Abstract
A 68-year-old man with occult hepatitis B virus (HBV) infection was diagnosed with malignant lymphoma and achieved complete remission after treatment with a chemotherapy regimen including rituximab for 5 months. Entecavir (ETV) was also used during and after chemotherapy and was ended at 14 months after chemotherapy. However, reactivation of HBV was observed in blood tests, which showed not only elevation of HBV-DNA but also HBsAg and HBeAg, at 27 months after the end of chemotherapy. After restarting ETV, the HBV-DNA levels immediately subsided. In addition, anti-HBs became and remained positive at 31 months after chemotherapy. ETV was re-discontinued at 36 months after chemotherapy.
Keywords: entecavir, HBV, reactivation, rituximab
Introduction
Hepatitis B virus (HBV) infection is a major health problem in many countries (1). Approximately 2 billion people worldwide have serologic markers for HBV, and nearly 300 million of these have chronic HBV infection. Most cases of HBV infection are identified by the presence of serum hepatitis B surface antigen (HBsAg), but some patients are positive for serum hepatitis B core antibody (anti-HBc) and have low levels of HBV-DNA but are negative for HBsAg. They are classified as having “occult HBV infection (OBI)” (2).
Reactivation of HBV under immunosuppressive treatment for malignant or autoimmune disease often becomes life threatening in HBsAg-positive patients (3, 4). It is recommended that such patients receive nucleotide analogue prophylaxis for 12 months after the end of immunosuppressive treatments (5). Although less frequently than in HBsAg-positive patients, OBI patients can also experience the reactivation of HBV under immunosuppressive conditions (3). Therefore, prophylaxis with nucleotide analogues is also recommended in OBI patients. However, it is unclear how long such patients should receive preventive treatment for HBV reactivation, and there are some reports of reactivation occurring in OBI patients more than 12 months after the end of immunosuppressive treatments (3, 6, 7).
We herein report a case of HBV reactivation in an OBI patient with non-Hodgkin’s lymphoma that occurred 24 months after rituximab discontinuation despite nucleotide analogue prophylaxis covering the 5 months of rituximab administration and the subsequent 14 months.
Case Report
A 68-year-old man visited our hospital because of rapid enlargement of the cervical lymph nodes in 2011. Although he had gone to a hospital regularly for treatment of hypertension and ischemic heart disease since the age of 60, he had never had an abnormal liver function test. His tonsils and cervical, axillary and abdominal lymph nodes were enlarged. A tonsil biopsy revealed malignant lymphoma (diffuse large B-cell type according to the WHO classification). Because the cytospin examination of the cerebrospinal fluid identified large atypical cells, he was diagnosed as clinical stage IVA and at high risk, according to the revised international prognostic index (R-IPI). His laboratory findings were negative for HBsAg [chemiluminescence enzyme immunoassay (CLEIA)] and anti-HBs (CLEIA) and positive for anti-HBc (CLEIA). His serum levels of HBV-DNA [real-time polymerase chain reaction (RT-PCR)] were 2.6 log copies/mL. Computed tomography showed a normal liver (Fig. 1). Therefore, he was also diagnosed with OBI. Entecavir (ETV) was instituted for the prevention of HBV reactivation due to chemotherapy. We performed R-THP-COP therapy [rituximab 375 mg/m2 (610 mg/body), cyclophosphamide 460 mg/m2 (750 mg/body), doxorubicin 30 mg/m2 (50 mg/body), vincristine 0.9 mg/m2 (1.4 mg/body) and prednisolone 1.0 mg/kg (60 mg/body)] with intrathecal administration (methotrexate 10 mg, predonisolone 20 mg and cytarabine 20 mg). R-THP-COP therapy was performed every 4 weeks, 6 times in total, 2 sessions of which were intrathecal administrations (Fig. 2). He achieved complete remission with chemotherapy at five months. During the period of chemotherapy, his serum levels of HBV-DNA remained undetectable.
Figure 1.
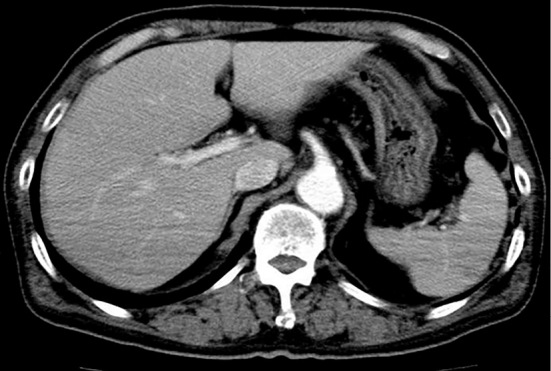
On contrast abdominal computed tomography, the liver and spleen were normal in size and shape.
Figure 2.
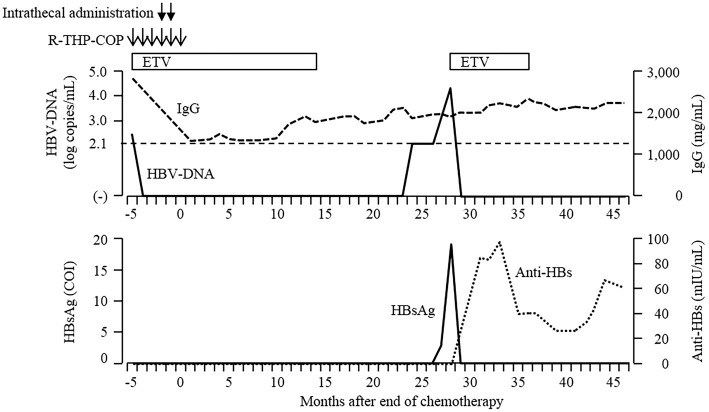
The patient’s clinical course. Serum HBsAg was negative, and HBV-DNA was present at 2.6 log copies/mL before chemotherapy. Entecavir (ETV) was used during R-THP-COP chemotherapy and the subsequent 14 months. Serum HBV-DNA levels remained undetectable, and serum gammaglobulin levels were within the normal range. ETV was discontinued at 14 months after the end of chemotherapy. However, serum HBV-DNA became positive at 24 months and increased to 3.3 log copies/mL at 27 months. In addition, serum HBsAg also reverted. After restarting ETV at 28 months, serum HBV-DNA and HBsAg immediately turned negative. Anti-HBs became positive for the first time at 31 months and remained positive at 46 months, whereas ETV was re-discontinued at 36 months.
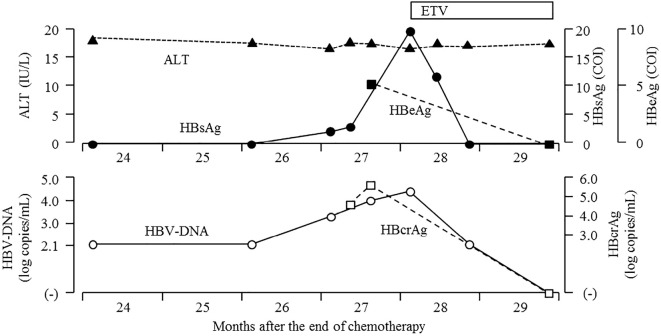
After chemotherapy, he continued ETV and was followed up regularly every 1-2 months (Fig. 2). ETV was discontinued at 14 months after the end of chemotherapy because the serum HBsAg, HBV-DNA and hepatitis B core-related antigen (HBcrAg, CLEIA) were all negative. After discontinuation of ETV, his serum HBV-DNA remained undetectable, and his serum gammaglobulin values were within the normal range for 10 months. However, his serum HBV-DNA levels became positive at 24 months after the end of chemotherapy (Fig. 2, 3), increasing to 3.3 log copies/mL. HBsAg and HBcrAg also became positive at 27 months after the end of chemotherapy. Two weeks later, laboratory data showed a further increase in the serum levels of HBV-DNA and reconversion of hepatitis B envelope antigen (HBeAg, CLEIA) without elevation of serum alanine aminotransferase (ALT) (Fig. 3). Although we recommended he restart ETV, the patient refused and requested a reexamination two weeks later. Laboratory data at 28 months after the end of chemotherapy showed normal liver function test findings and further elevations of the HBV-DNA and HBsAg levels (Table and Fig. 3). The genotype of HBV could not be identified because serum level of HBsAg was very low. He restarted ETV at 28 months after the end of chemotherapy; the serum HBV-DNA, HBsAg, HBeAg and HBcrAg findings immediately turned negative (Fig. 3). In addition, anti-HBs became positive for the first time at 31 months after the end of chemotherapy (Fig. 2). ETV was re-discontinued at 36 months after the end of chemotherapy, and anti-HBs has remained positive for 12 months.
Figure 3.
Details of the clinical course from 24 to 29 months after the end of chemotherapy. Serum HBV-DNA became positive at 24 months and increased to 3.3 log copies/mL at 27 months. HBsAg, HBeAg and HBcrAg reverted at the same time. Two weeks later, laboratory data showed a further increase in the serum HBV-DNA levels and reconversion of HBeAg without elevation of serum ALT levels. The patient started taking entecavir (ETV) again at 28 months. After restarting ETV, serum HBV-DNA, HBsAg, HBeAg and HBcrAg immediately turned negative.
Table.
Laboratory Findings at 28 Months after the End of Chemotherapy.
| Hematology | Serology | ||||
| WBC | 6,700 | /μL | CRP | 0.49 | mg/dL |
| Neutro. | 50.0 | % | IgG | 1,972 | mg/dL |
| Lympho. | 33.0 | % | IgA | 256 | mg/dL |
| RBC | 505×104 | /μL | IgM | 30 | mg/dL |
| Hb | 14.2 | g/dL | |||
| Ht | 42.3 | % | Tumor marker | ||
| Plt | 18.3×104 | /μL | sIL-2R | 1,381 | U/mL |
| Biochemistry | Coagulation | ||||
| TP | 8.0 | g/dL | PT% | 96.0 | % |
| Alb | 4.1 | g/dL | PT-INR | 1.02 | |
| T-bil | 0.4 | mg/dL | APTT | 39.6 | sec |
| AST | 21 | IU/L | Fibrinogen | 501 | mg/dL |
| ALT | 14 | IU/L | |||
| LDH | 105 | IU/L | HBV markers | ||
| ALP | 445 | IU/L | HBsAg | 19.5 | COI |
| GGT | 42 | IU/L | Anti-HBs | (-) | mIU/mL |
| T-cho | 188 | mg/dL | HBeAg | 9.6 | COI |
| BUN | 20 | mg/dL | Anti-HBe | 51.7 | % |
| Cre | 1.08 | mg/dL | HBV-DNA | 4.4 | log copies/mL |
| UA | 7.0 | mg/dL | HBcrAg | 5.6 | log U/mL |
| FPG | 106 | mg/dL | Genotype | indeterminate | |
| PC/CP type | wild/wild | ||||
WBC: white blood cell, RBC: red blood cell, Hb: hemoglobin, Ht: hematocrit, Plt: platelet, TP: total protein, Alb: albumin, T-bil: total bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, GGT: gamma glutamyl transpeptidase, T-cho: total cholesterol, TG: triglyceride, Amy: amylase, BUN: blood urea nitrogen, Cre: creatinine, FPG: fasting plasma glucose, CRP: C-reactive protein, sIL-2R: soluble interleukin-2 receptor, PT: prothrombin time, APTT activated partial thromboplastin time, HBsAg: hepatitis B surface antigen, Anti-HBs: hepatitis B surface antibody, HBeAg: hepatitis B e antigen, Anti-HBe: hepatitis B e antibody, HBcrAg: hepatitis B core related antigen, PC: precore, CP: core promotor
Discussion
Although several definitions of OBI have been proposed (8), the strict definition of OBI as the presence of HBV-DNA in the liver, regardless of the presence of serum HBV-DNA, without HBsAg was adopted in Italy in 2008 (9). The most accurate method for diagnosing OBI is the detection of HBV-DNA in DNA extracted from the liver. However, it is invasive and difficult to obtain hepatic HBV-DNA from the liver (8). As an alternative, recent RT-PCR-based assays for serum HBV-DNA have been shown to have adequate sensitivity (9). In clinical practice, OBI is defined as the presence of serum HBV-DNA without detectable HBsAg. Therefore, strictly defined OBI is divided into clinical OBI and resolved hepatitis B, with or without serum HBV-DNA, respectively (1). The present case was diagnosed with clinical OBI because the laboratory findings before the chemotherapy were negative for HBsAg and positive for HBV-DNA.
The natural course of chronic HBV infection depends on the interaction between virus replication and the host’s immune response (10, 11). It consists of an immune tolerance phase, an immune clearance phase, an inactive HBV carrier phase and a reactivation phase. In the inactive HBV carrier phase, HBV replication and production of HBsAg are gradually suppressed. Generally, serum HBV-DNA disappears first, followed by HBsAg in most cases. However, even when HBsAg decreases to undetectable levels, HBV-DNA often remains detectable, more so in the liver than in the serum (3). Therefore, most OBI patients diagnosed under the clinical definition are considered to be in the inactive carrier phase. Our case was also considered to be in the inactive carrier phase but not to have resolved hepatitis B, because serum HBV-DNA was positive before the chemotherapy.
OBI has some major clinical significance (8). First, there is a risk of OBI transmission through sexual activity or medical procedures (transfusion, needle sticking accidents or organ transplantation) (9). Second, OBI can be associated with the progression of hepatic fibrosis, especially in patients with chronic hepatitis due to hepatitis C virus (HCV) (12). Third, OBI may carry a risk of inducing hepatocellular carcinoma (13). Finally, OBI may reactivate in immunocompromised patients or those receiving chemotherapy (14-16). In OBI patients, HBV activity is strongly suppressed by the host’s immune surveillance and is preserved as covalently-closed-circular DNA (cccDNA) in the nuclei of the hepatocytes for a long period of time (17). However, this balance between the host and virus can be disrupted by any kind of immunosuppression. Reactivation of HBV in patients under immunosuppressive treatment is well-known and can be life-threatening in both HBsAg-positive (overt HBV infection) and clinical OBI patients (3, 4). Although viral reactivation in OBI patients is rarer than in HBsAg-positive patients, not only symptomatic hepatitis but also HBsAg re-seroconversion may occur (14-16). Therefore, recent major guidelines, such as AASLD (1), EASL (18), APASL (19) and JSH (5), recommend the administration of nucleotide analogues for prophylaxis of HBV reactivation not only in patients with overt infection but also in those with clinical OBI.
These guidelines also suggest extending the prophylaxis 12 months after the end of immunosuppressive treatment to prevent immunosuppressive therapy- or chemotherapy-induced reactivation of HBV infection (1, 5, 18, 19). Although our case had continued ETV for 14 months after the end of chemotherapy, reactivation of HBV was observed 24 months after the end of chemotherapy. There have been some reports of HBV reactivating later than 12 months, and all of them were treated with rituximab (3, 6, 7). Tonziello et al. (6) reported the reactivation of OBI with non-Hodgkin’s lymphoma at 20 months after rituximab discontinuation despite lamivudine prophylaxis covering the 4 months of rituximab administration and the subsequent 12 months. Rituximab, which is an anti-CD20 chimeric monoclonal antibody, depletes pre-plasma B-cells and often induces hypogammaglobulinemia (20). Furthermore, low IgG levels after rituximab therapy usually last six to nine months, but one case experienced long-term hypogammaglobulinemia for more than three years (21). Why late reactivation of HBV can occur in patients treated with rituximab is unclear. However, such late reactivation may explain why the immunosuppressive state induced by rituximab can continue for a long time. Sagnelli et al. (3) suggested that prospective studies are needed to confirm whether or not pharmacological prophylaxis should be extended to 18 months after the discontinuation of immunosuppressive treatment in patients receiving rituximab-based chemotherapy. A prospective study on the efficacy of extended prophylaxis after immunosuppressive treatment should be undertaken, especially in patients who receive rituximab-based chemotherapy.
There have been some reports that the absence or presence of low titers of baseline anti-HBs in clinical OBI is associated with a risk of HBV reactivation among lymphoma patients receiving rituximab-containing treatments (22-25). The present case was also negative for serum anti-HBs at baseline. Interestingly, the present case became positive for anti-HBs for the first time after restarting nucleotide analogue treatment (Fig. 3). Although the mechanisms of seroconversion during antiviral therapy are unknown, the reactivation of HBV after chemotherapy may affect the balance between virus replication and the host’s immune response.
In summary, clinical OBI patients can develop HBV reactivation even 27 months after discontinuation of immunosuppressive treatment. It is very important to conduct careful follow-up, being alert for HBV reactivation, after discontinuation of nucleotide analogue prophylaxis, and prompt antiviral treatment after the diagnosis of reactivation can be life-saving.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 50: 661-662, 2009. [DOI] [PubMed] [Google Scholar]
- 2. Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis 2: 479-486, 2002. [DOI] [PubMed] [Google Scholar]
- 3. Sagnelli E, Pisaturo M, Martini S, Filippini P, Sagnelli C, Coppola N. Clinical impact of occult hepatitis B virus infection in immunosuppressed patients. World J Hepatol 6: 384-393, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coppola N, Tonziello G, Pisaturo M, et al. Reactivation of overt and occult hepatitis B infection in various immunosuppressive settings. J Med Virol 83: 1909-1916, 2011. [DOI] [PubMed] [Google Scholar]
- 5. Tsubouchi H, Kumada H, Kiyosawa K, et al. Prevention of immunosuppressive therapy or chemotherapy-induced reactivation of hepatitis B virus infection: Joint report of the Intractable Liver Diseases Study Group of Japan and the Japanese Study Group of the Standard Antiviral Therapy for Viral Hepatitis. Kanzo 50: 38-42, 2009. [Google Scholar]
- 6. Tonziello G, Pisaturo M, Sica A, et al. Transient reactivation of occult hepatitis B virus infection despite lamivudine prophylaxis in a patient treated for non-Hodgkin lymphoma. Infection 41: 225-229, 2013. [DOI] [PubMed] [Google Scholar]
- 7. Garcia-Rodriguez MJ, Canales MA, Hernandez-Maraver D, Hernandez-Navarro F. Late reactivation of resolved hepatitis B virus infection: an increasing complication post rituximab-based regimens treatment? Am J Hematol 83: 673-675, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Kwak MS, Kim YJ. Occult hepatitis B virus infection. World J Hepatol 6: 860-869, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raimondo G, Allain JP, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol 49: 652-657, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Rehermann B, Ferrari C, Pasquinelli C, Chisari FV. The hepatitis B virus persists for decades after patients' recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med 2: 1104-1108, 1996. [DOI] [PubMed] [Google Scholar]
- 11. Zachou K, Sarantopoulos A, Gatselis NK, et al. Hepatitis B virus reactivation in hepatitis B virus surface antigen negative patients receiving immunosuppression: a hidden threat. World J Hepatol 5: 387-392, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Squadrito G, Cacciola I, Alibrandi A, Pollicino T, Raimondo G. Impact of occult hepatitis B virus infection on the outcome of chronic hepatitis C. J Hepatol 59: 696-700, 2013. [DOI] [PubMed] [Google Scholar]
- 13. Raimondo G, Pollicino T, Cacciola I, Squadrito G. Occult hepatitis B virus infection. J Hepatol 46: 160-170, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Kusumoto S, Tanaka Y, Mizokami M, Ueda R. Reactivation of hepatitis B virus following systemic chemotherapy for malignant lymphoma. Int J Hematol 90: 13-23, 2009. [DOI] [PubMed] [Google Scholar]
- 15. Onozawa M, Hashino S, Izumiyama K, et al. Progressive disappearance of anti-hepatitis B surface antigen antibody and reverse seroconversion after allogeneic hematopoietic stem cell transplantation in patients with previous hepatitis B virus infection. Transplantation 79: 616-619, 2005. [DOI] [PubMed] [Google Scholar]
- 16. Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology 43: 209-220, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Levrero M, Pollicino T, Petersen J, Belloni L, Raimondo G, Dandri M. Control of cccDNA function in hepatitis B virus infection. J Hepatol 51: 581-592, 2009. [DOI] [PubMed] [Google Scholar]
- 18. EASL clinical practice guidelines Management of chronic hepatitis B virus infection. J Hepatol 57: 167-185, 2012. [DOI] [PubMed] [Google Scholar]
- 19. Liaw YF, Kao JH, Piratvisuth T, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int 6: 531-561, 2012. [DOI] [PubMed] [Google Scholar]
- 20. Roberts DM, Jones RB, Smith RM, et al. Rituximab-associated hypogammaglobulinemia: incidence, predictors and outcomes in patients with multi-system autoimmune disease. J Autoimmun 57: 60-65, 2014. [DOI] [PubMed] [Google Scholar]
- 21. Miles SA, McGratten M. Persistent panhypogammaglobulinemia after CHOP-rituximab for HIV-related lymphoma. J Clin Oncol 23: 247-248, 2005. [DOI] [PubMed] [Google Scholar]
- 22. Yeo W, Chan TC, Leung NW, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol 27: 605-611, 2009. [DOI] [PubMed] [Google Scholar]
- 23. Niitsu N, Hagiwara Y, Tanae K, Kohri M, Takahashi N. Prospective analysis of hepatitis B virus reactivation in patients with diffuse large B-cell lymphoma after rituximab combination chemotherapy. J Clin Oncol 28: 5097-5100, 2010. Retraction in: J Clin Oncol 29: 3720, 2011. [DOI] [PubMed] [Google Scholar]
- 24. Matsue K, Kimura S, Takanashi Y, et al. Reactivation of hepatitis B virus after rituximab-containing treatment in patients with CD20-positive B-cell lymphoma. Cancer 116: 4769-4776, 2010. [DOI] [PubMed] [Google Scholar]
- 25. Pei SN, Ma MC, Wang MC, et al. Analysis of hepatitis B surface antibody titers in B cell lymphoma patients after rituximab therapy. Ann Hematol 91: 1007-1012, 2012. [DOI] [PubMed] [Google Scholar]