Abstract
The boron geochemistry of coral skeletons reflects the dissolved inorganic carbon (DIC) chemistry of the calcification fluid from which the skeletons precipitates and may be a valuable tool to investigate the effects of climate change on coral calcification. In this paper I calculate the predicted B/Ca of aragonite precipitating from seawater based fluids as a function of pH, [DIC] and [Ca2+]. I consider how different co-precipitating DIC species affect aragonite B/Ca and also estimate the impact of variations in the B(OH)4−/co-precipitating DIC aragonite partition coefficient (KD), which may be associated with changes in the DIC and Ca2+ chemistry of the calcification fluid. The coral skeletal B/Ca versus calcification fluid pH relationships reported previously can be reproduced by estimating B(OH)4− and co-precipitating DIC speciation as a function of pHCF and assuming that KD are constant i.e. unaffected by calcification fluid saturation state. Assuming that B(OH)4− co-precipitates with CO32−, then observed patterns can be reproduced by a fluid with approximately constant [DIC] i.e. increasing pHCF concentrates CO32−, as a function of DIC speciation. Assuming that B(OH)4− co-precipitates with HCO3− only or CO32− + HCO3− then the observed patterns can be reproduced if [DIC]CF and pHCF are positively related i.e. if DIC is increasingly concentrated in the calcification fluid at higher pHCF probably by CO2 diffusion into the calcification site.
Keywords: Geochemistry, Geology, Oceanography
1. Introduction
The boron geochemistry of coral skeletons offers a potential method to reconstruct the dissolved inorganic carbon (DIC) chemistry of the coral calcification fluid (Allison et al., 2014) and to determine how it responds to environmental change. Coral biomineralisation underpins the production of the coral reef structure and understanding the controls on the calcification process is key to predicting the impacts of increasing seawater temperatures (Hoegh-Guldberg et al., 2007) and pCO2 (ocean acidification, Caldeira and Wickett, 2003) on reef development.
Dissolved boron in seawater occurs primarily as boric acid, B(OH)3, and borate, B(OH)4−, and speciation is controlled by ambient pH (Hemming and Hanson, 1992). Most reports suggest that B(OH)4− is selectively incorporated into aragonite (Sen et al., 1994; Noireaux et al., 2015) substituting for CO32− in the lattice (Balan et al., 2016) and is depleted in 11B compared to B(OH)3 (Klochko et al., 2006). Hence aragonite δ11B reflects the pH of the fluid from which it precipitates (Allison et al., 2010) while [B] (usually measured as B/Ca) reflects both fluid pH and the concentration of the DIC species competing with B(OH)4− for inclusion in the carbonate (Allen et al., 2012).
Coral aragonite precipitates from an extracellular calcifying fluid enclosed in a semi-isolated space between the coral tissue and underlying skeleton (Clode and Marshall, 2002). The calcification fluid is derived from seawater (Tambutté et al., 2012) but corals actively increase its pH above that of seawater (Al-Horani et al., 2003; Venn et al., 2011; Venn et al., 2012) altering the fluid DIC chemistry. To date there has been no direct comparison of coral skeletal δ11B and calcifying fluid pH to confirm that skeletal δ11B records actual calcifying fluid pH. Fluid pH can be estimated by direct observation of pH sensitive dyes at the calcification site, The mean pH of dye-based observations in the light and dark of the branching coral Stylophora pistillata (Venn et al., 2011) is in good agreement with fluid pH estimates derived from skeletal δ11B of different individuals of the same coral species cultured at present day seawater pCO2 (Krief et al., 2010), when corrected for the typical ratio of light:dark calcification rates (Gattuso et al., 1999). Positive trends are observed between seawater pH and calcification fluid pH when fluid pH is either inferred from skeletal δ11B (Honisch et al., 2004; Reynaud et al., 2004) or directly measured (Venn et al., 2012). These collective observations suggest that skeletal δ11B reflects calcification fluid pH changes.
Increasing calcification fluid pH shifts the fluid DIC equilibrium in favour of carbonate (CO32−) at the expense of CO2 and bicarbonate (HCO3−) and creates a concentration gradient facilitating the diffusion of CO2 from the overlying coral tissue into the fluid (Erez, 1978). This CO2 can react to form more HCO3− and CO32−, thereby increasing calcification fluid DIC. Preliminary application of the skeletal B/Ca − calcification fluid DIC proxy suggested that [DIC] at the coral calcification site is increased above that of seawater and that bicarbonate contributes to the DIC pool used for calcification (Allison et al., 2014). The B(OH)4−:aragonite partition coefficient utilized in this study was estimated from the B/Ca analysis of a secondary aragonite cement in a fossil coral coupled with alkalinity measurements of pore fluid and δ11B of the cement (indicating pore fluid pH). Recent inorganic aragonite precipitation studies indicate that the borate:aragonite partition coefficient can be highly variable (Mavromatis et al., 2015; Holcomb et al., 2016) and is probably affected by the saturation state of the precipitating fluid (Holcomb et al., 2016). This complicates the interpretation of coral skeletal [B].
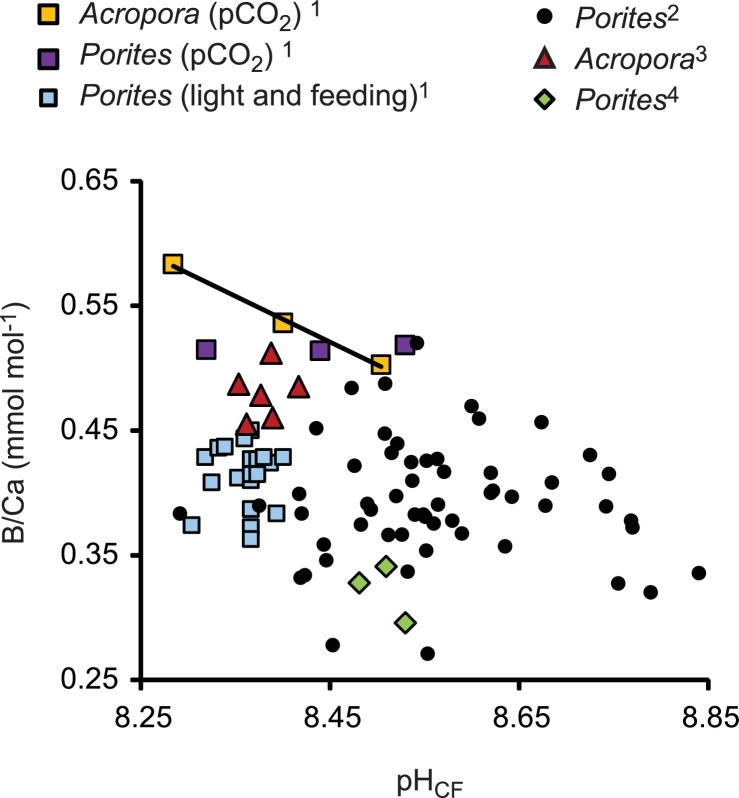
In this paper I explore how variations in calcification fluid DIC, [Ca2+] and pH affect skeletal B/Ca. Both boron and DIC speciation are pH dependent while variations in fluid DIC affect the saturation state of the fluid. I calculate the predicted B/Ca of aragonite precipitating from seawater based fluids with DIC, B and Ca2+ chemistries which are comparable to those of coral calcification fluids. Most coral datasets do not exhibit significant correlations between calcification fluid pH (inferred from δ11B) and skeletal B/Ca (Fig. 1) and it is timely to consider why this is. It is unclear which dissolved inorganic carbon (DIC) species is/are involved in aragonite precipitation. CO32− is predominantly incorporated in the crystal lattice (Von Euw et al., 2017) but HCO3− may also be involved in mineral precipitation (Wolthers et al., 2012; Mass et al., 2013; Von Euw et al., 2017). I consider how different co-precipitating DIC species affect aragonite B/Ca and also estimate the impact of variations in the B(OH)4−/co-precipitating DIC aragonite partition coefficient, which may be associated with changes in the DIC and Ca2+ chemistry of the calcification fluid.
Fig. 1.
Published relationships between coral calcification pHCF (inferred from δ11B) and skeletal B/Ca. Data sources are: 1 = Honisch et al. (2004); 2 = Allison et al. (2010), 3 = Dissard et al. (2012) and 4 = Allison et al. (2014). All data are corrected to the pH total scale. None of the datasets exhibit significant correlations between pHCF and B/Ca with the exception of Acropora sp. corals cultured over a range of seawater pCO2 (Honisch et al., 2004).
2. Methods
2.1. Impacts of coral processes on calcification fluid DIC chemistry
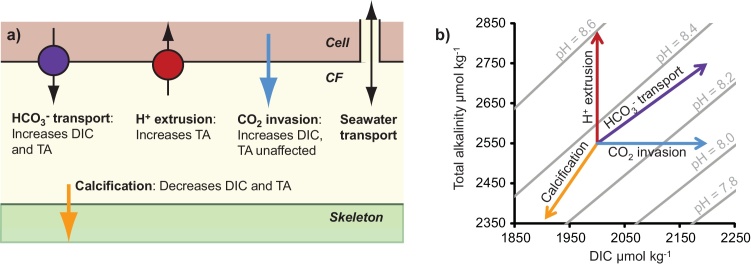
The key processes affecting calcification fluid DIC chemistry and their effects are summarized in Fig. 2. All pH values are reported on the total scale and the subscript CF denotes the DIC characteristics of the calcification fluid. Corals increase pHCF above that of seawater e.g. using the antiporter Ca-ATPase (Ip et al., 1991; Marshall, 1996) which pumps Ca2+ into the calcification site in exchange for 2H+. H+ extrusion increases the total alkalinity of the fluid but does not affect DIC. Total alkalinity is defined as the number of moles of hydrogen equivalent to the excess of proton acceptors over proton donors in the fluid (Zeebe and Wolf-Gladrow, 2001) so proton extrusion increases total alkalinity and decreases [H+] in a 1:1 mol ratio. Increasing pHCF favours the reaction of CO2 and H2O to form HCO3− (and H+) and facilitates the diffusion of CO2 into the calcification site. This increases fluid [DIC] but does not affect total alkalinity. Bicarbonate anion transporters (BATs) convey HCO3− into the calcification fluid, likely in exchange for Cl− (Zoccola et al., 2015). This increases total alkalinity and DIC in a 1:1 mol ratio and ultimately decreases pHCF. Aragonite precipitation removes DIC and total alkalinity from the calcification fluid in a 1:2 mole ratio (one mole of the CO32− ion ultimately incorporated in the aragonite contains one mole of carbon but is doubly charged so contributes 2 moles to total alkalinity) and also decreases pHCF.
Fig. 2.
(a) The key processes affecting coral calcification fluid DIC chemistry and (b) their effects on seawater DIC, total alkalinity and pHCF.
2.2. Calcification fluid chemistry and precipitation scenarios
I calculate the DIC chemistry of a seawater based calcification fluid and the B/Ca of aragonite precipitated from it under a range of different scenarios (summarised in Table 1) as a function of pHCF. The details of the scenarios are as follows:
Table 1.
Summary of scenarios used to estimate coral calcification fluid chemistry and aragonite B/Ca.
| Scenario | Detail of different scenarios |
|---|---|
| 1. Co-precipitating DIC species |
|
| 2. DICCF |
|
| 3. Seawater pCO2 |
|
| 4. B(OH)4−/co-precipitating DIC KD |
|
| 5. [Ca2+]CF |
|
2.2.1. Co-precipitating DIC species
I assume that either CO32− only, HCO3− only, or both CO32− and HCO3− co-precipitate with B(OH)4− in the aragonite (Table 1, scenario 1).
2.2.2. DICCF and seawater pCO2
I assume that the [DIC]CF is either low or high and that the fluid is overlain or surrounded by coral tissues and/or ambient seawater in equilibrium with either 400 μatm CO2 or 800 μatm CO2. I assume that [DIC]CF is either equivalent to that of seawater (the low DICCF scenario) or is higher than this (the high DICCF scenario), Table 1. In the case that [DIC]CF is equivalent to that of seawater, I effectively calculate the DIC of the calcification fluid as a closed system. I do not infer that the fluid is closed to CO2 diffusion but rather that additions of CO2 to the calcification fluid by diffusion are balanced by loss of DIC to precipitation. This is supported by direct measurements of pHCF and [CO32−]CF which indicate that DICCF is approximately constant and equal to that of ambient seawater (Cai et al., 2016). I assume that ambient seawater is in equilibrium with either 400 μatm CO2 (ambient seawater has pH 8 and [DIC] = 1796 μmol kg‐1) or 800 μatm CO2 (ambient seawater has pH 7.74 and [DIC] = 1911 μmol kg‐1). This doubling of seawater pCO2 indicates how DICCF is affected if the [CO2] of the coral tissue and body compartments is higher than that of ambient seawater (Cai et al., 2016). In the high DIC scenario, I assume that diffusion of CO2 into the calcification fluid increases [DIC]CF approximately twofold above that of seawater at typical coral calcification fluid pH and at a seawater pCO2 of 400 μatm CO2. The transport rate of CO2 across a membrane can be expressed by Fick’s first law of diffusion as:
| Flux = −P. A. ΔCw | (1) |
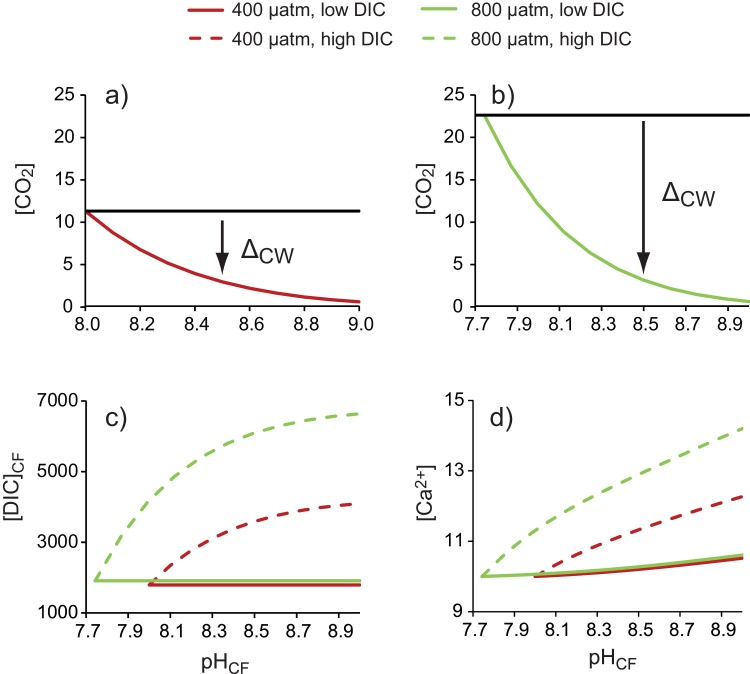
Where P = the membrane permeability, A = the diffusional area and ΔCw the difference of CO2 concentrations in the water phase immediately adjacent to the two sides of the membrane (Endeward et al., 2014). Assuming that P and A remain constant, the flux of CO2 into the calcification fluid is controlled by the CO2 concentration difference between the calcification fluid and the overlying coral tissue. I estimate this concentration difference assuming that the [CO2] of the overlying tissue is the same as ambient seawater (11.3 μmol kg−1 and 22.6 μmol kg−1 in the 400 and 800 μatm CO2 scenarios respectively) and that the [CO2] of the calcification fluid reflects that of ambient seawater brought to pHCF (Fig. 3a,b). I arbitrarily assume that CO2 diffusion doubles the [DIC]CF at pH 8.5 (the typical mean coral calcification fluid pH, Allison et al., 2014) in the 400 μatm CO2 scenario (i.e. adding 1796 μmol kg−1 to the DICCF) and scale the addition of DIC to the calcification fluid over the full pH range at 400 and 800 μatm CO2 as a linear function of ΔCw. This calculation is shown in detail in Appendix 1. I assume that additional H+ extrusion compensates for the pH decrease associated with the ingress of CO2 so that pHCF is unaffected by this CO2 invasion. I calculate the final [DIC]CF as the [DIC]seawater plus CO2 added by diffusion (Fig. 3c).
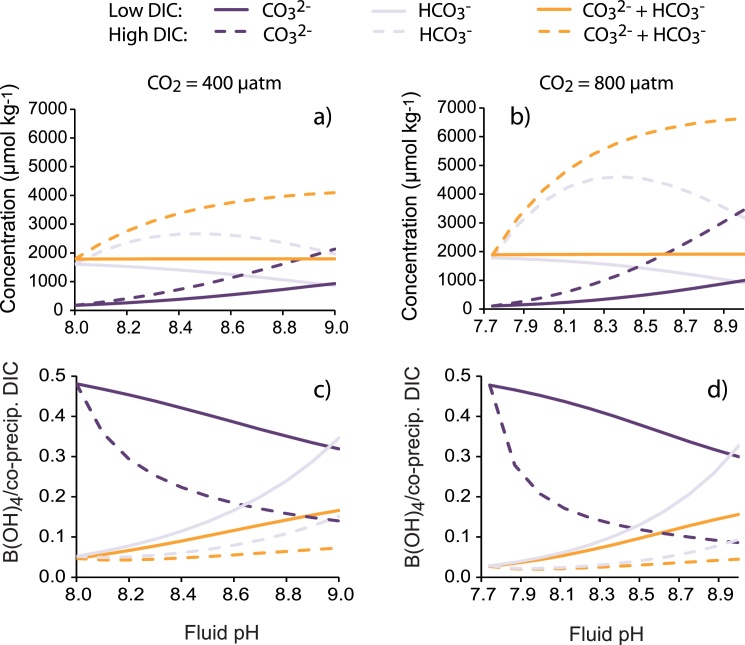
Fig. 3.
Estimated changes in fluid DIC (μmol kg-1) and [Ca2+] (mmol kg-1) as a function of pHCF. (a) and (b) [CO2] of a fluid with total [DIC] = 1796 μmol kg−1 (the typical [DIC] in seawater at equilibrium with 400 μatm) and 1911 μmol kg−1 (the typical [DIC] in seawater at equilibrium with 800 μatm CO2) respectively. Black horizontal lines indicate the [CO2] of an overlying ambient seawater at a) 400 μatm CO2 and b) 800 μatm CO2. These lines are extended across the entire pHCF range to ease visualisation of ΔCW although the pH of the overlying seawater is constant. ΔCW indicates the CO2 concentration gradient between the calcification fluid and ambient seawater at pH 8.5 which facilitates CO2 diffusion from seawater into the fluid. (c) Total fluid [DIC] after any CO2 diffusion (calculated by assuming CO2 diffusion doubles fluid [DIC] at pH 8.5 and 400 μatm CO2 and scaling all other CO2 additions by diffusion as a linear function of ΔCW at this pH. d) Fluid [Ca2+] calculated from fluid total alkalinity assuming that all proton extrusion is mediated by Ca-ATPase.
I estimate the concentrations of DIC species and total alkalinity in the calcification fluid by setting pHCF and [DIC]CF and calculating all other carbonate system parameters with CO2sys version 2.0 (Pierrot et al., 2006) using the equilibrium constants for carbonic acid from Mehrbach et al. (1973), refit by Dickson and Millero (1987) and for KHSO4 from Dickson (1990). Total fluid boron was set to 416 μmol kg−1 (Uppstrom, 1974). I assume constant temperature (25 °C) and salinity (35).
2.2.3. B(OH)4−/co-precipitating DIC aragonite KD
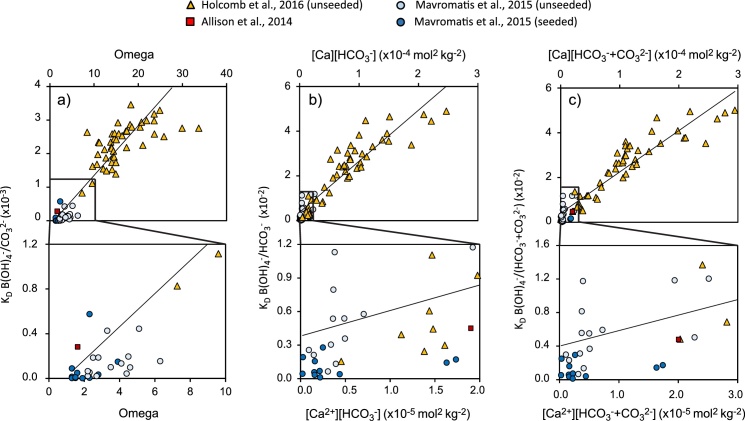
To estimate the B(OH)4−/co-precipitating DIC aragonite partition coefficients (hereafter abbreviated to KD) to apply in the calculations I combine data from two studies that measured boron partitioning in inorganic aragonite at ∼25 °C (Mavromatis et al., 2015; Holcomb et al., 2016). I exclude data collected from experiments conducted in the presence of buffers. I calculate the B(OH)4−/co-precipitating DIC ratios of the precipitation fluids and combine these with the B/Ca of the precipitated aragonites to estimate KD. Aragonite precipitation rate is typically positively correlated with the precipitation fluid saturation (Burton and Walter, 1987), an indication of the concentrations of solute ions in solution, and trace element partitioning may be affected by mineral precipitation rate (Watson, 1994; Elderfield et al., 1996). I plot the KD against the saturation of the precipitating fluid defined as Ωaragonite ([Ca2+][CO32−]/K*sparagonite) for scenario 1a, [Ca2+][HCO3−] for scenario 1b and [Ca2+][HCO3− + CO32−] for scenario 1c. The experiments of Mavromatis et al. (2015) and Holcomb et al. (2016) were conducted in 0.1–0.2 M NaCl solutions and seawater respectively. I have not corrected solute concentrations to activities. I have used stoichiometric equilibrium constants (conventionally denoted as K*), which correct for ionic strength and utilize ion concentrations rather than activities, for all calculations. There are no obvious offsets between the data of Holcomb et al. (2016) and Mavromatis et al. (2015) and all 3 partition coefficients are linearly positively correlated with fluid saturation (Fig. 4) according to the relationships:
| KD B(OH)4−/CO32− = 1.48 × 10−4omega − 1.30 × 10−4 (r2 = 0.86) | (2) |
| KD B(OH)4−/HCO3− = 1.85 × 102[Ca2+][HCO3−] + 4.01 × 10−3 (r2 = 0.89) | (3) |
| KD B(OH)4−/(CO32− + HCO3−) = 2.28 × 102[Ca2+][CO32− + HCO3−] + 3.81 × 10−3 (r2 = 0.87) | (4) |
Fig. 4.
B(OH)4−/co-precipitating DIC aragonite partition coefficients (calculated from Mavromatis et al., 2015 and Holcomb et al., 2016) assuming that B(OH)4− co-precipitates with (a) CO32− only, (b) HCO3− only and (c) CO32− + HCO3−, as a function of the saturation of the precipitating fluid defined as (a) Ωaragonite, (b) [Ca2+][HCO3−] and c) [Ca2+][CO32− + HCO3−], all concentrations in mol kg-1. The lower half of each figure is an expanded view of the boxed section in the upper figure. The partition coefficients determined from analysis of an inorganic cement in a fossil coral (Allison et al., 2014) are also shown.
The incorporation of boron in aragonite is not fully understood but this observation is consistent with the growth entrapment model (e.g. Watson, 1994) which suggests that trace elements are captured as impurities at the crystal surface before they can diffuse back into the surrounding fluid. B/Ca incorporation in calcite is positively correlated with crystal extension (Gabitov et al., 2014) and precipitation rate (Uchikawa et al., 2015). KD B(OH)4−/CO32− is also positively correlated with precipitation rate in aragonite but KD B(OH)4−/HCO3− and KD B(OH)4−/(CO32− + HCO3−) do not show this relationship (Holcomb et al., 2016), although precipitation rate is not necessarily a good indication of crystal extension rate. In the growth entrapment model, maximum entrapment is reached at high crystal growth rates and KD becomes independent of crystal growth rate. This may just be occurring at the highest fluid saturation states in Fig. 4.
In calculating aragonite B/Ca I assume that KD are either dependent on fluid saturation state or are constant (scenarios 4a and b, Table 1). In the first case I am inferring that higher fluid saturation stimulates high crystal extension rates leading to relatively high B incorporation. In the second case I am assuming that high fluid saturation does not affect crystal extension rate. Coral calcification rates are positively correlated with the saturation states of the calcification fluid (Allison et al., 2014) and seawater (Gattuso et al., 1998). However it does not automatically follow that the growth rates of coral skeletal crystals increase at high saturation states. In the massive Porites spp. corals, typically used for palaeoenvironmental reconstruction, linear extension of the skeleton occurs by the deposition of centres of calcification or centres of rapid accretion (Nothdurft and Webb, 2007) which are aligned perpendicular to the plane of the skeleton surface. Fasciculi, composed of bundles of acicular aragonite crystals radiate out from these centres and are aligned perpendicular to the centres and approximately parallel to the skeleton surface. These make up the bulk of the skeleton (Allison, 1996). Fast coral calcification rates could reflect rapid extension of fasciculi crystals but could equally well be explained by constant extension rates of a larger volume of fasciculi crystals.
In the case that B(OH)4−/co-precipitating DIC KD is dependent on fluid saturation state (scenario 4a) I calculate calcification fluid saturation as [Ca2+]CF[CO32−]CF/K*sparagonite for scenario 1a, [Ca2+]CF[HCO3−]CF for scenario 1b and [Ca2+]CF[HCO3− + CO32−]CF for scenario 1c and then calculate KD from Eqs. (2)–(4) (Fig. 5). In the case that KD is independent of fluid saturation, I calculate the saturation state for a typical coral fluid based on direct measurements of [CO32−]CF at pHCF = 8.55 (pH = 8.7 NBS scale, Cai et al., 2016), broadly comparable to the mean pHCF derived from δ11B of massive Porites spp. field corals (Allison et al., 2014). I calculate KD of 0.00105, 0.00636 and 0.00669 for B(OH)4−/CO32−, B(OH)4−/HCO3− and B(OH)4−/(CO32− + HCO3−) respectively. I apply these same KD to all scenarios in which I use a constant KD, irrespective of [co-precipitating DIC]CF, [Ca2+]CF or CO2 atmosphere.
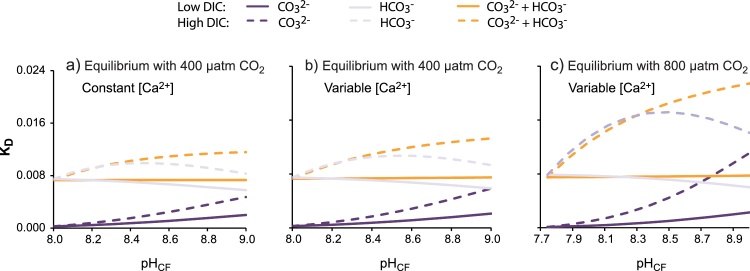
Fig. 5.
KD as a function of pHCF with (a) constant [Ca2+] and equilibrium with 400 μatm CO2, (b) variable [Ca2+] and equilibrium with 400 μatm CO2 and (c) variable [Ca2+] and equilibrium with 800 μatm CO2. Colours indicate different co-precipitating DIC species, solid lines indicate low DIC and dashed lines indicate high DIC.
2.2.4. [Ca2+]CF
[Ca2+]CF affects the saturation state of the calcification fluid and subsequently may influence the B(OH)4−:aragonite partition coefficient (see section 2.2.3). I adopt 2 approaches to estimate [Ca2+]CF. In scenario 5a I assume that all proton extrusion from the calcification fluid is mediated by Ca-ATPase. This enzyme pumps 2H+ out of the fluid for every Ca2+ pumped in, thereby increasing fluid total alkalinity and [Ca2+] in a 2:1 mole ratio. I use the total alkalinity of the calcification fluid (calculated in the DICCF and seawater pCO2 section) to infer the activity of the enzyme and to calculate fluid [Ca2+] (Fig. 3d) assuming that [Ca2+] of the fluid at pH 8 is that of ambient seawater (10 mmol kg‐1). Aragonite precipitation removes total alkalinity and Ca2+ in a 2:1 mole ratio so does not influence this approach. In scenario 5b I assume that proton extrusion is mediated by an alternative ATPase which does not affect fluid [Ca2+]. Direct measurements of coral calcification fluids suggest that fluid [Ca2+] is similar to seawater (within 5%, Al-Horani et al., 2003) and in scenario 5b I assume a constant [Ca2+]CF of 10 mmol kg−1.
2.2.5. Calculating aragonite B/Ca
I calculate the B/Ca of aragonite precipitated from the calcification fluid under the various scenarios. Aragonite B/Ca equates to aragonite B(OH)4−/CO32− as Ca and C are equimolar in CaCO3. I assume that dissolved boron is transported into the calcification fluid in seawater and that the total [B] of the fluid is the same as seawater i.e. 416 μmol kg−1 (Uppstrom, 1974). I assume this concentration is constant for all scenarios as aragonite precipitation has little effect on [B]CF due to the low B(OH)4−/co-precipitating DIC aragonite partition coefficients (Fig. 4). I estimate [B(OH)4−]CF using K*B = 2.527 × 10−9 (Dickson, 1990) and calculate fluid B(OH)4−/co-precipitating DIC. I multiply fluid B(OH)4−/co-precipitating DIC by KD to calculate aragonite B/Ca.
3. Results and discussion
3.1. [DIC]CF
δ11B of Porites spp. field corals suggests that mean coral pHCF is ∼8.5 (Allison et al., 2014). Here I assume that [DIC]CF is either comparable to that of seawater or is doubled by CO2 diffusion at pHCF = 8.5 and 400 μatm CO2. It is probable that these calculations reproduce the approximate DIC chemistry of the coral calcification fluid. Covariation of pHCF and DICCF suggests that [DIC]CF is broadly similar to that of seawater (Cai et al., 2016). While coral uptake of 45Ca and 14C in dual labelling experiments suggesting that the majority of skeletal carbon is derived from CO2 which diffuses into the calcification site rather than from seawater (Erez, 1978).
In scenarios where [DIC]CF increases above that of seawater I describe the CO2 concentration gradient (ΔCW) between the coral tissue and the calcification fluid assuming that the [CO2] of the overlying tissue is the same as ambient seawater and that fluid [CO2] reflects that of ambient seawater brought to pHCF. The [CO2] of a fluid brought to pHCF is exponentially related to pHCF i.e. at high pHCF fluid [CO2] becomes progressively smaller (Fig. 3a,b). Any increase in ΔCWat high pHCF is also progressively smaller and fluid [DIC] eventually begins to plateau. The final [DIC]CF ranges from 1796 μmol kg−1 and 1911 μmol kg−1 (the low DIC scenarios) to ∼4100 μmol kg−1 and ∼6600 μmol kg‐1 at pHCF 9 for the 400 and 800 μatm CO2 high DIC scenarios respectively (Fig. 3c). [DIC]CF is higher at 800 μatm CO2 as the larger ΔCW facilitates more CO2 diffusion into the calcification fluid.
3.2. [Ca2+]CF
I estimate the [Ca2+]CF assuming that either Ca-ATPase is responsible for all proton extrusion (resulting in pumping of Ca2+ into the calcification site) or that other, non-Ca pumping, enzymes fulfil this role (resulting in no change to [Ca2+]CF). In the first scenario [Ca2+]CF increases by 5% and 6% above that of seawater at 400 and 800 μatm CO2 respectively at low DIC and by 23% and 42% respectively at high DIC (Fig. 3d). The concentration increase is higher in the high DIC scenarios because increased Ca-ATPase activity is required to attain pHCF when more CO2 diffuses into the calcification fluid. Similarly [Ca2+]CF increases more at 800 μatm CO2 than at 400 μatm CO2 reflecting the higher Ca-ATPase activity to reach pHCF starting from an ambient seawater with pH ∼7.7.
3.3. Fluid B(OH)4−/[co-precipitating DIC]
Concentrations of individual co-precipitating DIC species under the different scenarios are illustrated in Fig. 6. In the low DIC scenarios I treat the calcification fluid as a closed system and the concentrations of co-precipitating DIC species are dependent on pHCF. [CO32−]CF is positively correlated with pHCF, [HCO3−]CF is negatively correlated and [CO32− + HCO3−]CF is almost constant (Fig. 6a,b solid lines). The high DIC scenarios (when CO2 invasion increases [DIC]CF above that of seawater) are associated with higher [co-precipitating DIC]CF than the low DIC at comparable pHCF. However while both [CO32−]CF and [CO32−+ HCO3−]CF are positively correlated with pHCF, [HCO3−]CF increases to a maximum at ∼ pH 8.3–8.5 and then begins to decrease again (Fig. 5a,b dotted lines). Above this pHCF, any further increase in [DIC]CF is relatively small (Fig. 3c) and the decrease in proportional abundance of HCO3− as pHCF increases outweighs any increase in total [DIC]CF.
Fig. 6.
Estimated calcification fluid DIC chemistry (a and b) and fluid B(OH)4−/co-precipitating DIC (c and d) as a function of fluid pH. (a) and (c) indicate scenarios at 400 μatm CO2 and b) and d) indicate scenarios at 800 μatm CO2. Colours indicate different co-precipitating DIC species, solid lines indicate low DIC and dashed lines indicate high DIC.
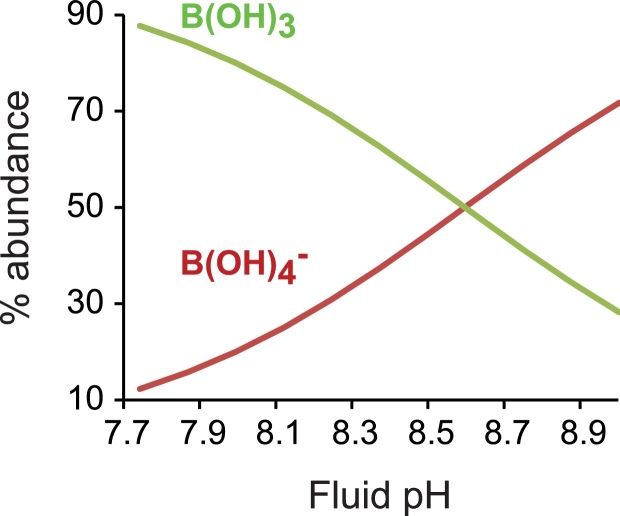
B(OH)3 and B(OH)4− speciation are illustrated in Fig. 7 and calcification fluid B(OH)4−/co-precipitating DIC ratios are illustrated in Fig. 6c,d. B(OH)4−/HCO3− and B(OH)4−/(CO32− + HCO3−) are positively correlated with pHCF in both the low and high DIC scenarios. As pHCF increases the increase in [B(OH)4−] (driven by the effect of pH on boron speciation) is larger than any increase in [HCO3−]CF and [CO32− + HCO3−]CF. In contrast, fluid B(OH)4−/CO32− is inversely correlated with pHCF at both low and high DIC. Here the proportional increase in [CO32−]CF at higher pHCF is larger than any increase in [B(OH)4−]CF. The high DIC scenarios generate lower B(OH)4−/co-precipitating DIC than the low DIC scenarios at comparable pH because [co-precipitating DIC]CF are higher in the high DIC scenarios. Likewise, [co-precipitating DIC] are higher at 800 μatm seawater pCO2 than at 400 μatm seawater pCO2 for comparable pHCF yielding lower B(OH)4−/[co-precipitating DIC] ratios at 800 μatm seawater pCO2 (Fig. 6c,d).
Fig. 7.
Percentage abundance of B(OH)3 and B(OH)4− as a function of pH (T = 25 °C, S = 35).
3.4. Aragonite B/Ca
I calculate the B/Ca of aragonite precipitating from the fluids using the B(OH)4−/co-precipitating DIC fluid compositions (Fig. 6c,d) and calculated KD (Fig. 5) defined under the different scenarios. I consider a maximum of 48 scenarios (3 co-precipitating scenarios x 2 DIC scenarios x 2CO2 atmospheres x 2 [Ca2+] scenarios x 2 KD scenarios, Table 1). 30 of these are illustrated in Fig. 8. I do not reproduce the data that combine variable [Ca2+]CF (scenario 5a) with a constant KD (scenario 4b) as in setting a constant KD I do not require [Ca2+]CF to estimate calcification fluid saturation state. The constant and variable [Ca2+]CF scenarios generate broadly similar patterns in skeletal B/Ca. I illustrate the impact of this change at 400 μatm seawater pCO2 but calculate expected aragonite B/Ca at 800 μatm seawater pCO2 using variable [Ca2+]CF only.
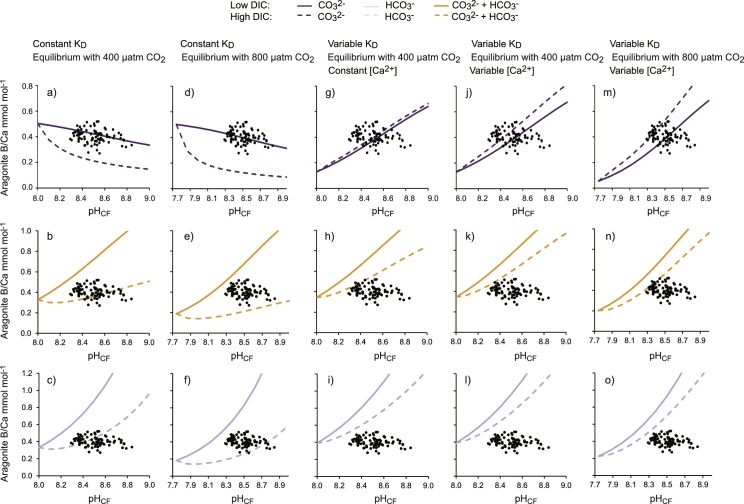
Fig. 8.
Predicted aragonite B/Ca as a function of pHCF under the different scenarios. Colours indicate different co-precipitating DIC species, solid lines indicate low DIC and dashed lines indicate high DIC. Published relationships between coral calcification pHCF and skeletal B/Ca in corals grown at ambient seawater pCO2 are overlain as black dots on each graph.
3.4.1. Impact of KD
Fig. 8a–f indicates aragonite B/Ca assuming that KD does not vary in response to calcification fluid saturation state. Patterns in calcification fluid B(OH)4−/co-precipitating DIC (Fig. 6c,d) are essentially preserved in aragonite B/Ca. Aragonite B/Ca is inversely correlated with pHCF if B(OH)4− co-precipitates with CO32− and is positively correlated with pHCF if B(OH)4− co-precipitates with HCO3− or (CO32−+ HCO3−). Higher [co-precipitating DIC]CF serves to dilute the calcification fluid B(OH)4− and generates lower skeletal B/Ca. For this reason the high DIC scenarios always generate lower skeletal B/Ca compared to the low DIC scenarios at comparable pHCF.
Assuming that KD varies in response to calcification fluid saturation state then these patterns change significantly (Fig. 8g–o). KD is positively correlated with fluid saturation state for all co-precipitating DIC species (Fig. 4). In the case that B(OH)4− co-precipitates with CO32−, [CO32−]CF is always relatively high at high pHCF resulting in high KD and enhanced B(OH)4− incorporation in the precipitating aragonite. Although fluid B(OH)4−/[CO32−]CF decreases at high pH (Fig. 6c), the increased B(OH)4− incorporation at high fluid saturation state overrides this dilution of fluid B(OH)4− by CO32−. Put simply, over the pHCF range 8 to 9 at 400 μatm seawater pCO2, B(OH)4−/CO32− decreases by x0.7 and x0.3 in the low and high DIC scenarios respectively (Fig. 6c) but KD increases x8 and x22 (assuming a variable [Ca2+]CF) over the same pH range (Fig. 5b). So in the case that B(OH)4− co-precipitates with CO32− and KD is variable, aragonite B/Ca and pHCF are always positively correlated (Fig. 8g,j,m). Higher aragonite B/Ca are generated in the high DIC scenarios than their low DIC counterparts for comparable pHCF because the increase in KD (promoting B(OH)4− incorporation in the aragonite) outweighs any B(OH)4− dilution by increased fluid [CO32−].
Variable KD have a more subtle effect on aragonite B/Ca if B(OH)4− co-precipitates with either HCO3− only or CO32− + HCO3−. Assuming that B(OH)4− co-precipitates with HCO3−, then at low DIC both [HCO3−]CF and KD are relatively high at low pHCF and relatively low at high pHCF (Fig. 6a,b and Fig. 5 respectively). As pHCF increases, the proportional increase in fluid B(OH)4−/HCO3− (Fig. 6c,d) exceeds the proportional decrease in KD and aragonite B/Ca and pHCF are positively correlated (Fig. 8i,l,o). This pattern is maintained at high DIC although in this case the proportional increase in KD (driven by higher [HCO3−]CF compared to the low DIC scenario) is outweighed by the proportional decrease in fluid B(OH)4−/HCO3− (driven by increased [HCO3−]CF). Thus the high DIC scenarios generate lower aragonite B/Ca than the low DIC scenarios at comparable pHCF. Predicted aragonite B/Ca is broadly similar if B(OH)4− co-precipitates with both CO32− + HCO3− as HCO3− is usually the dominant DIC species over the range of pHCF. Once again aragonite B/Ca is positively correlated with pHCF in all scenarios and high DIC scenarios generate lower aragonite B/Ca than their low DIC counterparts (Fig. 8h,k,n).
3.4.2. Impact of [Ca2+]CF
The constant and variable [Ca2+]CF scenarios generate broadly similar patterns in skeletal B/Ca (i.e. compare Fig. 8g–i with Fig. 8j–l respectively). [Ca2+]CF are higher under the variable [Ca2+] scenarios compared to constant [Ca2+]CF, as proton extrusion by Ca-ATPase serves to increase [Ca2+]CF. This causes small increases in fluid saturation state and therefore KD (Fig. 5a,b). These higher KD result in higher aragonite B/Ca but the effect is relatively subtle (compare Fig. 8g–i and j–l) as the proportional changes in [Ca2+]CF over all scenarios are small compared to changes in [co-precipitating DIC]CF.
3.4.3. Impact of seawater pCO2
Seawater pCO2 has a relatively minor impact on aragonite B/Ca. All [co-precipitating DIC]CF species are increased at 800 μatm seawater pCO2 compared to 400 μatm seawater pCO2 (Fig. 8). At constant KD these increases dilute the precipitating B(OH)4− and decrease aragonite B/Ca. Over all co-precipitation scenarios, changes between predicted aragonite B/Ca at 400 and 800 μatm seawater pCO2 are almost imperceptible at low DIC and more significant at high DIC. When KD is variable, the increases in [co-precipitating DIC]CF generate higher KD. If B(OH)4− co-precipitates with CO32−, then the effect of increasing KD outweighs the effect of decreased fluid B(OH)4−/[CO32−] and aragonite B/Ca is higher at 800 μatm compared to 400 μatm seawater pCO2. If B(OH)4− co-precipitates with HCO3− or both CO32− + HCO3− then the effect of decreased fluid B(OH)4−/[co-precipitating DIC] outweighs the effect of increasing KD and aragonite B/Ca is lower at 800 μatm compared to 400 μatm seawater pCO2. Under all co-precipitation scenarios at variable KD changes between predicted aragonite B/Ca at 400 and 800 μatm seawater pCO2 are very subtle.
3.4.4. Additional factors to consider
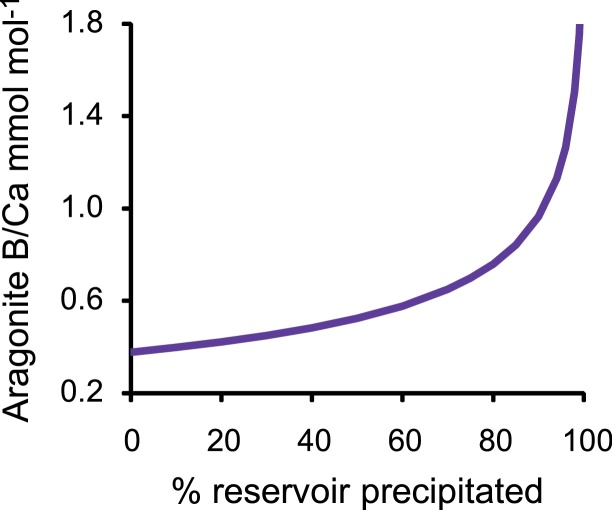
Rayleigh fractionation can occur when aragonite precipitates from an isolated fluid reservoir (Elderfield et al., 1996). As the KD for all B(OH)4−/co-precipitating DIC species are much smaller than 1, B(OH)4− is preferentially discriminated against during aragonite formation. As precipitation proceeds, the B(OH)4−/co-precipitating DIC of the fluid remaining in the reservoir, and of the aragonite precipitated from it, increases. The final B/Ca of the precipitated aragonite reflects the proportion of the reservoir used in precipitation (Fig. 9). If Rayleigh fractionation occurs in the coral calcification fluid then skeletal B/Ca will increase when a relatively large proportion of the fluid reservoir is precipitated. This is likely to occur at high fluid saturation states.
Fig. 9.
Aragonite B/Ca as a function of the % of the fluid reservoir utilised during precipitation assuming that B(OH)4− co-precipitates with CO32−. The B(OH)4−/CO32− aragonite partition coefficient is arbitarily set to 0.00105 when none of the reservoir is utilised.
Partition coefficients are usually lower in aragonites precipitated on seeds compared to unseeded material (Fig. 4) and the coefficients utilised here may overestimate the calcification fluid DIC of coral aragonite (which precipitates on existing skeletal aragonite).
In the scenario that (B(OH)4− co-precipitates with CO32−, in some instances the calcification fluid saturation states exceed the maximum state observed in the calculation of KD (Fig. 4). This occurs at pH 9 in the high DIC scenario at 400 μatm seawater pCO2 and ≥pH 8.6 in the high DIC scenario at 800 μatm seawater pCO2. I have assumed that KD is linearly correlated with calcification fluid saturation state and these high fluid saturation states generate KD (Fig. 5) that exceed the maximum values observed in Fig. 4. Under the growth entrapment model, KD approaches a constant value at high crystal extension rates (assumed to occur at high fluid saturation states) and in this case it is likely that I have overestimated aragonite B/Ca at high pHCF in the high DIC scenario at 800 μatm seawater pCO2.
3.5. Comparing modelled and observed coral skeletal B/Ca
The observed coral skeletal B/Ca from corals that grew under ambient CO2 conditions (i.e. all the data from Fig. 1 with the exception of corals cultured under altered seawater pCO2, Honisch et al., 2004) are superimposed onto the graphs in Fig. 8. I do not make any corrections for the different temperatures under which the corals grew. Temperature affects both DIC and boron speciation but the temperature range associated with the data represented in Fig. 1 is small (22–28 °C) and has a relatively minor effect on fluid B(OH)4−/co-precipitating DIC (<15%). Temperature also has no observable effect on boron partitioning in aragonite above fluid pH of 8.3 (Mavromatis et al., 2015).
Given the assumptions made in the calculations, e.g. in setting [DIC]CF and constant KD, I do not attempt to identify a scenario which duplicates the observed coral B/Ca values. However a comparison of the distribution of aragonite B/Ca that can be generated under the different scenarios is informative. Assuming that KD varies in response to fluid saturation state then all co-precipitation scenarios suggest that pHCF and aragonite B/Ca are positively correlated (Fig. 8g–o). Furthermore these scenarios generate relatively narrow ranges of potential B/Ca values (the area of each graph bounded by the low and high DIC scenario lines) irrespective of [DIC] and [Ca2+]. At high [co-precipitating DIC] the increase in KD (promoting the incorporation of higher concentrations of B(OH)4− in the precipitating aragonite) counteracts the dilution of fluid B(OH)4− by high co-precipitating DICCF. In corals the observed skeletal B/Ca is relative constant regardless of inferred pHCF (typically 8.3 to 8.8). This pattern cannot be reproduced by the variable KD scenarios either separately or in combination. I conclude that the coral data cannot be well described by any of the scenarios employing variable KD.
The scenarios utilizing constant KD generate wider ranges of aragonite B/Ca that are comparable to the skeletal B/Ca versus pHCF relationships observed in corals (Fig. 8a–f). The observation that coral data is best fitted assuming a constant KD suggests that while coral calcification fluid saturation state may be critical in controlling calcification rate (Gattuso et al., 1999), it is unlikely to affect skeletal extension rate. Under the constant KD scenarios skeletal B/Ca variations are driven by changes in pHCF (affecting boron and DIC speciation) and [DIC]CF (affecting [co-precipitating DIC]CF).
Most datasets do not exhibit significant correlations between coral pHCF and skeletal B/Ca (Fig. 1). In the case that B(OH)4− co-precipitates with CO32−, approximately constant skeletal B/Ca can be generated over a wide pHCF range by a calcification fluid with approximately constant [DIC], as in the low DIC scenario modelled here. Increasing pHCF shifts the DIC equilibrium (Fig. 6a) to increase [CO32−]CF which dilutes [B(OH)4−]CF (Fig. 6c). In the case that B(OH)4− co-precipitates with HCO3− only or CO32− + HCO3− then constant [DIC]CF scenarios generate positive correlations between pHCF and skeletal B/Ca (Fig. 8b,c,e,f) which are inconsistent with the observed coral data. To generate approximately constant skeletal B/Ca over a wide pHCF range under these co-precipitation scenarios requires that [DIC]CF increases at higher pHCF. Increasing pHCF serves to increase [DIC]CF probably by facilitating CO2 diffusion into the calcification site.
4. Conclusions
Observed coral skeletal B/Ca versus pHCF relationships can be reproduced by estimating B(OH)4− and co-precipitating DIC speciation as a function of pHCF and assuming that KD are constant i.e. unaffected by calcification fluid saturation state. Assuming that B(OH)4− co-precipitates with CO32−, then observed patterns can be reproduced by a fluid with approximately constant [DIC] i.e. increasing pHCF concentrates CO32−, as a function of DIC speciation. Assuming that B(OH)4− co-precipitates with HCO3− or CO32− + HCO3− then the observed patterns can be reproduced if [DIC]CF and pHCF are positively related i.e. if DIC is increasingly concentrated in the calcification fluid at higher pHCF probably by CO2 diffusion into the calcification site.
Declarations
Author contribution statement
Nicola Allison: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Competing interest statement
The authors declare no conflict of interest.
Funding statement
This work was supported by the UK Natural Environment Research Council (award NE/I022973/1).
Additional Information
No additional information is available for this paper.
Appendix A. Supplementary data
References
- Al-Horani F.A., Al-Moghrabi S.M., de Beer D. The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar. Biol. 2003;142:419–426. [Google Scholar]
- Allen K.A., Honisch B., Eggins S.M., Rosenthal Y. Environmental controls on B/Ca in calcite tests of the tropical planktic foraminifer species Globigerinoides ruber and Globigerinoides sacculifer. Earth Planet. Sci. Lett. 2012;351–352:270–280. [Google Scholar]
- Allison N. Geochemical anomalies in coral skeletons and their possible implications for palaeoenvironmental analysis. Mar. Chem. 1996;55:367–379. [Google Scholar]
- Allison N., Cohen I., Finch A.A., Erez J., Tudhope A.W. Corals concentrate dissolved inorganic carbon to facilitate calcification. Nat. Commun. 2014;5:5741. doi: 10.1038/ncomms6741. [DOI] [PubMed] [Google Scholar]
- Allison N., Finch A.A., EIMF δ11B, Sr, Mg and B in a modern Porites coral: the relationship between calcification site pH and skeletal chemistry. Geochim. Cosmochim. Acta. 2010;74:1790–1800. [Google Scholar]
- Balan E., Pietrucci F., Gervais C., Blanchard M., Schott J., Gaillardet J. First-principles study of boron speciation in calcite and aragonite. Geochim. Cosmochim. Acta. 2016:193. [Google Scholar]
- Burton E.A., Walter L.M. Relative precipitation rates of aragonite and Mg calcite from seawater: temperature or carbonate ion control. Geology. 1987;15:111–114. [Google Scholar]
- Cai W.-J., Ma Y., Hopkinson B.M., Grottoli A.G., Warner M.E., Ding Q., Hu X., Yuan X., Schoepf V., Xu H., Han C., Melman T.F., Hoadley K.D., Pettay D.T., Matsui Y., Baumann J.H., Levas S., Ying Y., Wang Y. Microelectrode characterization of coral daytime interior pH and carbonate chemistry. Nat. Commun. 2016;7:11144. doi: 10.1038/ncomms11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeira K., Wickett M.E. Anthropogenic carbon and ocean pH. Nature. 2003;425:365. doi: 10.1038/425365a. [DOI] [PubMed] [Google Scholar]
- Clode P.L., Marshall A.T. Low temperature FESEM of the calcifying interface of a scleractinian coral. Tissue Cell. 2002;34:187–198. doi: 10.1016/s0040-8166(02)00031-9. [DOI] [PubMed] [Google Scholar]
- Dickson A.G. Standard potential of the reaction: AgCl(s) + 12H2(g) = Ag(s) + HCl(aq) and the standard acidity constant of the ion HSO4− in synthetic sea water from 273.15 to 318.15 K. J. Chem. Thermodynam. 1990;22:113–127. [Google Scholar]
- Dickson A.G., Millero F.J. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep-Sea Res. 1987;34:1733–1743. [Google Scholar]
- Dissard D., Douvill E., Reynaud S., Juillet-Leclerc A., Montagna P., Louvat P., McCulloch M. Light and temperature effects on δ11B and B/Ca ratios of the zooxanthellate coral Acropora sp.: results from culturing experiments. Biogeosciences. 2012;9:4589–4605. [Google Scholar]
- Elderfield H., Bertram C.J., Erez J. Biomineralization model for the incorporation of trace elements into foraminiferal calcium carbonate. Earth Planet. Sci. Lett. 1996;142:409–423. [Google Scholar]
- Endeward V., Al-Samir S., Itel F., Gros G. How does carbon dioxide permeate cell membranes? A discussion of concepts, results and methods. Front. Physiol. 2014 doi: 10.3389/fphys.2013.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez J. Vital effect on the stable-isotope composition seen in foraminifera and coral skeletons. Nature. 1978;273:199–202. [Google Scholar]
- Gabitov R.I., Rollion-Bard C., Tripati A., Sadekov A. In situ study of boron partitioning between calcite and fluid at different crystal growth rates. Geochim. Cosmochim. Acta. 2014;137:81–92. [Google Scholar]
- Gattuso J.-P., Frankignoulle M., Bourge I., Romaine S., Buddemeier R.W. Effect of calcium carbonate saturation of seawater on coral calcification. Glob. Planet. Change. 1998;18:37–46. [Google Scholar]
- Gattuso J.-P., Allemand D., Frankignoulle M. Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: a review on interactions and control by carbonate chemistry. Am. Zool. 1999;39:160–183. [Google Scholar]
- Hemming N.G., Hanson G.N. Boron isotopic composition and concentration in modern marine carbonates. Geochim. Cosmochim. Acta. 1992;56:537–543. [Google Scholar]
- Hoegh-Guldberg O., Mumby P.J., Hooten A.J., Steneck R.S., Greenfield P., Gomez E., Harvell C.D., Sale P.F., Edwards A.J., Caldeira K., Knowlton N., Eakin C.M., Iglesias-Prieto R., Muthiga N., Bradbury R.H., Dubi A., Hatziolos M.E. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- Holcomb M., DeCarlo T.M., Gaetani G.A., McCulloch M. Factors affecting B/Ca ratios in synthetic aragonite. Chem. Geol. 2016;437:67–76. [Google Scholar]
- Honisch B., Hemming N.G., Grottoli A.G., Amat A., Hanson G.N., Bjima J. Assessing scleractinian corals as recorders for paleo-pH: empirical calibration and vital effects. Geochim. Cosmochim. Acta. 2004;68:3675–3685. [Google Scholar]
- Ip Y.K., Lim A.L.L., Lim R.W.L. Some properties of calcium-activated adenosine-triphosphatase from the hermatypic coral Galaxea fascicularis. Mar. Biol. 1991;111:191–197. [Google Scholar]
- Klochko K., Kaufman A.J., Yao W.S., Bryne R.H., Tossell J.A. Experimental measurement of boron isotope fractionation in seawater. Earth Planet. Sci. Lett. 2006;248:276–285. [Google Scholar]
- Krief S., Hendy E.J., Fine M., Yam R., Meibom A., Foster G.L., Shemesh A. Physiological and isotopic responses of scleractinian corals to ocean acidification. Geochim. Cosmochim. Acta. 2010;74:4988–5001. [Google Scholar]
- Marshall A.T. Calcification in hermatypic and ahermatypic corals. Science. 1996;271:637–639. [Google Scholar]
- Mass T., Drake J.L., Haramaty L., Dongun Kim J., Zelzion E., Bhattacharya D., Falkowski P.G. Cloning and characterization of four novel coral acid-rich proteins that precipitate carbonates in vitro. Curr. Biol. 2013 doi: 10.1016/j.cub.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Mavromatis V., Montouillout V., Noireaux J., Gaillardet J., Schott J. Characterization of boron incorporation and speciation in calcite and aragonite from co-precipitation experiments under controlled pH, temperature and precipitation rate. Geochim. Cosmochim. Acta. 2015;150:299–313. [Google Scholar]
- Mehrbach C., Culberson C.H., Hawley J.E., Pytkowicx R.M. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 1973;18:897–907. [Google Scholar]
- Noireaux J., Mavromatis V., Gaillardet J., Neuville D. Crystallographic control on the boron isotope paleo-pH proxy. Earth Planet. Sci. Lett. 2015;430:398–407. [Google Scholar]
- Nothdurft L.D., Webb G.E. Microstructure of common reef-building coral genera Acropora, Pocillopora, Goniastrea and Porites: constraints on spatial resolution in geochemical sampling. Facies. 2007;53:1–26. [Google Scholar]
- Pierrot D.E., Lewis D., Wallace W.R. Oak Ridge National Laboratory; 2006. MS Excel Program Developed for CO2 System Calculations. [Google Scholar]
- Reynaud S., Hemming N.G., Juillet-Leclerc A., Gattuso J.P. Effect of pCO2 and temperature on the boron isotopic composition of the zooxanthellate coral Acropora sp. Coral Reefs. 2004;23:539–546. [Google Scholar]
- Sen S., Stebbins J.F., Hemming N.G., Ghosh B. Coordination environments of B-impurities in calcite and aragonite polymorphs – a B-11 mas NMR-study. Am. Miner. 1994;79:819–825. [Google Scholar]
- Tambutté E., Tambutté S., Segonds N., Zoccola D., Venn A., Erez J., Allemand D. Calcein labelling and electrophysiology: insights on coral tissue permeability and calcification. Proc. R. Soc. B. 2012;279:19–27. doi: 10.1098/rspb.2011.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchikawa J., Penman D.E., Zachos J.C., Zeebe R.E. Experimental evidence for kinetic effects on B/Ca in synthetic calcite: implications for potential B(OH)4− and B(OH)3 incorporation. Geochim. Cosmochim. Acta. 2015;150:171–191. [Google Scholar]
- Uppstrom L.R. The boron/chlorinity ratio of the deep-sea water from the Pacific Ocean. Deep-Sea Res. 1974;21:161–162. [Google Scholar]
- Venn A.A., Tambutte E., Holcomb M., Allemand D., Tambutte S. Live tissue imaging shows reef corals elevate pH under their calcifying tissue relative to seawater. PLoS One. 2011;6:e20013. doi: 10.1371/journal.pone.0020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn A.A., Tambutte E., Holcomb M., Tambutte S. Impact of seawater acidification on pH at the tissue-skeleton interface and calcification in reef corals. Proc. Natl. Acad. Sci. 2012;110 doi: 10.1073/pnas.1216153110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Euw S., Zhang Q., Manichev V., Murali N., Gross J., Feldman L.C., Gustafsson T., Flach C., Mendelsohn R., Falkowski P.G. Biological control of aragonite formation in stony corals. Science. 2017;356:933–938. doi: 10.1126/science.aam6371. [DOI] [PubMed] [Google Scholar]
- Watson E.A. A conceptual model for near-surface kinetic controls on the trace-element and stable isotope composition of abiogenic calcite crystals. Geochim. Cosmochim. Acta. 1994;68:1473–1488. [Google Scholar]
- Wolthers M., Nehrke G., Gustafsson J.P., Van Cappellen P. Calcite growth kinetics: modeling the effect of solution stoichiometry. Geochim. Cosmochim. Acta. 2012;77:121–134. [Google Scholar]
- Zeebe R.E., Wolf-Gladrow D. Elsevier; Amsterdam, 2001: 2001. CO2 in Seawater: Equilibrium, Kinetics Isotopes. [Google Scholar]
- Zoccola D., Ganot P., Bertucci A., Caminiti-Segonds N., Techer N., Voolstra C.R., Aranda M., Tambutté E., Allemand D., Casey J.R., Tambutté S. Bicarbonate transporters in corals point towards a key step in the evolution of cnidarian calcification. Sci. Rep. 2015;5:9983. doi: 10.1038/srep09983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.