ABSTRACT
The aim of this study was to investigate the therapeutic effect of Sasa veitchii leaf extract (SE) on features of obesity induced by a high-fat diet (HFD), such as hyperglycemia, insulin resistance, and inflammatory response. Four-week-old male ddY mice were freely fed HFD or control normal diet for 12 weeks; half was given SE in addition twice per day in weeks 8–12. Glucose and insulin intolerance were estimated, and body weight measured, weekly throughout the study. Following the experiment, the mice were fasted for 16 h, euthanized, and plasma was collected. Liver and epididymal adipose tissue was collected and weighed. Treatment with SE significantly decreased body weight, adipose tissue weight, plasma glucose, insulin, leptin, and tumor necrosis factor α compared with HFD groups, and markedly reduced the impairment of glucose and insulin tolerance in obese mice. Furthermore, hepatic steatosis and hepatic insulin receptor substrate were improved by treatment with SE. Our findings demonstrate that SE may reduce obesity-induced glucose and insulin tolerance, not only by suppressing inflammatory responses but also by improving insulin signaling.
Key Words: obesity, insulin resistance, high-fat diet, Sasa veitchii
INTRODUCTION
Obesity is a major risk factor for diabetes, atherosclerosis, type 2 diabetes, cardiovascular diseases, hypertension, and lipid abnormalities, which together are referred to as the metabolic syndrome.1,2) In particular, obesity-induced inflammation plays a crucial role in the development of metabolic diseases.3,4) Several studies have shown that dysregulation of proteins such as cytokines, chemokines, and adipocytokines results in impaired insulin signaling and lipid metabolism.5-7) For example, tumor necrosis factor α (TNFα) impairs insulin signaling and induces insulin resistance.8) Mice rendered obese by a high-fat diet (HFD) are protected from insulin resistance and type 2 diabetes by blockage of TNFα activity.9) TNFα, interleukin (IL)-6, and monocyte chemoattractant protein 1 are associated with obesity-induced inflammation, which leads to hepatic insulin resistance and abnormal fat accumulation.10,11) On the other hand, adiponectin improves insulin resistance by suppressing hepatic glucose production and enhancing glucose uptake in muscle.12)
TNFα and IL-6 have been shown to be key mediators of hepatic inflammation, liver cell death, fibrosis, and liver regeneration after injury,13) indicating that metabolic dysregulation may be aggravated systemically by these adipose and hepatic tissue-derived factors. In this regard, reducing obesity-induced inflammation by targeting proteins derived from adipose and hepatic tissue may be a useful strategy for preventing obesity-induced metabolic pathologies.
Bamboo is a common perennial plant consisting of several species of the genus Sasa. It is widely distributed in Asian countries and its roots and leaves have been used in food and medicine. Sasa veitchii, which belongs to the Gramineae family, has recently been found to contain a large number of bioactive molecules such as polyphenol and flavones.14,15) The plant has been found to be useful in Asia for a long time: for example, in Japan, its leaves are used to wrap sushi sheets to protect against bacterial spoilage.
Studies have demonstrated that S. veitchii extract (SE) has antitumor,16) antiulcer,17) antiviral,18) antioxidant,19,20) and antiallergic properties.21) Others have reported anti-inflammatory and anti-obesity properties.22,23) However, it remains unclear whether SE can attenuate obesity-induced inflammation, and particularly whether it reduces obesity-related inflammatory complications such as insulin resistance.
In order to address these questions, we examined whether HFD-induced obesity responses, including insulin resistance, are altered by treatment with SE.
MATERIALS AND METHODS
Animal treatment
In the current study, we used ddY mice because we had used this strain in our previous study.21,24) Male 4-week-old ddY mice were purchased from Japan SLC (Shizuoka, Japan). Mice were housed under standard conditions of controlled temperature (24 ± 1°C), humidity (55 ± 5%), and light (12:12 h light/dark cycles), and provided with food and water ad libitum. All experiments were approved by the Institutional Animal Care and Experiment Committee of Kinjo Gakuin University (No. 109).
Experimental protocol
Mice were fed a HFD (Oriental Yeast Co., Tokyo, Japan; 60% calories, 5.06 kcal/g; protein [casein, L-cysteine] 23.0%, carbohydrate [corn, maltodextrin, sucrose] 25.3%, fat [lard, soybean oil] 35.0%, other) or normal diet (CE-2; Clea Japan, Inc., Tokyo, Japan; 3.59 kcal/g; protein [soybean waste, whitefish meal, yeast] 24.9%, carbohydrate [wheat flour, corn, Milo] 51.0%, fat [cereal germ, soybean oil] 4.6%, other) for a total of 12 weeks. After 8 weeks, the mice were divided into control groups (CE-2 and HFD), which received saline, and treatment groups that were given SE (Sunchlon Co. Ltd., Nagano, Japan) (CE-2 + SE and HFD + SE). The 0.2 mL dose of SE is equivalent to 0.564 g of S. veitchii leaves, as previously described.24) Treatment with saline or SE, 0.2 mL twice per day by oral gavage, lasted 4 weeks.
Body weight and food intake were measured every week throughout the study. Food intake per day was calculated as total food intake / number of mice. Following the experiment, mice from each group were fasted for 16 h, euthanized using pentobarbital, and bled for plasma samples, which were stored at –80 °C pending assays. Liver and epididymal adipose weights were determined. Separate samples from each liver were stored at –80 °C or fixed in 15% neutral buffered formalin (pH 7.2).
Plasma biochemical analysis
Plasma glucose, total cholesterol (T-CHO), and triglyceride (TG) levels were determined enzymatically using commercial assay kits (glucose CII-test, cholesterol E-test, and triglyceride E-test, respectively; Wako Pure Chemicals, Osaka, Japan) according to the manufacturer’s instructions and as previously described.25) Plasma insulin (Morinaga Co., Tokyo, Japan), leptin (R&D systems, Minneapolis, MN, USA), and TNFα (eBioscience, San Diego, CA, USA) were determined using commercially available ELISA kits, according to the manufacturer’s instructions. Calibration curves were prepared using standard solutions.
Histopathological findings
Staining method with hematoxylin and eosin (H&E) was previously described.26)
Oral glucose tolerance test (OGTT)
After 2 weeks of treatment with SE (10 weeks’ feeding with HFD or CE-2), the mice were fasted for 16 h and a basal blood sample (0 min) was collected from the tail vein. Blood samples were drawn 15, 30, 60, 90, and 120 min after oral administration of glucose (2 g/kg). Blood samples were centrifuged (3000 g for 10 min at 4 °C), and plasma samples were stored at –80 °C until analysis. Plasma glucose levels were measured as described above, and the area under the curve (AUC) for blood glucose was calculated.
Insulin tolerance test (ITT)
After 3 weeks of treatment with SE (11 weeks’ feeding with HFD or CE-2), a basal blood sample (0 min) was collected from the tail vein of each mouse. Blood samples were drawn 15, 30, 60, 90, and 120 min after intraperitoneal administration of human insulin (0.75 U/kg Humarin R; Japan Eli Lilly, Kobe, Japan). Blood samples were centrifuged (3000 g for 10 min at 4 °C), and plasma samples were stored at –80 °C until analysis. Plasma glucose levels were measured as described above.
Isolation of total RNA and qRT-PCR assay
Total RNA was extracted from 0.1 g liver sections using the ISOGEN II kit (Nippon Gene, Tokyo, Japan). qRT-PCR was performed with One Step SYBR PrimeScript PLUS RT-PCR kit (Perfect Real Time) (Takara Bio, Shiga, Japan) using an Applied Biosystems 7300 system (Applied Biosystems, Foster City, CA). PCR conditions were as previously described.25) Primer pairs are shown in Table 1. Relative expression of each mRNA was determined using the standard curve method. The amount of each target mRNA quantified was normalized against that of GAPDH-encoding mRNA.
Table 1.
Oligonucleotide primer sequences and PCR conditions for real-time RT-PCR
| Gene | Primer sequences | PCR Product |
|
|---|---|---|---|
| (Accession No.) | Sequence (5’ to 3’) | length (bp) | |
| IRS-1 | Forward | ATC AGT GGA TGG CAG TCC TG | 130 |
| (NM_010570) | Reverse | TGA GCA TCT AGA AGA AGG CAT GG | |
| IRS-2 | Forward | GCG GCC TCA TCT TCT TCA CT | 107 |
| (NM_001081212) | Reverse | TCA GAG AGT GGA GAG CGC TG | |
| GAPDH | Forward | TGG TGA AGG TCG GTG TGA AC | 98 |
| (NM_008084) | Reverse | GTC GTT GAT GGC AAC AAT CTC C |
Western blot analysis
0.1 g liver sections were homogenized with 900 μL ice-cold phosphate-buffered saline containing protease inhibitor (Nacalai Tesque, Kyoto, Japan) and centrifuged at 18,000 g for 20 min at 4 °C. The resulting supernatant for each sample was collected and protein level determined using the BCA protein kit (Nacalai Tesque). Protein samples (40 μg) were subjected to sodium dodecyl sulfate–polyacrylamide electrophoresis on a 7.5% gel and transferred to a polyvinylidene difluoride membrane. Rabbit anti-IRS1 monoclonal antibody and rabbit anti-IRS2 polyclonal antibody (Cell Signaling Technology, Beverly, MA, USA) were used as primary antibodies (1:1000 dilution) for immunoblotting. A peroxidase-conjugated anti-rabbit IgG was used as secondary antibody (1:5000 dilution). Mouse anti-β-actin monoclonal antibody (MBL, Aichi, Japan) was used as primary antibody (1:2000 dilution) for immunoblotting. A peroxidase-conjugated anti-mouse IgG was used as secondary antibody (1:5000 dilution). The immunoreactive bands were visualized with the ECL system (BioRad, Hercules, CA, USA).
Statistical analysis
Multiple comparisons were made by one-way analysis of variance (ANOVA) with post-hoc Tukey-Kramer’s test or two-way repeated-measures ANOVA. All statistical analyses were performed using SPSS 19.0 software (Chicago, IL, USA). Values of P<0.05 were considered statistically significant.
RESULTS
Effect of SE on body weight and food intake
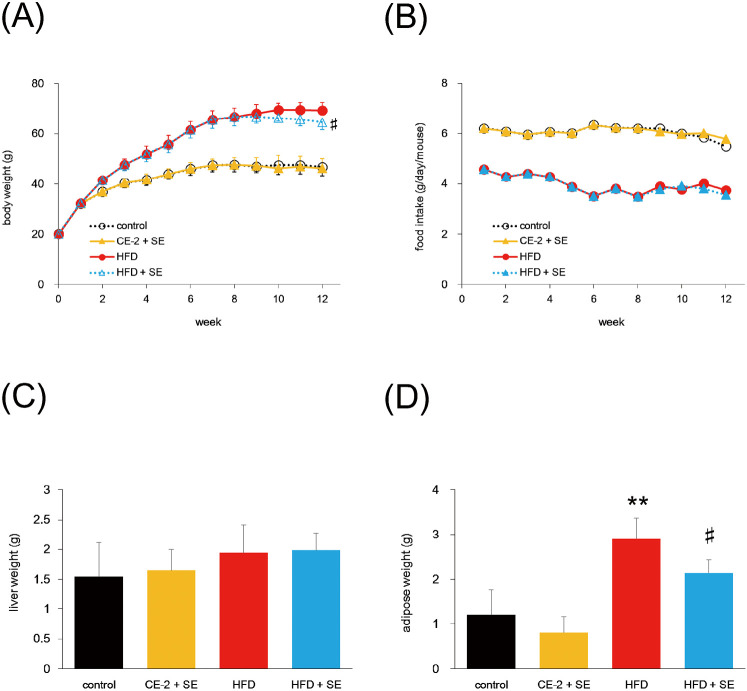
Body weight in the HFD group was greater than in the CE-2 group (Fig. 1A). In addition, body weight at week 12 was significantly lower in the HFD + SE group than the HFD group (P<0.05, Fig. 1A). Although the volume of food intake was lower in the HFD group than the CE-2 group (Fig. 1B), calorie intake was the almost same in the two groups (data not shown) and showed no significant change compared with saline and SE groups.
Fig. 1.
Effect of SE on body weight, food intake, liver weight, and epididymal adipose weight
Mice were fed a HFD or normal diet for 12 weeks. At 8 weeks of feeding, treatment with SE or saline were conducted for the last 4 weeks. Panel (A), (B), (C), and (D) indicate body weight, food intake, liver weight, and epididymal adipose weight, respectively. Food intake was calculated total food intake / number of mouse/ per day. The other data are plotted as mean ± S.D. and are representative of 6 mice per group. **P < 0.01 versus control group and #P < 0.05 versus HFD group.
Effect of SE on organ weight
To test whether organ weight is decreased by treatment with SE against HFD-induced body weight gain, we measured liver and epididymal adipose weight. Liver weight did not differ significantly between groups (Fig. 1C). In contrast, epididymal adipose weight in HFD mice was significantly decreased by SE treatment (Fig. 1D). Moreover, a tendency to reductions in other tissues, such as dorsal adipose tissue, was also observed on treatment with SE (data not shown).
Effect of SE on plasma biochemical parameters
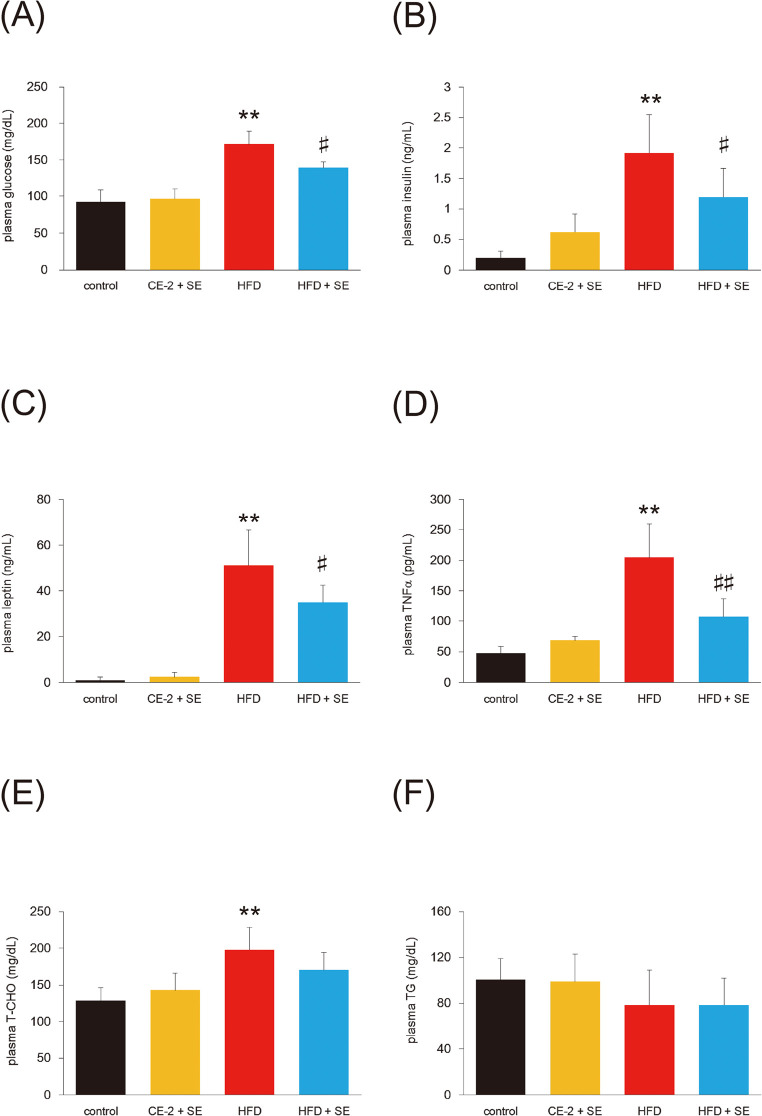
To test whether SE reduces obesity-induced hyperglycemia, we measured fasting glucose levels (Fig. 2A). Mice consuming the HFD showed increased plasma concentrations of glucose compared with CE-2 controls. Treatment with SE for 4 weeks significantly decreased HFD-induced hyperglycemia and plasma insulin (Fig. 2B).
Fig. 2.
Effect of SE on plasma biochemical parameters
Mice were fed a HFD or normal diet for 12 weeks. At 8 weeks of feeding, treatment with SE or saline were conducted for the last 4 weeks. Following the experiment, the mice from each group were fasted for 16 h and euthanized and bled for plasma. Panel (A), (B), (C), (D), (E), and (F) indicate plasma glucose, insulin, leptin, TNFα, T-CHO, and TG, respectively. Data are plotted as mean ± S.D. and are representative of 6 mice per group. **P < 0.01 versus control group and #P < 0.05 and ##P < 0.01 versus HFD group.
Systemic increases in leptin and TNFα are characteristic of obesity, and are responsible for the development of insulin resistance.27) As shown in Fig. 2C and 2D, plasma levels of leptin and TNFα were lower in the obese mice treated with SE than the untreated obese mice. We also examined plasma T-CHO (Fig. 2E) and TG (Fig. 2F). Although T-CHO was observed in mice fed the HFD, no significant reduction was observed on treatment with SE. The level of plasma TG was the same in all four groups.
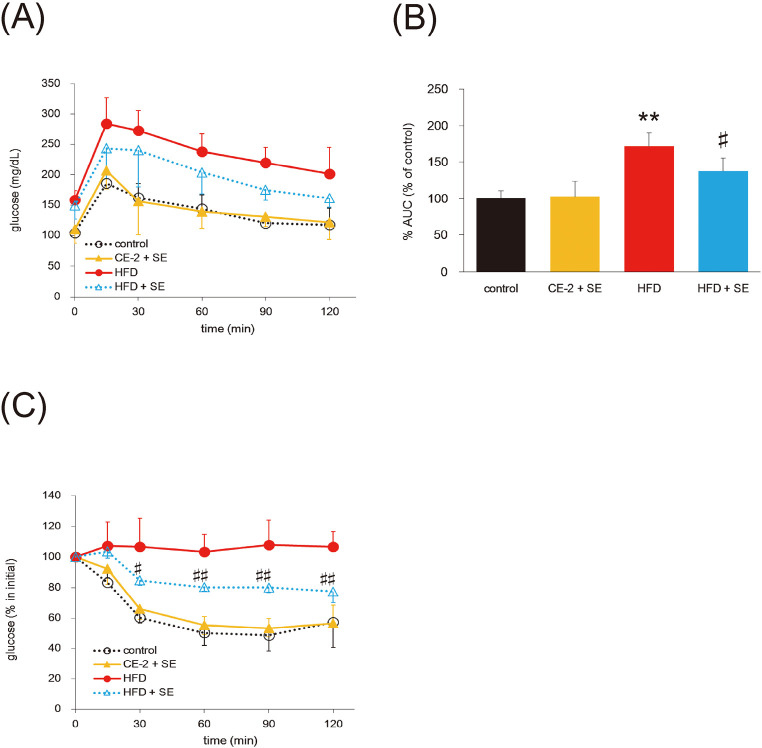
Effect of SE on glucose and insulin tolerance
To further analyze the effect of SE, we performed OGTT and ITT after 2 and 3 weeks of SE administration, respectively. After administration of 2 g/kg glucose, the plasma glucose levels were significantly higher at all times in the HFD group compared with the control group (Fig. 3A). Glucose levels in the HFD+SE group were slightly reduced compared with those in the HFD groups. The AUC was also lower in the HFD + SE group than in the HFD group (Fig. 3B).
Fig. 3.
Effect of SE on glucose tolerance and insulin tolerance
Mice were fed a HFD or normal diet for 12 weeks. At 8 weeks of feeding, treatment with SE or saline were conducted for the last 4 weeks. OGTT was tested for 10 weeks and ITT was for 11 weeks, respectively. Before OGTT, the mice were fasted for 16 h. Panel (A) and (C) indicate plasma glucose levels during an OGTT and ITT, respectively. Data (B) indicate AUC for plasma glucose. Data are plotted as mean ± S.D. and are representative of 6 mice per group. **P < 0.01 versus control group and #P < 0.05 and ##P < 0.01 versus HFD group.
Moreover, as shown in Fig. 3C, exogenous insulin had no effect on glucose levels in the HFD group. Mice in the HFD + SE group showed reduced blood glucose at 30, 60, 90, and 120 min compared with mice in the HFD group. These results indicate that SE improves glucose and insulin intolerance in HFD-induced obese mice.
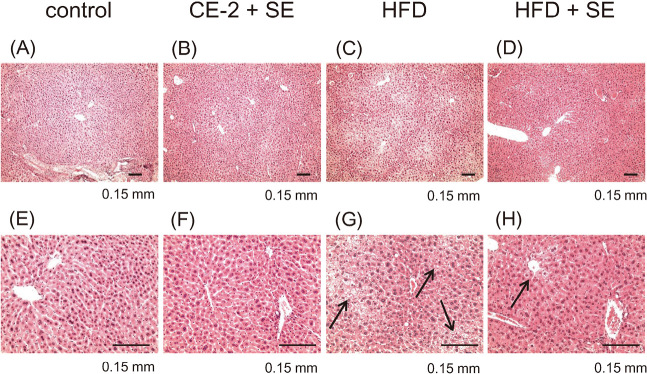
Effect of SE as assessed by morphology
In parallel with the measurement of plasma biochemical parameters, we conducted histopathological studies. H&E-stained liver sections from control and CE-2 + SE groups showed normal cell morphology and well-preserved cytoplasm in addition to a clear, plump nucleus (Fig. 4E, 4F). However, we observed extensive hepatic steatosis in mice fed with HFD (Fig. 4G), some but not all of which was ameliorated following treatment with SE (Fig. 4H).
Fig. 4.
Treatment with SE protects animals from HFD-induced hepatic steatosis, as assessed by H&E staining
Mice were fed a HFD or normal diet for 12 weeks. At 8 weeks of feeding, treatment with SE or saline were conducted for the last 4 weeks. Following the experiment, the mice from each group were euthanized and organ of livers were harvested at necropsy. Liver specimens were fixed and processed by standard methods, and sections were stained with H&E. Panel (A), (B), (C) and (D) indicate 4x magnified images and (E), (F), (G) and (H) indicate 10x magnified images, respectively. The image in (C) and (G) reveals hepatic steatosis in a HFD feeding mice, in contrast to the relatively normal hepatic structure seen in (D) and (H). Black arrows and bar indicate steatosis and 0.15 mm, respectively.
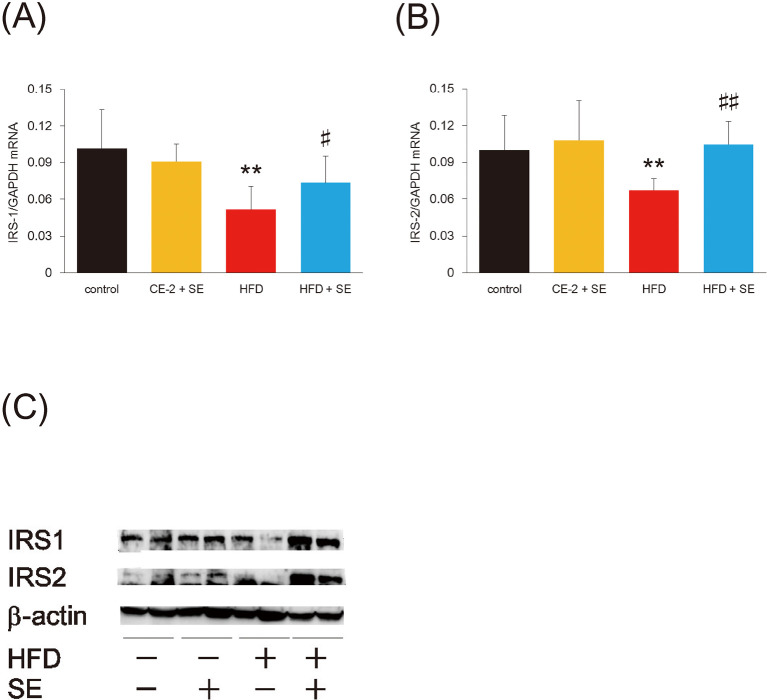
Effect of SE on hepatic insulin signaling, as assessed by qRT-PCR and western blotting
To determine whether SE alters insulin signaling, we measured hepatic insulin receptor substrate (IRS)-1 and -2 mRNA by RT-PCR: it decreased significantly in mice fed with HFD (Fig. 5A, 5B) and recovered after 4 weeks of treatment with SE. The same tendency was also observed in protein levels (Fig. 5C), suggesting that SE increases insulin sensitivity in the liver.
Fig. 5.
Treatment with SE improve insulin signaling but not gluconeogenic pathway in the liver
Mice were fed a HFD or normal diet for 12 weeks. At 8 weeks of feeding, treatment with SE or saline were conducted for the last 4 weeks. Following the experiment, the mice from each group were euthanized and total RNA or protein was prepared from the livers and used to measure each mRNA abundance by qRT-PCR or protein levels by western blotting, respectively. Data in (A) and (B) indicate IRS-1 and IRS-2 mRNA levels, respectively. These expressions were normalized to GAPDH mRNA. Data are plotted as mean ± S.D. and are representative of 6 mice per group. **P < 0.01 versus control group and #P < 0.05 and ##P < 0.01 versus HFD group. Data in (C) indicate IRS-1 and IRS-2 protein levels, respectively. As internal control, we measured β-actin.
DISCUSSION
We have investigated the effect of SE on obesity and insulin sensitivity and analyzed the underlying mechanism using mice with HFD-induced obesity. We found that SE significantly reduced body weight as well as adipose tissue and glucose levels. Furthermore, SE increased glucose and insulin intolerance compared with the HFD-induced obese mouse. These results suggest SE ameliorates adiposity, hyperglycemia, and insulin resistance in obese mice.
Obesity is typically viewed as resulting from increases in the number and size of adipocytes.28) The development of adipose tissue comprises two distinct processes. The first step is the formation of new adipocytes from precursor cells, which is followed by the formation of mature adipocytes accompanied by an increase in adipocyte size due to fat storage. In addition, several reports have indicated that plasma concentration of leptin correlates with fat weight.29) Here, we have shown reductions in both adipose weight and plasma leptin, suggesting SE has the potential to improve leptin insensitivity in obese mice. Moreover, although 4 weeks of administration with SE had no obvious change on food intake, body weight in the HFD + SE groups was significantly lower than in the HFD groups. These data suggest SE has the potential to increase energy consumption. As plant extracts such as capsaicin are known to modulate metabolic activities,30) the hypothesis is plausible. The current study did not measure parameters such as rectal temperature and motor activity: further investigation to determine these factors might be warranted.
In addition to adipocytes, hepatocytes are also needed to prevent metabolic syndrome: after a meal, hepatocytes store excess glucose as glycogen or convert it to fatty acids, while oxidizing fatty acids to provide energy for gluconeogenesis during fasting.31) Obesity-induced dysregulation of adipocytokines increases lipolysis, causing an increase in levels of free fatty acids.6) These metabolic changes not only impair hepatic lipid metabolism but also trigger hepatic insulin resistance, leading to systemic aggravation of metabolic dysfunction through hepatic steatosis and hepatic inflammation.32,33) Our results here indicate that 4 weeks of SE administration ameliorates some but not all hepatic steatosis, plasma TNFα, and glucose compared with the HFD group. These data suggest SE has potential to protect against hepatic glucose homeostasis and hepatic steatosis.
Next, we hypothesized that a critical mechanism of SE’s effect is insulin signaling. IRS-1 and -2 are key mediators of many responses in insulin-sensitive tissues, as the insulin signal is transduced largely through tyrosine phosphorylation of IRS-1 and -2 and scaffold proteins.34-36) Indeed, both expression of IRS and tyrosine phosphorylation of IRS are decreased by a HFD.27,37) The results we report here are consistent with these observations: mice in the HFD groups showed decreased IRS-1 and IRS-2, both in mRNA and protein levels. Moreover, 4 weeks of administration with SE recovered these two parameters. Hence, the main target of SE is insulin signaling.
In addition, OGTT and ITT are known to measure glucose and insulin sensitivities. Our current study indicates both tests were improved by treatment with SE. It has been reported that increase in body fat causes insulin and glucose resistance.8,9,27,29) Our investigation showed SE treatment to reduce body weight compared with HFD-fed mice. We assumed that the decrease in body weight is associated with adipose tissue reduction because liver weight did not change. Although significant change in body weight from baseline was not observed at the time we conducted OGTT (after 2 weeks of SE treatment) (P=0.144), plasma glucose levels had decreased slightly after the 2-week treatment (data not shown). These suggest that 2 weeks of SE treatment improved obesity-induced glucose tolerance. Moreover, ITT (3-week SE treatment, P=0.101) was more effective than OGTT. These differences might be explained by duration of SE treatment. Hence, the hypothesis, that reduction in adipose tissue causes improvement in glucose and insulin tolerance, was reasonable.
Most studies, including ours, utilize an extract obtained from S. veitchii leaves that has many components. Its bioactive or pharmacologically active substance has not been characterized. One candidate bioactive molecule is chlorophyll, because that is present in abundance. Chlorophyll has documented anti-inflammatory activity;38) as the amount of chlorophyll in the injections used in Subramoniam et al.’s study was low (2% w/v; 50 μL/paw in mice), a low level of chlorophyll in SE could contribute to suppress HFD-induced inflammatory response. Recently, many new flavonoids or polyphenols have also been discovered in S. veitchii,39) many of which have anti-inflammatory properties, making them candidate bioactive molecules. Additionally, other anti-inflammatory molecules have been found in plants.
Another possibility is a thiazolidinedione derivative. Indeed, representative thiazolidinedione drugs, such as pioglitazone, which is widely used for treatment of insulin resistance and type 2 diabetes, elicit anti-inflammatory effects by depressing levels of TNFα.41) Our data might support this hypothesis because pioglitazone is known to activate peroxisome proliferator activated receptor-γ, which is an indispensable modulator of adipocyte differentiation. Further analysis of Sasa components will lead to the identification of the active anti-inflammatory substance(s) in S. veitchii.
In conclusion, we found that oral administration of SE reduces adipose weight, plasma glucose levels, insulin resistance, and hepatic steatosis in HFD-induced obese mice. These effects were accompanied by changes in regulation of adipocytokine gene expression and activation of the insulin signaling pathway. Our findings suggest that SE is a useful tool for improving not only obesity-induced inflammation but also obesity-related metabolic disorders such as insulin resistance and liver diseases.
ACKNOWLEDGMENTS
This research was financially supported by Kinjo Gakuin University Research Grant. We are grateful to Sunchlon Co., Ltd. for providing the compositional the data for SE.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCE
- 1).Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest, 2000; 106: 473–481. [DOI] [PMC free article] [PubMed]
- 2).Janoschek R, Bae-Gartz I, Vohlen C, Alcazar MA, Dinger K, Appel S, et al. Dietary intervention in obese dams protects male offspring from WAT induction of TRPV4, adiposity, and hyperinsulinemia. Obesity (Silver Spring), 2016; 24: 1266–1273. [DOI] [PubMed]
- 3).Fernandez-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev, 2003; 24: 278–301. [DOI] [PubMed]
- 4).Kim SH, Park HS, Hong MJ, Yoo JY, Lee H, Lee JA, et al. Tongqiaohuoxue Decoction Ameliorates Obesity-induced Inflammation and the Prothrombotic State by Regulating Adiponectin and Plasminogen Activator Inhibitor-1. J Ethnopharmacol, 2016; 192: 201–209. [DOI] [PubMed]
- 5).Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev, 2001; 2: 239–254. [DOI] [PubMed]
- 6).Fasshauer M, Paschke R. Regulation of adipocytokines and insulin resistance. Diabetologia, 2003; 46: 1594–1603. [DOI] [PubMed]
- 7).Jadeja RN, Thounaojam MC, Ramani UV, Devkar RV, Ramachandran AV. Anti-obesity potential of Clerodendron glandulosum.Coleb leaf aqueous extract. J Ethnopharmacol, 2011; 135: 338–343. [DOI] [PubMed]
- 8).Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science, 1993; 259: 87–91. [DOI] [PubMed]
- 9).Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature, 1997; 389: 610–614. [DOI] [PubMed]
- 10).Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A, 2003; 100: 7265–7270. [DOI] [PMC free article] [PubMed]
- 11).Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest, 2006; 116: 1784–1792. [DOI] [PMC free article] [PubMed]
- 12).Miranda PJ, DeFronzo RA, Califf RM, Guyton JR. Metabolic syndrome: definition, pathophysiology, and mechanisms. Am Heart J, 2005; 149: 33–45. [DOI] [PubMed]
- 13).Kolarski V, Todorov A, Petrova D. [Cytokines and the liver in health and disease]. Vutr Boles, 2000; 32: 19–24. [PubMed]
- 14).Zhang Y, Jiao J, Liu C, Wu X, Zhang Y. Isolation and purification of four flavone C-glycosides from antioxidant of bamboo leaves by macroporous resin column chromatography and preparative high-performance liquid chromatography. Food Chemistry, 2008; 107: 1326–1336.
- 15).Zulkafli ZD, Wang H, Miyashita F, Utsumi N, Tamura K. Cosolvent-modified supercritical carbon dioxide extraction of phenolic compounds from bamboo leaves (Sasa palmata). J Supercrit Fluids, 2014; 94: 123–129.
- 16).Seki T, Kida K, Maeda H. Immunostimulation-Mediated Anti-tumor Activity of Bamboo (Sasa senanensis) Leaf Extracts Obtained Under ‘Vigorous’ Condition. Evid Based Complement Alternat Med, 2010; 7: 447–457. [DOI] [PMC free article] [PubMed]
- 17).Hirose K, Onishi H, Sasatsu M, Takeshita K, Kouzuma K, Isowa K, et al. In vivo evaluation of Kumazasa extract and chitosan films containing the extract against deep skin ulcer modl in rats. Biol Pharm Bull, 2007; 30: 2406–2411. [DOI] [PubMed]
- 18).Hayashi K, Lee J-B, Kurosaki Y, Nozawa M, Asai S, Takeshita K, et al. Evaluation of fractions and isolated polysaccharides from Sasa veitchii for their preventive effects on influenza A virus infection. J Funct Foods, 2014; 10: 25–34.
- 19).Okada Y, Okajima H, Takeshita K, Kanamori M. Kinetic study of Sasa veitchii extract as a radical scavenger and an antioxidant. J Food Sci, 2012; 77: C1211–1217. [DOI] [PubMed]
- 20).Jiao J, Zhang Y, Liu C, Liu J, Wu X. Separation and purification of tricin from an antioxidant product derived from bamboo leaves. J Agric Food Chem, 2007; 55: 10086–10092. [DOI] [PubMed]
- 21).Usuda H, Fujii H, Nonogaki T. Sasa veitchii extracts suppress 2,4-dinitrofluorobenzene-induced contact hypersensitivity in mice. Food and Agricult Immunol, 2016; 27: 523–534.
- 22).Yang JH, Lim HS, Heo YR. Sasa borealis leaves extract improves insulin resistance by modulating inflammatory cytokine secretion in high fat diet-induced obese C57/BL6J mice. Nutr Res Pract, 2010; 4: 99–105. [DOI] [PMC free article] [PubMed]
- 23).Van Hoyweghen L, De Bosscher K, Haegeman G, Deforce D, Heyerick A. In vitro inhibition of the transcription factor NF-kappaB and cyclooxygenase by bamboo extracts. Phytother Res, 2014; 28: 224–230. [DOI] [PubMed]
- 24).Yoshioka H, Tanaka M, Fujii H, Nonogaki T. Sasa veitchii extract suppresses carbon tetrachloride-induced hepato- and nephrotoxicity in mice. Environ Health Prev Med, 2016; 21: 554–562. [DOI] [PMC free article] [PubMed]
- 25).Yoshioka H, Onosaka S. Zinc sulfate pretreatment prevents carbon tetrachloride-induced lethal toxicity through metallothionein-mediated suppression of lipid peroxidation in mice. Fundam Toxicol Sci, 2016; 3: 151–156.
- 26).Yoshioka H, Usuda H, Nonogaki T, Onosaka S. Carbon tetrachloride-induced lethality in mouse is prevented by multiple pretreatment with zinc sulfate. J Toxicol Sci, 2016; 41: 55–63. [DOI] [PubMed]
- 27).Kang JH, Goto T, Han IS, Kawada T, Kim YM, Yu R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity (Silver Spring), 2010; 18: 780–787. [DOI] [PubMed]
- 28).Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev, 1998; 78: 783–809. [DOI] [PubMed]
- 29).Sato M, Kawakami T, Kondoh M, Takiguchi M, Kadota Y, Himeno S, et al. Development of high-fat-diet-induced obesity in female metallothionein-null mice. FASEB J, 2010; 24: 2375–2384. [DOI] [PubMed]
- 30).Kang JH, Tsuyoshi G, Le Ngoc H, Kim HM, Tu TH, Noh HJ, et al. Dietary capsaicin attenuates metabolic dysregulation in genetically obese diabetic mice. J Med Food, 2011; 14: 310–315. [DOI] [PubMed]
- 31).Gribble FM. Metabolism: a higher power for insulin. Nature, 2005; 434: 965–966. [DOI] [PubMed]
- 32).Koppe SW. Obesity and the liver: nonalcoholic fatty liver disease. Transl Res, 2014; 164: 312–322. [DOI] [PubMed]
- 33).Kim CS, Kwon Y, Choe SY, Hong SM, Yoo H, Goto T, et al. Quercetin reduces obesity-induced hepatosteatosis by enhancing mitochondrial oxidative metabolism via heme oxygenase-1. Nutr Metab (Lond), 2015; 12: 33. [DOI] [PMC free article] [PubMed]
- 34).Valverde AM, Burks DJ, Fabregat I, Fisher TL, Carretero J, White MF, Benito M. Molecular mechanisms of insulin resistance in IRS-2-deficient hepatocytes. Diabetes, 2003; 52: 2239–2248. [DOI] [PubMed]
- 35).Dong X, Park S, Lin X, Copps K, Yi X, White MF. Irs1 and Irs2 signaling is essential for hepatic glucose homeostasis and systemic growth. J Clin Invest, 2006; 116: 101–114. [DOI] [PMC free article] [PubMed]
- 36).Guo S, Copps KD, Dong X, Park S, Cheng Z, Pocai A, et al. The Irs1 branch of the insulin signaling cascade plays a dominant role in hepatic nutrient homeostasis. Mol Cell Biol, 2009; 29: 5070–5083. [DOI] [PMC free article] [PubMed]
- 37).de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett, 2008; 582: 97–105. [DOI] [PMC free article] [PubMed]
- 38).Subramoniam A, Asha VV, Nair SA, Sasidharan SP, Sureshkumar PK, Rajendran KN, et al. Chlorophyll revisited: anti-inflammatory activities of chlorophyll a and inhibition of expression of TNF-alpha gene by the same. Inflammation, 2012; 35: 959–966. [DOI] [PubMed]
- 39).Sakagami H. Biological activities and possible dental application of three major groups of polyphenols. J Pharmacol Sci, 2014; 126: 92–106. [DOI] [PubMed]
- 40).Dobreva ZG, Popov BN, Georgieva SY, Stanilova SA. Immunostimulatory activities of Haberlea rhodopensis leaf extract on the specific antibody response: protective effects against γ-radiation-induced immunosuppression. Food and Agricult Immunol, 2015; 26: 381–393.
- 41).Ghanim H, Dhindsa S, Aljada A, Chaudhuri A, Viswanathan P, Dandona P. Low-dose rosiglitazone exerts an antiinflammatory effect with an increase in adiponectin independently of free fatty acid fall and insulin sensitization in obese type 2 diabetics. J Clin Endocrinol Metab, 2006; 91: 3553–3558. [DOI] [PubMed]