Abstract
Sarcoidosis predominantly affects the lungs, intrathoracic lymph nodes, and eyes; it less frequently affects the musculoskeletal system. We herein report a case of paraneoplastic sarcoidosis in a patient presenting with multiple myeloma. The patient developed ocular sarcoidosis and showed an increased 18F-fluorodeoxyglucose uptake in the mediastinal lymph nodes and vertebral column. A lymph node specimen showed the histological features of sarcoidosis, while an examination of the vertebral tumor revealed myeloma. Although the simultaneous occurrence of sarcoidosis and myeloma is extremely rare, our case indicates the importance of exculing any underlying malignancies before establishing a diagnosis of skeletal sarcoidosis when bone lesions are observed at unusual sites.
Keywords: sarcoidosis, bone, multiple myeloma
Introduction
Sarcoidosis is a systemic granulomatous disorder that commonly affects young adults. Patients frequently present with bilateral hilar lymphadenopathy, pulmonary infiltrates, and skin and/or eye lesions (1). A few patients develop skeletal sarcoidosis, which typically manifests as asymptomatic small lytic lesions in the middle and distal phalanges (2, 3). Sarcoidosis is believed to be caused by an abnormal antigen-specific immune response, but the antigenic stimulus that initiates the disease process remains to be elucidated.
An association between sarcoidosis and malignant disease has been documented (4, 5). The onset of sarcoidosis may either precede or follow the diagnosis of malignant disease. Less frequently, a paraneoplastic relationship exists between sarcoidosis and the underlying malignancy (paraneoplastic sarcoidosis) (5). Patients with a simultaneous hematologic malignancy and sarcoidosis have an increased risk of lymphoma (sarcoidosis-lymphoma syndrome) (6). The concurrent presence of sarcoidosis and multiple myeloma is extremely rare, and only 13 such cases have so far been reported in the literature (7-9). We herein report a case of the simultaneous occurrence of sarcoidosis and multiple myeloma.
Case Report
A 76-year-old man presented with a 1-week history of thoracic back pain. Physical examination revealed marked tenderness over the thoracic spine at the T6 and T11 levels. Blood test results showed a hemoglobin concentration of 11.7 g/dL, a white blood cell count of 3.3×109/L (54% neutrophils, 4% eosinophils, 6% monocytes, and 36% lymphocytes), and platelet count of 266×109/L. The carcinoembryonic antigen, squamous cell carcinoma antigen, and neuron specific enolase concentrations were within the reference ranges. The serum levels of IgG, IgA, and IgM were 1,842 mg/dL (reference range, 870-1,700 mg/dL), 102 mg/dL (reference range, 110-410 mg/dL), and 29 mg/dL (reference range, 33-190 mg/dL), respectively. The serum level of angiotensin-converting enzyme (ACE) was 11.0 U/L (reference range, 8.3-21.4 U/L). Computed tomography (CT) of the chest demonstrated a right upper lobe mass that was located adjacent to the pleura exhibiting irregular margins, air bronchograms, and vascular convergence (Fig. 1a). Although mediastinal lymphadenopathy was not detected on CT, 18F-fluorodeoxyglucose (FDG) positron emission tomography showed an increased FDG uptake in the bilateral hilar and mediastinal lymph nodes (LNs) (Fig. 1b) as well as in the pulmonary mass. In addition, multifocal FDG-avid lesions were present in the vertebral column. Magnetic resonance images showed large destructive lesions of the T6 and T11 vertebral bodies (Fig. 1c). Several small osteolytic lesions were also identified. The patient was suspected to have lung cancer with metastatic bone disease.
Figure 1.
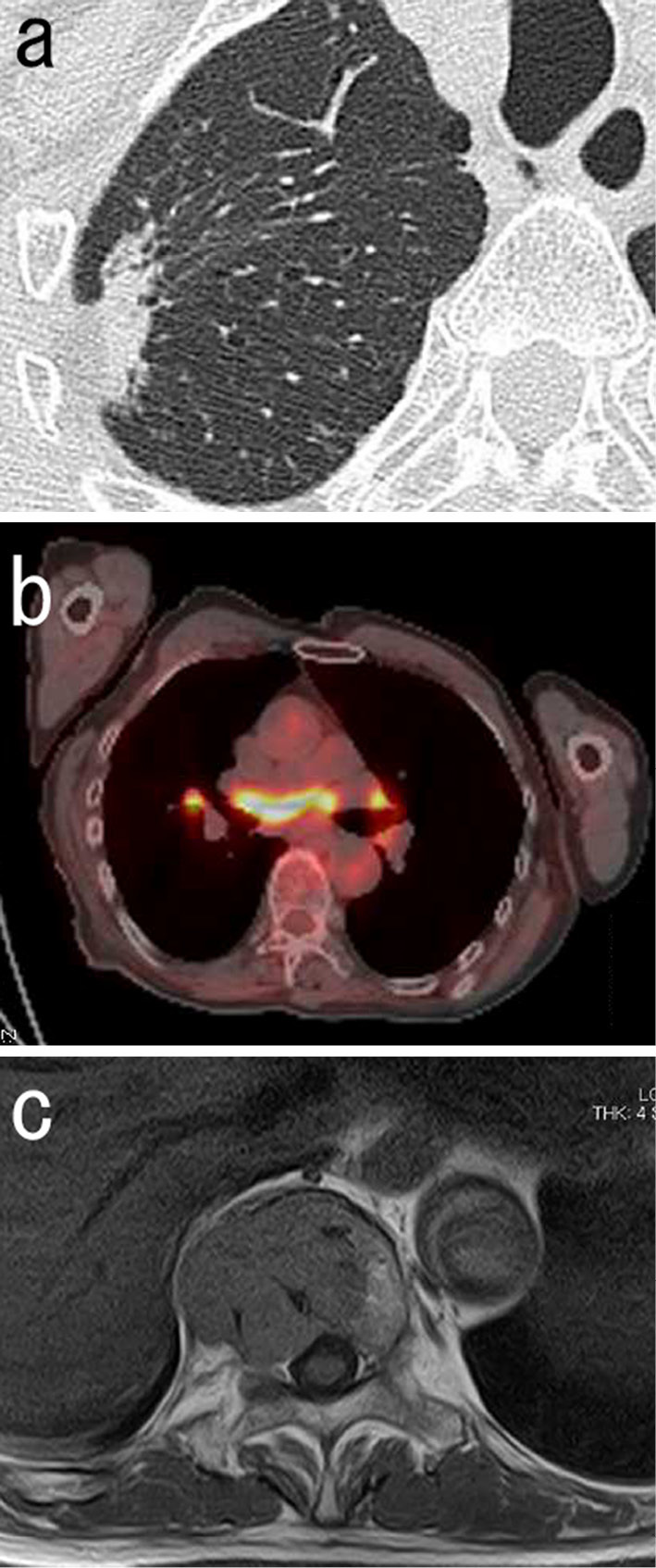
Imaging findings. a: A chest computed tomographic scan showing a right upper lobe mass. b: 18F-fluorodeoxyglucose positron emission tomography scan combined with computed tomographic scan showing an increased 18F-fluorodeoxyglucose uptake in the bilateral hilar and mediastinal lymph nodes. c: Magnetic resonance image showing a destructive lesion of the T11 vertebral body.
The patient underwent video-assisted thoracoscopic right upper lobectomy with mediastinal LN sampling. A LN specimen showed numerous non-necrotizing granulomas composed of epithelioid cells and multinucleated giant cells (Fig. 2a). Some giant cells contained asteroid bodies within their cytoplasm. The lung specimen showed histological features of nonspecific interstitial fibrosis in the absence of granulomatous lesions. There was no histological evidence of malignancy in these specimens. Although the patient did not complain of any ocular symptoms, iris nodules were found in the left eye, suggestive of ocular sarcoidosis. Therefore, a diagnosis of sarcoidosis was made. Whether the right upper lobe mass was involved in the pathogenesis of the sarcoidosis was unclear. However, the normal serum KL-6 concentration (351 IU/L; reference range, <500 IU/L) and the normal-appearing lung parenchyma outside the pulmonary mass excluded the presence of active interstitial lung disease.
Figure 2.
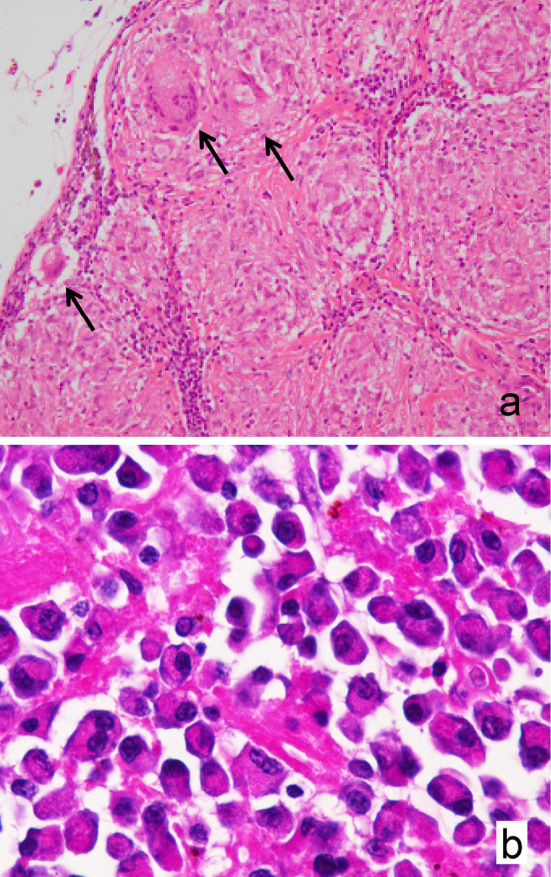
Histopathological findings. a: A lymph node specimen showing numerous non-necrotizing granulomas composed of epithelioid cells and multinucleated giant cells with asteroid bodies (arrows) [original magnification ×100, Hematoxylin and Eosin (H&E) staining]. b: A bone biopsy specimen showing monotonous plasma cell infiltration (original magnification ×400, H&E staining).
Posterior fixation surgery was performed at T10 to T12, and an examination of a tumor biopsy specimen showed the monotonous and destructive infiltration of CD138 and kappa light chain-positive plasma cells (Fig. 2b). Serum immunoelectrophoresis revealed an IgG kappa monoclonal band and an increased serum free light chain kappa/lambda ratio (12.10; normal, 0.26-1.65). A bone marrow film showed 7.2% plasma cells. The patient was therefore diagnosed with active multiple myeloma based on the biopsy-proven bony plasmacytoma and multiple osteolytic lesions.
Palliative radiotherapy was performed for the vertebral bone lesions and chemotherapy was performed with bortezomib and dexamethasone, leading to a stabilization of the myeloma and a resolution of the ocular sarcoidosis. Pulmonary toxicity associated with the bortezomib therapy was not observed.
Discussion
An association of sarcoidosis with lymphoma has been sporadically reported and this phenomenon is known as sarcoidosis-lymphoma syndrome (6). In contrast, the association between sarcoidosis and myeloma is still poorly understood, although some authors have suggested that myeloma-associated sarcoidosis occurs in older people (median age of 56 years) and that the diagnosis of sarcoidosis precedes the development of myeloma by several years (median interval of approximately 6 years) (7). Considering that almost all patients with myeloma evolve from a long-lasting (median, 10 years) and asymptomatic premalignant stage termed monoclonal gammopathy of undetermined significance (10), myeloma-associated sarcoidosis could represent a specific disease entity caused by an abnormal immune response to myeloma cells. Because myeloma-associated lesions have been observed in distant LNs rather than locally draining LNs (7-9), circulating plasma cells or myeloma-derived soluble factors could trigger the development of sarcoidosis. From a clinical viewpoint, our findings suggest that FDG-avid LNs do not always represent sites of active myeloma. Finally, the diagnosis of multiple myeloma was made after examination of the bone biopsy specimen. Although skeletal involvement has been reported in a minority of patients with sarcoidosis, vertebral sarcoidosis is extremely rare (11, 12). Our experience therefore appear to indicate the importance of histological evidence and the exclusion of underlying malignancies before establishing a diagnosis of skeletal sarcoidosis when bone lesions are observed at unusual sites, such as the long bones or axial skeleton.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet 383: 1155-1167, 2014. [DOI] [PubMed] [Google Scholar]
- 2. Zisman DA, Shorr AF, Lynch JP 3rd. Sarcoidosis involving the musculoskeletal system. Semin Respir Crit Care Med 23: 555-570, 2002. [DOI] [PubMed] [Google Scholar]
- 3. Wilcox A, Bharadwaj P, Sharma OP. Bone sarcoidosis. Curr Opin Rheumatol 12: 321-330, 2000. [DOI] [PubMed] [Google Scholar]
- 4. Askling J, Grunewald J, Eklund A, Hillerdal G, Ekbom A. Increased risk for cancer following sarcoidosis. Am J Respir Crit Care Med 160: 1668-1672, 1999. [DOI] [PubMed] [Google Scholar]
- 5. Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol 25: 326-333, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Chalayer É, Bachy E, Occelli P, et al. Sarcoidosis and lymphoma: a comparative study. QJM 108: 871-878, 2015. [DOI] [PubMed] [Google Scholar]
- 7. Sen F, Mann KP, Medeiros LJ. Multiple myeloma in association with sarcoidosis. Arch Pathol Lab Med 126: 365-368, 2002. [DOI] [PubMed] [Google Scholar]
- 8. Dachs R, Horn A, Koornhof H, de Jager L, Maqungo S, Roche S. Double pathology, sarcoidosis associated with multiple myeloma: a case report. J Bone Oncol 3: 61-65, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nair V, Prajapat D, Talwar D. Sarcoidosis and multiple myeloma: concurrent presentation of an unusual association. Lung India 33: 75-78, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med 346: 564-569, 2002. [DOI] [PubMed] [Google Scholar]
- 11. Kuzyshyn H, Feinstein D, Kolasinski SL, Eid H. Osseous sarcoidosis: a case series. Rheumatol Int 35: 925-933, 2015. [DOI] [PubMed] [Google Scholar]
- 12. Lefere M, Larbi A, Malghem J, Vande Berg B, Dallaudière B. Vertebral sarcoidosis: long-term follow-up with MRI. Skeletal Radiol 43: 1185-1190, 2014. [DOI] [PubMed] [Google Scholar]


