Abstract
Evidence from animal studies suggests that stress-induced increases in Nrf2-regulated antioxidant gene expression, a critical mechanism of cellular protection, declines with aging. This study examined whether this also occurs in humans. We measured the basal and inducible levels of Nrf2-regulated antioxidant genes in human bronchial epithelial (HBE) cells from subjects of young adult (21–29 years) and older (60–69 years) non-smokers, and explored factors affecting expresion. The basal expression of three representative Nrf2-regulated genes, the catalytic and modulator subunits of glutamate cysteine ligase (GCLC and GCLM, respectively), and NAD(P)H quinone oxidoreductase 1 (NQO1), was higher in cells from the older donors compared with cells from the young adult donors. Upon exposure to the Nrf2 activator, sulforaphane (SF), the expression of these antioxidant genes was increased in cells from both the young adults and the older donors; however, the induction by SF in older donor cells was significantly less than that seen in young adult cells. In addition, the activation of an EpRE-driven reporter by SF was lower in cells from older donors compared to cells from young adults. The basal expression of Nrf2 protein was also lower in cells from older donors than cells from young adults. Furthermore, we found that the basal expression of both Bach1 and c-Myc, two Nrf2 suppressors, was higher in cells from older adults than from young adult donors. In summary, our data suggest that, as in other species, basal expression of Nrf2-regulated genes increases with aging, while inducibility declines with aging. The increased expression of Nrf2 suppressors such as Bach1 and c-Myc may contribute to the impaired inducibility of the Nrf2-regulated antioxidant genes with aging in human bronchial epithelial cells.
1. Introduction
Antioxidant enzymes play an important role in protecting cells against oxidative stress, including the apparent increase of oxidative stress in aging [30]. Activation of nuclear factor E2-related factor 2 (Nrf2) signaling provides a critical mechanism to counteract oxidative stress through its ability to increase the expression of genes containing the electrophile response element (EpRE, also called the antioxidant response element or ARE) in the promoter region. EpRE sequences can be found in many genes, but only some of them function in transcriptional activation through Nrf2 binding [34]. Nevertheless, Nrf2 signaling is involved in the regulation of many antioxidant/detoxifying genes, including heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase 1 (NQO-1) [29] and both subunits of glutamate cysteine ligase (GCLC and GCLM) which catalyzes the first step in glutathione synthesis [31], [6], and is a key factor in for minimizing oxidative stress.
Sulforaphane (SF), an isocyanate found in cruciferous vegetables, is one of the most potent naturally-occurring inducers of phase II enzymes and is widely acknowledged to provide chemo-preventive benefits in humans [3], [36]. In a clinical trial, SF was found to improve the behavior of young men with autism spectrum disorder compared to placebo recipients due to its ability to up-regulate genes that protect aerobic cells against oxidative stress [24]. Whether SF treatment functions differently in aging humans than in young adults has not been reported, previously. Our previous studies showed that the inducibility of Nrf2-dependent antioxidant enzymes was already markedly depressed at middle age in the lungs, liver and cerebellum of mice [32], [35], and that the loss of inducibility correlated with increased expression of the Nrf2 inhibitors, Bach1 and c-Myc. Bach1 is a classic suppressor of EpRE-mediated gene expression, as it competes with Nrf2 for binding to the same EpRE cis-element in several genes [20]. An observed increase in Bach1 in aged mice may explain, in part, the loss of inducibility of some Nrf2-regulated genes [35]. Thus, it was important to investigate the potential change of the Nrf2-Bach1 dynamic relationship with age in humans. The oncogene product, c-Myc also interferes with Nrf2 signaling and its target gene expression, but by a different mechanism than reported for Bach1 [11]. Regulation of the EpRE/Nrf2 signaling pathway by c-Myc appears to be through both interaction with the EpRE binding complex and increased degradation of Nrf2 [11].
Although many have observed a decline in Nrf2 signaling with aging [21], [28], [32], most of the investigations were based on animal models, and it remained unclear if this phenotypic aging alteration also occurred in humans. Using primary human bronchial epithelial cells from donors of various ages, we investigated the expression of Nrf2-regulated antioxidant genes and the induction of these genes and Nrf2 signaling by SF, and explored the underlying mechanism of the changes with age.
2. Materials and methods
2.1. Reagents
Unless otherwise noted, all chemicals were from Sigma (St. Louis, MO, USA). NE-PER (Nuclear Protein Extraction reagent) was from Thermal Fisher Scientific (Rockford, IL, USA). RNA extraction kit was from Qiagen (Qiagen Inc, CA, USA). All the antibodies except c-Myc were from Santa Cruz (Santa Cruz, CA, USA). c-Myc protein antibody was from Cell Signaling (Cell Signaling Technology, Beverly, MA, USA). Reverse transcription reagents and SYBR Green PCR Master Mix were from Applied Biosystems (Rockford, IL, USA). The stripping buffer for western blots was from Millipore Inc. (Bedford, MA, USA).
2.2. Cell culture
Primary human bronchial epithelial (HBE) cells and small airway epithelial cells (SAE) were obtained from Lonza (Lonza, MD, USA) or Lifeline Cell Technology LLC (Frederick, MD). Donor information provided by Lonza and Lifeline Cell Technology were listed in Table 1S in Supplemental data. Cells were cultured on collagen coated flasks or petri-dishes in BEGM basal medium supplemented with cell growth factors at 37 °C in 5% CO2. All the experiments were conducted using cells with a passage number less than 5.
2.3. RT-PCR
RNA was extracted with RNeasy mini kit following the protocol provided by manufacturer. Total RNA was treated with DNA-free reagent to remove genomic DNA contamination. After reserve transcription, mRNA was quantified by RT-PCR as previously described [33]. Sequences for primers amplifying β-actin, GCLC, GCLM, and NQO-1 were as reported before, for amplification of Bach1 and c-Myc, the sequences of the primers used were: Bach1 antisense CCTGGCCTACGATTCTTGAG; sense TGCGATGTCACCATCTTTGT; c-Myc antisense TGTCGTTGAGAGGGTAGGGGAAGA; sense GAGAGGCAGAGGGAGCGAGCGGGC.
2.4. Western blot analysis
Briefly, cell cytosolic and nuclear lysate was extracted with NE-PER (Thermo Scientific). Twenty micrograms of protein was heated for 10 min at 95 °C in 5× loading buffer containing SDS (Tris base, PH 6.5, glycerol, DTT and pyronin y), electrophoresed on 4–20% Tris-glycine acrylamide gel (Invitrogen, Carlsbad, CA, USA) and then electroblotted onto polyvinylidene difluoride (PVDF) membranes (Millipore, Corporation, Bedford, MA, USA). Membrane was blocked with 5% fat-free milk and then incubated overnight at 4 °C with primary antibody in 5% milk solution. After completion of each blotting, membrane was stripped with stripping buffer (Millipore) and reused for the next new blotting. After being washed with TBS containing 0.05% Tween 20 (TTBS), the membrane was incubated with secondary antibody (Goat anti-rabbit IgG) at room temperature for 2 h, and then treated with an enhanced chemiluminescence reagent mixture (ECL Plus, Amersham, Arlington Heights, IL, USA) for 5 min. The target bands were imaged using the Syngene PXi6 imaging system (Syngene, Cambridge, UK). The blots were analyzed with ImageJ [4].
2.5. Transfection and reporter assay
Cells (80% confluence) were transfected with EpRE-luciferase reporter [34] using lipofectamine 2000 transfection reagent (Thermal Fisher Scientific, Rockford, IL, USA) in 24-well plates. Twenty-four hours after transfection, the medium was replaced and cells were treated with or without SF. After treatment for 24 h, luciferase activity was measured with procedures described previously [33].
2.6. Statistical analysis
A comparative ΔΔCT method was used for the relative mRNA quantitation [14]. Data are expressed as mean ± standard error. Prism 6 was used for statistical analysis, with statistical significance at p < 0.05. Two sample t-test to test for significant effect of treatment of mRNA levels and protein levels.
3. Results
3.1. Demographics and characteristics of HBE cell donors
A limitation of this study was the availability of primary purified HBE cells. We originally selected cells from only male non-smokers, who were free of lung disease. These came from two groups: three young adult males (28/29) and three older (WHO definition) (67/69) samples. This represented similar age groups to our previous studies with mice in which we compared young adult (6-month old) and older (21-month old) mice [35]. Within each of the two groups, variation of baseline and induction among the individuals were small. But, as groups of 3 seemed small, we purchased additional samples from the same company (Lonza) and other two vials of cells from young adults from a different company (Lifeline Cell Technology) as described in Methods. Because only one sample from a non-smoker over age 60 was available, that sample was added even though it was from a female donor. For statistical analyses of phase II mRNA, we grouped the five young adults (21–29) and compared them with the group of four older donors (60–69). Due to a lack of material however, we compared proteins from only the three 28–29 and three 67–69 year-old donors.
3.2. Variation of the basal expression of Nrf2-regulated genes with aging
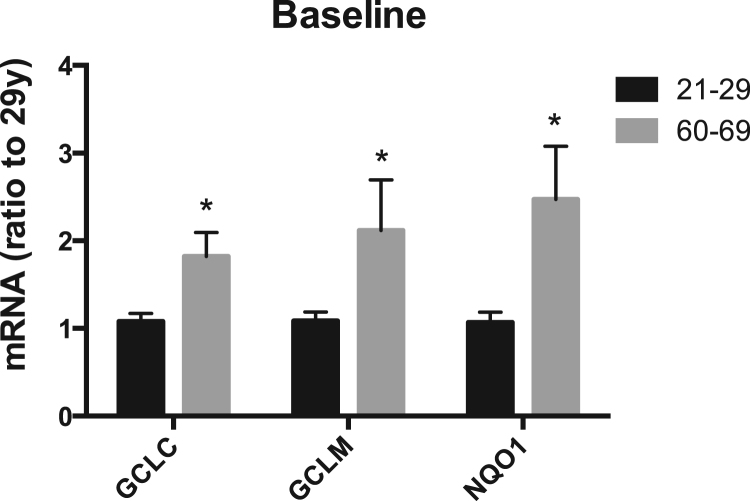
We first determined the basal mRNA levels of these three representative Nrf2-regulated antioxidant genes in HBE cells. Heme oxygenase 1 was also measured, but found to have very low expression in the HBE cells. The basal mRNA levels of NQO1, GCLC, and GCLM in cells from the older donors were significantly higher than those from the young adults (Fig. 1). This finding paralleled results that we published previously for mice [35]. Mean levels of GCLC, GCLM and NQO1 were not changed significantly by inclusion of data from cells of the 60-year-old female (p < 0.05 for all comparisons). Basal expression of the above-mentioned phase II genes in cells of the entire age range (13–69) is presented in the supplemental materials. Cells from adolescents exhibited comparatively higher basal GCLC and NQO1 gene expression than those in young adult cells (Fig. 1s). As lungs are still developing during adolescence, we have not included these two samples in the comparisons. Similarly, while results with the one 57-year-old donor were similar to those from older cells, they were also excluded from the comparisons.
Fig. 1.
Variation of basal mRNA levels of Nrf2-regulated antioxidant genes (GCLC, GCLM and NQO1) between HBE cells from young adults (age 21–29) and older donors (age 60–69). Data were normalized to that of 29 years of age. Bars represent means ± SD. * p < 0.05, n = 5 for young adult group and n = 4 for the older group.
3.3. SF induction of Nrf2-regulated antioxidant genes
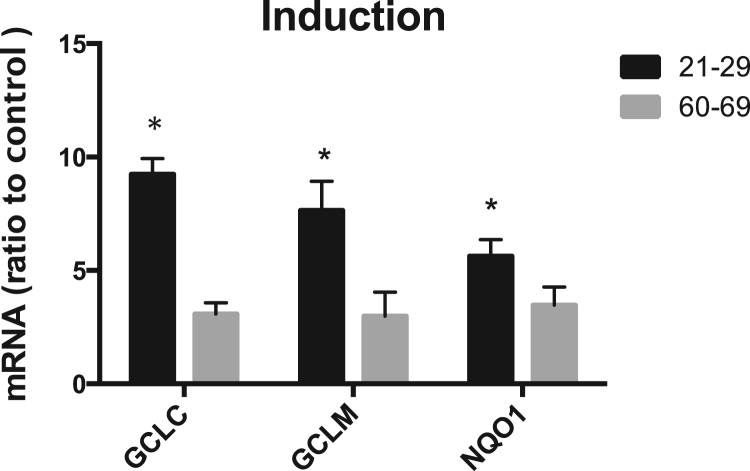
To determine the effect of aging on the inducibility of Nrf2-regulated antioxidant genes, HBE cells were treated with sulforaphane (SF), a potent Nrf2 activator [27]. Upon treatment, the mRNA level of all three representative genes regulated by Nrf2 was significantly increased in HBE cells from all individuals; however, the induction was significantly greater in cells from young adults compared to that from older donors (Fig. 2), indicating an age-dependent decline of the inducibility of these Nrf2-regulated genes with aging. This also resembled what was reported by us for chronic exposure to nanoparticles in mice [35]. The mean ± S.E for the SF induction of GCLM, GCLC and NQO1 in cells from older donors, including the age 60 female, were 2.99 ± 0.50, 3.08 ± 0.49 and 3.47 ± 0.40 times higher than untreated cells, respectively. Excluding the sample from the 60-year old female resulted in values of 3.08 ± 0.28, 2.98 ± 0.61 and 3.47 ± 0.46 for GCLM, GCLC and NQO1, respectively. The p values were less than 0.05 for all comparisons. Phase II gene induction following SF treatment in cells of the entire age range (13–69) is presented in the supplement materials. As mentioned above, we excluded samples from outside the young adult and older donors in our analyses.
Fig. 2.
Induction of Nrf2-regulated genes by sulforaphane (SF) in cells from young adults (age 21–29) and older donors (age 60–69). HBE cells were treated with 2.5 μM SF for 12 h and the mRNA level of GCLC, GCLM and NQO1 was measured with real time PCR assay. A statistical significance was observed between young adult and older. * p < 0.05, n = 5 for young adult group and n = 4 for the older group.
3.4. Change in Bach1 and c-Myc expression with aging
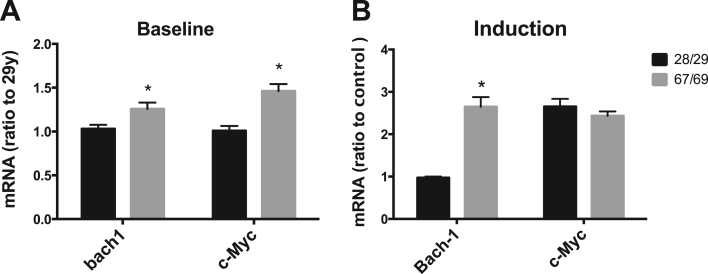
As Bach1 [26] and c-Myc [10] were previously found to inhibit Nrf2-dependent transcription, we determined the mRNA expression of Bach1 and c-Myc in HBE cells from the donors. Basal expression of Bach 1 and c-Myc was significantly higher, by 20% and 40%respectively, in the older donors’ cells compared to cells from the young adults (Fig. 3A). Following the treatment with SF for 12 h, Bach1 was induced to a small but significant extent in cells from young adult donors and more markedly increased in cells from older donors (Fig. 3B). In contrast, a significant induction of c-Myc by SF was observed in cells regardless of age (Fig. 3B). Basal Bach1 and c-Myc expression in cells of the entire age range (13–69) is presented in supplement (Fig. 3s).
Fig. 3.
Basal and inducible expression of Bach1 and c-Myc with aging. HBE cells were treated with 2.5 μM SF for 12 h and the mRNA level of Bach1 and c-Myc was measured with real time PCR assay, and statistical analysis was done to compare the age-dependent change of the basal (A) and SF-induced (B) expression in cells of age 28/29 and 67/69. * p < 0.05, n = 3 for the young adult group and n = 3 for the older group.
3.5. Decreased Nrf2-EpRE activity with aging
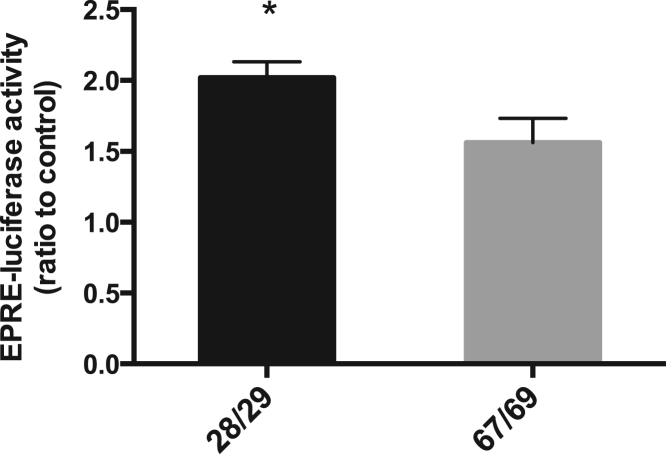
To confirm the involvement of Nrf2 signaling in the age-related decline of antioxidant gene induction, HBE cells from young adults and older donors were transfected with an EpRE-driven reporter and then stimulated with or without SF for 24 h. The EpRE reporter activity was significantly increased upon SF exposure in cells from both young adult and older donors; however, the SF-induced increase in EpRE-luciferase activity was 20% lower in cells from the older donors compared to cells from young adults, indicating that Nrf2-dependent gene inducibility was impaired in the older donors (Fig. 4).
Fig. 4.
Induction of EpRE-luciferase activity was decreased in the older donor cells. HBE cells from young adult (age 28/29) and older (67/69) donors were transfected with EpRE-luciferase reporter and 24 h later the cells were treated with/without 2.5 μM SF for 24 h, and then luciferase activity was measured. * p < 0.05, n = 3 for the young adult group and n = 3 for the older group.
3.6. Changes in nuclear protein levels of Nrf2 and its suppressors with aging
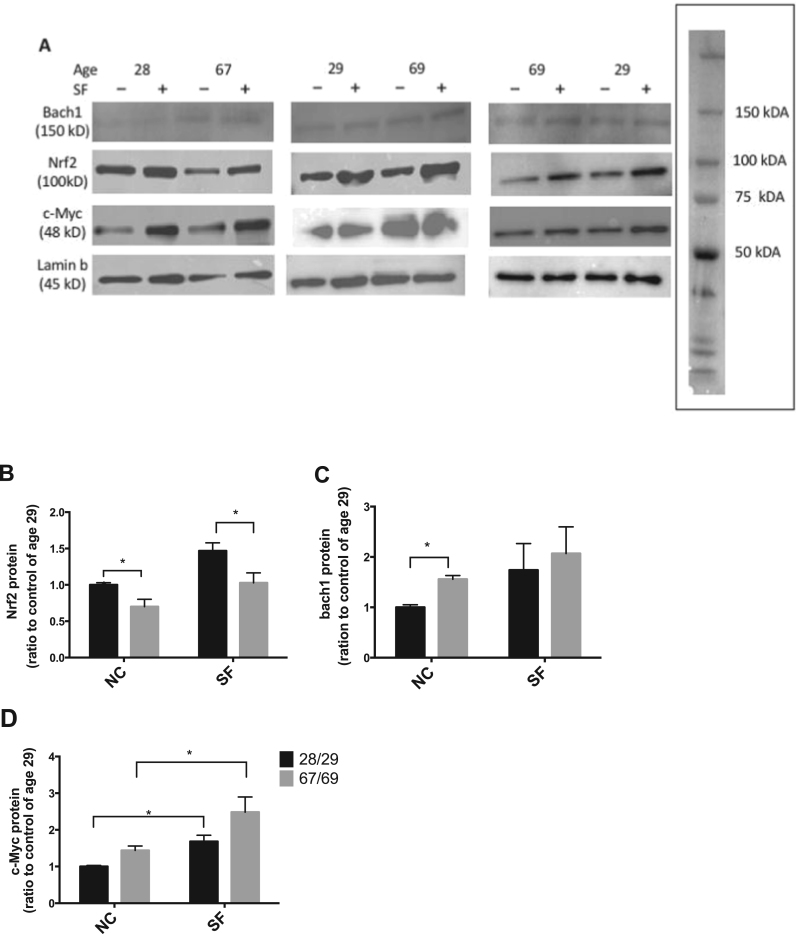
To explore the underlying mechanism of age-related decline in the induction of Nrf2-regulated genes, we determined the activation of Nrf2 signaling and the nuclear protein levels of its suppressors Bach1 and c-Myc in HBE cells from young adults and older donors. Similar to what we observed at the mRNA level, basal nuclear protein levels of both Bach 1 and c-Myc were greater in cells from the older donors versus young adults. Exposure to SF for 1 h did not significantly increase nuclear Bach1 levels in cells from either older donors or young adults, while nuclear c-Myc showed a significant increase in cells from both age groups. However, no significant difference was observed between older donors and young adults in the increase of nuclear c-Myc following SF treatment (Fig. 3B).
Activation of Nrf2 in response to oxidative stress or chemical inducers results in its translocation to the nucleus and this is commonly used as an indicator of the activation of Nrf2 signaling [16], [7]. Basal nuclear Nrf2 protein levels in cells from young adults were 1.34 times as high as in cells from the older donors (Fig. 5B). Representative western blots from each individual are shown in Fig. 5A. Upon SF exposure, there was a significant increase in nuclear Nrf2 protein levels in cells from both age groups; however, Nrf2 nuclear translocation after SF exposure was higher in cells from young adults compared with older donors (Fig. 5B). Basal nuclear Bach1 protein levels in cells from older donors were 1.5 times higher than in cells from young adult donors. An increase in Bach1 protein expression was observed in the older donor cells after SF treatment but not in the cells from young adult donors (Fig. 5C). Basal nuclear c-Myc protein was also higher in the cells from older donors and significantly increased in cells of both older donors and young adults after SF treatment (Fig. 5D). However, the difference in induction of c-Myc upon SF stimulation was not significant. These results were consistent with the changes in basal and induced Bach1 and c-Myc with age at the mRNA level.
Fig. 5.
Aging effect on the basal and SF-activated nuclear protein expression of Nrf2 and its suppressors. Cells from three young adult (28/29) and three older (67/79) donors were treated with/without SF (2.5 μM) for 1 h, and the nuclear protein level of target proteins was determined with western blotting. (A) Representative western blot of nuclear Nrf2, Bach1 and c-Myc protein levels in non-treated and treated HBE cells from 6 donors. The western blots were run as pairs from young adult and older donors. The box shows a representative blot for molecular weight markers. Densitometric quantification was done by photon counting in a Syngene PXi6 imaging system for (B) Nrf2 (C) Bach1 and (D) c-Myc proteins in nucleus between cells from young adults and older. * p < 0.05, n = 3 for the young adult group and n = 3 for the older group.
4. Discussion
Cumulative oxidative damage to macromolecules has been postulated as a mechanism responsible for the aging-related phenotype [5]. A major mechanism in cellular defense against oxidative/electrophilic stress is activation of the Nrf2/EpRE signaling pathway, which controls the expression of genes that are involved in detoxification and elimination of reactive oxidants and electrophilic agents [16]. Using HBE cells from human donors of different ages, we demonstrated here that although the basal expression of Nrf2-regulated antioxidant genes was higher in the older donor cells, the induction of these genes and EpRE-driven reporter declined with aging, suggesting an impaired Nrf2 signaling in the elderly. The mechanism underlying this decline may involve Bach1 and c-Myc, two Nrf2 suppressors, as the basal expression of both was significantly higher in the older donor cells than in cells from young adults. Furthermore, SF inducible expression of Bach1 was almost three-fold higher in cells from older donors than from their younger counterparts.
The basal expression of Nrf2-regulated antioxidant genes was higher in the HBE cells from the older human donors compared to that from young adults (Fig. 1). This is consistent with a previous study where we found that the basal levels of Nrf2-regulated antioxidant enzymes in middle-aged mice were elevated compared to that in young animals while the inducibility of these genes was significantly depressed or absent in the middle-aged mice [35]. Evidence from others also has demonstrated an increase in the basal level of Nrf2-regulated genes [21], [8]. It remains unclear whether this occurs at the transcriptional or post-transcriptional level. As the basal nuclear Nrf2 protein level was lower in the older donor cells compared to the young adults (Fig. 5B), it seems unlikely that Nrf2 alone was responsible for the higher basal expression of these genes. Previous studies reported that both Nrf2 and AP1 were potentially involved in the induction of GCL genes [9]. At the transcriptional level, these antioxidant genes could also be regulated through EpRE binding transcription factors other than Nrf2, such as Nrf1 [12], [17], c-Jun and other AP-1 family proteins [15], [1], and small Maf proteins, or through cis-elements other than EpRE, such as AP-1 cis-element [13]. Since only limited information is available about the expression of other transcription factors and/or cis-element activity with aging, their involvement in the change of the basal gene expression remains to be determined. Nonetheless, the increased basal expression of Nrf2-regulated antioxidant genes might be mediated by signaling molecules other than Nrf2.
The current study demonstrates that both Nrf2 signaling, and induction of Nrf2-regulated antioxidant genes, are impaired in primary HBE cells from older human donors, as evidenced by the significant decrease in gene induction and EpRE-driven reporter activation upon SF exposure (Fig. 2, Fig. 4 respectively). To our knowledge, this is the first evidence based on human primary cells showing aging-related decline of Nrf2 signaling. These data confirmed previous studies based on animals including mice, Drosophila melanogaster flies, and Caenorhabditis elegans worms, that Nrf2 (orthologue) signaling is disrupted during aging and leads to a decreased endogenous antioxidant response [19], [2], [23], [25]. Nrf2 signaling could be regulated at multiple levels and involve various competitors and regulators [32]. Here we found that c-Myc and Bach1, two Nrf2 suppressors [18], [22], [11], were increased in the older donor HBE cells at basal level compared to that from young adults (Fig. 3, Fig. 5). An increase in c-Myc could result in faster Nrf2 turnover [11] and decrease the levels of Nrf2 in the nucleus, as observed here (Fig. 5). On the other hand, Bach1, as an Nrf2 competitor binding to EpRE cis-element, could further decrease Nrf2/EpRE activity and induction of genes upon exposure to SF or other oxidative stressors. It is possible that other regulators could also underlie the aging-related decline in Nrf2 signaling. The roles of Bach1 and c-Myc induction during the activation of Nrf2 are not fully elucidated. Previous data suggested that Bach1 was not increased by stimuli in tissues of 6 month old mice, but was induced in 21 month old mice [35]. Our results suggest that treatment with SF was able to induce a similar c-Myc accumulation in both young adult and older cells.
In conclusion, we found that although the basal expression of the Nrf2-regulated antioxidant genes was increased in the HBE cells from older human donors, the inducibility of these genes was impaired compared to that seen in HBE from young adult donors. We also showed that the increased nuclear level of both c-Myc and Bach1 in the cells of older donors might be involved in the aging-related decline in Nrf2 signaling, especially since Bach1 actually exhibited greater inducibility with donor age.
The induction of Nrf2-regulated antioxidant and detoxifying enzymes is considered a major adaptive response to endogenous and exogenous toxicants [15], and is clearly a major component of overall adaptive homeostasis capacity [37]. Adaptive Homeostasis. Molecular Aspects of Medicine 49, 1–7). The depressed induction of Nrf2 and Nrf2-regulated genes in older cells may underlie the increased susceptibility of the elderly to oxidative stress. The higher basal level of antioxidant enzymes and Nrf2 suppressing genes indicates that older people may establish a new, and higher, steady state level of antioxidant enzyme expression that improves basal capacity to cope with oxidation but is then less able to further increase antioxidant enzymes in response to stress. Our findings also suggest that therapeutic approaches based on activating Nrf2 may not be as effective in older individuals as in young adults because of increased activities of the Nrf2 suppressors Bach1 and c-Myc. Therefore, suppression of Bach1 and c-Myc may be a more effective method for future treatments specifically aimed at older individuals.
Acknowledgements
This work was supported by grants ES023864, ES003598, and AG052374 from the US National Institutes of Health. We thank undergraduate students, Gabi Lopez and Manali Begur who contributed to the experimental work.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.08.014.
Appendix A. Supplementary material
Supplementary material
References
- 1.Curran T., Franza B.R. Fos and Jun: the AP-1 connection. Cell. 1988;55:395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- 2.Duan W., Zhang R., Guo Y., Jiang Y., Huang Y., Jiang H., Li C. Nrf2 activity is lost in the spinal cord and its astrocytes of aged mice. Vitr. Cell. Dev. Biol. - Anim. 2009;45:388–397. doi: 10.1007/s11626-009-9194-5. [DOI] [PubMed] [Google Scholar]
- 3.Elbarbry F., Elrody N. Potential health benefits of sulforaphane: a review of the experimental, clinical and epidemiological evidences and underlying mechanisms. J. Med. Plants Res. 2011;5:473–484. [Google Scholar]
- 4.Gassmann M., Grenacher B., Rohde B., Vogel J. Quantifying Western blots: pitfalls of densitometry. Electrophoresis. 2009;30:1845–1855. doi: 10.1002/elps.200800720. [DOI] [PubMed] [Google Scholar]
- 5.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 6.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y., Oyake T., Hayashi N., Satoh K., Hatayama I., Yamamoto M., Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 7.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kireev R.A., Tresguerres A.C., Garcia C., Borras C., Ariznavarreta C., Vara E., Vina J., Tresguerres J.A. Hormonal regulation of pro-inflammatory and lipid peroxidation processes in liver of old ovariectomized female rats. Biogerontology. 2010;11:229–243. doi: 10.1007/s10522-009-9242-2. [DOI] [PubMed] [Google Scholar]
- 9.Lee J.-M., Johnson J.A. An important role of Nrf2-ARE pathway in the cellular defense mechanism. BMB Rep. 2004;37:139–143. doi: 10.5483/bmbrep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- 10.Levy S., Forman H.J. C-Myc is a Nrf2-interacting protein that negatively regulates phase II genes through their electrophile responsive elements. IUBMB Life. 2010;62:237–246. doi: 10.1002/iub.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy S., Forman H.J. C‐Myc is a Nrf2‐interacting protein that negatively regulates phase II genes through their electrophile responsive elements. IUBMB life. 2010;62:237–246. doi: 10.1002/iub.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis K.N., Wason E., Edrey Y.H., Kristan D.M., Nevo E., Buffenstein R. Regulation of Nrf2 signaling and longevity in naturally long-lived rodents. Proc. Natl. Acad. Sci. 2015;112:3722–3727. doi: 10.1073/pnas.1417566112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y., Jaiswal A.K. Regulation of human NAD(P)H: quinone oxidoreductase gene. Role of AP1 binding site contained within human antioxidant response element. J. Biol. Chem. 1992;267:15097–15104. [PubMed] [Google Scholar]
- 14.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen T., Huang H., Pickett C.B. Transcriptional regulation of the antioxidant response element Activation by Nrf2 and repression by MafK. J. Biol. Chem. 2000;275:15466–15473. doi: 10.1074/jbc.M000361200. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen T., Nioi P., Pickett C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohtsuji M., Katsuoka F., Kobayashi A., Aburatani H., Hayes J.D., Yamamoto M. Nrf1 and Nrf2 play distinct roles in activation of antioxidant response element-dependent genes. J. Biol. Chem. 2008;283:33554–33562. doi: 10.1074/jbc.M804597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okada S., Muto A., Ogawa E., Nakanome A., Katoh Y., Ikawa S., Aiba S., Igarashi K., Okuyama R. Bach1-dependent and -independent Regulation of Heme Oxygenase-1 in Keratinocytes. J. Biol. Chem. 2010;285:23581–23589. doi: 10.1074/jbc.M109.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickering A.M., Staab T.A., Tower J., Sieburth D., Davies K.J. A conserved role for the 20S proteasome and Nrf2 transcription factor in oxidative stress adaptation in mammals, Caenorhabditis elegans and Drosophila melanogaster. J. Exp. Biol. 2013;216:543–553. doi: 10.1242/jeb.074757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reichard J.F., Motz G.T., Puga A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acids Res. 2007;35:7074–7086. doi: 10.1093/nar/gkm638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sachdeva M.M., Cano M., Handa J.T. Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Exp. Eye Res. 2014;119:111–114. doi: 10.1016/j.exer.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shan Y., Lambrecht R.W., Donohue S.E., Bonkovsky H.L. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J. 2006;20:2651–2653. doi: 10.1096/fj.06-6346fje. [DOI] [PubMed] [Google Scholar]
- 23.Shih P.-H., Yen G.-C. Differential expressions of antioxidant status in aging rats: the role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology. 2007;8:71–80. doi: 10.1007/s10522-006-9033-y. [DOI] [PubMed] [Google Scholar]
- 24.Singh K., Connors S.L., Macklin E.A., Smith K.D., Fahey J.W., Talalay P., Zimmerman A.W. Sulforaphane treatment of autism spectrum disorder (ASD) Proc. Natl. Acad. Sci. 2014;111:15550–15555. doi: 10.1073/pnas.1416940111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suh J.H., Shenvi S.V., Dixon B.M., Liu H., Jaiswal A.K., Liu R.M., Hagen T.M. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc. Natl. Acad. Sci. USA. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun J., Hoshino H., Takaku K., Nakajima O., Muto A., Suzuki H., Tashiro S., Takahashi S., Shibahara S., Alam J. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase‐1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thimmulappa R.K., Mai K.H., Srisuma S., Kensler T.W., Yamamoto M., Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 28.Valcarcel-Ares M.N., Gautam T., Warrington J.P., Bailey-Downs L., Sosnowska D., De Cabo R., Losonczy G., Sonntag W.E., Ungvari Z., Csiszar A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J. Gerontol. Ser. A: Biomed. Sci. Med. Sci. 2012;67:821–829. doi: 10.1093/gerona/glr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venugopal R., Jaiswal A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H: quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. USA. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Y.-H., Lee H.-C. Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Exp. Biol. Med. 2002;227:671–682. doi: 10.1177/153537020222700901. [DOI] [PubMed] [Google Scholar]
- 31.Yang H., Magilnick N., Lee C., Kalmaz D., Ou X., Chan J.Y., Lu S.C. Nrf1 and Nrf2 regulate rat glutamate-cysteine ligase catalytic subunit transcription indirectly via NF-κB and AP-1. Mol. Cell. Biol. 2005;25:5933–5946. doi: 10.1128/MCB.25.14.5933-5946.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H., Davies K.J., Forman H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015;88:314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H., Dickinson D.A., Liu R.-M., Forman H.J. 4-Hydroxynonenal increases γ-glutamyl transpeptidase gene expression through mitogen-activated protein kinase pathways. Free Radic. Biol. Med. 2005;38:463–471. doi: 10.1016/j.freeradbiomed.2004.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H., Forman H.J. Reexamination of the electrophile response element sequences and context reveals a lack of consensus in gene function. Biochim. Et Biophys. Acta (BBA) - Gene Regul. Mech. 2010;1799:496–501. doi: 10.1016/j.bbagrm.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H., Liu H., Davies K.J., Sioutas C., Finch C.E., Morgan T.E., Forman H.J. Nrf2-regulated phase II enzymes are induced by chronic ambient nanoparticle exposure in young mice with age-related impairments. Free Radic. Biol. Med. 2012;52:2038–2046. doi: 10.1016/j.freeradbiomed.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y., Talalay P., Cho C.-G., Posner G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc. Natl. Acad. Sci. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies K.J.A. Adaptive homeostasis. Mol. Asp. Med. 2016;49:1–7. doi: 10.1016/j.mam.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material