Abstract
This unit describes the published Matrigel Mattress method. Briefly, we describe the application of the mattress, human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) Matrigel Mattress re-plating, and hiPSC-CM mattress maintenance. Adherence to this protocol will yield individual robust shortening hiPSC-CMs, which can be used for downstream applications.
Keywords: Matrigel, hiPSC-CMs, myocytes, cardiac, stem cells
Assessing function in hiSPC-CMs
Cardiovascular disease is the leading cause of mortality worldwide (Anonymous, 2016). In the United States, heart failure is projected to increase by 46% from 2012–2030 thus affecting over 8 million Americans adults (Heidenreich et al., 2013). Ergo, there is an urgent need for appropriate model systems to investigate disease pathophysiology and cardiovascular biology. Advances in hiPSC biology have led to reliable and efficient methods of differentiating hiPSCs into hiPSC-CMs for research and disease modeling (Burridge et al., 2014; Hinson et al., 2015; Mummery et al., 2012). Initially, the majority of physiological readouts using hiPSC-CMs have focused on electrophysiology and calcium handling measurements. Functional assessment of single cell contractility (cell shortening) has been limited due to the lack of cell shortening due to the aberrant spherical morphology of hiPSC-CMs cultured on standard substrates. To circumvent this issue, biophysical approaches such as atomic force microscopy, traction force microscopy, and micropost deflection have been employed to assess the contractile forces of single cell hiPSC-CMs (Ahola et al., 2014; Liu et al., 2012; Rodriguez et al., 2014). Although quantitative, these methods are technically challenging and require specialized lab equipment, which limits their adoptability.
Alternatively, tunable synthetic polymers coupled to printed biological substrates have been used to quantify hiPSC-CM single cell contractility (Ribeiro et al., 2015). Our method, the Matrigel Mattress, takes advantage of Matrigel’s natural and environmental stiffness (450 Pa)(Li et al., 1994; Soofi et al., 2009) which alleviates the need for microcontacted prints on biomaterials. In this unit, we describe the simple 1 hour protocol for generating the Matrigel Mattress, which generates individual, shortening, hiSPC-CMs within 3–5 days post-mattress plating (Feaster et al., 2015).
Generating Matrigel Mattresses (~1 hour)
The protocol outlines the hiPSC-CM dissociation, Matrigel Mattress application, and hiPSC-CMs re-plating steps. Successful completion of the protocol will yield functional cardiomyocytes that will be ready for downstream applications in 3–5 days post-plating.
Note: The protocol detailed below must be performed using proper aseptic technique in a class II, type A2 biological safety cabinet.
Note: This protocol describes the dissociation from cultured differentiated hiSPC-CMs at day 30 of cardiac induction.
Note: Frozen hiPSC-CMs should be thawed and cultured for 3–5 days before dissociation.
Note: Check status of cultured hiPSC-CMs before dissociation by assessing viability and contractility.
Note: hiPSC-CM purity is important, as non-CMs will also adhere to the Matrigel mattress. We recommend metabolic selection via glucose deprivation at day 10 of induction for two days to remove contaminating cell types as in Feaster et. al.(Feaster et al., 2015) (Media composition, RPMI 1640 (Life Technologies, 11879–020) supplemented with 2% B27-minus insulin (Life Technologies, A1895601).
Note: The protocol described outlines the removal and re-plating of hiSPC-CMs from a 35 mm cell culture dish containing approximately 2 × 106 hiPSC-CMs.
Note: The protocol describes the generation of 3 Matrigel Mattress dishes (Delta TPG DISH)
Materials
HiPSC-CMs in culture
HiPSC-CM media (See Recipe)
Matrigel Growth Factor Reduced Basement Membrane Matrix (Corning, Product ##356230 )
TrypLE Express (Life Technologies, 12604013)
D-PBS without CaCl2 or MgCl2 (Life Technologies, 14190144
Falcon™ 15mL Conical Centrifuge Tubes (Fisher Scientific)
Small Cell Scraper (Corning, #3010)
2μl Gilson pipet with tips
Timer
Light microscope
Incubator
Delta TPG DISH (Fisher Scientific, Delta TPG DISH (50/Pack) 0.17mm black, 12-071-34) for experiments (typically 3 dishes per group)
Reagents and Solutions
HiPSC-CM media: RPMI 1640 with glucose (Invitrogen, cat# 11875); 2% B27 with insulin (Invitrogen, cat#17504-044); 1 % Pen-Strep (Invitrogen, cat#17504-044).
HiPSC-CM dissociation Timing: 30 minutes
Note: Thaw an aliquot of pure undiluted Matrigel on ice before beginning
-
1
Wash hiPSC-CMs by adding 2 mL D-PBS without to the 35 mm dish.
-
2
Aspirate solution.
-
3
Add 1 mL TrypLE Express (room temperature) to the 35 mm dish and incubate at 37°C for 15 minutes (Perform step 4 during incubation).
-
4
Add 10 mL of hiPSC-CMs culture media to a 15 mL conical centrifuge tube.
-
5
After incubation, gently remove TrypLE Express from the dish and transfer it to the 15 mL conical centrifuge tube.
-
6
Add 1 mL of hiPSC-CM media to the culture dish.
-
7
Gently pipette hiPSC-CM media and eject it onto the cell culture dish surface to dislodge remaining adherent cells.
-
8
Repeat 3 times.
-
9
Use a cell scraper to dislodge any attached hiPSC-CMs.
-
10
Transfer entire contents of the dish to the 15 mL conical centrifuge tube.
-
11
Add 3 mL of hiPSC-CM media the 15 mL conical centrifuge tube to bring the total volume to 15 mL.
-
12
Place the 15 mL conical tube into the centrifuge and spin for 5 minutes at 200g.
-
13
Decant the conical tube. Be careful not to disrupt the cell pellet.
-
14
Re-suspend the pellet using 3 mL of cardiomyocyte media.
-
15
Bring to a final volume of 5 mL
-
16
Count the cardiomyocytes using a hemacytometer.
-
17
Adjust to 2 × 105 cardiomyocytes per 1 mL of cardiomyocte media.
-
18
Incubate cardiomyocytes at room temp during Mattress preparation.
Matrigel Mattress Preparation and hiSPC-CM re-plating: Timing: 30 minutes
Note: It may be helpful to practice ahead of time. Also to prepare a smaller number of Mattresses to start (~4) until proper technique is established.
-
19
Mix the Matrigel by carefully flicking the microcentrifuge tube.
-
20
Place the Martigel tube on ice.
-
21
Start a timer with application of the first Mattress
-
22
Chill the pipet tip by pipetting 1μL of chilled Matrigel up and down 2–3 times
-
23
Apply 1 μL of undiluted Matrigel over the length of 20 mm line. (Fig 1)
Be sure to pipette a 45-degree angle.
Apply 4, 1μL lines in parallel per dish. Each line (1 μL) represents one Mattress.
Figure 1.
Sample Delta TPG dish with 4 Matrigel Mattresses drawn. Mattresses are indicated by red arrows.
Note: Aim to spend 2 minutes preparing 12 Mattresses.
Note: To help with orientation during experimentation, plate all Mattress lines in parallel.
Note: Mattresses shorter than 10 mm are too thick resulting in an unstable Mattress
Note: Work quickly to avoid Matrigel solidifying in the pipette tip. If Matrigel solidifies in the pipette tip, discard the tip, re-chill a new pipette tip, and continue.
-
24
Incubate the Mattresses at room temperature for 8–10 minutes.
Note: An 8 minute incubation allows for enough time for the Matrigel to solidify. Incubation longer than 10 minutes results in a stiff Mattress which impedes cell shortening. Mattresses incubated under 8 minutes are unstable.
-
25
Add ~40,000 cardiomyocytes in 200 μL hiPSC-CM media directly to each dish.
Note: Addition of cardiomyocytes and media halts Mattress polymerization.
-
26
Incubate at room temperature for 5 minutes. (Do not disturb the dish as the cells are adhering to the mattress)
-
27
Using a light microscope, visually inspect the dish to ensure the cells are not too dense. i.e. hiPSC-CMs do not touch. If the hiPSC-CMs are touching, make new Matrigel Mattresses and repeat plating at a lower density.
-
28
Gently pipette 1 mL of hiPSC-CM media onto each dish.
-
29
Incubate at 37°C for 3–5 days until cell shortening begins
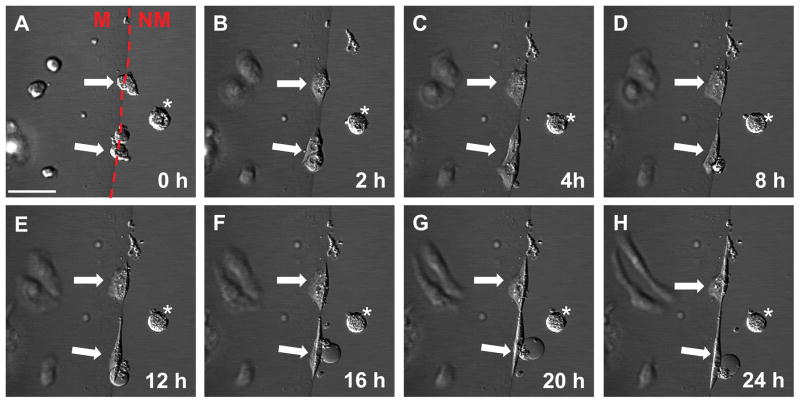
Note: Visually inspect for morphology changes. Cell rounding should begin immediately. By 12 hours cardiomyocytes should exhibit distinct morphologic changes and robust cell shortening at 24 hours. (Fig 2)
Figure 2.
Time-lapse imaging of hiPSC-CMs on the Mattrigel Mattress. Mattress hiPSC-CMs adopt elongated morphology ~12 hours post-plating. Arrows indicate hiPSC-CMs on the Mattress. Asterisk indicate cell not on the mattress. The red dashed line separates the Matrigel mattress from non-mattress area. M indicates mattress, NM indicates non-mattress, scale bar is 50 μm.
-
30
Change the hiPSC-CM media
Commentary
Background Information
Matrigel is a solubilized basement membrane matrix extracted from the Engelbreth-Holm-Swarm mouse sarcoma (Kleinman et al., 1982). This tumor is rich in extracellular proteins such as procollagen IV, laminin and heparin sulfate proteoglycans (Kleinman et al., 1982). This tumor is also rich in growth factors such as transforming growth factor β (TGF-β), epidermal growth factor (EGF), insulin-like growth factor 1, bovine fibroblast growth factor (bFGF), and platelet-derived growth factor (PDGF) (Vukicevic et al., 1992). Although, we don’t know the exact mechanism that causes the change in hiPSC-CM morphology and function, but we postulate a combination of both growth factors and membrane hydrogel stiffness, that allows for the robust hiPSC-CM shortening. Future research should identify which combination of factors and substrates mimic the Matrigel Mattress in order to establish a chemical and synthetic hydrogel to promote functional maturation.
Critical Parameters
It is critical to identify hiPSC-CM health, as poorly differentiated cells may not adopt the Matrigel Mattress phenotype. Be sure to evaluate the hiPSC-CMs for rhythmic beating, purity and viability.
Troubleshooting
Refer to Table 1 for common Matrigel Mattress problems and solutions.
Table 1.
List of potential Matrigel Mattress problems and solutions.
| Problem | Possible Reason | Solution |
|---|---|---|
| Matrigel solidifies in pipette tip | Matrigel has reached room temperature | Work quickly |
| Mattress in oblique line | Pipetting too quickly | Slow down |
| Poor quality mattresses | Preparing too many mattress simultaneously | Prepare less until proper technique is established |
| Mattress implodes | Short incubation | Incubate mattresses for 8–10 minutes prior to seeding hiPSC-CMs |
| hiPSC-CMs do not contract on mattress | Mattresses are too thick or thin | Apply Matrigel at a thickness of 0.4 mm |
| Cells dissociate from mattress | Perfusion or aspiration too strong during daily media changes | Decrease aspiration strength |
| Mattress ruptures or dislodges from dish | Media forcefully applied to dish | Gently add media to the corner of the dish; not directly on the mattress |
Anticipated Results
This protocol yields individually contracting cardiomyocytes within 3–5 days on the Matrigel Mattress.
Time Considerations
HiPSC-CM dissociation: Timing: 30 minutes
Matrigel Mattress Preparation and hiSPC-CM re-plating: Timing: 30 minutes
Significance Statement.
The Matrigel Mattress is an innovative platform that promotes single cell shortening in hiPSC-CMs.
Acknowledgments
This work was supported by the National Institutes of Health grants 5R01HL104040, 5R01HL095813, and P50GM115305 to C.C. H. The project publication described was supported by CTSA award No. UL1TR000445 to A.G.C. from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. Live cell imaging were performed in part through the use of the VUMC Cell Imaging Shared Resource (supported by NIH grants CA68485, DK20593, DK58404, DK59637 and EY08126).
Literature Cited
- Ahola A, Kiviaho AL, Larsson K, Honkanen M, Aalto-Setala K, Hyttinen J. Video image-based analysis of single human induced pluripotent stem cell derived cardiomyocyte beating dynamics using digital image correlation. Biomedical engineering online. 2014;13:39. doi: 10.1186/1475-925X-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, Plews JR, Abilez OJ, Cui B, Gold JD, Wu JC. Chemically defined generation of human cardiomyocytes. Nature methods. 2014;11:855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feaster TK, Cadar AG, Wang L, Williams CH, Chun YW, Hempel JE, Bloodworth N, Merryman WD, Lim CC, Wu JC, Knollmann BC, Hong CC. Matrigel Mattress: A Method for the Generation of Single Contracting Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circulation research. 2015;117:995–1000. doi: 10.1161/CIRCRESAHA.115.307580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG American Heart Association Advocacy Coordinating C, Council on Arteriosclerosis T, Vascular B, Council on Cardiovascular R, Intervention Council on Clinical C. Council on E. Prevention and Stroke C. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circulation. Heart failure. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JT, Chopra A, Nafissi N, Polacheck WJ, Benson CC, Swist S, Gorham J, Yang L, Schafer S, Sheng CC, Haghighi A, Homsy J, Hubner N, Church G, Cook SA, Linke WA, Chen CS, Seidman JG, Seidman CE. HEART DISEASE. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science. 2015;349:982–986. doi: 10.1126/science.aaa5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Li X, Tsai P, Wieder ED, Kribben A, Van Putten V, Schrier RW, Nemenoff RA. Vascular smooth muscle cells grown on Matrigel. A model of the contractile phenotype with decreased activation of mitogen-activated protein kinase. The Journal of biological chemistry. 1994;269:19653–19658. [PubMed] [Google Scholar]
- Liu J, Sun N, Bruce MA, Wu JC, Butte MJ. Atomic force mechanobiology of pluripotent stem cell-derived cardiomyocytes. PloS one. 2012;7:e37559. doi: 10.1371/journal.pone.0037559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circulation research. 2012;111:344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro AJ, Ang YS, Fu JD, Rivas RN, Mohamed TM, Higgs GC, Srivastava D, Pruitt BL. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:12705–12710. doi: 10.1073/pnas.1508073112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez ML, Graham BT, Pabon LM, Han SJ, Murry CE, Sniadecki NJ. Measuring the contractile forces of human induced pluripotent stem cell-derived cardiomyocytes with arrays of microposts. Journal of biomechanical engineering. 2014;136:051005. doi: 10.1115/1.4027145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soofi SS, Last JA, Liliensiek SJ, Nealey PF, Murphy CJ. The elastic modulus of Matrigel as determined by atomic force microscopy. Journal of structural biology. 2009;167:216–219. doi: 10.1016/j.jsb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Experimental cell research. 1992;202:1–8. doi: 10.1016/0014-4827(92)90397-q. [DOI] [PubMed] [Google Scholar]