Abstract
The DNA fragment coding for the signal peptide of the OmpA protein, a major outer membrane protein of Escherichia coli, has been inserted into the high-level expression vectors, pIN-III. A foreign DNA fragment can be cloned in any one of the three reading frames at the unique EcoRI, HindIII or BamHI sites immediately after the ompA signal peptide coding sequence. The cloned foreign gene is under the control of both the lpp promoter and the lac promoter-operator. The expression of the gene is regulated by the lac repressor produced by the same vectors. Using the pIN-III-ompA vector, the DNA fragment coding for only the mature portion of beta-lactamase was inserted into the EcoRI site. Upon induction of gene expression, beta-lactamase was secreted into the periplasmic space. The ompA signal peptide was correctly removed resulting in the production of beta-lactamase with four extra amino acid residues (Gly-Ile-Pro-Gly) at its amino terminus due to the linker sequence in the vector. After a 3-h induction, beta-lactamase was accumulated to 20% of total cellular protein without any detectable accumulation of pro-beta-lactamase. Using oligonucleotide-directed site-specific mutagenesis, we have also removed the linker sequence and upon induction of gene expression, beta-lactamase with the authentic NH2-terminal sequence was produced, in even larger amounts than the beta-lactamase with the linker sequence.
Full text
PDF
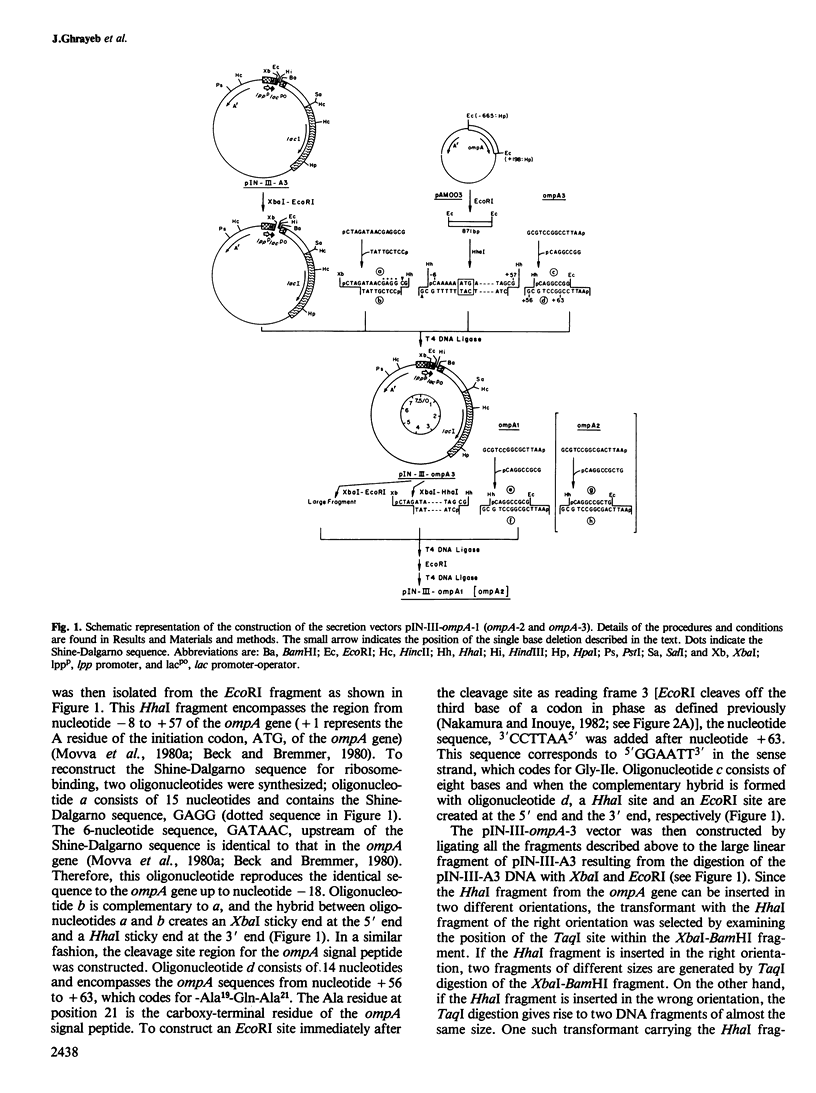
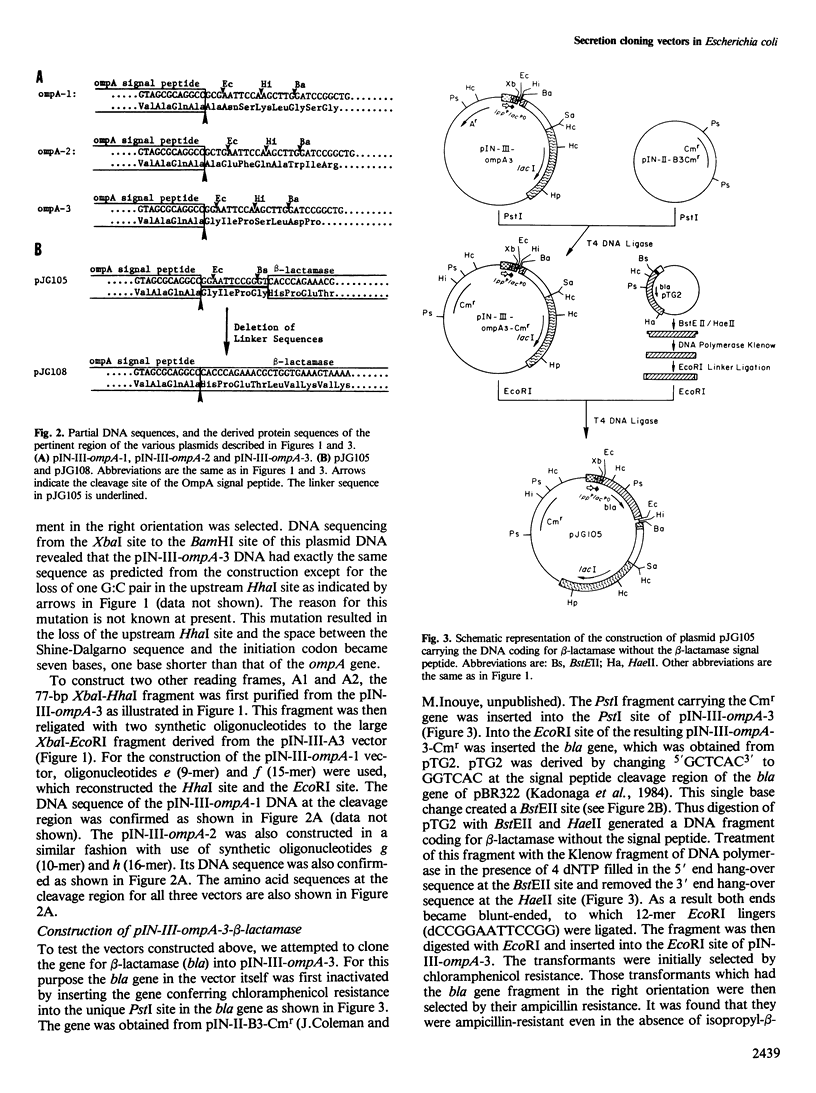
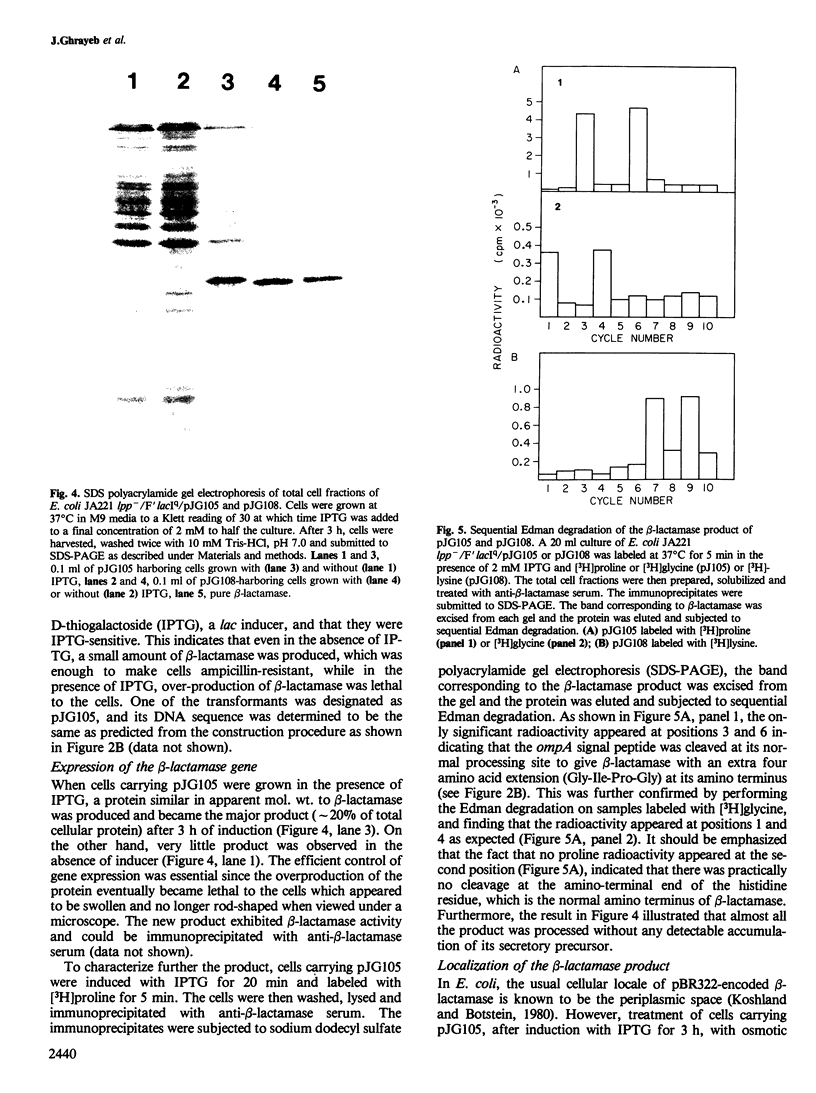


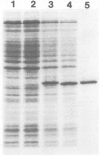
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck E., Bremer E. Nucleotide sequence of the gene ompA coding the outer membrane protein II of Escherichia coli K-12. Nucleic Acids Res. 1980 Jul 11;8(13):3011–3027. doi: 10.1093/nar/8.13.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghrayeb J., Inouye M. Nine amino acid residues at the NH2-terminal of lipoprotein are sufficient for its modification, processing, and localization in the outer membrane of Escherichia coli. J Biol Chem. 1984 Jan 10;259(1):463–467. [PubMed] [Google Scholar]
- Hsiung H., Inouye S., West J., Sturm B., Inouye M. Further improvements on the phosphotriester synthesis of deoxyribooligonucleotides and the oligonucleotide directed site-specific mutagenesis of E. coli lipoprotein gene. Nucleic Acids Res. 1983 May 25;11(10):3227–3239. doi: 10.1093/nar/11.10.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Franceschini T., Sato M., Itakura K., Inouye M. Prolipoprotein signal peptidase of Escherichia coli requires a cysteine residue at the cleavage site. EMBO J. 1983;2(1):87–91. doi: 10.1002/j.1460-2075.1983.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Soberon X., Franceschini T., Nakamura K., Itakura K., Inouye M. Role of positive charge on the amino-terminal region of the signal peptide in protein secretion across the membrane. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3438–3441. doi: 10.1073/pnas.79.11.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Wang S., Sekizawa J., Halegoua S., Inouye M. Amino acid sequence for the peptide extension on the prolipoprotein of the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1004–1008. doi: 10.1073/pnas.74.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T., Gautier A. E., Straus D. R., Charles A. D., Edge M. D., Knowles J. R. The role of the beta-lactamase signal sequence in the secretion of proteins by Escherichia coli. J Biol Chem. 1984 Feb 25;259(4):2149–2154. [PubMed] [Google Scholar]
- Koshland D., Botstein D. Secretion of beta-lactamase requires the carboxy end of the protein. Cell. 1980 Jul;20(3):749–760. doi: 10.1016/0092-8674(80)90321-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movva N. R., Nakamura K., Inouye M. Amino acid sequence of the signal peptide of ompA protein, a major outer membrane protein of Escherichia coli. J Biol Chem. 1980 Jan 10;255(1):27–29. [PubMed] [Google Scholar]
- Movva N. R., Nakamura K., Inouye M. Gene structure of the OmpA protein, a major surface protein of Escherichia coli required for cell-cell interaction. J Mol Biol. 1980 Nov 5;143(3):317–328. doi: 10.1016/0022-2836(80)90193-x. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Inouye M. Construction of versatile expression cloning vehicles using the lipoprotein gene of Escherichia coli. EMBO J. 1982;1(6):771–775. doi: 10.1002/j.1460-2075.1982.tb01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Inouye M. DNA sequence of the gene for the outer membrane lipoprotein of E. coli: an extremely AT-rich promoter. Cell. 1979 Dec;18(4):1109–1117. doi: 10.1016/0092-8674(79)90224-1. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Pollitt S., Zalkin H. Role of primary structure and disulfide bond formation in beta-lactamase secretion. J Bacteriol. 1983 Jan;153(1):27–32. doi: 10.1128/jb.153.1.27-32.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Wu H. C. Post-translational modification and processing of Escherichia coli prolipoprotein in vitro. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2255–2259. doi: 10.1073/pnas.79.7.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasuk G. P., Inouye S., Ito H., Itakura K., Inouye M. Effects of the complete removal of basic amino acid residues from the signal peptide on secretion of lipoprotein in Escherichia coli. J Biol Chem. 1983 Jun 10;258(11):7141–7148. [PubMed] [Google Scholar]