Saturday from 8 a.m. to 8 p.m., with patient and physician calls staggered by 4 weeks. To most closely replicate clinical reality, callers began asking questions to the pharmacy staffer answering the phone and did not specifically ask to speak to a pharmacist. The University of Hawaii Institutional Review Board deemed this study to be nonhuman subjects research.
Statistical analyses were performed using EPI Info 7 [6]. Chi-square and Fisher’s exact tests were used to determine differences in responses received by patient and physician callers.
3. Results
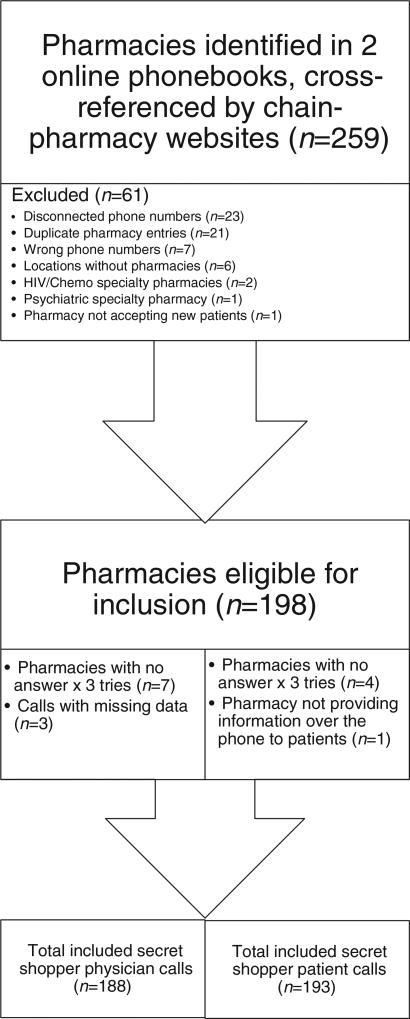
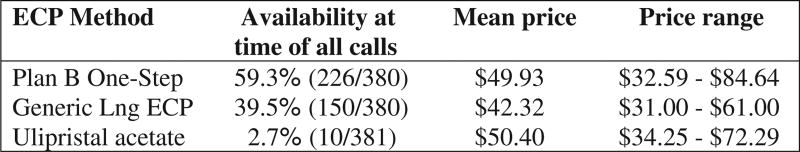
Of 259 pharmacies initially identified, 193 patient calls and 188 physician calls were included in the analysis (Fig. 1). UPA was reported to be immediately available to 2.1% (4/193) of patient callers and 3.2% (6/188) of physician callers (p = 0.5). If UPA was not in stock in 19.1% (36/189) of patients calls, it was reported to be orderable, compared to 30.8% (56/182) of physician calls (p = 0.01). The mean time until UPA availability in these cases was 35.1 h (16–88 h) for patient callers and 35.3 h (16–168 h) for physician callers (p = 0. 5). In contrast, LNG ECPs were available at 82.4% of pharmacies contacted by both patients and physicians (p = 0.1). Plan B One-Step was the most commonly available LNG ECP with 59.5% (226/380) of pharmacies carrying the medication, compared to 39.5% (150/380) offering generic LNG ECP products. There was no statistically significant price difference between UPA and brand name Plan B One-Step (US$50.40 vs. US$49.93), while generic LNG ECPs were less expensive (US$42.32) (Fig. 2).
Fig. 1.
Inclusion/Exclusion criteria.
Fig. 2.
Method, availability and prices reported during all patient and physician calls.
4. Discussion
Despite its increased efficacy and potential for increased cost-effectiveness relative to LNG ECPs, the availability of UPA in Hawaiian pharmacies is extremely limited. Our finding of same-day availability of UPA in only 2.1% of patient calls is even more limited than the 7% found in a study of two counties in western Massachusetts, the only other published study on UPA pharmacy availability [7]. Such limited access is particularly concerning given Hawaii’s geographic isolation.
Strengths of this study include close replication of authentic clinical conditions through a secret shopper approach and initiation of interviews with the pharmacy staff person answering the phone, as well as the ability to compare responses received by patient and physician callers. Our data collection period also encompassed an interval during which UPA had a transition in distributors, which may, temporarily, have further reduced its availability. However, this additional potential access barrier did reflect real-world conditions. Our study assessed access through retail pharmacies serving the general public. There is the potential for additional access to UPA through clinics, private medical offices and through on-line pharmacies though this was beyond the scope of our study.
While many factors influence the stocking and dispensing practices of pharmacies, actual or perceived lack of demand from physicians likely contributes. Recently published data by Batur et al. demonstrates near universal awareness of LNG ECP as an emergency contraception method amongst providers across specialties caring for women of reproductive age, while familiarity with UPA is at 50% for reproductive health providers and 15% for emergency medicine [8]. Increased education of clinicians, pharmacists and patients regarding the range of ECPs available and the important differences between them may assist in increasing demand for UPA, as well as motivation for prescribers to partner with pharmacies in navigating distribution and other systems-based barriers. Physician advocacy to pharmacies to stock the medication as a means of pharmacy education outreach has the potential to increase UPA access in Hawaii.
Advanced prescription of ECPs as advocated by the American Congress of Obstetricians and Gynecologists is vital to optimizing women’s potential to reduce unplanned pregnancy [9]. However, in the case of UPA in Hawaii, even an advanced prescription is insufficient to ensure access.
Footnotes
Research funding provided by Society of Family Planning Trainee Grant.
References
- 1.Lalitkumar PGL, Berger C, Gemzell-Danielsson K. Emergency contraception. Best Practice & Research Clinical Endocrinology & Metabolism. 2013;27 doi: 10.1016/j.beem.2012.09.003. 91–01. [DOI] [PubMed] [Google Scholar]
- 2.Orleans RJ. NDA22-474. Ella (ulipristal acetate 30 mg) US Food and Drug Administration; 2010. Clinical review. [Google Scholar]
- 3.Glasier AF, Cameron ST, Fine PM, Logan SJ, Casale W, Van Horn J, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555–62. doi: 10.1016/S0140-6736(10)60101-8. [DOI] [PubMed] [Google Scholar]
- 4.Glasier AF, Cameron ST, Blithe D, Scherrer B, Mathe H, Levy D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84:363–7. doi: 10.1016/j.contraception.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Bayer LL, Edelman A, Caughey AB, Rodriguez MI. The price of emergency contraception in the United States: what is the cost-effectiveness of ulipristal acetate versus single-dose levonorgestrel? Contraception. 2013;87(3):385–90. doi: 10.1016/j.contraception.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 6.Dean AG, Arner TG, Sunki GG, Friedman R, Lantinga M, Sangam S, Zubieta JC, Sullivan KM, Brendel KA, Gao Z, Fontaine N, Shu M, Fuller G, Smith DC, Nitschke DA, Fagan RF. Epi Info™, a database and statistics program for public health professionals. Atlanta, GA, USA: CDC; 2011. [Google Scholar]
- 7.Brant A, White K, St Marie P. Pharmacy availability of ulipristal acetate emergency contraception: an audit study. Contraception. 2014;90(3):338–9. [Google Scholar]
- 8.Batur P, Cleland K, McNamara M, et al. Emergency contraception: a multispecialty survey of clinician knowledge and practices. Contraception. 2016;93(2):145–52. doi: 10.1016/j.contraception.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American College of Obstetricians and Gynecologists. ACOG committee opinion no. 542: access to emergency contraception. Obstet Gynecol. 2012;120:1250–3. doi: 10.1097/aog.0b013e318277c960. [DOI] [PubMed] [Google Scholar]