Abstract
A single dose of pegfilgrastim or the daily administration of colony-stimulating factors can be used to prevent febrile neutropenia. This may delay the detection of rapidly progressive infections among cancer patients undergoing chemotherapy. We report a case of Pseudomonas aeruginosa bacteremic pneumonia that occurred in a patient receiving pegfilgrastim.
Keywords: pegfilgrastim, Pseudomonas aeruginosa, bacteremic pneumonia, solid cancer, Gram stain
Introduction
With the spread of outpatient chemotherapy, pegfilgrastim has been used for the prevention of febrile neutropenia (FN) because its prolonged half-life permits single-dose administration. However, cancer patients receiving chemotherapy are always at risk of invasive infections regardless of the presence of neutropenia.
Pseudomonas aeruginosa bacteremic pneumonia (PABP) is an infection that is usually fatal, which generally occurs in neutropenic patients undergoing chemotherapy. This is the first report of a case of PABP in a solid cancer patient without neutropenia following outpatient chemotherapy and pegfilgrastim treatment.
Case Report
A 69-year-old man presented with high fever and general fatigue. He had been diagnosed with stage IV pancreatic cancer with multiple lung metastases two and a half years previously. He had been treated with gemcitabine (GEM) for two years and then TS-1 for 1 month. He developed FN after his first course of third-line chemotherapy with navelbine plus GEM due to progressive disease. Thereafter, he received outpatient chemotherapy along with pegfilgrastim. Fourteen days after the fifth course of third-line chemotherapy, he visited the hospital on the date of his scheduled outpatient appointment. He had a high fever (39°C) and general fatigue and was admitted to hospital with a suspected lung abscess.
On admission, the patient had no cough, sputum, dyspnea, chest pain, headache, or vomiting. He had no known contact with sick individuals. A physical examination revealed a body temperature of 39.0°C, a pulse rate of 66 beats/min, a respiratory rate of 18 breaths/min, and blood pressure of 90/56 mmHg. Lungs auscultation was clear, with the exception of decreased breath sounds in the right lung. No heart murmur was audible. There was mild pitting edema in his lower legs. Chest radiography on arrival showed consolidation in the upper field of the right lung (Fig. 1). Computed tomography (CT) disclosed a cavitary lesion, measuring 45×43 mm, with surrounding inflammation in the right upper lobe (Fig. 2). The laboratory data showed that he did not have neutropenia (white blood cell [WBC] count, 44,700/μL [neutrophils, 93%]). The other values were as follows: hemoglobin, 8.7 g/dL; platelets, 239,000/μL; aspirate aminotransferase, 24 U/L; alanine aminotransferase, 15 U/L; lactic dehydrogenase, 272 U/L; total protein, 5.3 g/dL; albumin, 2.1 g/dL; creatinine, 0.87 mg/dL; serum urea nitrogen, 26 mg/dL; and C-reactive protein, 7.9 mg/dL.
Figure 1.
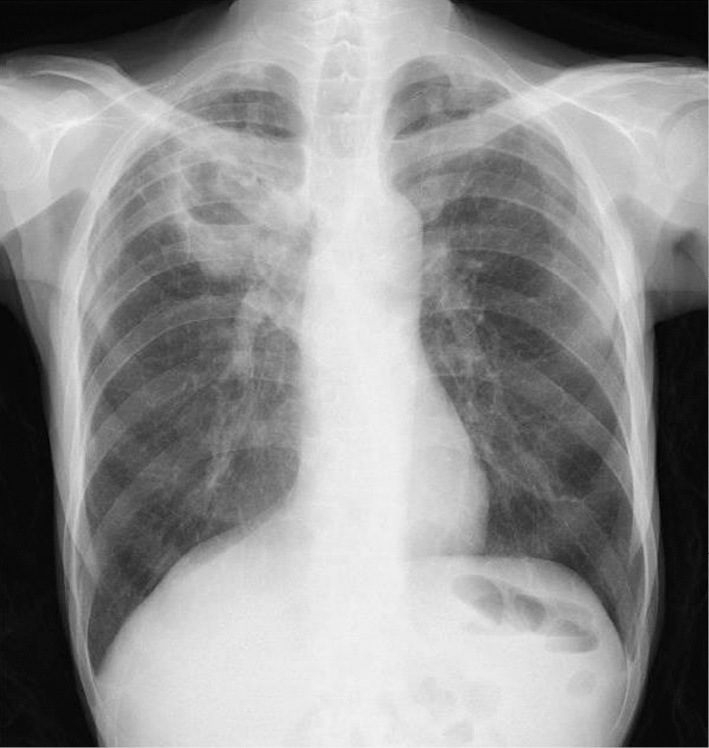
A chest radiograph taken on admission.
Figure 2.
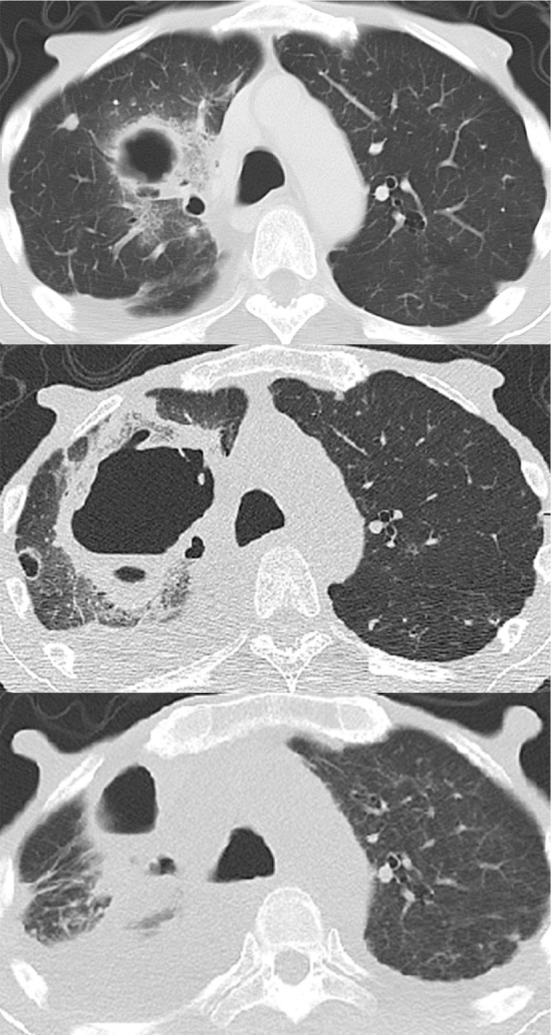
Computed tomography images taken on admission (top), 3 days after admission (middle), and 7 weeks after admission (bottom).
He was initially treated with intravenous ampicillin/sulbactam (12 g/day) as a pulmonary abscess was suspected after blood and sputum culturing. Sputum Gram staining (Miller and Jones sputum classification: P1) revealed abundant Gram-negative bacilli and few WBCs (Fig. 3). Respiratory symptoms, such as purulent and bloody sputum, cough, dyspnea, and chest pain, appeared on the day after admission and supplemental oxygen was required. His fever persisted and the cavitary lesion rapidly grew within only a few days (Fig. 2). Two sets of blood and sputum cultures were positive for P. aeruginosa after 3 days of incubation. The pathogen was susceptible to all of the anti-pseudomonal tested antibiotics. The patient was diagnosed with PABP based on these clinical and microbiological findings. Thus, ampicillin/sulbactam was immediately changed to piperacillin/tazobactam (18 g/day). Blood culturing, which was performed 6 days later, revealed no organisms in two sets of blood cultures. At 4 weeks after the treatment, the patient's respiratory symptoms gradually improved and the cavitary lesion became smaller. He was discharged at 12 weeks after admission.
Figure 3.

Sputum Gram staining.
Discussion
Granulocyte colony-stimulating factors (G-CSFs) reduce the duration and severity of neutropenia and the risk of FN and may improve survival. According to the American Society of Clinical Oncology, pegfilgrastim and filgrastim (conventional G-CSFs that are administered daily) are equally recommended for the prevention of FN (1). Some systematic reviews have suggested that pegfilgrastim is similar or more effective than filgrastim in reducing the risk of FN (2, 3).
Pseudomonas aeruginosa is one of the most frequent pathogens implicated in nosocomial infection and causes a variety of infections that are associated with considerable morbidity and mortality in immunocompromised hosts (4). The mortality rate of cancer patients with P. aeruginosa bacteremia is 18-25% regardless of the presence of neutropenia (5-7). PABP is frequently fatal, with a mortality rate of 51-57% (7-9). The classical clinical features of PABP include the initial absence of respiratory symptoms—with the exception of fever—followed by the rapid progression of pneumonia and abscess formation at 48 hours after the disease onset (10, 11). The presence of pulmonary cavities, which are rare in neutropenic patients, are a poor prognostic sign (5, 12). In this case, the patient developed fever without neutropenia and abscess formation, which suggests that a few days had passed since the onset of PABP. We presume that patients receiving pegfilgrastim undergo fewer medical examinations because they have fewer hospital visits in comparison to patients who receive conventional filgrastim. Thus, the administration of pegfilgrastim may cause physicians to overlook the early symptoms and delay the diagnosis and initial treatment of infectious diseases such as PABP, the clinical presentation of which is initially non-typical. A review study showed lower incidences of FN and FN-related hospitalization among patients who received pegfilgrastim in comparison to those who received filgrastim (2); however, proper attention is necessary to detect invasive infections in patients without neutropenia. Clinicians should advise patients to visit the hospital immediately if they feel unwell.
Consistent with some reports that have shown that PABP can occur in patients with solid organ cancer—irrespective of the presence of neutropenia (5, 6, 11)—our case suggests that disseminated P. aeruginosa infection may occur in cancer patients recovering from myelosuppression after chemotherapy. G-CSFs are known to enhance the production and maturation of neutrophils. In this case, the numbers of WBCs and neutrophils in the peripheral blood were markedly elevated on admission. However, we believe that neutrophil dysfunction might have been present because neutrophil dysfunction has been reported to occur in association with hyper-activation in association with the use of G-CSFs (13). Neutrophils stimulated by G-CSFs showed cytoskeletal rearrangement related to F-actin polymerization. These modifications in the neutrophils are probably related to a reduction in chemotaxis (13, 14). In addition, malignancy and malnutrition cause functional defects in chemotaxis and microbicidal activity (15). In the present case, sputum Gram staining revealed abundant gram-negative bacilli, but we did not detect phagocytosis by neutrophils. This phenomenon may be associated with neutrophil dysfunction due to the use of G-CSFs.
It is not clear whether empirical treatment with anti-pseudomonas is necessary for febrile patients without neutropenia after chemotherapy. Furthermore, the prediction of the risk of pneumonia caused by multidrug-resistant (MDR) pathogens, including P. aeruginosa is essential for determining the empirical therapy. According to the 2005 American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) guidelines, treatment with extended-spectrum antibiotics is recommended for patients with hospital-acquired pneumonia, ventilator-associated pneumonia, or healthcare-associated pneumonia (HCAP) in patients with at least one of the risk factors for MDR pathogens (16). The predictive risk factors for pneumonia may be insufficient for patients with MDR pathogens. Several studies have proposed various predictive risk factors and prediction scores for MDR pathogens (17-21). In a previous study, in which clinical prediction scores were derived and validated, the drug resistance in pneumonia (DRIP) score was a better predictor of the risk of pneumonia due to MDR pathogens than the HCAP criteria (the ATS/IDSA guidelines) (DRIP vs. the HCAP criteria: area under the receiver operator curve [AUROC], 0.88 [95% confidence interval (CI): 0.82-0.93] vs. 0.72 [95% CI: 0.64-0.79]) (17). The patient in the present case was diagnosed with HCAP, had no risk factors for MDR pathogens (according to the ATS/IDSA guidelines), and his DRIP score indicated that he did not have a high risk of infection with MDR pathogens. Thus, initial treatment with extended-spectrum antibiotics was not recommended. However, pneumonia due to MDR pathogens was suspected based on the results of sputum Gram staining. In this case, sputum Gram staining prior to antimicrobial treatment showed abundant Gram-negative bacilli, suggesting that P. aeruginosa was present in the upper airway. A prospective observational study in Japan reported that the sensitivity and specificity of sputum Gram staining in patients with HCAP due to P. aeruginosa were 22.2% and 99.8%, respectively (22). Sputum Gram staining may be useful in the early diagnosis of PABP regardless of a patient's respiratory symptoms. Empirical treatment with anti-pseudomonas agents should be considered for febrile patients undergoing cancer chemotherapy regardless of the presence of neutropenia. To the best of our knowledge, this is the first report of a case of PABP in a patient receiving pegfilgrastim. Further studies are necessary to investigate the epidemiology of infections in patients receiving outpatient chemotherapy plus pegfilgrastim.
In conclusion, the administration of pegfilgrastim may lead to a delay in the detection of the early symptoms of rapidly progressive infections, such as PABP, and physicians must pay close attention to detect invasive infections in patients without neutropenia. Empirical treatment with anti-pseudomona agents should be considered for febrile cancer patients, particularly those in whom sputum Gram staining reveals Gram-negative bacilli, which can be presumed to represent P. aeruginosa infection.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
This case was presented at a poster session (Presentation number 803) for the American Thoracic Society (ATS) 2016, San Francisco, May 13 to 18, 2016.
References
- 1. Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33: 3199-3212, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Pfeil AM, Allcott K, Pettengell R, von Minckwitz G, Schwenkglenks M, Szabo Z. Efficacy, effectiveness and safety of long-acting granulocyte colony-stimulating factors for prophylaxis of chemotherapy-induced neutropenia in patients with cancer: a systematic review. Support Care Cancer 23: 525-545, 2015. [DOI] [PubMed] [Google Scholar]
- 3. Mitchell S, Li X, Woods M, et al. Comparative effectiveness of granulocyte colony-stimulating factors to prevent febrile neutropenia and related complications in cancer patients in clinical practice: A systematic review. J Oncol Pharm Pract 2016 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 4. Bennett JE, Dolin R, Blaser MJ In: Mandell, Douglas, and Bennett's principles and practice of infections diseases. 8th ed Elsevier, Philadelphia, PA, 2015: 2518-2531. [Google Scholar]
- 5. Chatzinikolaou I, Abi-Said D, Bodey GP, Rolston KV, Tarrand JJ, Samonis G. Recent experience with Pseudomonas aeruginosa bacteremia in patients with cancer: Retrospective analysis of 245 episodes. Arch Intern Med 160: 501-509, 2000. [DOI] [PubMed] [Google Scholar]
- 6. Joo EJ, Kang CI, Ha YE, et al. Clinical predictors of Pseudomonas aeruginosa bacteremia among Gram-negative bacterial infections in non-neutropenic patients with solid tumor. J Infect 63: 207-214, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Todeschini G, Franchini M, Tecchio C, et al. Improved prognosis of Pseudomonas aeruginosa bacteremia in 127 consecutive neutropenic patients with hematologic malignancies. Int J Infect Dis 3: 99-104, 1998-1999. [DOI] [PubMed] [Google Scholar]
- 8. Park SY, Park HJ, Moon SM, et al. Impact of adequate empirical combination therapy on mortality from bacteremic Pseudomonas aeruginosa pneumonia. BMC Infect Dis 12: 308, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carratalà J, Rosón B, Fernández-Sevilla A, Alcaide F, Gudiol F. Bacteremic pneumonia in neutropenic patients with cancer: causes, empirical antibiotic therapy, and outcome. Arch Intern Med 158: 868-872, 1998. [DOI] [PubMed] [Google Scholar]
- 10. Iannini PB, Claffey T, Quintiliani R. Bacteremic Pseudomonas pneumonia. JAMA 230: 558-561, 1974. [DOI] [PubMed] [Google Scholar]
- 11. Fujita T, Gu Y, Kishida N, Okinaka K, Ohmagari N. Two cases of bacteremic pneumonia caused by Pseudomonas aeruginosa in solid-organ cancer patients. Kansenshogaku Zasshi 84: 588-591, 2010(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 12.In: Infections in Hematology. Maschmeyer G, Rolston KV, Eds. Springer, New York, 2015: 3-15. [Google Scholar]
- 13. Fazzi R, Orciuolo E, Trombi L, et al. PEG-Filgrastim activity on granulocyte functions. Leuk Res 31: 1453-1455, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Invernizzi R, Grasso D, Travaglino E, et al. Biological effects of pegfilgrastim on circulating neutrophils in breast cancer patients undergoing dose-dense chemotherapy. Oncology 75: 237-244, 2008. [DOI] [PubMed] [Google Scholar]
- 15. Kasper DL, Fauci AS, Hauser SL, et al. In: Harrison's principle of internal medicine. 19th ed McGraw-Hill Education, New York, NY, 2015: 413-421. [Google Scholar]
- 16. American Thoracic Society; Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171: 388-416, 2005. [DOI] [PubMed] [Google Scholar]
- 17. Webb BJ, Dascomb K, Stenehjem E, et al. Derivation and multicenter validation of the drug resistance in pneumonia clinical prediction score. Antimicrob Agents Chemother 60: 2652-2663, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shindo Y, Ito R, Kobayashi D, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med 188: 985-995, 2013. [DOI] [PubMed] [Google Scholar]
- 19. Aliberti S, Di Pasquale M, Zanaboni AM, et al. Stratifying risk factors for multidrug-resistant pathogens in hospitalized patients coming from the community with pneumonia. Clin Infect Dis 54: 470-478, 2012. [DOI] [PubMed] [Google Scholar]
- 20. Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Prediction of infection due to antibiotic-resistant bacteria by select risk factors for health care-associated pneumonia. Arch Intern Med 168: 2205-2210, 2008. [DOI] [PubMed] [Google Scholar]
- 21. Prina E, Ranzani OT, Polverino E, et al. Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann Am Thorac Soc 12: 153-160, 2015. [DOI] [PubMed] [Google Scholar]
- 22. Fukuyama H, Yamashiro S, Kinjo K, Tamaki H, Kishaba T. Validation of sputum Gram stain for treatment of community-acquired pneumonia and healthcare-associated pneumonia: a prospective observational study. BMC Infect Dis 14: 534, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


