Abstract
Endobronchial ultrasound endoscopy is a state of the art diagnostic endoscopic procedure for the thorax. Firstly it was designed mainly for the staging of lung cancer and of course for the diagnosis of suspicious findings in large central airways. The main limitation of the equipment is the diameter of the instrument and therefore it can only be guided through large airways. However; the diameter of the working channel also provides a large tissue sample nowadays with the 19G biopsy needle. We will provide our experience with the 22G needle of the endobronchial convex-probe in several medical situations of the thorax.
Keywords: EBUS, Pentax, Hitachi, Endobronchial ultrasound, Sarcoidosis, Lymphoma, Lung cancer, Thoracic malignancies
1. Introduction
Bronchoscopy used to be the first endoscopic procedure to be performed for diagnosis of suspicious findings in the thorax. Bronchoscopy is performed with mild sedation or local anesthesia (lidocaine) based on the centers' experience and equipment. However; there are several cases where a more advanced equipment was necessary in order to diagnosed central findings next to large vessels without endobronchial findings. Therefore the convex-probe endobronchial sound (EBUS) was designed [1]. This equipment was also designed in order to supplement the positron emission tomography (PET-CT) by taking biopsies from the lymph nodes of 7 different positions within the thorax. Until now PET-CT cannot replace mediastinoscopy and therefore lymph node biopsy is necessary with the convex – probe EBUS [2], [3]. We will present a case series where diagnosis was performed with a convex-probe EBUS 22G needle, we will try and focus on the pathological findings/technique and materials used.
2. Material and methods
EBUS guided tissue material undergone by three-steps procedure. First step is the infiltration of visible tissues that can be selected by a niddle surgical forchep. This material is treated like histological specimen and followed by graduated over-night dehydration and then embedded in paraffin. Three micron tissue sections were taken for histological examination after Haematoxylin/Eosin (H/E) stained. The second step is the centrifugation of the rest material that provides a viscous deposit, full of small tissue fragments, that can be selected by a pipette and create a cell block. This material is also treated like a tissue one, followed by overnight dehydration and embedded in paraffin. Tissue sections can be taken for histological examination. The third step is, the treatment of the rest complete liquid material, as a cytological material. This is centrifugated in a cytostatic centrifuge and the precipitate is coated on positive charged slides and stained by “Papanikolaou” (PAP) stain. More unstained slides can by prepared for cytochemical evaluation. The material that result by the two first steps, is a histological material, that can provide numerous sections for histological and immunohistochemical procedures. It is also suitable for molecular tests due to the adequate amount of DNA that can be extracted of the paraffin embedded tissues. All the biopsies were performed with a 22G Mediglobe needle (Fig. 1). In our case series we used a Pentax Convex-Probe-Endobronchial Ultrasound (Fig. 2).
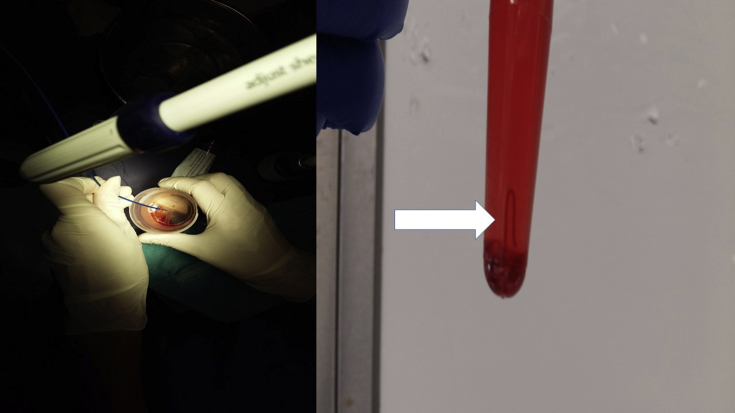
Fig. 1.
On the left side: after the biopsy, cleaning the 22G needle, on the right side: a tissue core from the lesion (Figures by Paul Zarogoulidis).
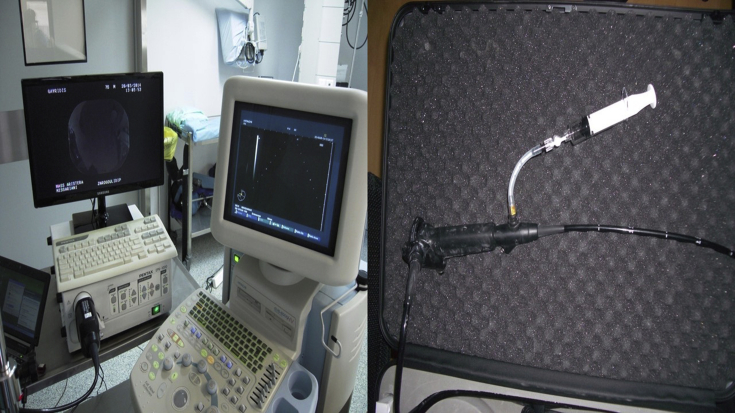
Fig. 2.
On the left side: the equipment of Pentax, an EPK-1000 and EUB-6500HV, on the right side: a EB-1970UK EBUS endoscope (Figures by Paul Zarogoulidis equipment in the Private Hospital “Saint Luke'’).
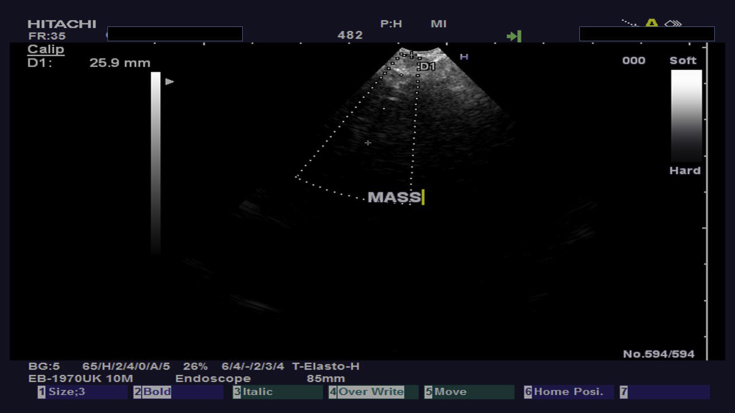
3. Case 1 (b-cell lymphoma)
A 45 year old woman was refered to a tertiary hospital for investigation of pulmonary hypertension. Upon CT of the chest lymphnode enlargement was observed ≤3cm and endobronchial ultrasound was performed. Fig. 3, Fig. 4. The FNA material of the station 4 R included numerous lymphoid cells. These cells were small to medium sized and provided a monotonous appearance. The cytoplasm was minimal and the nucleus rounded to oval shaped partially convoluted or dark with inconspicuous nucleolies. The features of the lymphoid cells were like lymphoplsmatoid or monocytoid cells (Fig. 5). The immunohistochemical examination revealed that the neoplastic cells were negative for Cytokeratin 8/18 and synaptophysin, excluded the diagnosis of an epithelial neoplasm or neuroendocrine carcinoma. The majority of neoplastic cells were positive for CD20 (Fig. 6), a B-cell lymphoid marker, supporting the diagnosis of a low grade, non-Hodgkin lymphoma of B-cell origin.
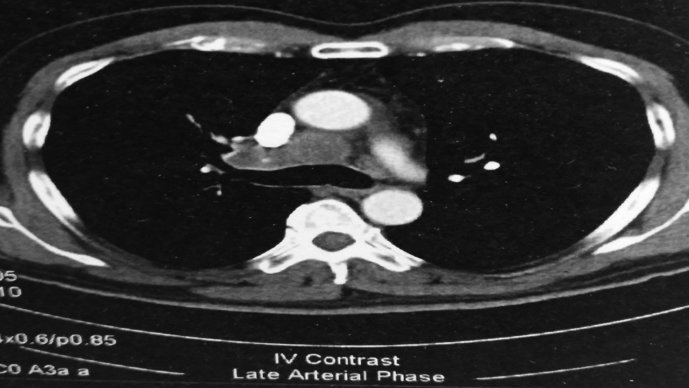
Fig. 3.
CT of the thorax with an enlarged station 4R.
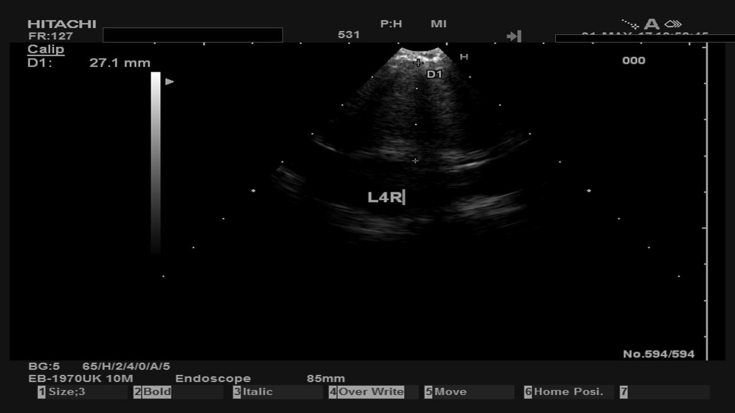
Fig. 4.
The previously demonstrated in Fig. 3 station 4R during biopsy.
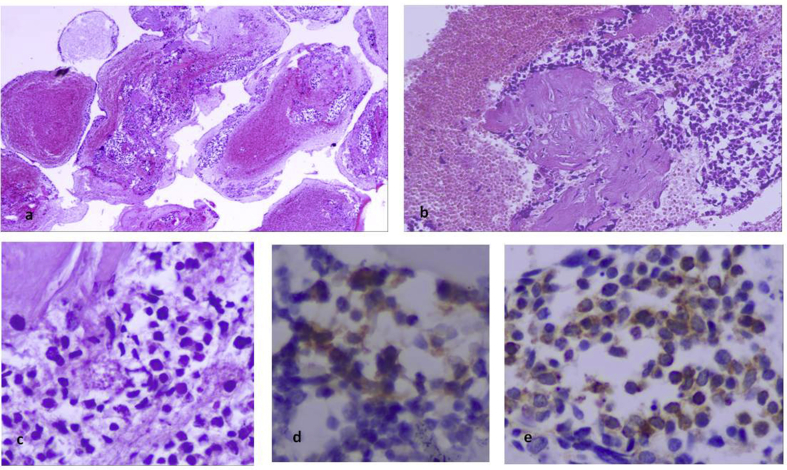
Fig. 5.
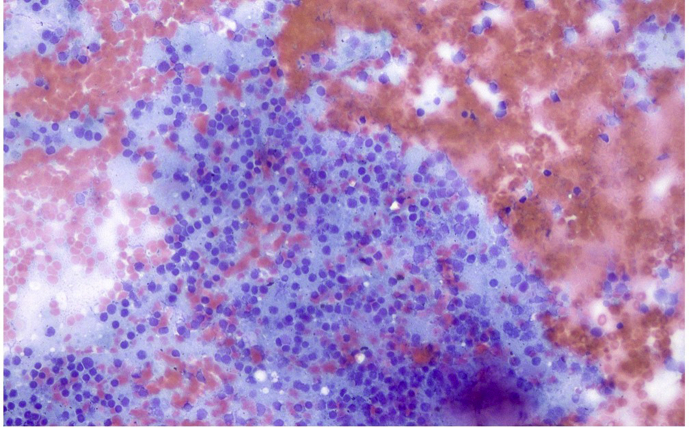
Subcarinal lymph node aspiration and cell block preparation. a) Haematoxylin/Eosin (H/E) stain in cell block of lymph node material contained numerous lymphoid aggregates (magnification x 40). b) in high power view (magnification x 400) the lymphoid cells were small in size with convoluted or “gyroid” nucleus and abundant clear cytoplasm. c,d) after conventional cytological preparation (Papanikolaou stain) the lymphoid cells were uniform with basophilic dark nucleus (magnification x 200, x 400).
Fig. 6.
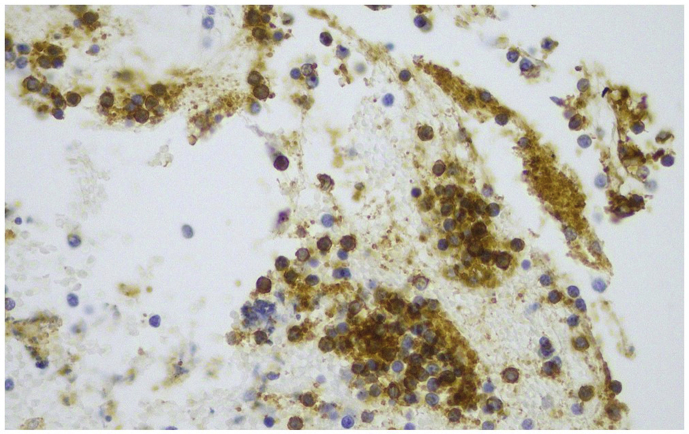
Immunostain for CD20. The neoplastic lymphoid cells were almost totally positive for CD20 (magnification x 400).
4. Case 2 (lung adenocarcinoma)
A 62 year old heavy smoker was diagnosed with a 5cm mass in the right lower lobe and an enlarged 11Linferior lymph node 2.5cm with CT of the thorax. EBUS was performed and lung cancer (adenocarcinoma) was diagnosed (Fig. 7). The FNA material from the lung tumor and the lymph node provided cell block and cytological aspiration. Both materials included numerous inflammatory cells and large neoplastic cells. In cell block (H/E stain), the neoplastic cells performed unequal size with polymorphic nucleus and formed large aggregates with fibrous stroma between them and small sized vessels (Fig. 8a and b). In cytological aspiration (PAP stain) individuals neoplastic cells were found, in close-bonded, small groups. The nucleus was large with eosinophilic nucleoli (Fig. 8c). The immunohistochemical examination confirmed the neoplastic features of an adenocarcinoma with the positivity of CK 7 (Fig. 9a and b) and the lung origin with the positivity of TTF-1 (Fig. 9c and d).
Fig. 7.
Enlarged 11Linferior lymph node.
Fig. 8.
Lung adenocarcinoma. a)Haematoxylin/Eosin (H/E) stain in cell block preparation of the lung tumor, revealed aggregations of large neoplastic cells (magnification x 100). b) Their cytoplasm varied in size and enclosed polymorphous nucleus. The cytoplasm was amphophilic (magnification x 100). c) Papanikolaou stain in lymph node aspiration with neoplastic cells similar of those in lung tumor. The nucleus was enlarged with conspicuous nucleoli (magnification x 400).
Fig. 9.
Immunostains in lung adenocarcinoma. a) Strong cytoplasmic positivity for CK7 immunostain of neoplastic cells in cell block preparation (magnification x 100). b) atypical adenoid structures were positive for CK 7 (magnification x 100). c, d) the majority of neoplastic cells performed nuclear positivity for TTF-1 (magnification x 200).
5. Case 3 (T-cell lymphoma)
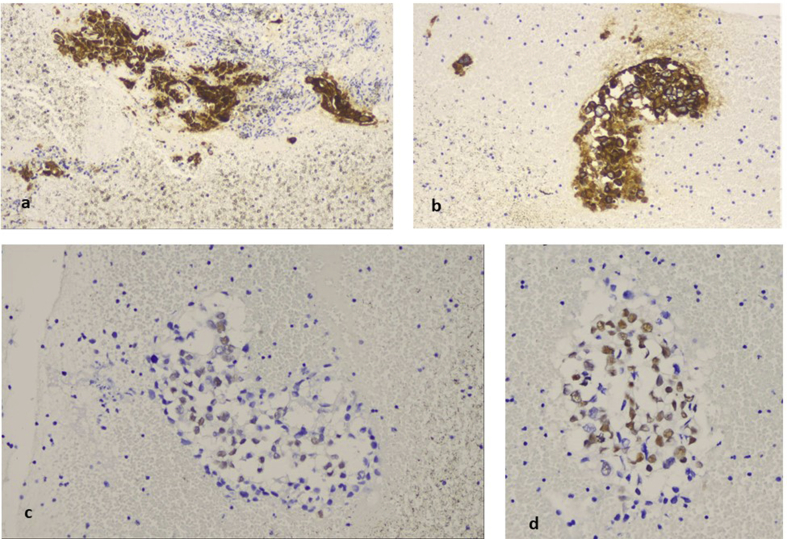
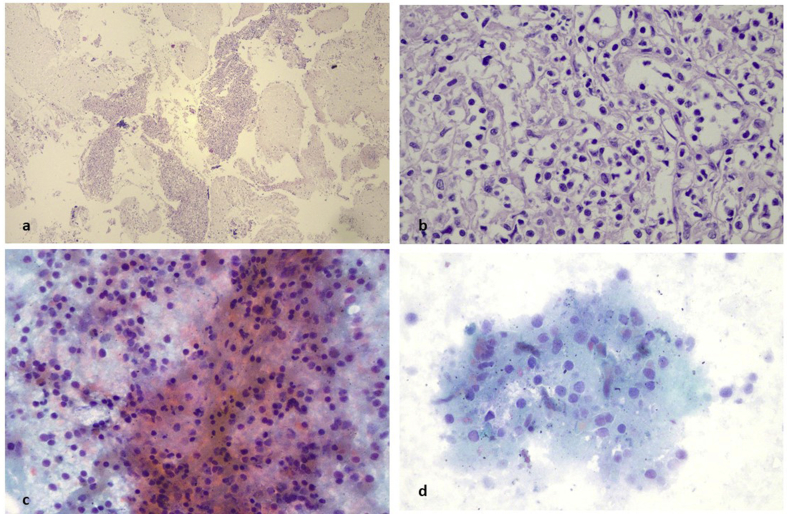
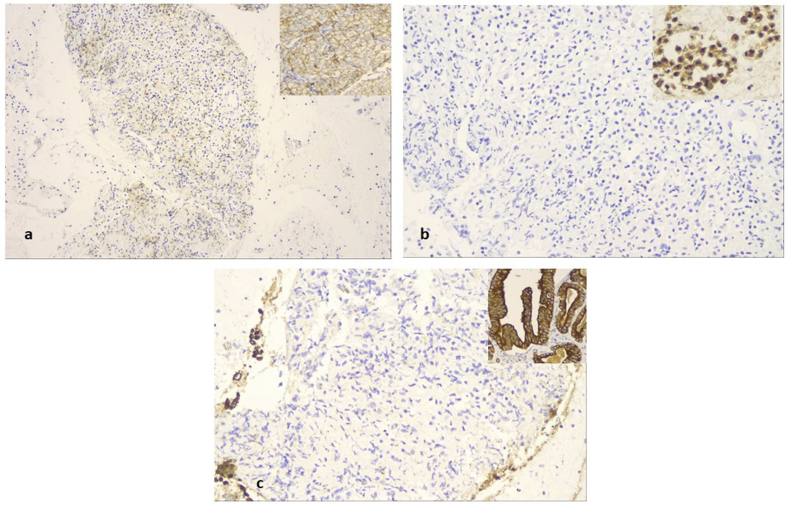
A 52 year old woman was presented with cough in the outpatient cabinet, and a CT of the thorax presented an enlarged subcarinal lymphnode and a paraesophagial mass that was also paratracheal and therefore EBUS was performed (Fig. 10). EBUS guided aspiration of the subcarina lymph node and the paraesophagial lump provided cell block material. The H/E stain revealed lymphoid aggregates with loose stroma and inconspicuous vessels. The lymphoid cells were small to medium sized with small amount of clear cytoplasm and oval shaped or “gyroid” nucleus. Some of them were convoluted (Fig. 11a and b). PAP stain in cytological preparation showed lymphoid cells with uniform, basophilic dark nucleus and scattered inflammatory cells (Fig. 11c and d). The lymphoid aggregates was almost totally positive in CD3, while rare cells were positive for CD20, indicating that the lymphoid population was of T-cell origin (Fig. 12a and b). Immunostains for CD56 synaptophysin and Pancytokeratin, were negative and excluded the diagnosis of a small cell carcinoma, an differentiated carcinoma or a thymoma. All the immunostains were performed with external positive controls for each marker, in all slides (Fig. 13a,b,c). The domination of lymphoid cells with atypical morphological features and positivity in CD3, raised the diagnosis of an underline non Hodgkin lymphoma, of T-cell origin.
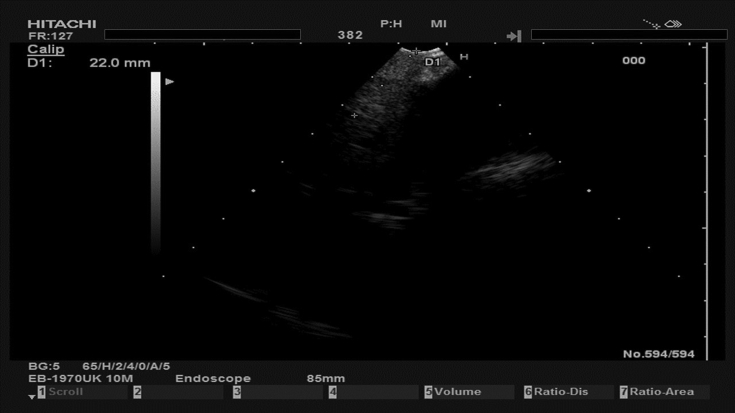
Fig. 10.
Subcarinal lymph node 22mm.
Fig. 11.
Subcarina lymph node aspiration and cell block preparation. a) Haematoxylin/Eosin (H/E) stain in cell block of lymph node material contained numerous lymphoid aggregates (magnification x 40). b) in high power view (magnification x 400) the lymphoid cells were small in size with convoluted or “gyroid” nucleus and abundant clear cytoplasm. c,d) after conventional cytological preparation (Papanikolaou stain) the lymphoid cells were uniform with basophilic dark nucleus (magnification x 200, x 400).
Fig. 12.
Immunohistochemistry for CD3, CD20 in cell block material. a)The majority of lymphoid cells were of T-cell origin and strongly positive for CD3, b) while CD20 positive lymphocytes (B cells) were practically absent (magnification x 200).
Fig. 13.
Immunohistochemistry for CD56, synaptophysin and CK7 in cell block material a)Immunostain for CD56, b) synaptophysin and c) CK7 were negative (magnification x 200). Upper right sided inserts are external positive controls for the above markers.
6. Case 4 (ectopus thyroid)
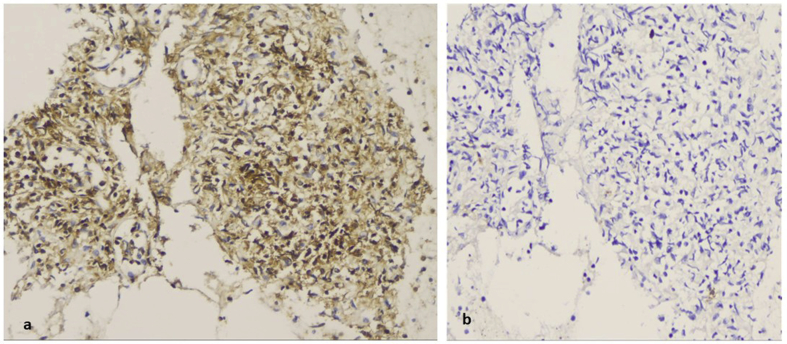
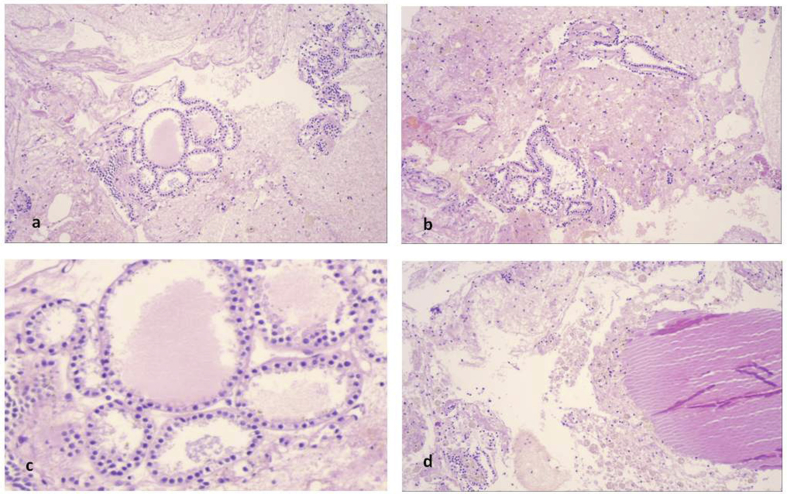
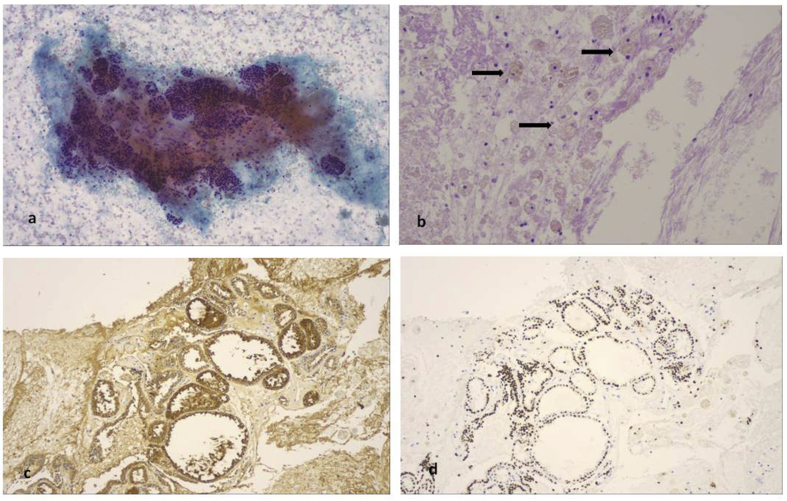
A 65 year old patient underwent cholecystectomy, and difficult intubation was observed during intubation. A flexible bronchoscope (STORZ) was used for intubation. The anesthesiologists observed that the trachea had an “S” shape and CT of the thorax was performed. A large mass surrounding the trachea was observed deforming its regular shape. EBUS was performed and the diagnosis was ectopus thyroid (Fig. 14). Cell block and cytological aspiration of the paratrachea tumor, included numerous inflammatory cells and abundant stroma with follicular structures lined by low columnar or cuboidal cells with hyperplastic features. In the lumens was found an amorfous eosinophilic material, resembling colloid without peripheral vacuolization (Fig. 15a,b,c). This material was acellular and was expanded within the matrix (Fig. 15d). In cytologic aspiration the follicular structures composed of hyperplastic cuboidal epithelial cells without atypia (Fig. 16a). Additionally numerous haemosiderin-macrophages were found in stroma (Fig. 16b). These cells were almost totally positive for anti-thyreosphairin protein and TTF-1 indicating thyroid origin of these structures (Fig. 16 c,d).
Fig. 14.
Large mass surrounding the trachea.
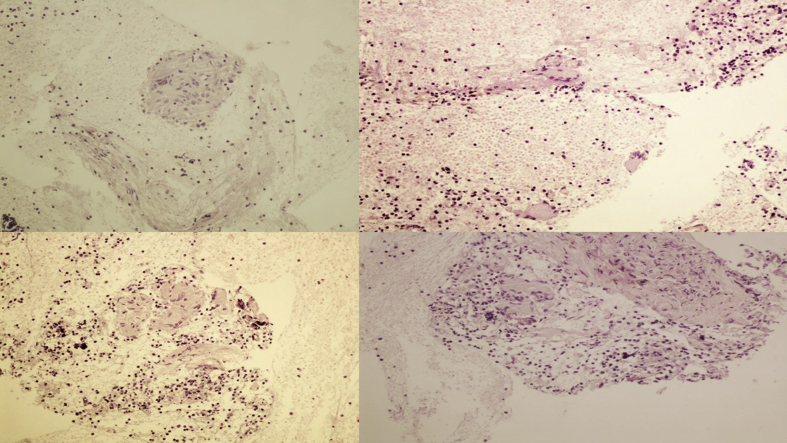
Fig. 15.
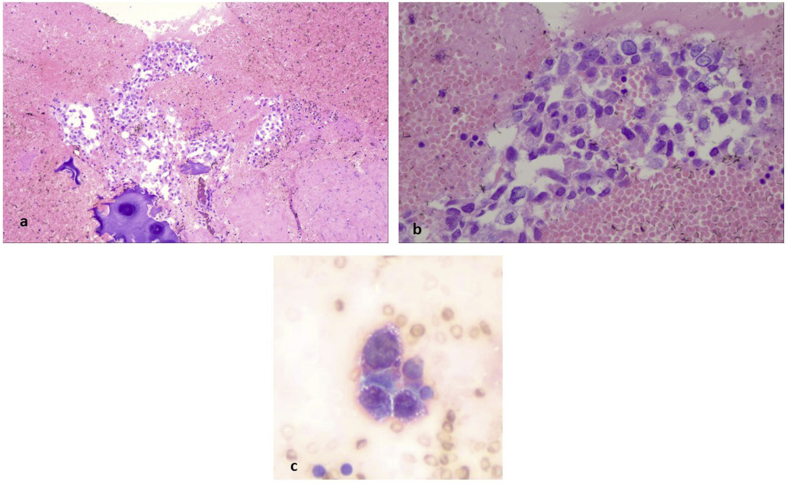
Paratranchea tumor. a, b) Cell block contained abundant stroma, haemorages areas, inflammatory cells and follicular structures in variable size and configuration (magnification x 100). c) the epithelial cells were uniform in size, cuboidal with amphophilic cytoplasm and lumens contained an eosinophilic amorfous material resembling colloid (magnification x 400) which was also exaggerated in the surrounding matrix (d) (magnification x 100).
Fig. 16.
Cytological aspiration and immunohistochemistry. a) Well formed follicular structures were also found in cytological aspiration (PAP stain, magnification x 100), b) as well as numerous macrophages with abundant haemosidinin granules in their cytoplasm (arrows, magnification x 100). In serial sections of the cell block tissue, the same follicular structures were both positive for (c) anti-thyreosphairin protein and (d) TTF-1 (magnification x 100).
7. Case 5 (thymoma)
A 60 year old man was presented as an outpatient for evaluation, he had a recent CT of the thorax with a large mass ≥10cm in the front part of the mediastinum. EBUS was performed and the biopsy revealed thymoma (Fig. 17). Fine niddle aspiration of the paratranchea lump revealed well-formed groups of cells with organoid formations (Fig. 18a). Thick fibrous septae bordered or separated the above groups of cells (Fig. 18b). The neoplastic population constituted of two types of cells. The first type, was mainly medium-sidz cells with an amount of cytoplasm and oval shaped nucleus which exhibited epithelial-like features. The second type, was scattered mature small lymphocytes, among the epithelial-like cells (Fig. 18c). The first type of epithelial-like cells was positive for CD117 and Cytokeratin AE1/AE3 (Fig. 18c and d) but was negative for p63 and CD5 (Fig. 18e) CKAE1/AE3 magnification x 600). This immunoprofile is compatible to thymocytes and the combination of morphology and topography leaded to the diagnosis of thymoma.
Fig. 17.
CT of the thorax with a large mass ≥10cm.
Fig. 18.
Thymoma. a) Cell block section of EBUS guided aspiration of a paratrachea lump, revealed matrix-riched and haemorragic background with small-medium sized neoplastic cells in substance organoid formations (magnification x 400, b) the neoplastic groups bordered with thick tissue septae (magnification x 100) and (c) constituted of cells with medium sized cytoplasm and epithelial-like features with small lymphocytes between them (magnification x 600), d) these epithelial-like cells were thymocytes and exhibited positivity for CD 117 and e) CKAE1/AE3 (magnification x 600).
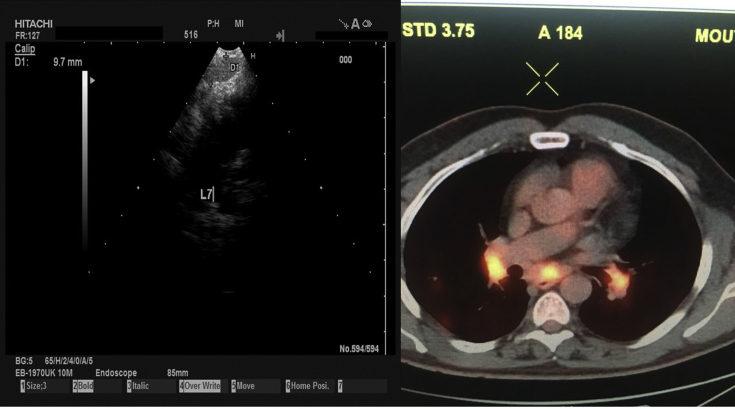
8. Case 6 (sarcoidosis)
A 42 year old non-smoker woman presented with persistent cough, positron emission tomography-CT revealed lymphadenopathy of the mesothorax (lymph nodes ≤3cm). EBUS was performed and sarcoidosis was diagnosed (Fig. 19). Sarcoidosis is a systemic disease, that can be seen in any organ. Fine needle aspiration (FNA) biopsy can detect characteristic non – necrotizing granulomas consisting of epithelioid histiocytes, admixed with background of reactive lymphocytes and scattered plasma cells. Sarcoidosis is a diagnosis of exclusion, but aspiration biopsies in the proper clinical setting containing prominent granulomatous inflammation without necrosis are very helpful in conforming the presence of sarcoidal information in patients with lymphadenitis. (Figure 20).
Fig. 19.
On the left lymph node station number 7 and on the right, PET-CT with lymphadenopathy of the mesothorax (lymph nodes ≤3cm.
Fig. 20.
Non-necrotizing granulomas.
9. Discussion
Endobronchial ultrasound is an excellent tool which can access central lesions. There are specific lymphnode stations that can be accessed and of course any lesion that is next to a large airway and can be accessed by the EBUS then transbronchial biopsy can be performed [2]. Some users might also try and perform a biopsy through the esophagus for lesions that are easily accessible such as station number 7 and of course in centers with experience in this procedure [4], [5]. Elastography is an imaging method that can asses the strain ratio of a lesion and it is used in some centers as an additional tool in order to know before biopsy results if we have a benign/malignant or just inflammation of a lesion [6], [7], [8]. This method is considered a rapid on site evaluation, however; the efficiency and sensitivity are still not 100%. Our group established the efficiency and sensitivity of elastography up to 91%, this method is still under evaluation [6]. Rapid on site evaluation is a technique that can be also used, however; it has its limitations [9]. The needles that can be used nowadays through the ebus working channel; 22G, 21G and 19 G. 21G needle has better results than that of 22G [10]. Unfortunately we cannot access all stations or lesions with a 19G needle and the reason is either the high probability to cause damage to a vessel or due to technical aspects during the process. In any case we prefer large core biopsies. A major issue that has to be dealt appropriately before performing any biopsy procedure is to have the appropriate diagnostic laboratory which consists of a pathologist and cytologist. These colleagues must have any additional clinical information in order to make the appropriate immunohistochemistry. In any case when there is high suspicion of lymphoma and we have negative biopsy results with the EBUS methodology then mediastinoscopy should be performed, although several types of lymphoma have been diagnosed with EBUS biopsy [11], [12], [13], [14], [15], [16], [17]. In our case series we present our experience with several different clinical situations except tuberculosis. We consider that there are other methods much less interventional in order to diagnose this infection than taking lymph node biopsies. However; one could obtain biopsies from lymph nodes when taking bronchial lavage for tuberculosis inspection. Certainly there going to be such similarities in the samples with sarcoidosis, however; the clinical findings/imaging and the results of the bronchial lavage along with the mantoux test most of the times are sufficient. We provide several information that any colleague can use in his/her daily practise.
Conflict of interest
None to Declare.
Acknowledgments
All biopsies were performed by Dr. Paul Zarogoulidis and his equipment.
References
- 1.Kern M., Kerner T., Tank S. Sedation for advanced procedures in the bronchoscopy suite: proceduralist or anesthesiologist? Curr. Opin. Anaesthesiol. 2017;30(4):490–495. doi: 10.1097/ACO.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 2.Oezkan F., Khan A., Zarogoulidis P., Hohenforst-Schmidt W., Theegarten D., Yasufuku K., Nakajima T., Freitag L., Darwiche K. Efficient utilization of EBUS-TBNA samples for both diagnosis and molecular analyses. OncoTargets Ther. 2014;7:2061–2065. doi: 10.2147/OTT.S72974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sivapalan P., Naur T.M.H., Colella S., Richter Larsen K., Konge L., Frost Clementsen P. Impact of EBUS-TBNA on PET-CT imaging of mediastinal nodes. J. Bronchol. Intervent. Pulmonol. 2017;24(3):188–192. doi: 10.1097/LBR.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 4.Kang H.J., Hwangbo B., Lee G.K., Nam B.H., Lee H.S., Kim M.S., Lee J.M., Zo J.I., Lee H.S., Han J.Y. EBUS-centred versus EUS-centred mediastinal staging in lung cancer: a randomised controlled trial. Thorax. 2014;69(3):261–268. doi: 10.1136/thoraxjnl-2013-203881. [DOI] [PubMed] [Google Scholar]
- 5.Dietrich C.F., Annema J.T., Clementsen P., Cui X.W., Borst M.M., Jenssen C. Ultrasound techniques in the evaluation of the mediastinum, part I: endoscopic ultrasound (EUS), endobronchial ultrasound (EBUS) and transcutaneous mediastinal ultrasound (TMUS), introduction into ultrasound techniques. J. Thorac. Dis. 2015;7(9):E311–E325. doi: 10.3978/j.issn.2072-1439.2015.09.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang H., Huang Z., Wang Q., Wang X., Dong Y., Zhang W., Zarogoulidis P., Man Y.G., Hohenforst Schmidt W., Bai C. Effectiveness of the benign and malignant diagnosis of mediastinal and hilar lymph nodes by endobronchial ultrasound elastography. J. Cancer. 2017;8(10):1843–1848. doi: 10.7150/jca.19819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu Y., Shi H., Su C., Chen X., Zhang S., Li W., Wu F., Gao G., Wang H., Chu H., Zhou C., Zhou F., Ren S. The role of endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Oncotarget. 2017 doi: 10.18632/oncotarget.19031. (e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He H.Y., Chen J.L., Ma H., Zhu J., Wu D.D., Lv X.D. Value of endobronchial ultrasound elastography in diagnosis of central lung lesions. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017;23:3269–3275. doi: 10.12659/MSM.901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain D., Allen T.C., Aisner D.L., Beasley M.B., Cagle P.T., Capelozzi V.L., Hariri L.P., Lantuejoul S., Miller R., Mino-Kenudson M., Monaco S.E., Moreira A., Raparia K., Rekhtman N., Roden A.C., Roy-Chowdhuri S., da Cunha Santos G., Thunnissen E., Troncone G., Vivero M. Rapid on-site evaluation of endobronchial ultrasound-guided transbronchial needle aspirations for the diagnosis of lung cancer: a perspective from members of the pulmonary pathology society. Arch. Pathol. Lab. Med. 2017 doi: 10.5858/arpa.2017-0114-SA. (e-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 10.Yarmus L.B., Akulian J., Lechtzin N., Yasin F., Kamdar B., Ernst A., Ost D.E., Ray C., Greenhill S.R., Jimenez C.A., Filner J., Feller-Kopman D., American College of Chest Physicians Quality Improvement Registry E., Evaluation P. Comparison of 21-gauge and 22-gauge aspiration needle in endobronchial ultrasound-guided transbronchial needle aspiration: results of the american college of chest physicians quality improvement registry, education, and evaluation registry. Chest. 2013;143(4):1036–1043. doi: 10.1378/chest.12-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senturk A., Babaoglu E., Kilic H., Hezer H., Dogan H.T., Hasanoglu H.C., Bilaceroglu S. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Asian Pac. J. Cancer Prev. APJCP. 2014;15(10):4169–4173. doi: 10.7314/apjcp.2014.15.10.4169. [DOI] [PubMed] [Google Scholar]
- 12.Korrungruang P., Oki M., Saka H., Kogure Y., Tsuboi R., Oka S., Nakahata M., Hori K., Murakami Y., Ise Y., Ahmed S.N., Kitagawa C. Endobronchial ultrasound-guided transbronchial needle aspiration is useful as an initial procedure for the diagnosis of lymphoma. Respir. Investig. 2016;54(1):29–34. doi: 10.1016/j.resinv.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa B.S., Bernstein M., Siddiqi N., Pastis N.J. Diagnosing Hodgkin lymphoma from an endobronchial ultrasound core needle biopsy. J. Bronchol. Intervent. Pulmonol. 2016;23(4):336–339. doi: 10.1097/LBR.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 14.Jin M., Wakely P.E., Jr. Endoscopic/endobronchial ultrasound-guided fine needle aspiration and ancillary techniques, particularly flow cytometry, in diagnosing deep-seated lymphomas. Acta Cytol. 2016;60(4):326–335. doi: 10.1159/000447253. [DOI] [PubMed] [Google Scholar]
- 15.Erer O.F., Erol S., Anar C., Aydogdu Z., Ozkan S.A. Diagnostic yield of EBUS-TBNA for lymphoma and review of the literature. Endosc. Ultrasound. 2016 doi: 10.4103/2303-9027.180762. (e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baltayiannis N., Michail C., Lazaridis G., Anagnostopoulos D., Baka S., Mpoukovinas I., Karavasilis V., Lampaki S., Papaiwannou A., Karavergou A., Kioumis I., Pitsiou G., Katsikogiannis N., Tsakiridis K., Rapti A., Trakada G., Zissimopoulos A., Zarogoulidis K., Zarogoulidis P. Minimally invasive procedures. Ann. Transl. Med. 2015;3(4):55. doi: 10.3978/j.issn.2305-5839.2015.03.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y., Huang H., Li Q., Browning R.F., Parrish S., Turner J.F., Jr., Zarogoulidis K., Kougioumtzi I., Dryllis G., Kioumis I., Pitsiou G., Papaiwannou A., Lampaki S., Machairiotis N., Katsikogiannis N., Madesis A., Karaiskos T., Li Z., Zarogoulidis P. Transbronchial lung biopsy and pneumothorax. J. Thorac. Dis. 2014;6(Suppl 4):S443–S447. doi: 10.3978/j.issn.2072-1439.2014.08.48. [DOI] [PMC free article] [PubMed] [Google Scholar]