Abstract
Here we present our standard protocol for studying the binding of kinetochore proteins to microtubules as a paradigm for designing single-molecule total internal reflection fluorescence (TIRF) microscopy experiments. Several aspects of this protocol require empirical optimization, including the method for anchoring the polymer or substrate to the coverslip, the type and amount of blocking protein to prevent nonspecific protein adsorption to the glass, the appropriate protein concentration, the laser power, and the duration of imaging. Our method uses bovine serum albumin and κ-casein as blocking agents to coat any imperfections in the coverslip silanization and thereby prevent protein adsorption to the coverslip. Protein concentration and duration of imaging must be optimized for each experiment and protein of interest. Ideally, a range is determined that allows for resolution of single complexes binding to microtubules to ensure proper measurement of kinetic off rates and diffusion along microtubules. Excessively high concentrations may lead to overlapping binding of proteins on microtubules, making it impossible to resolve single binding events. The duration of imaging must be long enough to capture very low off rates (long residence time on microtubules) and we typically image at 10 frames/sec for 200 sec. The laser power can be adjusted to prevent photobleaching, but must be high enough to achieve a sufficient signal/noise ratio.
Materials
It is essential that you consult the appropriate Material Safety Data Sheets and your institution's Environmental Health and Safety Office for proper handling of equipment and hazardous material used in this protocol.
Reagents
Alexa-568-labeled GMPCPP seeds (Hyman et al. 1991)
Alexa-568-labeled, paclitaxel-stabilized microtubules (Hyman et al. 1991)
BB80 (BRB80 <R> containing 8 mg/mL BSA)
BB80T (BB80 containing 10 μM taxol)
Bovine serum albumin (BSA; 40 mg/mL stock solution; filter sterilized)
κ-Casein (5 mg/mL stock solution; filter sterilized; optional)
Catalase (1.75 mg/mL stock solution)
Dithiothreitol (DTT; 250 mM stock solution)
Ethanol
Glucose (1.25 M stock solution)
Glucose oxidase (10 mg/mL stock solution)
GTP (100 mM stock solution, pH 7.0)
Microtubule growth buffer (GB; BB80 containing 1 mM GTP)
Purified protein of interest
Rigor kinesin
Tubulin (bovine; 1:100 Alexa-568-labeled to unlabeled)
Equipment
Adaptors for peristaltic pump (custom-made)
Adhesive transfer tape (3M F9473PC)
Coverslips (glass; 22 × 60 mm; silanized) (see Protocol: Coverslip Cleaning and Functionalization for Total Internal Reflection Fluorescence Microscopy<prot085548> Kudalkar et al. [2015])
Drill (with diamond bit)
Double-sided tape
Razor blade
Slides (glass; 3 × 1 in)
Vacuum grease
Method
This protocol for utilizing TIRF microscopy to study the binding of kinetochore proteins to microtubules at the single-molecule level is adapted from Gestaut et al. (2010).
Assembly of Flow Chamber
The design of the flow chamber depends on whether the experiment is to be performed with the peristaltic pump method (see Steps 1–8) or the sealed slide method (see Steps 9–12). The peristaltic pump method is used to introduce reactants to the flow chamber during imaging on the microscope and the sealed slide method is used to introduce reactants prior to imaging. Both methods utilize the same procedure for washes, adherence of rigor kinesin, and introduction of microtubules and proteins. If the reactants are introduced prior to imaging, the entire protocol is performed at the bench using an aspirator to draw liquid through the chambers and then the slide is sealed with either clear nail polish or vacuum grease.
I. Flow Chamber for Peristaltic Pump Method
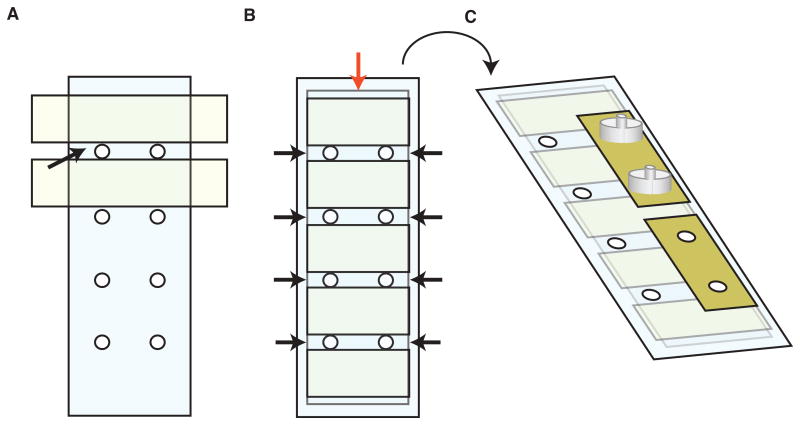
Drill eight holes into a 3 × 1 in glass slide using a diamond bit—two sets of four holes on the long axis of the slide, directly across from one another (Fig. 1A).
Clean the drilled glass slide with ethanol. Place double-sided tape crosswise between the drilled holes leaving approximately 3 mm between each piece of tape to create four equivalently sized flow chambers.
Place a silanized coverslip lengthwise over the middle of the slide. Ensure all eight holes are covered to create four perfusion chambers. Gently press down on the surface of the coverslip to ensure a tight seal (Fig. 1B).
Remove the excess tape on the sides of the coverslip using a razor blade.
Using a cotton-tipped applicator, seal the chambers by gently pressing vacuum grease into each opening on the outer edges of the slide until it reaches the holes. Wipe off excess grease with ethanol.
Flip the glass slide over so the coverslip is underneath. Apply adhesive tape to four of the holes on this side of the slide and, using forceps, remove circles of tape that cover each hole (Fig. 1C).
Place custom-made adaptors on the tape, centering each one with the holes in the slide. Press gently to ensure a good seal (Fig. 1C).
Make a small circle around each remaining hole using vacuum grease to create a pool for buffers.
Figure 1.
Assembly of flow chamber for peristaltic pump method. (A) Apply double-sided tape to a glass slide pre-drilled with two sets of four holes in a lengthwise arrangement. Position the tape to create four 3-mm-wide chambers (black arrows). (B) Position a cleaned coverslip (red arrow) in the middle of the slide and press firmly to seal against the tape. Trim the tape on both sides using a razor blade so it is flush with the coverslip edge. Push vacuum grease into the sides of chambers (black arrows) to seal. Do not cover the holes. (C) Flip the slide over so coverslip is face down. Apply 3M adhesive tape to cover the holes on one edge of slide (yellow). Using forceps, remove the tape covering each hole to create an open channel. Center custom-made flow adaptors (grey) on top of each hole and press firmly to seal.
II. Flow Chamber for Sealed Slide Method
-
9
Clean a 3 × 1 in glass slide thoroughly with ethanol.
-
10
Apply several pieces of double-sided tape crosswise to the slide, leaving a gap of approximately 3 mm in between the pieces to create chambers.
-
11
Place a silanized coverslip crosswise on the slide leaving an equal-sized ledge on either side of the slide. Gently press down to ensure a tight and even seal between the coverslip and tape.
-
12
Using a razor blade, trim off excess tape from either side of the coverslip so it is flush with the edge.
Binding Interactions using Paclitaxel-Stabilized Microtubules
Direct adsorption of microtubules to the coverslip can interfere with their ability to grow and shorten (if using non-stabilized microtubules) and could potentially hinder protein binding. To attach microtubules to the coverslip without direct adherence to the glass, we employ an established method that utilizes a mutated kinesin (“rigor kinesin”) that is competent to bind, but not release, microtubules (Rice et al. 1999). We optimize the concentration of rigor kinesin to ensure that microtubules are stably tethered to the coverslip, but the kinesin does not interfere with experimental protein binding.
-
13
Flow 100 μL of Milli-Q purified water through the chamber twice.
-
14
Flow 25 μL of rigor kinesin (diluted in BB80T) through the chamber and incubate for 5 min. Determine the dilution factor for rigor kinesin empirically for each coverslip preparation to ensure proper anchoring and coverage of microtubules. Apply 50 μL of BB80T to the edge or hole of the flow chamber during incubation to prevent the chamber from drying.
-
15
Flow 50 μL of BB80T through the chamber.
-
16
Flow 15 μL of Alexa-568-labeled, paclitaxel-stabilized microtubules diluted in BB80T through the chamber. Determine the dilution factor empirically to achieve an appropriate amount of microtubule coverage (typically about 3–7 nonoverlapping microtubules per field is optimal for analysis). Incubate for 5 min. Apply 50 μL of BB80T to the edge or hole of the flow chamber during incubation to prevent the chamber from drying.
-
17
During the microtubule incubation, prepare the experimental reaction, which typically contains 10–100 pM protein, 25 mM glucose, 5 mM DTT, and oxygen scavengers 200 μg /mL glucose oxidase and 35 μg /mL catalase Adjust the volume to 50 μL with BB80T.
To prevent protein loss to the tube (a problem that can occur when working in the pM concentration range), dilute the protein of interest immediately before adding to the reaction mixture.
-
18
Flow 50 μL of BB80T through the chamber and then 50 μL of reaction mixture. Image immediately on both 488 nm and 561 nm channels (see Protocol: Data Analysis for Total Internal Reflection Fluorescence Microscopy<prot085571> [Asbury 2015]).
Binding Interactions using Dynamic Microtubules
-
19
Follow Steps 13 and 14 of the protocol using stabilized microtubules.
The concentration of rigor kinesin may be increased to ensure proper anchoring of growing microtubule extensions.
-
20
Flow 50 μL of GB through the chamber.
-
21
Flow 15 μL of Alexa-568-labeled GMPCPP seeds diluted in BB80 through the chamber. Incubate for 1 min. Optimize the concentration of seeds to ensure proper coverslip coverage.
-
22
Flow 50 μL of GB through the chamber.
-
23
Prepare the tubulin mix, which contains 2 mg/mL bovine tubulin (1:100 Alexa-568-labeled to unlabeled), 25 mM glucose, 5 mM DTT, and oxygen scavengers 200 μg /mL glucose oxidase and 35 μg /mL catalase. Adjust the volume to 50 μL with BB80. Flow 50 μL of tubulin mix through the chamber. Incubate for 15 min to allow microtubules to extend off the Alexa-568 GMPCPP seeds.
-
24
During the incubation, focus the microscope on the channel.
Once the reaction mix is added (Step 25), the microtubules will immediately begin to depolymerize. Therefore, it is essential to have the field of view already in focus so as to begin imaging promptly upon addition of the reaction mix.
-
25
Prepare the reaction mix containing 10 pM to 1 nM protein, 200 μg /mL glucose oxidase, 35 μg /mL catalase, 25 mM glucose, and 5 mM DTT. Adjust the volume to 50 μL with BB80. Flow 50 μL of reaction mix through the chamber. Begin imaging immediately (see Protocol: Data Analysis for Total Internal Reflection Fluorescence Microscopy<prot085571> [Asbury 2015]).
References
- Asbury CL. Data analysis for total internal reflection fluorescence microscopy. Cold Spring Harb Protoc. 2015 doi: 10.1101/pdb.prot085571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestaut DR, Cooper J, Asbury CL, Davis TN, Wordeman L. Reconstitution and functional analysis of kinetochore subcomplexes. Methods Cell Biol. 2010;95:641–656. doi: 10.1016/S0091-679X(10)95032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman A, Drechsel D, Kellogg D, Salser S, Sawin K, Steffen P, Wordeman L, Mitchison T. Preparation of modified tubulins. Methods Enzymol. 1991;196:478–485. doi: 10.1016/0076-6879(91)96041-o. [DOI] [PubMed] [Google Scholar]
- Kudalkar EM, Deng Y, Davis TN, Asbury CL. Coverslip cleaning and functionalization for total internal reflection fluorescence microscopy. Cold Spring Harb Protoc. 2015 doi: 10.1101/pdb.prot085548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S, Lin AW, Safer D, Hart CL, Naber N, Carragher BO, Cain SM, Pechatnikova E, Wilson-Kubalek EM, Whittaker M, et al. A structural change in the kinesin motor protein that drives motility. Nature. 1999;402:778–784. doi: 10.1038/45483. [DOI] [PubMed] [Google Scholar]