Abstract
Highly crystalline single-domain magnetite Fe3O4 nanoparticles (NPs) are important, not only for fundamental understanding of magnetic behaviour, but also for their considerable potential applications in biomedicine and industry. Fe3O4 NPs with sizes of 10–300 nm were systematically investigated to reveal the fundamental relationship between the crystal domain structure and the magnetic properties. The examined Fe3O4 NPs were prepared under well-controlled crystal growth conditions using a large-scale liquid precipitation method. The crystallite size of cube-like NPs estimated from X-ray diffraction pattern increased linearly as the particle size (estimated by transmission electron microscopy) increased from 10 to 64.7 nm, which indicates that the NPs have a single-domain structure. This was further confirmed by the uniform lattice fringes. The critical size of approximately 76 nm was obtained by correlating particle size with both crystallite size and magnetic coercivity; this was reported for the first time in this study. The coercivity of cube-like Fe3O4 NPs increased to a maximum of 190 Oe at the critical size, which suggests strong exchange interactions during spin alignment. Compared with cube-like NPs, sphere-like NPs have lower magnetic coercivity and remanence values, which is caused by the different orientations of their polycrystalline structure.
Introduction
Magnetite (Fe3O4) nanoparticles (NPs) have attracted considerable interest as a result of their attractive properties, such as their strong magnetic moment, biocompatibility, chemical stability, and magnetoelectric properties1. Numerous reports have described their advances in nanotechnology and their development of a wide range of applications, such as medical application2, catalysis3, Li batteries4, printer toners5, and wastewater treatment6. For the broad application of these NPs, control of the crystal size, shape, and domain structure is important for defining their chemical and physical properties7. This was demonstrated in our previous study8 of photoluminescent NPs, for which the performance was attributable to the domain size rather than the particle size. The particle size, which originates from the magnetic domain structure, has also been recognised as a key factor in the application of magnetic NPs.
The relationship between particle size and the magnetic properties, such as the coercivity (Hc), of Fe3O4 NPs has been widely reported. The critical size of magnetic NPs, which indicates the transition from a single- to multi-domain structure, was evaluated by the change in Hc with respect to the particle size. However, the critical size for Fe3O4 NPs has not yet been systematically demonstrated because this value depends on the crystal structure, which can have spherical, cubic, or multiple phases. Therefore, investigation of the critical size on the basis of the crystal structure is necessary.
The particle size required to achieve superparamagnetism in Fe3O4 NPs is widely estimated to be below 20 nm6, 9–11, whereas the critical size for forming a multi-domain structure has been theoretically estimated to be 76 nm for cubic12 and 128 nm for spherical Fe3O4 NPs13. However, the critical size for cubic Fe3O4 NPs has been experimentally determined to be higher than 160 nm14. Much smaller critical sizes of 30–46 nm15–18 have also been reported for cubic Fe3O4 NPs. Multi-granule Fe3O4 NPs with sizes of 16–512 nm showed a transitional size of approximately 120 nm19. Although the effects of size and shape on the behaviour of magnetic particles have been known for more than half a century10, the quantitative effects on Zn0.4Fe2.6O4 NPs remained undiscovered in the size range of 20–140 nm until 201220, when the critical size was found to be approximately 60 nm, and the saturation magnetisation (Ms) was found to be lower for spherical particles than for cubic ones.
Owing to the experimental difficulty in controlling particle sizes over a wide size range21, a systematic investigation of the magnetic domain structures of the most commonly used Fe3O4 NPs is still lacking, although such an investigation is needed to meet the currently increasing requirements for various applications. The current study therefore investigates the size dependence of the magnetic properties of Fe3O4 NPs with sizes of 10‒300 nm. The study includes cube- and sphere-like NPs that were produced under well-controlled crystal growth conditions on a large scale. The critical size of highly crystalline cube-like Fe3O4 NPs was examined through the correlation between the particle size measured by transmission electron microscopy (TEM) and the crystallite size estimated by X-ray diffraction (XRD). The value was further confirmed by observing the lattice fringes and examining the dependence of Hc on the particle size. The high Hc value obtained in this study is discussed in detail in terms of the spin interactions in a single-domain structure.
Methods
Two types of highly crystalline Fe3O4 NPs, i.e., cube-like and sphere-like ones, were synthesised on a large scale under precise control of the Fe2+ concentration, pH, temperature, and aeration rate. The cube-like Fe3O4 NPs were prepared by a two-stage oxidation reaction, which is described in patent No. US 5843610A (Toda Kogyo Co., Ltd., Japan)22. In contrast, the sphere-like Fe3O4 NPs were prepared by a one-stage oxidation reaction, which is described in patent No. US 4992191A (Toda Kogyo Co., Ltd., Japan)23. All data generated or analyzed during this study are included in this article and its Supplementary Information files.
The morphologies of the prepared NPs were analysed using field-emission scanning electron microscopy (FE SEM; Hitachi S-5000, Tokyo, Japan) and TEM (JEM-2010, 200 kV, JEOL Ltd., Tokyo, Japan). The crystallite size and chemical composition of the prepared NP samples were examined by XRD (RINT2000, Rigaku Denki Co. Ltd., Tokyo, Japan), using Cu Kα radiation with a scanning range of 2θ 10–80°. Their magnetic performance was assessed using a superconducting quantum interference device (SQUID, Quantum Design, Tokyo, Japan), operated at 300 K. The prepared cube-like NPs with particle sizes (d p) of 9.6, 19.6, 24.4, 31.9, 45.3, 64.7, 130, 243, and 287 nm were named as C1, C2, C3, C4, C5, C6, C7, C8, and C9, respectively. The sphere-like NPs with d p of 93.3 and 121 nm were named as S1 and S2, respectively.
Results and Discussion
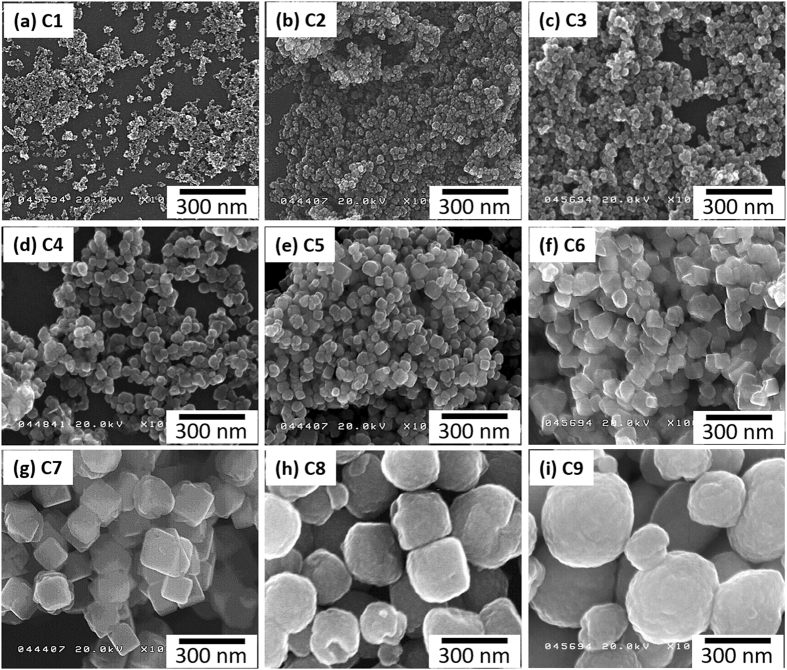
SEM images of the cube-like Fe3O4 NPs are shown in Fig. 1. The d p of these particles are 9.6, 19.6, 24.4, 31.9, 45.3, 64.7, 130, 243, and 287 nm for C1‒C9, respectively, and their size distributions are given in Supplementary Fig. S1. The high-resolution TEM (HRTEM) images of the cube-like Fe3O4 NPs in Supplementary Fig. S2 show the lattice fringes of the NPs, and indicate that the NPs have a single crystalline structure up to a d p of 64.7 nm. In contrast, the C7–C9 NPs, with diameters larger than 100 nm, show a polycrystalline structure. SEM images and size distributions of the sphere-like Fe3O4 NPs are shown in Supplementary Fig. S3(a–d). The values of d p were 93.3 and 121 nm for S1 and S2, respectively. All of the Fe3O4 NPs exhibit quasi-narrow size distributions compared with previously reported particles7, 14.
Figure 1.
Scanning electron microscopy images of cube-like Fe3O4 nanoparticles with various particle sizes. (a) 9.6, (b) 19.6, (c) 24.4, (d) 31.9, (e) 45.3, (f) 64.7, (g) 130, (h) 243, and (i) 287 nm, which are named as C1‒C9, respectively.
The XRD patterns of cube-like Fe3O4 NPs are shown in Fig. 2. The diffraction peaks of 2θ correspond to the [111], [311], [222], [400], [422], [511], and [440] planes, which indicate the pure cubic phase of the Fe3O4 NPs. The sharp peaks indicate the highly crystalline nature of the Fe3O4 NPs. The XRD patterns of the sphere-like NPs, shown in Supplementary Fig. S3(e), also demonstrate the high crystallinity of these particles. Impurity peaks and transition phases were not observed, which indicates that the particles prepared by the liquid precipitation method were pure, both chemically and in their crystalline phase. The crystallite sizes (d c) of all the Fe3O4 NPs were estimated by the Scherrer formula using the highest intensity XRD peak24, namely [311], and compared with the d p obtained from the TEM analysis, as listed in Supplementary Table S1.
Figure 2.

X-ray diffraction patterns of cube-like Fe3O4 nanoparticles with various particle sizes.
The relationship between d p and d c is shown in Fig. 3. The straight line indicates a single crystalline particle, whereas the particles corresponding to points that fall below this line are polycrystalline. Notably, the d c and corresponding d p have almost the same value for particles with a diameter of 10 to around 80 nm, which indicates a single-domain structure. The particles with diameters of larger than 80 nm have a constant d c, which indicates a multi-domain structure. The critical size of these cube-like NPs was calculated as 78 ± 9 nm from the relationship between d p and d c. The critical size is usually obtained from the change in magnetic properties, such as the relationship of Hc and d p, and this is the first time it has been obtained from the relationship between d p and d c for Fe3O4 NPs. The same tendency was observed by Lee et al.19 in 2015 for multi-granule Fe3O4 NPs, which have a smaller d c than our NPs. The HRTEM images shown in Supplementary Fig. S2 confirm that NPs with a diameter of up to 64.7 nm have a single crystalline structure, which is shown by the single direction of the lattice fringes. This result is consistent with those obtained for particles produced by a colloidal chemical synthetic route7, 14.
Figure 3.
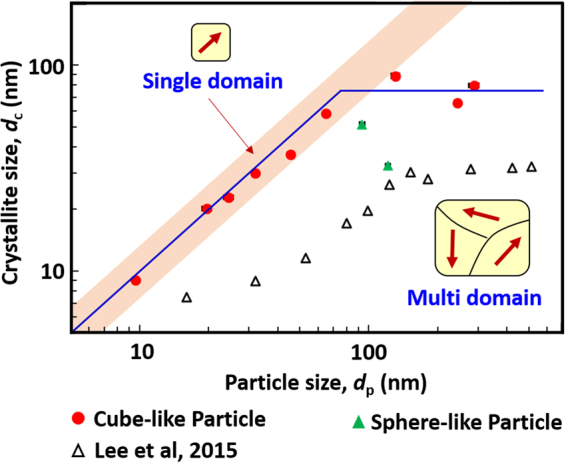
Relationship between average particle size and crystallite size. The solid line shows the trend in the relationship for cube-like Fe3O4 nanoparticles. The black open triangles were replotted from Table 1 in ref. 19 for multi-granule Fe3O4 nanoparticles.
The sphere-like NPs show different crystal properties. For these NPs, the d c is much smaller than the d p, and also much smaller than those of the cube-like NPs with a similar d p, as shown in Supplementary Table S1 and Fig. 3. Supplementary Fig. S4 shows a detailed comparison of the cube-like C7 and sphere-like S2 NPs, which have a similar average d p. The dark-field TEM image and electron diffraction pattern show that the C7 NP is polycrystalline with a single orientation. However, a polycrystalline structure containing different orientations was observed in the S2 NPs. This was further confirmed from the HRTEM images by the existence of different directions of the lattice fringe in a single S2 NP (Supplementary Fig. S4(g–i)). Sphere-like NPs commonly consist of agglomerates of variously sized cubic NPs5, 14, 25. The different morphological structure arises from the different preparation processes11, as described in the patent22, 23.
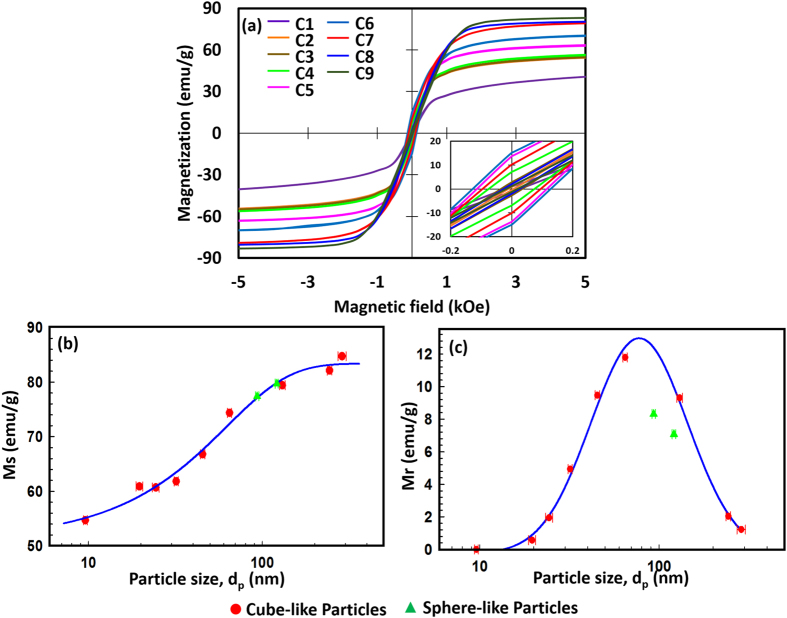
The hysteresis loops for these particles, shown in Fig. 4(a), show the ferrimagnetic nature of the Fe3O4 NPs. The cube-like Fe3O4 NPs possess high Ms values, which are affected by the d p as shown in Fig. 4(b). The Ms value, obtained by applying the law of approach to saturation26, increases with increasing d p for all samples, including the sphere-like NPs. This trend is consistent with those found in other reports on Fe3O4 NPs with diameters lower than 100 nm5, 16, 27, 28. The Ms value increased from 54.7 emu/g (9.6-nm NPs) to 84.7 emu/g (287-nm NPs), which is close to the theoretically estimated Ms for bulk Fe3O4 (92 emu/g). The Hc and remanent magnetisation (Mr), which are also affected by d p, are shown in Figs 4(c) and 5. These values increase from around 0 for the 9.6-nm NPs, which are known to be superparamagnetic26, 29, 30, to a maximum value of around 190 Oe (Hc) and 13 emu/g (Mr) at a d p of around 80 nm, and then decrease continuously with further increases in d p. The trends in these two parameters are consistent with previous theoretical estimations10, 19, 31, and they have similar characteristics to those obtained for Zn0.4Fe2.6O4 NPs20. The initial increase in Hc with domain size corresponds to the sixth power of the domain size. The high Hc value may be caused by the strong spin interactions in highly crystalline Fe3O4 NPs during spin alignment, which has previously been observed in soft magnetic NPs32.
Figure 4.
(a) Hysteresis loops of cube-like Fe3O4 nanoparticles, and particle size dependence of (b) saturation magnetization and (c) remanent magnetization for all Fe3O4 nanoparticles.
Figure 5.
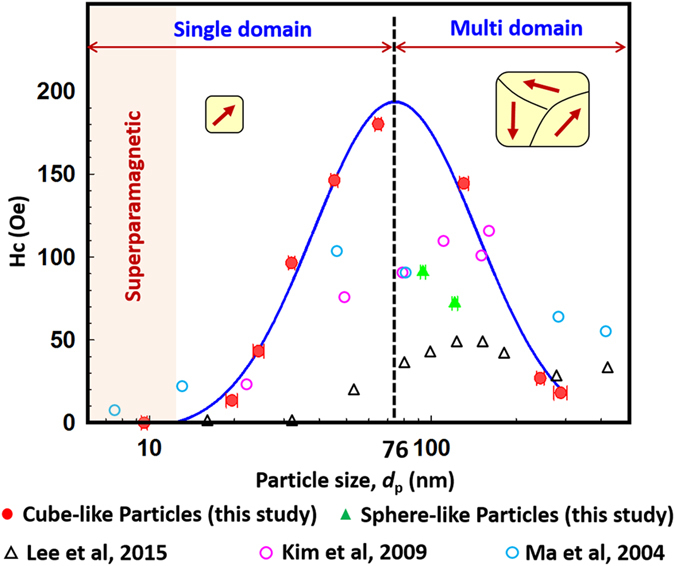
Magnetic performance of Fe3O4 nanoparticles measured at 300 K: particle size dependence of the coercivity (Hc). The black open triangles, violet open circles, and blue open circles were replotted from refs 14, 15 and 19, respectively.
By fitting the measured Mr and Hc values using a log-normal distribution function, shown by the dark blue solid line, the critical sizes for the maximum Mr and Hc values were determined to be 77 ± 2 nm and 75 ± 3 nm, respectively. On average, therefore, the critical value for the transition is about 76 ± 4 nm. This value is consistent with the critical size of 76 nm estimated theoretically for the transition from single- to multi-domain behavior12. Furthermore, the critical size of about 76 nm is almost the same as the transition size obtained from the relationship of d c and d p, as shown in Fig. 3. Single-domain cube-like Fe3O4 NPs, such as those with a size of 64.7 nm, can be applied effectively as starting materials for many real products, especially for new rare-earth-free magnets with high magnetic moments, by transformation into α″-Fe16N2 NPs and subsequent dispersion and assembly under a magnetic field33–38.
The crystalline properties of the NPs affect their magnetic properties, especially Hc. The different crystalline properties of the cube- and sphere-like NPs result in their different magnetic performances, as shown in Figs 4(c) and 5. The measured Mr and Hc values for the sphere-like NPs (S1 and S2) are both lower than the corresponding fitted values for the cube-like NPs. This may be caused by their composite small crystallite size. A comparison of the hysteresis loops of the two NPs with similar d p (C7 and S2) in Supplementary Fig. S5 shows the difference between the two samples, although their Ms values are similar (79.4 and 79.7 emu/g as listed in Supplementary Table S1). The multiple orientations of the polycrystalline in the sphere-like NPs, which lead to the multiple orientations of their easy axes, is considered to be the reason for their lower Mr and Hc values compared with those of the cube-like NPs. This is consistent with a previous study on the particle-size and shape dependence of Fe3O4 NPs5. However, further theoretical explanation and experimental investigation are still required.
Conclusions
The magnetic properties, including the Ms, Mr, and Hc, of Fe3O4 NPs are highly influenced by the particle size and domain structure. The Ms increases with increasing particle size, regardless of the crystal structure and particle shape. After exceeding the superparamagnetic limit, the Hc and Mr values increase with increasing particle size up to a maximum value of about 190 Oe and 13 emu/g, respectively, at the critical size of 76 nm. Above this critical size, the Hc and Mr values decrease with further increases in the particle size, and the cube-like Fe3O4 NPs change from a single- to multi-domain structure. The multiple orientations of the crystallites within the multi-domain-structured NPs lead to the decrease in the Hc value. These findings suggest that considerable attention should be given to the particle size and crystalline properties of Fe3O4 NPs, which have potential biomedical and industrial applications. These applications require that magnetic particles are sized appropriately to achieve a good balance between effective surface area and satisfactory magnetic performance.
Electronic supplementary material
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 26709061 and 16K13642. This work was partly supported by the Center for Functional Nano Oxide at Hiroshima University. The authors also gratefully acknowledge the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan for providing scholarships (C. W. K.). The authors further thank Prof. Takahiro Onimaru, Prof. Toshiro Takabatake, and Prof. Hiroshi Fukuoka from Hiroshima University. We also thank Dr. Makoto Maeda of NBARD, Hiroshima University, for his assistance with the TEM analysis.
Author Contributions
Contributions of each Author is as follows: Qing Li: Writer of the manuscript and conducted the analyses. Christina W. Kartikowati: Writer of the manuscript and conducted the analyses. Shinji Horie: Synthesis of nanoparticles. Takashi Ogi: Supervision on manuscript writing and analyses. Toru Iwaki: Supervision on manuscript writing and analyses. Kikuo Okuyama: Supervision on manuscript writing and analyses.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-09897-5
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yoo K, Jeon BG, Chun SH, et al. Quantitative measurements of size-dependent magnetoelectric coupling in Fe3O4 nanoparticles. Nano Lett. 2016;16:7408–7413. doi: 10.1021/acs.nanolett.6b02978. [DOI] [PubMed] [Google Scholar]
- 2.Ling D, Hyeon T. Chemical design of biocompatible iron oxide nanoparticles for medical applications. Small. 2013;9:1450–1466. doi: 10.1002/smll.201202111. [DOI] [PubMed] [Google Scholar]
- 3.Nie S, Starodub E, Monti M, et al. Insight into magnetite’s redox catalysis from observing surface morphology during oxidation. J. Am. Chem. Soc. 2013;135:10091–10098. doi: 10.1021/ja402599t. [DOI] [PubMed] [Google Scholar]
- 4.Yamada T, Morita K, Kume K, Yoshikawa H, Awaga K. The solid-state electrochemical reduction process of magnetite in Li batteries: in situ magnetic measurements toward electrochemical magnets. J. Mater. Chem. C. 2014;2 doi: 10.1039/C4TC00299G. [DOI] [Google Scholar]
- 5.Meisen U, Kathrein H. The influence of particle size, shape and particle size distribution on properties of magnetites for the production of toners. J. Imaging Sci. Technol. 2000;44:508–513. [Google Scholar]
- 6.Yavuz CT, Mayo JT, Yu WW, et al. Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science. 2006;314:964–967. doi: 10.1126/science.1131475. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Kwon SG, Park JG, Hyeon T. Size dependence of metal-insulator transition in stoichiometric Fe3O4 nanocrystals. Nano Lett. 2015;15:4337–4342. doi: 10.1021/acs.nanolett.5b00331. [DOI] [PubMed] [Google Scholar]
- 8.Wang WN, Widiyastuti W, Ogi T, Lenggoro IW, Okuyama K. Correlations between crystallite: Particle size and photoluminescence properties of submicrometer phosphors. Chem. Mater. 2007;19:1723–1730. doi: 10.1021/cm062887p. [DOI] [Google Scholar]
- 9.Jun YW, Huh YM, Choi JS, et al. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J. Am. Chem. Soc. 2005;127:5732–5733. doi: 10.1021/ja0422155. [DOI] [PubMed] [Google Scholar]
- 10.Huber DL. Synthesis, properties, and applications of iron nanoparticles. Small. 2005;1:482–501. doi: 10.1002/smll.200500006. [DOI] [PubMed] [Google Scholar]
- 11.Ozel F, Kockar H. Growth and characterizations of magnetic nanoparticles under hydrothermal conditions: Reaction time and temperature. J. Magn. Magn. Mater. 2015;373:213–216. doi: 10.1016/j.jmmm.2014.02.072. [DOI] [Google Scholar]
- 12.Butler RF, Banerjee SK. Theoretical single-domain grain size range in magnetite and titanomagnetite. J. Geophys. Res. 1975;80:4049–4058. doi: 10.1029/JB080i029p04049. [DOI] [Google Scholar]
- 13.Leslie-Pelecky DL, Rieke RD. Magnetic properties of nanostructured materials. Chem. Mater. 1996;8:1770–1783. doi: 10.1021/cm960077f. [DOI] [Google Scholar]
- 14.Kim D, Lee N, Park M, et al. Synthesis of uniform ferrimagnetic magnetite nanocubes. J. Am. Chem. Soc. 2009;131:454–455. doi: 10.1021/ja8086906. [DOI] [PubMed] [Google Scholar]
- 15.Ma M, Wu Y, Zhou J, et al. Size dependence of specific power absorption of Fe3O4 particles in AC magnetic field. J. Magn. Magn. Mater. 2004;268:33–39. doi: 10.1016/S0304-8853(03)00426-8. [DOI] [Google Scholar]
- 16.Iida H, Takayanagi K, Nakanishi T, Osaka T. Synthesis of Fe3O4 nanoparticles with various sizes and magnetic properties by controlled hydrolysis. J. Colloid Interface Sci. 2007;314:274–280. doi: 10.1016/j.jcis.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 17.Santoyo Salazar J, Perez L, de Abril O, et al. Magnetic iron oxide nanoparticles in 10–40 nm range: Composition in terms of magnetite/maghemite ratio and effect on the magnetic properties. Chem. Mater. 2011;23:1379–1386. doi: 10.1021/cm103188a. [DOI] [Google Scholar]
- 18.Upadhyay S, Parekh K, Pandey B. Influence of crystallite size on the magnetic properties of Fe3O4 nanoparticles. J. Alloys Compd. 2016;678:478–485. doi: 10.1016/j.jallcom.2016.03.279. [DOI] [Google Scholar]
- 19.Lee JS, Myung Cha J, Young Yoon H, Lee JK, Kim YK. Magnetic multi-granule nanoclusters: A model system that exhibits universal size effect of magnetic coercivity. Sci. Rep. 2015;5 doi: 10.1038/srep12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noh SH, Na W, Jang JT, et al. Nanoscale magnetism control via surface and exchange anisotropy for optimized ferrimagnetic hysteresis. Nano Lett. 2012;12:3716–3721. doi: 10.1021/nl301499u. [DOI] [PubMed] [Google Scholar]
- 21.Yoo K, Jeon B-G, Chun SH, et al. Quantitative Measurements of Size-Dependent Magnetoelectric Coupling in Fe3O4 Nanoparticles. Nano Lett. 2016;16:7408–7413. doi: 10.1021/acs.nanolett.6b02978. [DOI] [PubMed] [Google Scholar]
- 22.Uchida, N., Fujioka, K., Aoki, K., Misawa, H. & Kozawa, M. Magnetic particles for magnetic toner and process for producing the same. Japan patent (1998).
- 23.Mori, K., Kawabata, M., Kunishige, M., Horiishi, N. & Toda, K. Sphere-like magnetite particles and a process for producing the same. Japan patent (1991).
- 24.Sun S, Zheng H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002;124:8204–8205. doi: 10.1021/ja026501x. [DOI] [PubMed] [Google Scholar]
- 25.Baumgartner J, Dey A, Bomans PH, et al. Nucleation and growth of magnetite from solution. Nat. Mater. 2013;12:310–314. doi: 10.1038/nmat3558. [DOI] [PubMed] [Google Scholar]
- 26.Klokkenburg M, Vonk C, Claesson EM, et al. Direct imaging of zero-field dipolar structures in colloidal dispersions of synthetic magnetite. J. Am. Chem. Soc. 2004;126:16706–16707. doi: 10.1021/ja0456252. [DOI] [PubMed] [Google Scholar]
- 27.Goya GF, Berquó TS, Fonseca FC, Morales MP. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Appl. Phys. 2003;94:3520–3528. doi: 10.1063/1.1599959. [DOI] [Google Scholar]
- 28.Caruntu D, Caruntu G, O’Connor CJ. Magnetic properties of variable-sized Fe3O4 nanoparticles synthesized from non-aqueous homogeneous solutions of polyols. J. Phys. D: Appl. Phys. 2007;40 doi: 10.1088/0022-3727/40/19/001. [DOI] [Google Scholar]
- 29.Kovalenko MV, Bodnarchuk MI, Lechner RT, et al. Fatty acid salts as stabilizers in size- and shape-controlled nanocrystal synthesis: The case of inverse spinel iron oxide. J. Am. Chem. Soc. 2007;129:6352–6353. doi: 10.1021/ja0692478. [DOI] [PubMed] [Google Scholar]
- 30.Kandasamy G, Maity D. Recent advances in superparamagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int. J. Pharm. 2015;496:191–218. doi: 10.1016/j.ijpharm.2015.10.058. [DOI] [PubMed] [Google Scholar]
- 31.Néel L. Propriétés d’un ferromagnétique cubique en grains fins. CR Acad. Sci. Paris. 1947;224:1488–1490. [Google Scholar]
- 32.Herzer G. Nanocrystalline soft magnetic materials. J. Magn. Magn. Mater. 1996;157/158:133–136. doi: 10.1016/0304-8853(95)01126-9. [DOI] [Google Scholar]
- 33.Ogi T, Li Q, Horie S, et al. High-purity core-shell α″-Fe16N2/Al2O3 nanoparticles synthesized from α-hematite for rare-earth-free magnet applications. Adv. Powder Technol. 2016;27:2520–2525. doi: 10.1016/j.apt.2016.09.017. [DOI] [Google Scholar]
- 34.Ogi T, Zulhijah R, Iwaki T, Okuyama K. Recent progress in nanoparticle dispersion using bead mill. KONA Powder and Particle Journal. 2017;34:3–23. doi: 10.14356/kona.2017004. [DOI] [Google Scholar]
- 35.Kartikowati, C. W., Suhendi, A. & Zulhijah, R. et al. Effect of magnetic field strength on the alignment of α″-Fe16N2 nanoparticle films. Nanoscale8, 2648–2655 (2016). [DOI] [PubMed]
- 36.Ogi T, Nandiyanto ABD, Okuyama K. Nanostructuring strategies in functional fine-particle synthesis towards resource and energy saving applications. Adv. Powder Technol. 2014;25:3–17. doi: 10.1016/j.apt.2013.11.005. [DOI] [Google Scholar]
- 37.Kartikowati CW, Suhendi A, Zulhijah R, et al. Preparation and evaluation of magnetic nanocomposite fibers containing α″-Fe16N2 and α-Fe nanoparticles in polyvinylpyrrolidone via magneto-electrospinning. Nanotechnology. 2016;27 doi: 10.1088/0957-4484/27/2/025601. [DOI] [PubMed] [Google Scholar]
- 38.Suhendi A, Kartikowati CW, Zulhijah R, et al. Preparation and characterization of magnetic films of well-dispersed single domain of core–shell α″-Fe16N2/Al2O3 nanoparticles. Adv. Powder Technol. 2015;26:1618–1623. doi: 10.1016/j.apt.2015.09.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.