Abstract
Poloxamers are triblock copolymers with a center block of hydrophobic polypropylene oxide (PPO) flanked by two hydrophilic polyethyleneoxide (PEO) blocks. Among this family of copolymers, poloxamer 407 is a non-ionic surfactant with reversible gelation properties above a particular polymer concentration and a particular temperature. Easy preparation of poloxamer 407 based sterile injectable formulations have made this copolymer a good candidate for drug delivery, specifically when controlled release of the drug is required. Previously, the applications of compendial poloxamer 407 preparations were demonstrated; however, low viscosity, poor elasticity, and sol-to-gel transition temperature (Tsol-gel) over a wide temperature range were observed. A purification process was introduced to eliminate impurities and low molecular weight copolymer molecules from the compendial poloxamer 407 resulting in higher viscosity values with Tsol-gel in a narrow temperature range. Here, poloxamer 407 was purified based on the proposed process and the rheological and analytical evaluation of the purified poloxamer 407 was conducted and compared to unpurified, compendial poloxamer 407. Then, the impact of poloxamer 407 concentration on gel formation was evaluated. For drug delivery applications, the effect of relevant buffer salts and the effect of addition of ethanol to the poloxamer 407 solutions were rheologically evaluated.
Keywords: Bioengineering, Materials Science
1. Introduction
Poloxamers (Pluronic®) are a family of triblock copolymers with a center block of hydrophobic polypropylene oxide (PPO) flanked by two hydrophilic polyethyleneoxide (PEO) blocks [1, 2]. Among the poloxamers, Poloxamer 407 with registered trademarks of Pluronic® F127 by BASF Laboratories and Synperonic PE/F127® by ICP Laboratories is a non-ionic surfactant with a wide range of applications [2, 3]. The formal molecular weight of poloxamer 407 is approximately 12.6 kDa with the lengths of two PEO blocks of 101 repeat units and a PPO block of 56 repeat units [2, 4]. Poloxamer 407 is known as an “inactive ingredient” by U.S. Food and Drug Administration (FDA) for a variety of drug products such as oral solutions, suspensions, inhalation formulations, intravenous (I.V.), ophthalmic or topical formulations [2].
Concentrated aqueous solutions of poloxamer 407 undergo thermoreversible gelation which makes this copolymer an interesting formulation choice for optimizing drug formulations and drug delivery applications [2, 5, 6, 7, 8, 9, 10]. In two separate studies, the application of poloxamer 407 for development of sustained release formulation of lidocaine was introduced [11, 12]. Ricci and coworkers showed that lidocaine release rate could be controlled by changing the poloxamer 407 concentration of the formulation. Their work also confirmed that the use of poloxamer 407 gels for delivery of lidocaine extends the residence time of the drug at the site of injection and sustains the release of the drug resulting in an increase in therapeutic efficacy [11]. In the second study, poloxamer 407 was introduced for intraperitoneal administration of both lidocaine and dexamethasone for management of post-operative pain [12]. Akkari and coworkers demonstrated a binary poloxamer 407/188 gel formulation as a delivery system for infiltrative local anesthesia for applications in the treatment of post-operative pain using ropivaciane as a model drug. They showed that the application of a poloxamer based formulation reduced in-vitro cytotoxic effect and increased the duration of analgesia without inducing in-vivo inflammation signs after local injection [13]. Poloxamer 407 was also used as a solubilizing agent for the formulation development of tolfenamic acid. The outcome of the study indicated the possibility of both systemic and topical administration of tolfenamic acid in an aqueous solution or gel format as a result of poloxamer 407 concentration [14]. A poloxamer 407 gel formulation was developed for intranasal delivery of naratriptan hydrochloride for treatment of intractable migraine. This study showed controllable sustained release of the drug from the formulation with no signs of damage to columnar epithelial cells in the animal model suggesting the non-toxic nature of the formulation [15]. Xuan and coworkers used poloxamer 407 for intramuscular delivery of piroxicam. They confirmed that the delivery of piroxicam using poloxamer 407 would be practically useful in that it allowed sustained release for the drug, resulting in better bioavailability [16]. Poloxamer 407 could also be used as a gelling system to improve the bioavailability of ophthalmic pharmaceutical formulations [17].
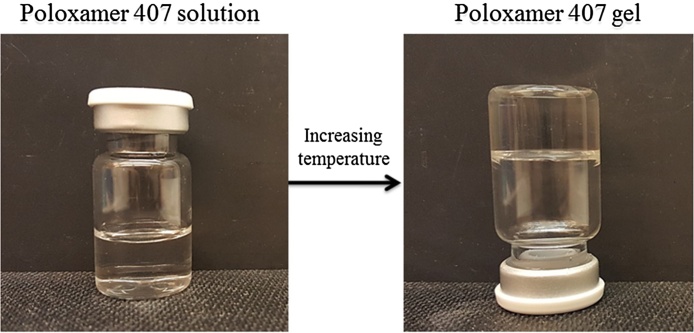
The gelation phenomenon is reversible and characterized by a sol-gel transition temperature (Tsol-gel). Below Tsol-gel, poloxamer 407 aqueous solutions remain fluid and the solution turns to a semi-solid material above this temperature [2, 3, 5]. The thermogelation is due to hydrophobic interactions between the poloxamer 407 copolymer chains. By elevating the temperature, the poloxamer 407 copolymer chains start to aggregate into a micellar structure. The formation of micelle structures is a result of the dehydration of the hydrophobic PPO repeat units and defines the initial step of gelation [2]. Tsol-gel is concentration dependent and increases by a reduction of the poloxamer 407 concentration in aqueous solution until a lower level is reached at which point poloxamer 407 does not gel anymore (Fig. 1) [2, 18]. Moreover, addition of active pharmaceutical ingredients, salts, excipients, and other compounds to the poloxamer 407 based formulations can increase or decrease the Tsol-gel [2].
Fig. 1.
Sol-to-gel transition of poloxamer 407. Tsol-gel in thermo-sensitive gels is dependent on the polymer concentration.
Ease of formulation preparation, solubilization ability as a surfactant, a final aqueous solution and the ability for sterile filtration have made this copolymer an interesting candidate for developing sterile products [2, 19]. Poloxamer 407 thermogels are thus good candidates for the delivery of small molecules, peptides, and biological molecules, specifically when controlled release of the drug is required [20]. Previously, compendial poloxamer 407 was shown to have low viscosity, poor elasticity, and Tsol-gel over a wide temperature range [21]. Reeve and Hinsberg described a process to eliminate impurities and low molecular weight copolymer molecules from compendial poloxamer 407 resulting in a narrow molecular weight distribution. They showed that after performing this purification process, higher viscosity values with Tsol-gel in a narrow temperature range could be observed [21]; however, no further characterization was performed. The purified poloxamer 407 material might potentially be a better formulation choice with superior properties as compared to the unpurified, compendial poloxamer 407 for biomedical and pharmaceutical applications [21]. The purified poloxamer 407 has been used for development of a sustained release formulation of dexamethasone in inner ear cochlear fluids by Otonomy Inc. [22].
This contribution describes the rheological and analytical evaluation of purified poloxamer 407 and compares it to unpurified poloxamer 407. Moreover, we evaluated the impact of addition of buffer salts to the poloxamer 407 solution, poloxamer 407 concentration, and the effect of ethanol addition to poloxamer 407 solutions and their effect on viscosity, Tsol-gel, and maximum recorded storage modulus.
2. Materials and methods
2.1. Materials
Ammonium sulfate ((NH4)2SO4) and poloxamer 407 NF were purchased from Sigma Aldrich. Dichloromethane (DCM) and undenatured ethanol USP were obtained from Spectrum Chemicals. Phosphate Buffered Saline pH 7.2 (PBS 1X) was obtained from Life Technologies. Tris-Buffered Saline pH 7.2 (Tris 1X) was made in the lab.
2.2. Methods
2.2.1. Purification of poloxamer 407
2.2.1.1. Process of purification
An aqueous-aqueous extraction procedure based on US6,761,824 was employed to purify poloxamer 407 [21]. First, poloxamer 407 (100 g) was added to distilled water (900 mL) in a 2L flask and stirred for 7 hours at 5 °C until the poloxamer 407 was fully dissolved. Then, a cooled ammonium sulfate solution (1000 mL, 25% w/v filtered through a 0.22 μm filter) was slowly added to the solution at 5 °C while mixing until a turbid solution was obtained. Next, the solution was transferred to a 2L separation funnel and kept overnight at 5 °C until two distinct phases developed. The lower phase was discarded and the upper phase was transferred to a 2L glass flask. Distilled water was added to the solution while mixing until the volume of the solution increased to 1L and the solution cooled back to 5 °C. Next, cooled ammonium sulfate solution (800 mL, 5 °C, 25% w/v filtered through a 0.22 μm filter) was slowly added to the poloxamer 407 solution until a turbid solution was observed. The turbid solution was transferred to the separation funnel and kept overnight at 5 °C until two distinct phases were obtained. The lower phase was again discarded and the upper phase was transferred back to a 2L glass flask. The volume of the solution was increased to 1 liter by adding distilled water while stirring and cooled to 5 °C. Cooled ammonium sulfate solution (800 mL, 5 °C, 25% w/v filtered through a 0.22 μm filter) was slowly added to the solution until a turbid solution was achieved. As previously done, the lower phase was discarded and the upper phase was transferred to a glass flask. 500 mL dichloromethane (DCM) was then added to the flask and stirred well. The mixture was transferred to the separation funnel and kept overnight at 5 °C until two distinct phases were achieved. The lower phase was collected and another 500 mL of DCM added to the separation funnel and mixed well. The mixture was kept overnight until two separated phases were obtained. This cycle was repeated one more time. After this final step, DCM was removed via a Rotavap until a concentrated poloxamer 407 solution was obtained. Finally, the purified poloxamer 407 was completely dried in a vacuum oven overnight at 30 °C.
2.2.1.2. Purification confirmation by HPLC-ELSD
The separation of the polymer species was based on chemical composition and not molecular volume or shape as measured by GPC. Due to the hydrophobic nature of the PPO repeat units, reserved phase HPLC was used to separate the polymer. It can be inferred that the species that elute prior to the main peak are representative of polymer chains with lower numbers of hydrophobic units and therefore represent polymer species that are shorter in length. Unpurified and purified poloxamer 407 was characterized using hydrophobicity of the polymer using an Agilent 1100HPLC system with a Sedex 75 evaporative light scattering detector using a Luna C8 column (Phenomenex, 5 μm 100 Å, 4.6 × 150 mm). Samples (50 μl, 1 mg/mL) were separated using a step-gradient of Mobile Phase A (50 mM ammonium acetate, pH 4) and Mobile Phase B (methanol) that was at 30% B for 2 min; 100% B for 10 min; and then 30% B for 6 min at a flow rate of 1 mL/minute and a column temperature of 28 °C. The ELSD was run at 50 °C drift tube temperature, 3.5 bar pressure and a gain of 3.
2.2.1.3. Purification confirmation by compendial testing
Three lots of the purified poloxamer 407 were characterized as described by the U.S. Pharmacopeia (USP) methods (USP29-NF24 Page 3392) described below and compared to unpurified poloxamer 407:
-
(A)
Spectrophotometric Identification Test- USP <197> by Infrared (IR) absorption spectrum exhibiting the maxima identical to the wavelength of the corresponding to the USP reference standard.
-
(B)
The average molecular weight of the polymer (range from 9840 to 14600) was determined by using phthalic anhydride titration as described in USP29-NF24.
-
(C)
The relative amount of polymer unsaturation (Specification: 0.048 +/− 0.017 mEq/g) was determined using Mercuric acetate solution as described in USP29-NF24.
-
(D)
The relative amount of methylene chloride was measured by the GC method described in USP Class 2 Residual Solvent <467> − (Procedure C). This confirmed the levels were no more than (NMT) 600 ppm.
2.2.1.4. Purification confirmation by rheological evaluation
Sol-to-gel transition temperature (Tsol-gel), maximum recorded storage modulus, and viscosity measurements for unpurified and purified poloxamer 407 solutions were conducted using a cone-plate geometry (radius 49.9 mm, 1° angle) and 800 μL sample volume with an MCR-301 torsional rheometer (Anton Paar) that detects torque (T) in the range 0.1 mNm to 200 mN. Samples, free of visible air bubbles, were prepared before setting the final measurement gap in the geometry by careful pipetting and visual inspection of the limited exposed surface. A hood covered the cone-plate geometry to mitigate evaporation.
A temperature sweep (10–40 °C) was conducted to record storage (G’) and loss modulus (G”) of the poloxamer 407 solutions during gelation. Tsol-gel was defined as the temperature at which the storage modulus (G’) is half way between the values of the storage modulus for the solution and the gel. Based on the preliminary strain sweep test results, a strain amplitude of 0.1% was used for the temperature sweep tests to apply adequate torque and linear viscoelastic behavior. The maximum storage modulus was recorded for each sample as an indication of the strength of the formed gel.
The viscosity of the samples was measured over a shear rate sweep of 1–1000 s−1. The testing was conducted at 10 °C and the viscosity at shear rate of 1000 s−1 was reported to compare the samples. Reproducibility of the measurements was tested by running temperature and shear rate sweep in triplicate (results not reported).
Three lots of purified poloxamer 407 and one lot of starting, unpurified poloxamer 407 NF were employed for rheological evaluations. For sample preparation, 17.9% (w/v) poloxamer 407 was dissolved in phosphate buffered saline (1X PBS) overnight at 5 °C while stirring. Then, the temperature sweep and shear rate sweep were performed for each prepared sample using the rheometer to measure Tsol-gel, maximum recorded storage modulus, and viscosity of the poloxamer 407 samples.
2.2.2. The effect of poloxamer 407 concentration on gel formation
In order to develop formulations using poloxamer 407, first the impact of the poloxamer 407 concentration on the gel formation was evaluated. Purified poloxamer 407 at concentrations of 10.5%, 11.6%, 12.6%, 13.7%, 14.7%, 15.8%, 16.8%, 17.9%, and 18.9% (w/v) were dissolved in 1X PBS overnight at 5 °C. Then, the effect of concentration on gel formation and viscosity were evaluated using the rheometer as described in Section 2.2.1.4.
2.2.3. The influence of salt addition on gel formation
As described previously, addition of salt to poloxamer 407 formulations can impact Tsol-gel. To evaluate the impact of salt addition on gel formation, purified poloxamer 407 at 17.9% w/v was dissolved in 1X PBS, distilled water, or 1X Tris buffer overnight at 5 °C. Then, the rheometer was used as described to investigate the effect of salt addition on gel formation and the viscosity of prepared poloxamer 407 solutions.
2.2.4. The influence of ethanol addition on gel formation
Water miscible organic solvent such as ethanol can be used as a co-solvent to incorporate active pharmaceutical ingredients into poloxamer 407 formulations for drug delivery applications. The addition of organic solvent may have an impact on gel formation, Tsol-gel, and the viscosity of poloxamer 407 based formulations. Here, ethanol at 2.5%, 5%, 7.5%, 10%, 15%, 20%, and 25% (v/v) was added to a 17.9% (w/v) poloxamer 407 solution in 1X PBS. Gel formation and the viscosity of the prepared samples were evaluated as described in Section 2.2.1.4.
3. Results and discussion
3.1. Purification of poloxamer 407
3.1.1. Process of purification
As described in Section 2.2, an aqueous-aqueous extraction procedure based on US6,761,824 was used to purify poloxamer 407 [21]. First, poloxamer 407 was fully dissolved in distilled water after 7 hours at 5 °C. Slow addition of cooled ammonium sulfate to the poloxamer 407 solution resulted in a turbid solution. By keeping this turbid solution in a separation funnel overnight at 5 °C, two distinct phases developed. The lower phase containing impurities was discarded. The upper phase containing poloxamer 407 was transferred to a glass flask and distilled water was added to the solution while mixing until the volume of the solution increased to 1L at 5 °C. This cycle of cooled ammonium sulfate solution addition to poloxamer solution and phase separation at 5 °C was carried out two more times to make sure the impurities were removed from poloxamer 407. After last phase separation and collecting the upper phase, dichloromethane (DCM) was added and mixed to the poloxamer 407 solution to extract poloxamer 407 from the solution. This mixture was transferred to the separation funnel and kept overnight at 5 °C until two phases were achieved. The lower (DCM containing poloxamer 407) was collected. Again, DCM was added to the remaining solution in the separation funnel and mixed well. The mixture was kept overnight at 5 °C to obtain two separate phases. This cycle was carried out one more time to make sure poloxamer 407 was fully extracted from solution into DCM. Finally, the DCM was removed from poloxamer 407 via a Rotavap and the poloxamer 407 was dried under vacuum overnight at 30 °C. The result of the purification process was a white powder collected and stored at room temperature.
3.1.2. Purification confirmation by RP-HPLC-ELSD
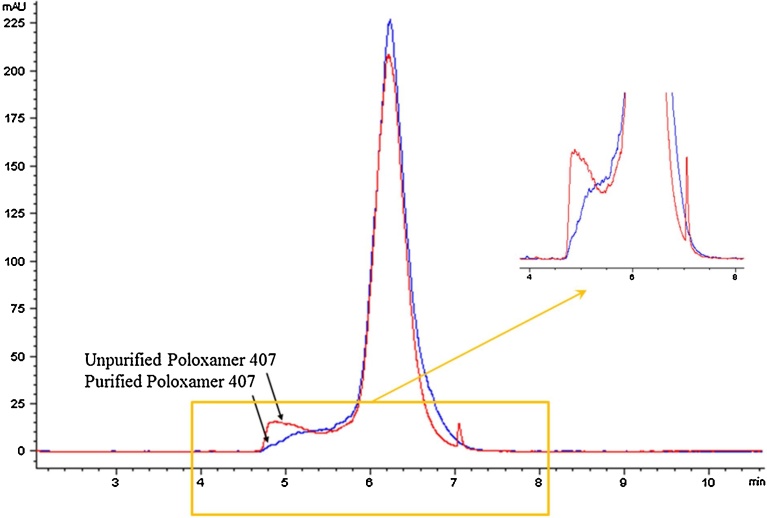
Reverse phase HPLC with ELSD was used to compare unpurified and purified poloxamer 407 and to confirm the purification step. We selected to separate the polymer based on chemical composition and not molecular volume or shape as measured by Gel Permeation Chromatography (GPC). Due to the hydrophobic nature of the PPO repeat units, reserved phase HPLC was used to separate the polymer. It can be inferred that the species that elute prior to the main peak are representative of polymer chains with lower numbers of hydrophobic units and therefore represent polymer species that are shorter in length. Prepared unpurified and purified samples were injected into the HPLC column and generated data were collected. Fig. 2 shows the HPLC-ELSD results for both unpurified and purified poloxamer 407. The five minutes shoulder of the unpurified poloxamer sample is minimized indicting that lower molecular weight impurities from the starting poloxamer 407 were eliminated [21].
Fig. 2.
HPLC-ELSD Poloxamer 407 purification results.
3.1.3. Purification confirmation by compendial testing
To evaluate the purification process and confirm its reproducibility, three lots of purified poloxamer 407 were prepared, characterized by the USP methods, and compared to unpurified poloxamer 407. As indicated in Table 1, the USP characterization of the three purified poloxamer 407 lots shows that the properties are comparable to each other and differ from the unpurified starting polymer. Table 1 shows that the average molecular weight increased, indicating that lower molecular weight impurities were removed during the purification process and the unsaturation levels were also decreased because of the purification, confirming successful purification by elimination of lower molecular weight impurities. Moreover, IR results showed no change because of purification process indicating that the purification process did not impact on the chemical structure of the poloxamer 407. The results of the purification were still within USP compendia ranges for poloxamer 407 for most criteria other than unsaturation, which decreased below compendia levels. The minor changes in attributes between the purified polymers and the unpurified starting material translated into divergent rheological properties as will be described in Sections 3.1.3. Overall, purification of the polymer results in a product that can be reproducibly manufacturered and characterized for clinical applications.
Table 1.
Characterization of three lots of purified poloxamer 407 and comparison to unpurified poloxamer 407 based on USP methods.
| Poloxamer lot | Identification by <USP197> (IR) | Average molecular weight (Da) | Unsaturation (mEq/g) | Class 2 Residual Solvent <467> (DCM) |
|---|---|---|---|---|
| USP specification | Meets | 9840–14600 | 0.048 ± 0.017 | <600 ppm |
| Unpurified | Meets | 10,988 | 0.057 | <600 ppm |
| Purified lot #1 | Meets | 12,590 | 0.025 | <600 ppm |
| Purified lot #2 | Meets | 11,956 | 0.019 | <600 ppm |
| Purified lot #3 | Meets | 12,128 | 0.020 | <600 ppm |
3.1.4. Purification confirmation by rheological evaluation
Poloxamer 407 purification was also confirmed via rheological evaluation. Three lots of purified poloxamer 407 and one lot of starting, unpurified poloxamer 407, were used for rheological evaluations. All the samples were prepared at 17.9% (w/v) poloxamer 407 concentration in 1X PBS. Temperature sweeps were performed to obtain Tsol-gel for the purified and unpurified polymer and maximum recorded storage modulus and shear rate sweep was conducted to measure the viscosity.
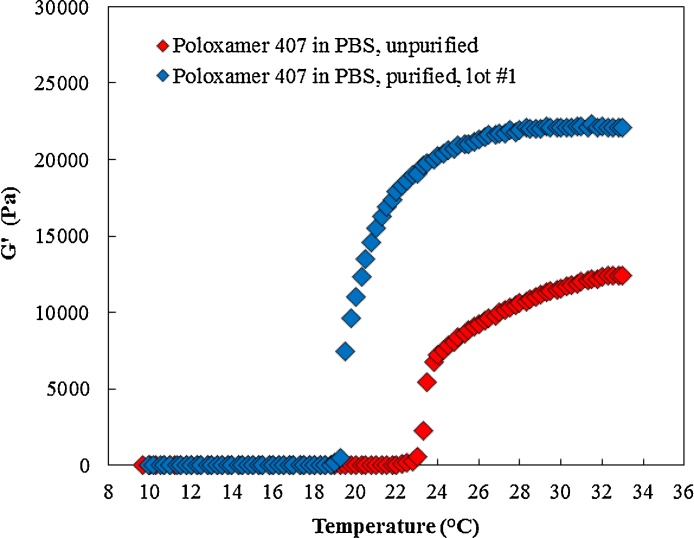
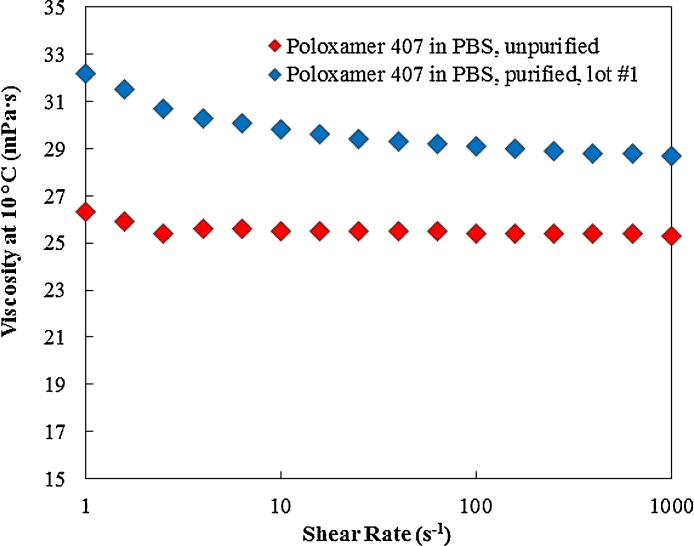
Fig. 3 and Table 2 show the Tsol-gel and maximum recorded storage modulus (G’) for unpurified and purified poloxamer 407. Purification reduced the Tsol-gel from 23.4 °C to 20.9 °C and increased the maximum recorded storage modulus from 12.9 kPa to 22.3 kPa. Moreover, the viscosity results shown in Fig. 4 and Table 2 indicate that the viscosity of purified poloxamer 407 is greater than the viscosity of unpurified poloxamer 407 at the tested condition. These results confirmed that the purification was successful in removing lower molecular weight impurities from the starting poloxamer 407 that have an impact on gelation. The purified poloxamer 407 underwent sol-to-gel transition at lower temperature and the formed gel had a higher maximum recorded storage modulus as compared to unpurified poloxamer 407.
Fig. 3.
The impact of purification on gel formation. Purified poloxamer 407 goes into sol-to-gel transition at lower temperature. The maximum recorded storage modulus increased as a result of purification.
Table 2.
Recorded Tsol-gel, maximum storage modulus, and viscosity of the unpurified and purified poloxamer 407 samples.
| Sample ID | Tsol-gel (°C) | Maximum recorded storage modulus (kPa) | Viscosity (mPa s) at 1000 (s−1) & 10 °C |
|---|---|---|---|
| Poloxamer 407 in PBS, unpurified | 23.4 | 12.9 | 25.3 |
| Poloxamer 407 in PBS, purified, lot #1 | 20.9 | 22.3 | 28.7 |
Fig. 4.
The impact of purification on viscosity of poloxamer 407 solution at 10 °C. Higher viscosity values were recorded for the purified poloxamer 407 as compared to unpurified poloxamer 407.
The change in rheological properties of purified poloxamer 407 as compared to unpurified poloxamer 407 confirmed that purification process removed the impurities from poloxamer 407. These impurities could be diblock copolymers of PPO and PEO which are not fully synthesized or removed during manufacturing process of poloxamer 407. These impurities could disrupt hydrophobic interactions during sol-to-gel transition of poloxamer 407 resulting in gelation at higher temperature. Removing such impurities from poloxamer 407 could result in elimination of this distribution from hydrophobic interactions of poloxamer 407 copolymer chains and gelation at lower temperature. As showed here, the purification reduced the Tsol-gel indicating that the impurities were removed from poloxamer 407. The absence of such impurities could lead to stronger hydrophobic interactions resulting formation of an stronger gel. Here, we observed the increase in maximum recorded storage modulus. Same as sol-to-gel transition, the viscosity of poloxamer 407 could be impacted by elimination of such impurities. Removing diblock copolymers of PPO and PEO from poloxamer 407 could increase the interactions between poloxamer 407 polymer chains leading to increase the viscosity. Here, we confirmed that the purification process increased the viscosity of poloxamer 407.
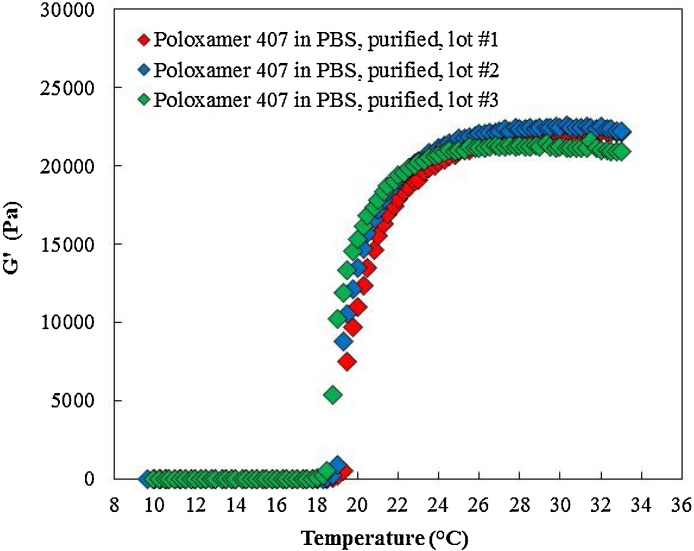
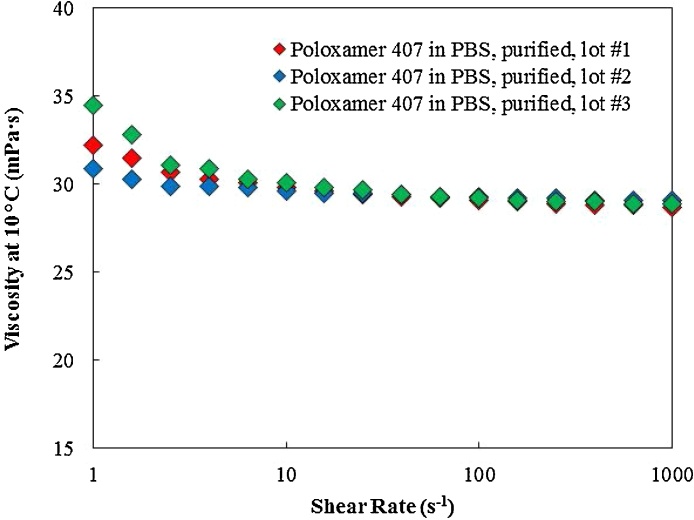
For lot-to-lot comparison and reproducibility evaluation, three purified poloxamer 407 batches were prepared and tested from the same starting lot of poloxamer 407. Fig. 5, Fig. 6, and Table 3 present the rheological results and comparison of the three purified lots. The average Tsol-gel recorded at 20.03 °C with a 2.5% coefficient of variation. Additionally, the average maximum recorded storage modulus for the three purified lots obtained at 22.5 kPa with a 3.0% coefficient of variation. Finally, the recorded viscosity at 1000 s−1 and 10 °C was 29.1 mPa s with a 1.4% coefficient of variation. The results indicated that the purification process was reproducible with a maximum of 3% variation in rheological properties between the three lots.
Fig. 5.
Tsol-gel and maximum storage modulus of three lots of purified poloxamer 407. The Tsol-gel and maximum storage modulus for three purified lots of poloxamer 407 were comparable.
Fig. 6.
Viscosity of three lots of purified poloxamer 407 measured at 10 °C over a shear rate sweep. The viscosity for three purified lots of poloxamer 407 were comparable.
Table 3.
Recorded Tsol-gel, maximum storage modulus, and viscosity of the three lots of purified poloxamer 407 samples.
| Sample ID | Tsol-gel (°C) | Maximum recorded storage modulus (kPa) | Viscosity (mPa s) at 1000 (s−1) & 10 °C |
|---|---|---|---|
| Poloxamer 407 in PBS, purified, lot #1 | 20.9 | 22.3 | 28.7 |
| Poloxamer 407 in PBS, purified, lot #2 | 20.25 | 22.6 | 29.1 |
| Poloxamer 407 in PBS, purified, lot #3 | 19.9 | 21.6 | 28.9 |
| Average | 20.03 | 22.5 | 29.1 |
| Coefficient Variation | 2.5% | 3.0% | 1.4% |
3.2. The effect of poloxamer 407 concentration on gel formation
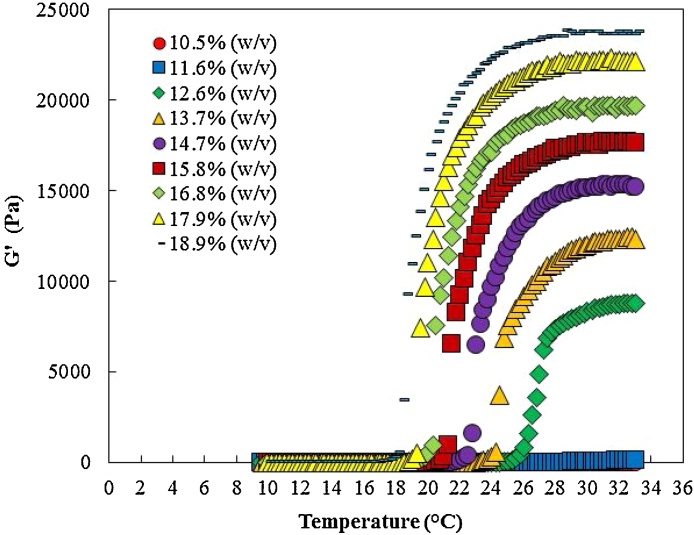
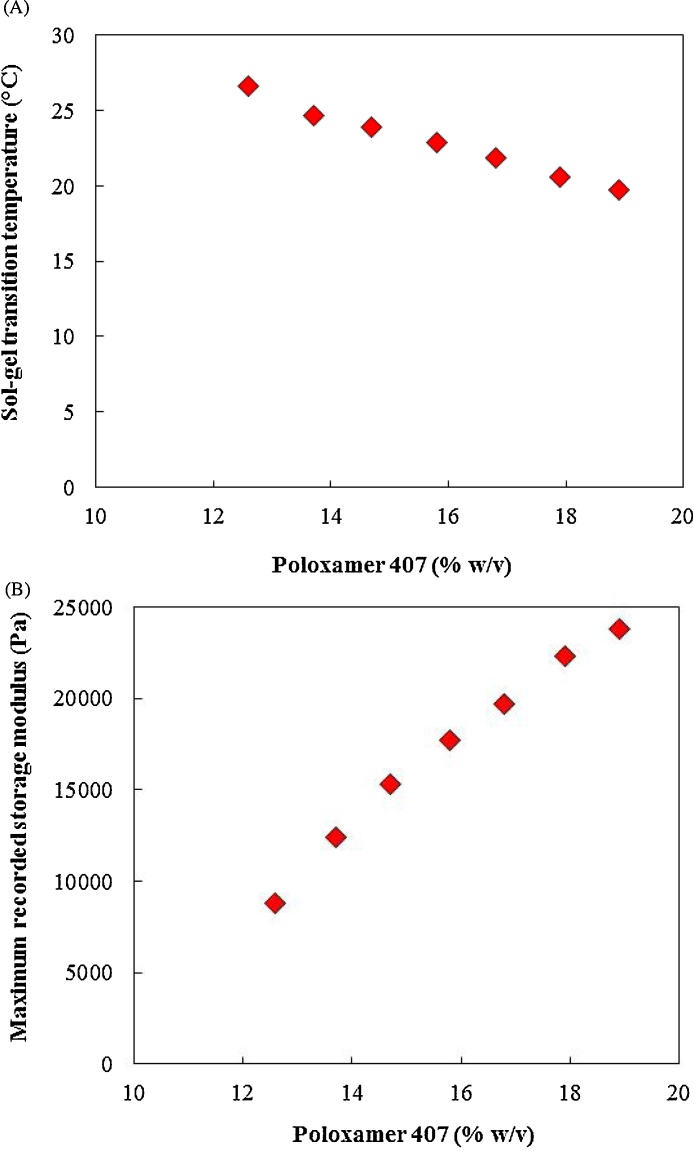
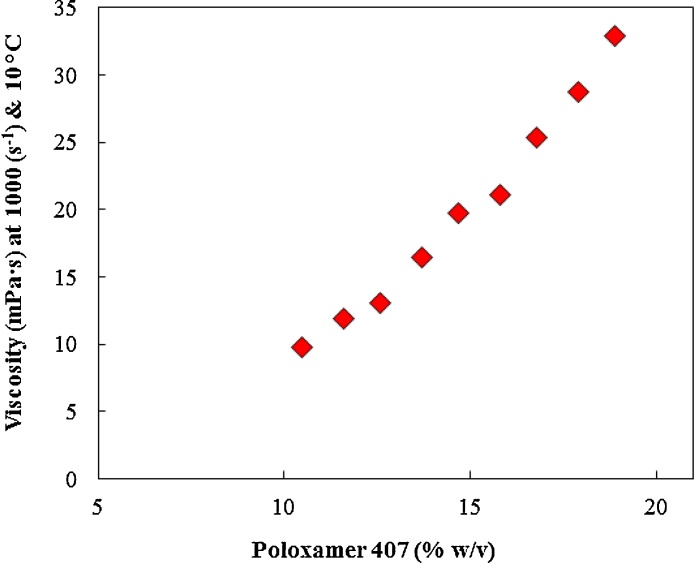
Fig. 7 shows the impact of poloxamer 407 concentration on gel formation. The results demonstrated that no gel was formed at 11.6% w/v and 10.5% w/v poloxamer 407 concentrations. Fig. 8 indicates that increasing the poloxamer 407 concentration decreased the Tsol-gel and increased the maximum recorded storage modulus. Higher poloxamer 407 concentration resulted in gel formation at lower temperatures. Moreover, the increase in the poloxamer 407 concentration increased the maximum recorded storage modulus. This indicated that at higher poloxamer 407 concentrations stronger gel were formed.
Fig. 7.
The impact of poloxamer 407 concentration on gel formation.
Fig. 8.
The influence of poloxamer 407 concentration on (A) Tsol-gel transition temperature and (B) maximum recorded storage modulus.
Fig. 9 shows the effect of poloxamer 407 concentration on viscosity at 10 °C. This result demonstrated that the increase in poloxamer 407 concentration increased the visocsity at 10 °C as a result of more physical entanglement.
Fig. 9.
The influence of poloxamer 407 concentration on viscosity at 10 °C.
3.3. The influence of salt addition on gel formation
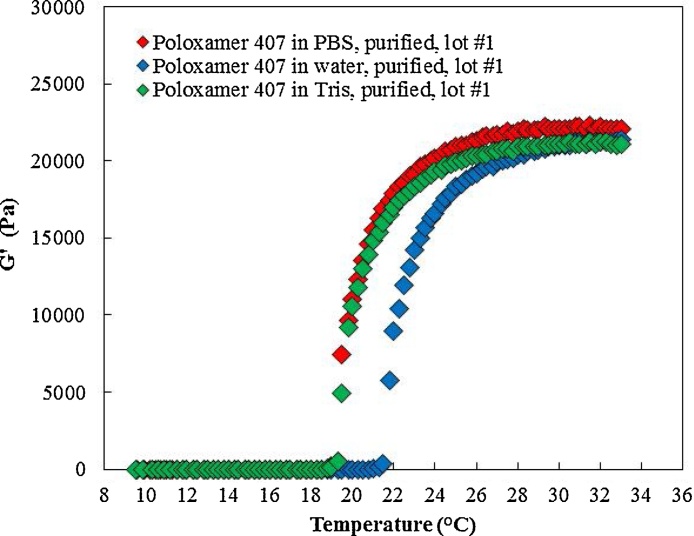
As discussed in the introduction section, gel formation and Tsol-gel can be impacted by addition of active pharmaceutical ingredients, salts, excipients, and other compounds. Here, we evaluated the impact of addition of typical buffer salts to the poloxamer 407. Purified poloxamer 407 was dissolved in 1X PBS, distilled water, or 1X tris buffer at 17.9 (w/v) concentration. Then, the rheometer was used to evaluate the prepared samples.
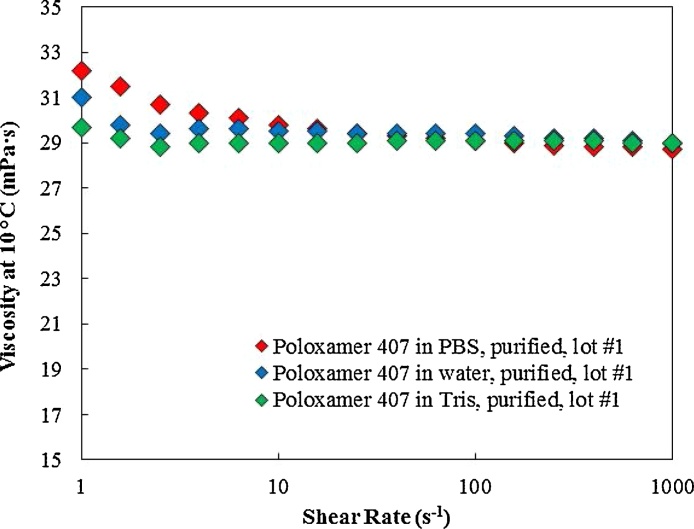
Fig. 10, Fig. 11, and Table 4 show the rheological properties of poloxamer 407 (17.9 w/v) in 1X PBS, distilled water, and 1X tris buffer. Addition of salt (1X PBS or 1X tris) reduced the Tsol-gel but did not have an impact on maximum recorded storage modulus and viscosity. The presence of the salt in the poloxamer 407 solution resulted in formation of micelles (and eventually gel formation) at lower temperature, however, it did not influence the poloxamer 407 polymer interaction which would cause a change in maximum recorded storage modulus. As shown in Table 4, the viscosity was not impacted by addition of salt and it remained constant at ∼ 29 mPa s at 1000 (s−1) at 10 °C for the tested samples.
Fig. 10.
Tsol-gel and maximum storage modulus of poloxamer 407 dissolved in 1X PBS, distilled water, and 1X Tris buffer. The presence of salt in poloxamer 407 aqueous solution reduced the Tsol-gel but did not impact on maximum storage modulus.
Fig. 11.
Viscosity of poloxamer 407 dissolved in 1X PBS, distilled water, and 1X Tris buffer measured at 10 °C. The viscosity of poloxamer 407 aqueous solutions were not impacted by addition of salt.
Table 4.
Recorded Tsol-gel, maximum storage modulus, and viscosity of poloxamer 407 dissolved in 1X PBS, distilled water, and 1X Tris buffer.
| Sample ID | Tsol-gel (°C) | Maximum recorded storage modulus (Pa) | Viscosity (mPa s) at 1000 (s−1) & 10 °C |
|---|---|---|---|
| Poloxamer 407 in 1X PBS, purified | 20.9 | 22300 | 28.7 |
| Poloxamer 407 in water, purified | 23.0 | 21500 | 29 |
| Poloxamer 407 in 1X tris, purified | 21.2 | 21200 | 29 |
In formulation development, addition of salts to make solutions isotonic is an important parameter. The experiments conducted here demonstrated that there was no impact on maximum storage modulus and viscosity, when using PBS or Tris buffers, while there was a modest reduction in the Tsol-gel. This finding probably extends to other typically used formulation salts and is especially important in drug delivery applications of such poloxamer solutions in protein delivery [23, 24, 25].
3.4. Effect of ethanol addition to poloxamer 407
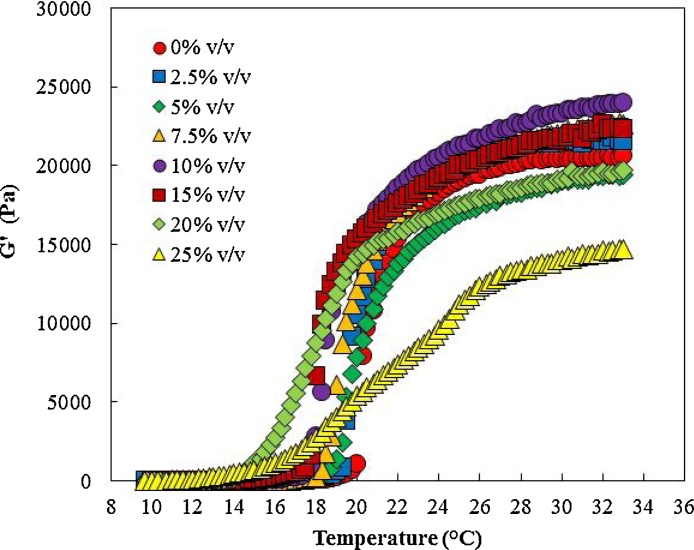
Ethanol and other water miscible organic solvents can be used to incorporate poorly water soluble active pharmacutical ingredients into poloxamer 407 aqueous solutions for drug delivery applications. Previously, the effect of dimethyl sulfoxide (DMSO), a water missible organic solvent, on poloxamer 407 gel formation and drug release was evalauted [26]. Here, we used ethanol as another commonly used and benign water miscible organic solvent. To evaluate the effect of ethanol addition, ethanol was added to the poloxamer 407 solution at different concentrations from 0%, 2.5%, 5%, 7.5%, 10%, 15%, 20%, and 25% (v/v) to evaluate its impact on gel formation, Tsol-gel, strength of the formed gel, and the viscosity of the poloxamer 407 solution. Then, the prepared samples were characterized by rheometer.
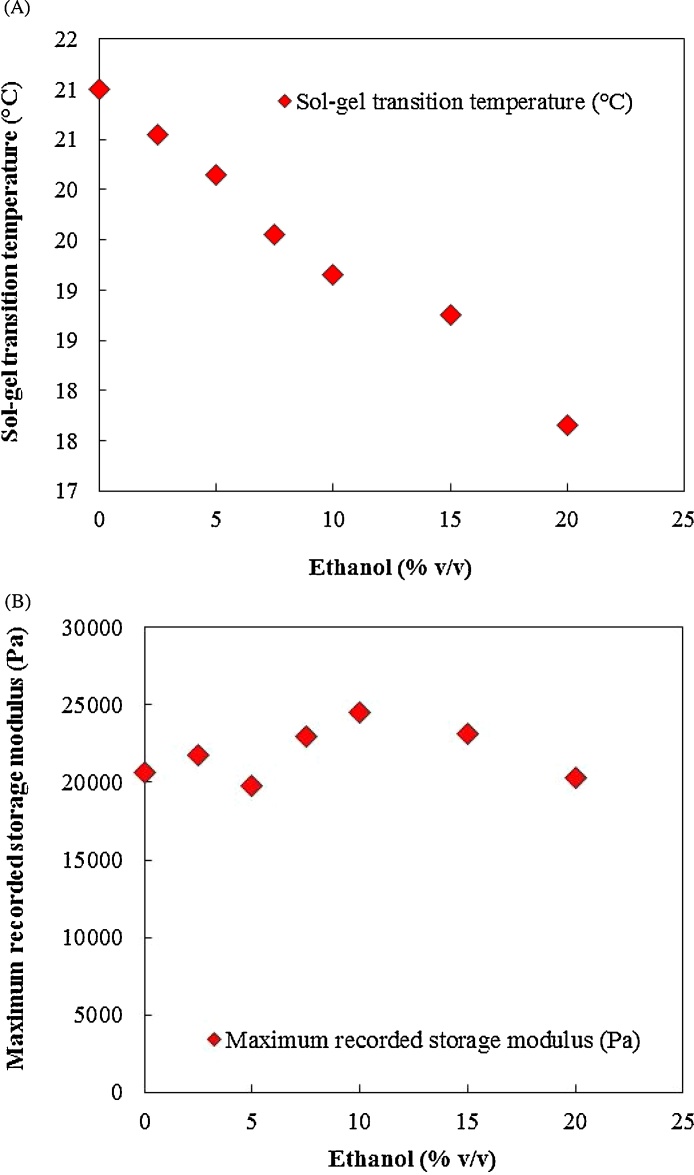
Fig. 12, Fig. 13 present the effect of increasing ethanol concentration on gel formation. Increasing ethanol from 0% (v/v) to 20% (v/v) decreased the Tsol-gel but did not have an impact on maximum recorded storage modulus. Increasing ethanol concentration further to 25% (v/v) prevented gel formation. This could be as a result of prevention of poloxamer 407 hydrophobic interactions and micelle formation at 25% (v/v) ethanol concentration.
Fig. 12.
The impact of ethanol concentration on gel formation.
Fig. 13.
The influence of ethanol concentration on (A) Tsol-gel and (B) maximum recorded storage modulus. Increasing ethanol decreased Tsol-gelbut did not impact on maximum storage modulus.
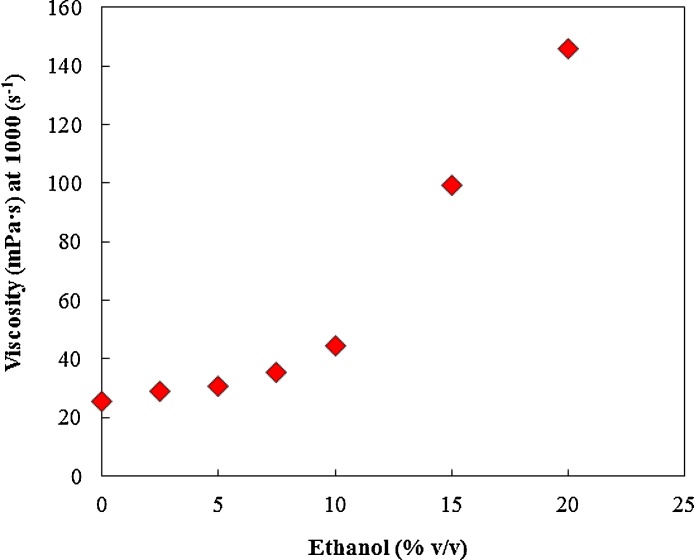
Fig. 14 shows the impact of ethanol concentration on viscosity of poloxamer 407 at 10 °C with increasing ethanol concentration leading to increased viscosity of poloxamer 407 solutions.
Fig. 14.
The influence of ethanol concentration on viscosity of poloxamer 407 solution at 10 °C. Increasing ethanol increased the viscosity of poloxamer 407 aqueous solution.
Thus, addition of ethanol impacted on the rheological behavior of poloxamer 407 aqueous solutions until an upper limit of about 20% v/v. Many small molecule drugs are poorly soluble in aqueous solutions, making intra-tumoral injections of such drugs challenging [27]. Addition of water-miscible solvents can overcome some of these formulation challenges, but it still leaves open a controlled drug release challenge. This drug delivery challenge could be overcome when using theremo-sensitive poloxamer solutions [27]. For example, ethanol can be used as a co-solvent for incorporation and controlled precipitation of water insoluble active pharmaceutical ingredients within poloxamer 407 aqueous solution for drug delivery applications. We identified the maximum ethanol percentage that could be added as a co-solvent to the poloxamer 407 aqueous solution so that the poloxamer 407 can still undergo sol-to-gel transition. These findings will help the development of sustained release formulations of small molecular drugs based on poloxamer 407 thermo-sensitive gel.
4. Conclusion
Compendial poloxamer 407 is a widely used polymer for drug delivery applications due to its ease of use and ease of formulation with a large variety of molecules from small molecular weight drugs to biologics. Here, poloxamer 407 was purified based on a published process[21] and the rheological and analytical evaluation of the purified poloxamer 407 were conducted and compared to unpurified, compendial poloxamer 407. The rheological and analytical evaluation confirmed that the purification process was successful in improving the rheological properties of the resulting purified polymer with sharply defined Tsol-gel and higher resulting viscosity. The impact of poloxamer 407 concentration on gel formation was evaluated and it was confirmed that increasing poloxamer 407 concentration impacted on Tsol-gel, maximum recorded storage modulus, and viscosity with lower amounts of the purified poloxamer 407 needed to reach comparable levels to the unpurified, compendial poloxamer 407.
To properly formulate a variety of drugs, additives such as buffers and organic co-solvents might be needed. Addition of typical buffer salts, phosphate and tromethamine, also impacted on the rheological properties of poloxamer 407 aqueous solution resulting in reduction of Tsol-gel. We showed that Tsol-gel can be reduced by addition of salt without any impact on maximum storage modulus and viscosity. The lowering of Tsol-gel due to the buffer salts should have no effect on the formulations incorporating biological molecules which require the presence of salts (buffer) in the formulation as the final viscosity did not change and presumably the diffusion properties of the gel did not change.
For unsoluble small molecules organic co-solvents might be needed for their formulation in these aqueous polymer solutions. The rheological effects of ethanol addition to the poloxamer 407 aqueous solution were evaluated and it was demonstrated that increasing ethanol concentration reduced Tsol-gel and increased the viscosity of poloxamer 407 solution. Up to 20% v/v ethanol addition as a co-solvent was tolerated and sol-gel transition still took place. Similar to DMSO, ethanol can be used as a co-solvent to incorporate and precipitation of water insoluble active pharmaceutical ingredients within in poloxamer 407 in a controlled manner. At high ethanol concentration (25% v/v), gel formation was disrupted.
Purified poloxamer 407 can potentially be used for development of sustained release systems and delivery of all types of active pharmaceutical ingredient including small molecules, peptides, and proteins with higher control on rheological properties as compared to unpurified compendial poloxamer 407. In case of buffer salts which are normally used to stabilize compounds such as biological molecules, the impact of the buffer should be evaluated on gel formation. Finally, co-solvents such as ethanol and DMSO can be employed to introduce water insoluble compounds such as small molecules into poloxamer 407 for development of sustained release formulations.
Declarations
Author contribution statement
Amir Fakhari: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Marta Corcoran, Alexander Schwarz: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Competing interest statement
The authors declare no conflict of interest.
Funding statement
This work was supported by MedImmune LLC.
Additional Information
No additional information is available for this paper.
References
- 1.Fakhari A., Berkland C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 2013;9(7):7081–7092. doi: 10.1016/j.actbio.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dumortier G. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006;23(12):2709–2728. doi: 10.1007/s11095-006-9104-4. [DOI] [PubMed] [Google Scholar]
- 3.Yu G.-E. Micellisation and gelation of triblock copoly (oxyethylene/oxypropylene/oxyethylene), F127. J. Chem. Soc. Faraday Trans. 1992;88(17):2537–2544. [Google Scholar]
- 4.Peretti K.L. A highly active, isospecific cobalt catalyst for propylene oxide polymerization. J. Am. Chem. Soc. 2005;127(33):11566–11567. doi: 10.1021/ja053451y. [DOI] [PubMed] [Google Scholar]
- 5.Pereira G.G. Formulation and characterization of poloxamer 407®: thermoreversible gel containing polymeric microparticles and hyaluronic acid. Química Nova. 2013;36(8):1121–1125. [Google Scholar]
- 6.Branco M.C., Schneider J.P. Self-assembling materials for therapeutic delivery. Acta Biomater. 2009;5(3):817–831. doi: 10.1016/j.actbio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabanov A.V., Batrakova E.V., Alakhov V.Y. Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control. Release. 2002;82(2):189–212. doi: 10.1016/s0168-3659(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 8.Kojarunchitt T. Development and characterisation of modified poloxamer 407 thermoresponsive depot systems containing cubosomes. Int. J. Pharm. 2011;408(1):20–26. doi: 10.1016/j.ijpharm.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 9.Akash M.S.H., Rehman K. Recent progress in biomedical applications of Pluronic (PF127): pharmaceutical perspectives. J. Control. Release. 2015;209:120–138. doi: 10.1016/j.jconrel.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 10.Das N., Madan P., Lin S. Statistical optimization of insulin-loaded Pluronic F-127 gels for buccal delivery of basal insulin. Pharm. Dev. Technol. 2012;17(3):363–374. doi: 10.3109/10837450.2010.542164. [DOI] [PubMed] [Google Scholar]
- 11.Ricci E. Sustained release of lidocaine from Poloxamer 407 gels. Int. J. Pharm. 2005;288(2):235–244. doi: 10.1016/j.ijpharm.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 12.Arbelaez-Camargo D. Preformulation and characterization of a lidocaine hydrochloride and dexamethasone sodium phosphate thermo-reversible and bioadhesive long-acting gel for intraperitoneal administration. Int. J. Pharm. 2016;498(1):142–152. doi: 10.1016/j.ijpharm.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Akkari A.C. Poloxamer 407/188 binary thermosensitive hydrogels as delivery systems for infiltrative local anesthesia: physico-chemical characterization and pharmacological evaluation. Mater. Sci. Eng.: C. 2016;68:299–307. doi: 10.1016/j.msec.2016.05.088. [DOI] [PubMed] [Google Scholar]
- 14.Cafaggi S. Poloxamer 407 as a solubilising agent for tolfenamic acid and as a base for a gel formulation. Eur. J. Pharm. Sci. 2008;35(1):19–29. doi: 10.1016/j.ejps.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Shelke S. Formulation and evaluation of thermoreversible mucoadhesive in-situ gel for intranasal delivery of naratriptan hydrochloride. J. Drug Deliv. Sci. Technol. 2015;29:238–244. [Google Scholar]
- 16.Xuan J.-J. Rheological characterization and in vivo evaluation of thermosensitive poloxamer-based hydrogel for intramuscular injection of piroxicam. Int. J. Pharm. 2010;395(1):317–323. doi: 10.1016/j.ijpharm.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 17.Almeida H. In situ gelling systems: a strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov. Today. 2014;19(4):400–412. doi: 10.1016/j.drudis.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Fakhari A., Subramony J.A. Engineered in-situ depot-forming hydrogels for intratumoral drug delivery. J. Control. Release. 2015;220:465–475. doi: 10.1016/j.jconrel.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Loh X.J. Springer; 2015. In-Situ Gelling Polymers. [Google Scholar]
- 20.Fakhari A. Thermosensitive gel based formulation for intra-tumoral delivery of toll-like receptor 7/8 dual agonist, MEDI9197. J. Pharm. Sci. 2017 doi: 10.1016/j.xphs.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 21.L.E. Reeve, M.G. Hinsberg, Process for the fractionation of polymers, 2004, Google Patents. US6977045 B2.
- 22.Wang X. Dose-dependent sustained release of dexamethasone in inner ear cochlear fluids using a novel local delivery approach. Audiol. Neurotol. 2009;14(6):393–401. doi: 10.1159/000241896. [DOI] [PubMed] [Google Scholar]
- 23.Stratton L.P. Drug delivery matrix containing native protein precipitates suspended in a poloxamer gel. J. Pharm. Sci. 1997;86(9):1006–1010. doi: 10.1021/js970034d. [DOI] [PubMed] [Google Scholar]
- 24.Akash M.S.H., Rehman K., Chen S. Pluronic F127-based thermosensitive gels for delivery of therapeutic proteins and peptides. Polym. Rev. 2014;54(4):573–597. [Google Scholar]
- 25.Akash M.S.H. Assessment of release kinetics, stability and polymer interaction of poloxamer 407-based thermosensitive gel of interleukin-1 receptor antagonist. Pharm. Dev. Technol. 2014;19(3):278–284. doi: 10.3109/10837450.2013.775158. [DOI] [PubMed] [Google Scholar]
- 26.Ur-Rehman T., Tavelin S., Gröbner G. Effect of DMSO on micellization, gelation and drug release profile of Poloxamer 407. Int. J. Pharm. 2010;394(1):92–98. doi: 10.1016/j.ijpharm.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Fakhari A. Thermosensitive gel-based formulation for intratumoral delivery of toll-like receptor 7/8 dual agonist, MEDI9197. J. Pharm. Sci. 2017;106(8):2037–2045. doi: 10.1016/j.xphs.2017.04.041. [DOI] [PubMed] [Google Scholar]