Abstract
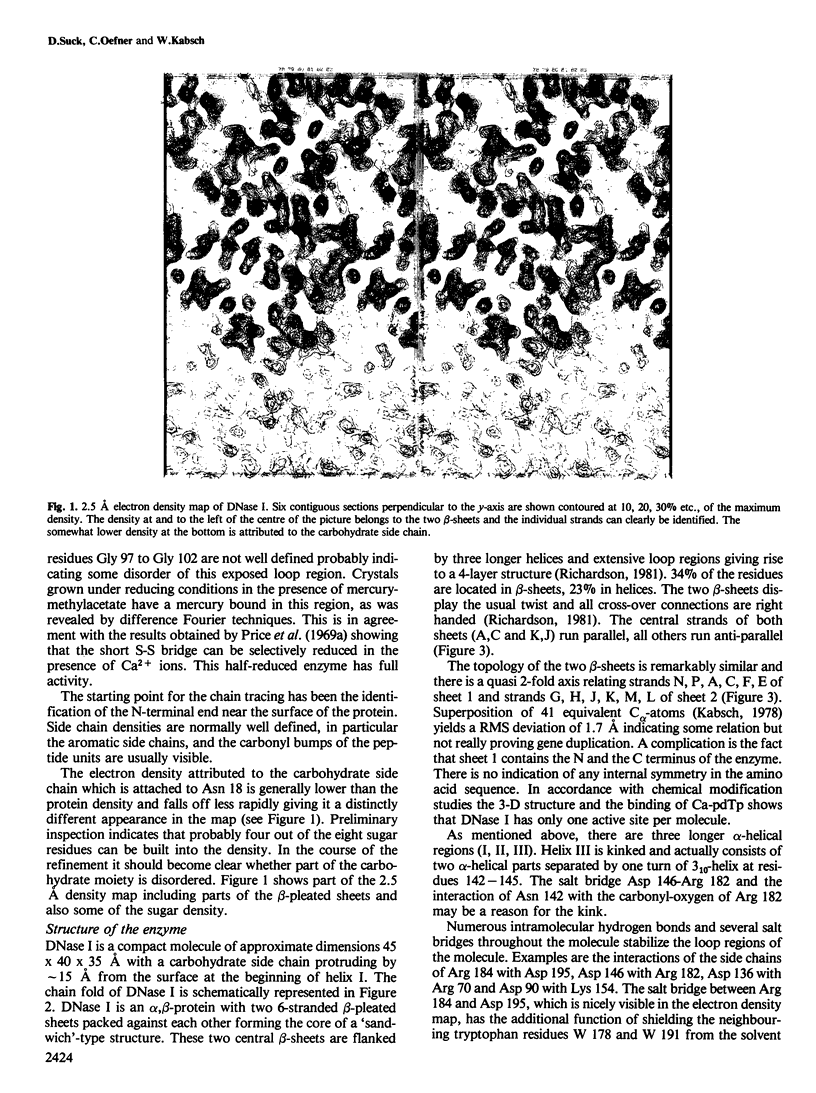
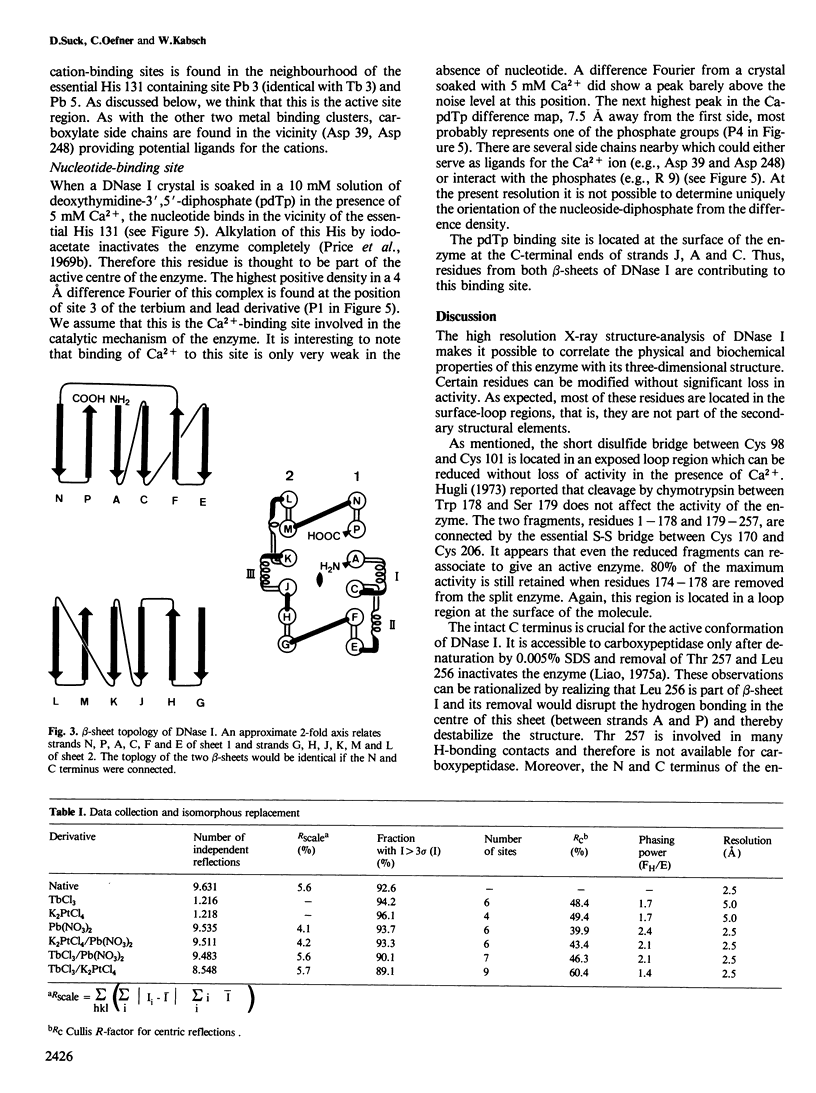
The three-dimensional structure of bovine pancreatic deoxyribonuclease I (DNase I) has been determined at 2.5 A resolution by X-ray diffraction from single crystals. An atomic model was fitted into the electron density using a graphics display system. DNase I is an alpha, beta-protein with two 6-stranded beta-pleated sheets packed against each other forming the core of a 'sandwich'-type structure. The two predominantly anti-parallel beta-sheets are flanked by three longer alpha-helices and extensive loop regions. The carbohydrate side chain attached to Asn 18 is protruding by approximately 15 A from the otherwise compact molecule of approximate dimensions 45 A X 40 A. The binding site of CA2+-deoxythymidine-3',5'-biphosphate (Ca-pdTp) has been determined by difference Fourier techniques confirming biochemical results that the active centre is close to His 131. Ca-pdTp binds at the surface of the enzyme between the two beta-pleated sheets and seems to interact with several charged amino acid side chains. Active site geometry and folding pattern of DNase I are quite different from staphylococcal nuclease, the only other Ca2+-dependent deoxyribonuclease whose structure is known at high resolution. The electron density map indicates that two Ca2+ ions are bound to the enzyme under crystallization conditions.
Full text
PDF
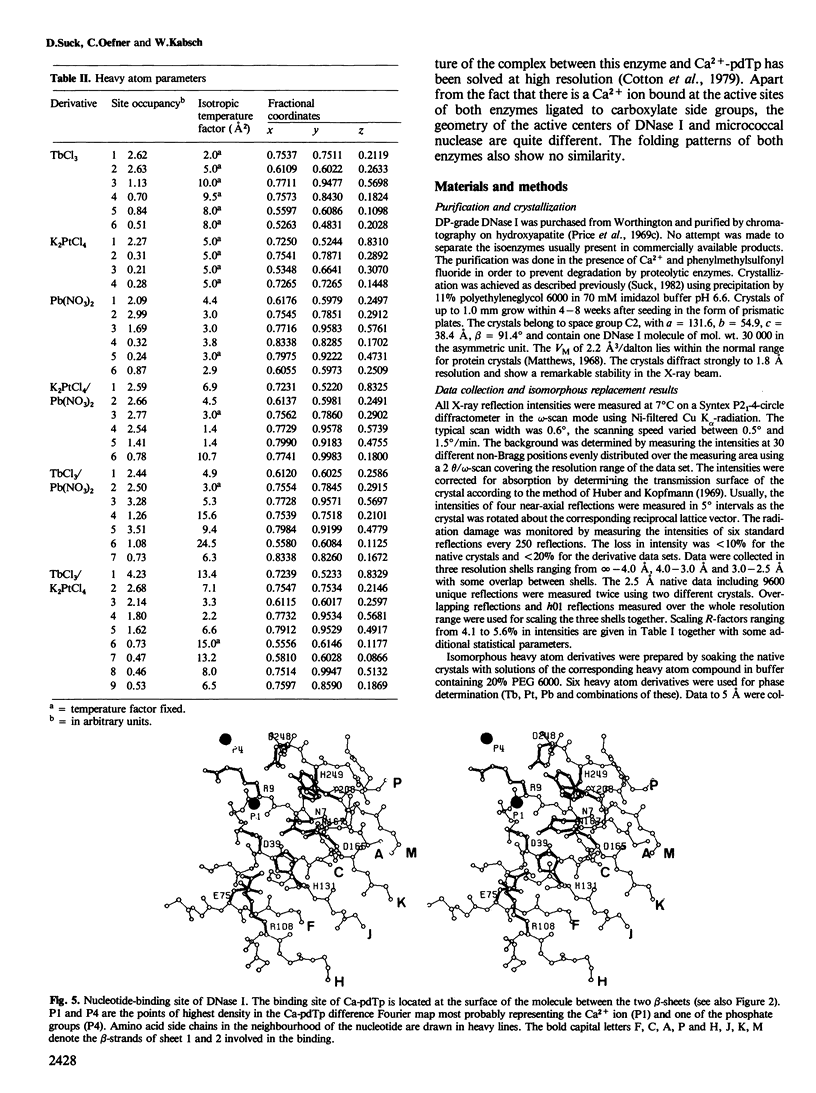
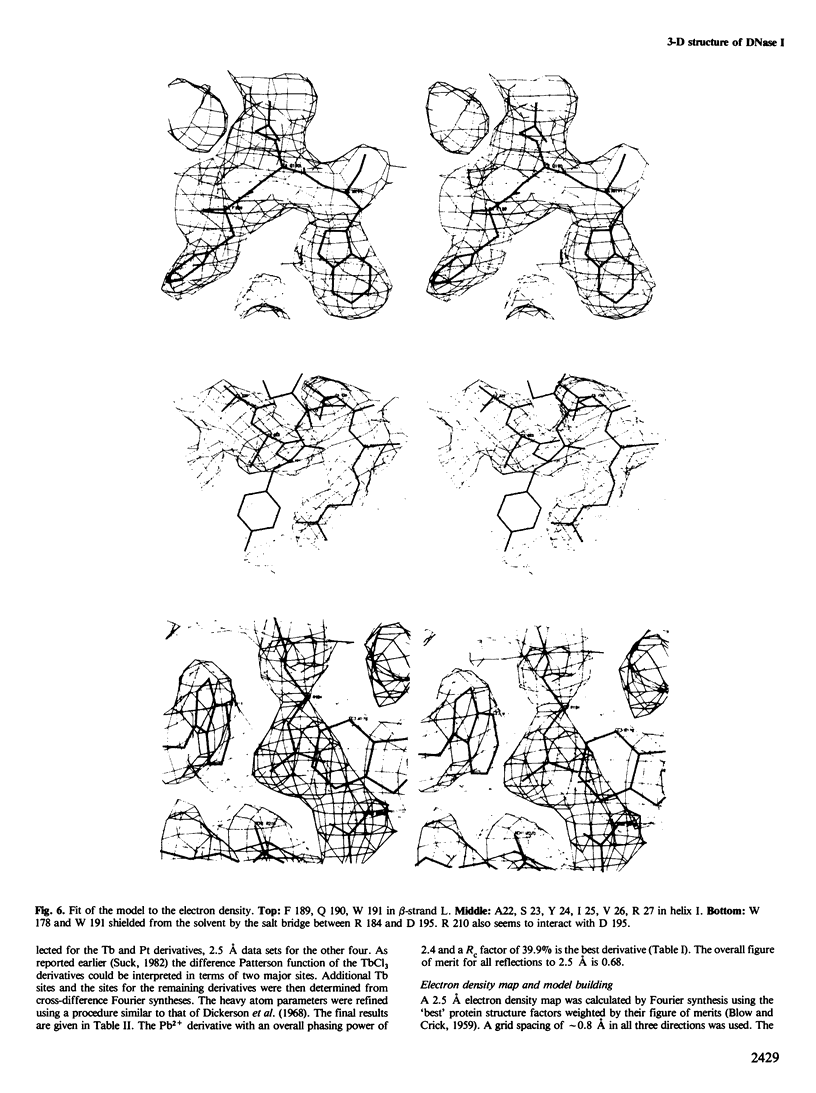

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe A., Liao T. H. The immunological and structural comparisons of deoxyribonucleases I. Glycosylation differences between bovine pancreatic and parotid deoxyribonucleases. J Biol Chem. 1983 Sep 10;258(17):10283–10288. [PubMed] [Google Scholar]
- BOLLUM F. J. DEGRADATION OF THE HOMOPOLYMER COMPLEXES POLYDEOXYADENYLATE-POLYDEOXYTHYMIDYLATE, POLYDEOXYINOSINATE-POLYDEOXYCYTIDYLATE, AND POLYDEOXYGUANYLATE-POLYDEOXYCYTIDYLATE BY DEOXYRIBONUCLEASE I. J Biol Chem. 1965 Jun;240:2599–2601. [PubMed] [Google Scholar]
- Bause E., Hettkamp H., Legler G. Conformational aspects of N-glycosylation of proteins. Studies with linear and cyclic peptides as probes. Biochem J. 1982 Jun 1;203(3):761–768. doi: 10.1042/bj2030761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi A., Gaillard C., Bernardi G. The specificity of five DNAases as studied by the analysis of 5'-terminal doublets. Eur J Biochem. 1975 Apr 1;52(3):451–457. doi: 10.1111/j.1432-1033.1975.tb04013.x. [DOI] [PubMed] [Google Scholar]
- Bernardi G., Ehrlich S. D., Thiery J. P. The specificity of deoxyribonucleases and their use in nucleotide sequence studies. Nat New Biol. 1973 Nov 14;246(150):36–40. doi: 10.1038/newbio246036a0. [DOI] [PubMed] [Google Scholar]
- Campbell V. W., Jackson D. A. The effect of divalent cations on the mode of action of DNase I. The initial reaction products produced from covalently closed circular DNA. J Biol Chem. 1980 Apr 25;255(8):3726–3735. [PubMed] [Google Scholar]
- Cotton F. A., Hazen E. E., Jr, Legg M. J. Staphylococcal nuclease: proposed mechanism of action based on structure of enzyme-thymidine 3',5'-bisphosphate-calcium ion complex at 1.5-A resolution. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2551–2555. doi: 10.1073/pnas.76.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R. Structure of a B-DNA dodecamer. II. Influence of base sequence on helix structure. J Mol Biol. 1981 Jul 15;149(4):761–786. doi: 10.1016/0022-2836(81)90357-0. [DOI] [PubMed] [Google Scholar]
- Hugli T. E. The preparation and characterization of an active derivative of bovine pancreatic deoxyribonuclease A formed by selective cleavage with alpha-chymotrypsin. J Biol Chem. 1973 Mar 10;248(5):1712–1718. [PubMed] [Google Scholar]
- Junowicz E., Spencer J. H. Studies on bovine pancreatic deoxyribonuclease A. I. General properties and activation with different bivalent metals. Biochim Biophys Acta. 1973 Jun 8;312(1):72–84. [PubMed] [Google Scholar]
- KUNITZ M. Crystalline desoxyribonuclease; digestion of thymus nucleic acid; the kinetics of the reaction. J Gen Physiol. 1950 Mar;33(4):363–377. doi: 10.1085/jgp.33.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug A., Lutter L. C. The helical periodicity of DNA on the nucleosome. Nucleic Acids Res. 1981 Sep 11;9(17):4267–4283. doi: 10.1093/nar/9.17.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Lindberg U. Actin is the naturally occurring inhibitor of deoxyribonuclease I. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4742–4746. doi: 10.1073/pnas.71.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao T. H., Salnikow J., Moore S., Stein W. H. Bovine pancreatic deoxyribonuclease A. Isolation of cyanogen bromide peptides; complete covalent structure of the polypeptide chain. J Biol Chem. 1973 Feb 25;248(4):1489–1495. [PubMed] [Google Scholar]
- Liao T. Deoxythymidine 3', 5'-di-p-nitrophenyl phosphate as a synthetic substrate for bovine pancreatic deoxyribonuclease. J Biol Chem. 1975 May 25;250(10):3721–3724. [PubMed] [Google Scholar]
- Liao T. Reversible inactivation of pancreatic deoxyribonuclease A by sodium dodecyl sulfate. Removal of COOH-terminal residues from the denatured protein by carboxypeptidase A. J Biol Chem. 1975 May 25;250(10):3831–3836. [PubMed] [Google Scholar]
- Lomonossoff G. P., Butler P. J., Klug A. Sequence-dependent variation in the conformation of DNA. J Mol Biol. 1981 Jul 15;149(4):745–760. doi: 10.1016/0022-2836(81)90356-9. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- Melgar E., Goldthwait D. A. Deoxyribonucleic acid nucleases. II. The effects of metals on the mechanism of action of deoxyribonuclease I. J Biol Chem. 1968 Sep 10;243(17):4409–4416. [PubMed] [Google Scholar]
- Noll M. Internal structure of the chromatin subunit. Nucleic Acids Res. 1974 Nov;1(11):1573–1578. doi: 10.1093/nar/1.11.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P. A. Characterization of Ca ++ and Mg ++ binding to bovine pancreatic deoxyribonuclease A. J Biol Chem. 1972 May 10;247(9):2895–2899. [PubMed] [Google Scholar]
- Price P. A., Liu T. Y., Stein W. H., Moore S. Properties of chromatographically purified bovine pancreatic deoxyribonuclease. J Biol Chem. 1969 Feb 10;244(3):917–923. [PubMed] [Google Scholar]
- Price P. A., Moore S., Stein W. H. Alkylation of a histidine residue at the active site of bovine pancreatic deoxyribonuclease. J Biol Chem. 1969 Feb 10;244(3):924–928. [PubMed] [Google Scholar]
- Price P. A., Stein W. H., Moore S. Effect of divalent cations on the reduction and re-formation of the disulfide bonds of deoxyribonuclease. J Biol Chem. 1969 Feb 10;244(3):929–932. [PubMed] [Google Scholar]
- Price P. A. The essential role of Ca2+ in the activity of bovine pancreatic deoxyribonuclease. J Biol Chem. 1975 Mar 25;250(6):1981–1986. [PubMed] [Google Scholar]
- RALPH R. K., SMITH R. A., KHORANA H. G. Studies on polynucleotides. XV. Enzymic degradation. The mode of action of pancreatic deoxyribonuclease on thymidine, deoxycytidine, and deoxyadenosine polynucleotides. Biochemistry. 1962 Jan;1:131–137. doi: 10.1021/bi00907a020. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Salnikow J., Moore S., Stein W. H. Comparison of the multiple forms of bovine pancreatic deoxyribonuclease. J Biol Chem. 1970 Nov 10;245(21):5685–5690. [PubMed] [Google Scholar]
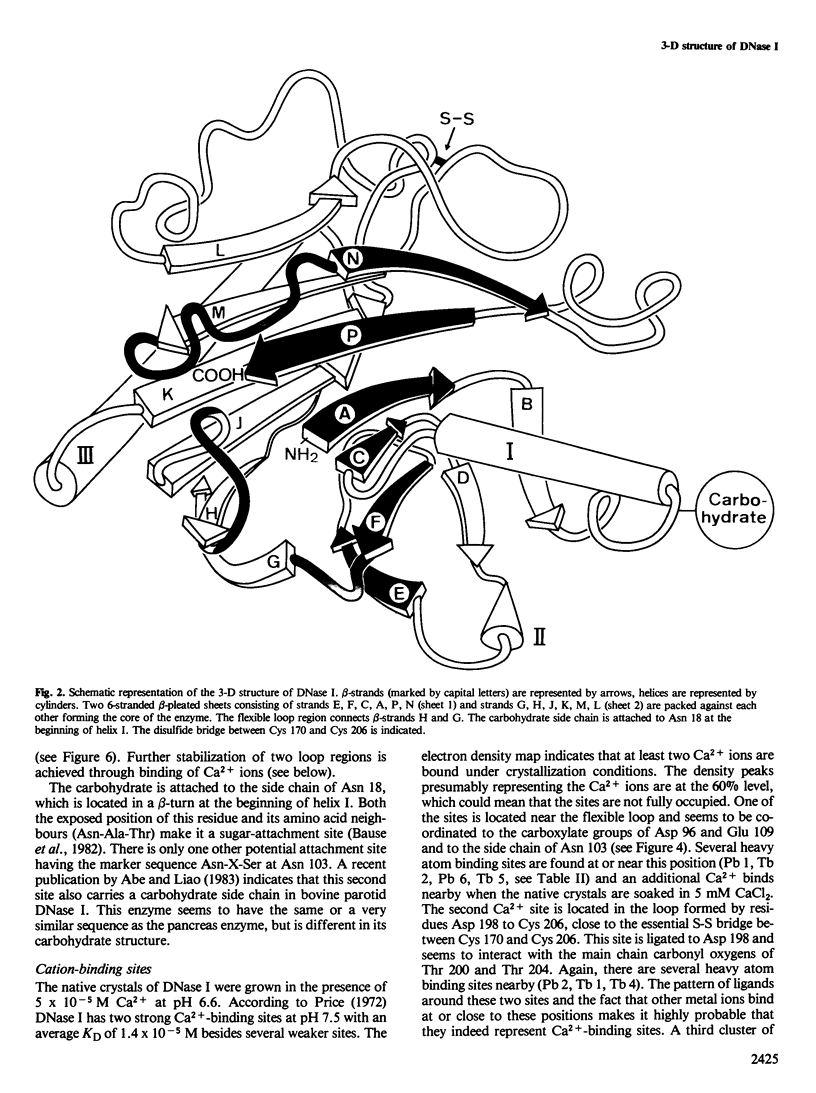
- Sauer R. T., Yocum R. R., Doolittle R. F., Lewis M., Pabo C. O. Homology among DNA-binding proteins suggests use of a conserved super-secondary structure. Nature. 1982 Jul 29;298(5873):447–451. doi: 10.1038/298447a0. [DOI] [PubMed] [Google Scholar]
- Steitz T. A., Ohlendorf D. H., McKay D. B., Anderson W. F., Matthews B. W. Structural similarity in the DNA-binding domains of catabolite gene activator and cro repressor proteins. Proc Natl Acad Sci U S A. 1982 May;79(10):3097–3100. doi: 10.1073/pnas.79.10.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suck D. Crystallization and preliminary crystallographic data of bovine pancreatic deoxyribonuclease I. J Mol Biol. 1982 Dec 5;162(2):511–513. doi: 10.1016/0022-2836(82)90542-3. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Ohlendorf D. H., Anderson W. F., Matthews B. W. DNA-binding proteins. Science. 1983 Sep 9;221(4615):1020–1026. doi: 10.1126/science.6308768. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Coleman N. F. Pancreatic deoxyribonuclease. The role of dimerization in the reversible thermal inactivation at acid pH. J Biol Chem. 1971 Jan 25;246(2):309–317. [PubMed] [Google Scholar]




