Abstract
Metastasis-associated recurrence is the main cause for the poor prognosis of hepatocellular carcinoma (HCC). However, the detailed molecular mechanisms underlying HCC metastasis remain elusive. Though some data indicated the oncogenic role of Sorcin in tumors, the prognostic value and biological role of Sorcin in HCC is still unknown. In this study, it demonstrated that Sorcin expression levels were significantly upregulated in HCC tumor tissues compared with matched adjacent nontumorous liver tissues and normal liver tissues, and such expression level correlated with HCC metastasis. High Sorcin expression was significantly correlated with aggressive clinicopathological characteristics such as multiple tumor nodules, high Edmondson-Steiner grade, microvascular invasion, advanced TNM stage and advanced BCLC stage (all P < 0.05). HCC patients with high Sorcin expression had both shorter survival and higher recurrence than those with low Sorcin expression (all P < 0.05). Sorcin expression was an independent and significant risk factor for survival and recurrence of HCC patients. Results of functional experiments showed that Sorcin could promote HCC cell proliferation, migration, and invasion in vitro, and facilitate HCC growth and metastasis in vivo. Mechanistically, Sorcin exerted its role by activating extracellular signal-regulated kinase (ERK) pathway and promoted metastasis by facilitating epithelial-mesenchymal transition (EMT) in HCC.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignant tumor and the third cause of cancer-related deaths worldwide1. Surgical resection is still the prime therapeutic strategy to treat HCC2, 3. Despite advancement in surgical resection, the prognosis of HCC remains bleak, with a 5-year overall survival rate approximately 30% after radical hepatic resection4. Metastasis-associated recurrence, by dissemination of metastatic HCC cells, is the main cause for the poor prognosis3, 5, 6. However, the molecular mechanisms underlying HCC metastasis are not well characterized. Therefore, further investigations into its molecular mechanisms are of great importance in identifying new prognostic biomarkers and developing novel therapeutics for HCC.
Sorcin, a 22-kDa calcium (Ca2+)-binding cytoplasmic protein, was initially discovered in multidrug resistant cells7. Previous studies show that Sorcin plays a role in regulating Ca2+ signaling8, which is involved in chemoresistance, cytoskeletal reorganization, cell migration, and cancer metastasis9. Sorcin overexpression has been observed in multiple human cancers, including human gastric, colorectal, lung cancer et al.10–12. Recently, the study indicated that Sorcin could enhance cancer cell migration, invasion, and epithelial-mesenchymal transition (EMT) in breast and colorectal cancer11, 13. In light of its oncogenic role in other tumors, we hypothesized that Sorcin might also play a role in the metastasis of HCC. However, the role of Sorcin in the metastasis of HCC has never been explored.
In this study, we investigated the clinical relevance and the biological role of Sorcin in human HCC. We observed a significantly up-regulated expression of Sorcin in HCC and high Sorcin expression was an independent prognosticator for survival and recurrence. Then, results of functional experiments indicated that Sorcin promoted cell migration and invasion in vitro as well as metastasis in vivo. Mechanically, we further demonstrated that Sorcin exerted its function by activating extracellular signal-regulated kinase (ERK) pathway and inducing EMT in HCC.
Results
Sorcin Expression Is Significantly Elevated and Associated with Metastasis in HCC
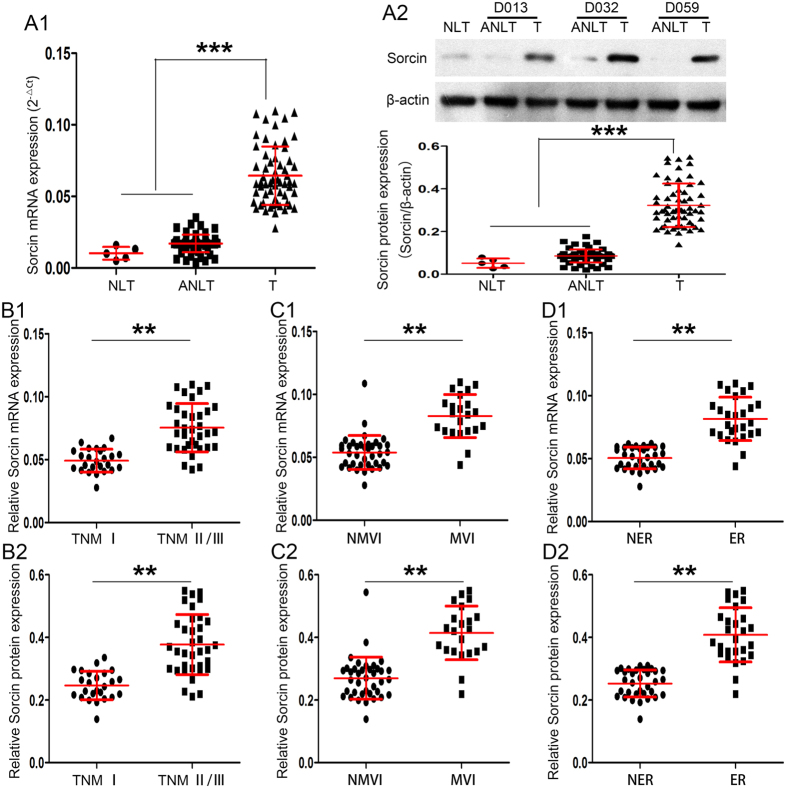
Firstly, the expression of Sorcin was determined in sixty pairs of HCC samples including tumors (Ts) and their corresponding adjacent nontumorous liver tissues (ANLTs) in screening cohort (Supplementary Fig. 1 and Supplementary Table 1) by real-time PCR (RT-PCR) and western blot. Five normal liver tissues (NLTs) from hepatic hemangioma patients were set as loading control. Results showed Sorcin mRNA and protein expression in Ts were markedly higher than in ANLTs or NLTs (P < 0.001; Fig. 1A1,2). The expression levels of Sorcin in HCC tissues were significantly higher in advanced stage patients (TNM stages II/III) than in early stage patients (TNM stage I) (P < 0.01; Fig. 1B1,2). Notably, Sorcin expression was significantly higher in tumors with microvascular invasion (MVI) than tumors without MVI (P < 0.01; Fig. 1C1,2). HCC tissues from patients with early tumor recurrences (within 2 years) also exhibited higher Sorcin expression than that from patients without early tumor recurrences (P < 0.01; Fig. 1D1,2). Taken together, these data proved that the expression of Sorcin was significantly elevated in HCC tissues and may be associated with metastasis in HCC.
Figure 1.
Sorcin Expression Is Significantly Elevated and Associated with Metastasis in HCC. (A) Sorcin mRNA and protein expression in Ts were markedly higher than in their corresponding ANLTs or NLTs. (A1) Real-time PCR (RT-PCR) and (A2) western blot were used to determine the expression of Sorcin in sixty pairs of Ts and ANLTs in screening cohort. Five NLTs from hepatic hemangioma patients were set as loading control. NLT, ANLT, T represent normal liver tissue, adjacent nontumorous liver tissue, tumor tissue. (B) Sorcin mRNA (B1) and protein (B2) expression in HCC tissues from advanced stage patients were significantly higher (TNM stages II/III) than in those from early stage patients (TNM stage I). (C) Sorcin mRNA (C1) and protein (C2) expression was significantly higher in tumors with microvascular invasion (MVI) than those without MVI. MVI, microvascular invasion; NMVI, without microvascular invasion. (D) Sorcin mRNA (D1) and protein (D2) expression in HCC tissues from patients with early tumor recurrences (within 2 years) was higher than those from patients without early tumor recurrences. ER, NER represent HCC with early recurrence, HCC without early recurrence respectively. **P < 0.01, ***P < 0.001.
High Sorcin Expression Correlates with Aggressive Clinicopathological Characteristics and Predicts Poor Prognosis in HCC Patients
Next, we assessed the association of Sorcin expression with the prognosis of HCC patients according to ReMARK guidelines for reporting prognostic biomarkers in cancer14. Immunohistochemical (IHC) assays of HCC samples from training cohort (Supplementary Table 1 and Supplementary Fig. 1) showed Sorcin expression was mainly located in the cytoplasm (Fig. 2A). The expression level of Sorcin was significantly higher in Ts than that in ANLTs (Fig. 2B), and high Sorcin expression was found in 66.25% (106/160) in Ts, as compared with only 8.13% (13/160) in ANLTs from training cohort (P < 0.001; Fig. 2C1). HCC patients were divided into two groups: the high Sorcin expression group and low Sorcin expression group based on IHC staining score of HCC tissues. Analyzing the relationship of Sorcin expression with clinicopathologic features showed that high Sorcin expression significantly correlated with aggressive clinicopathological variables such as multiple nodules (P = 0.001; Table 1), larger tumor size (P < 0.001; Table 1), microvascular invasion (P < 0.001; Table 1), higher Edmondson-Stainer grade (P = 0.002; Table 1), and advanced TNM (P < 0.001; Table 1) or BCLC stage (P < 0.001; Table 1). However, high Sorcin expression did not correlate with clinicopathological variables including gender, age, HBV infection, serum AFP level, presence of cirrhosis and presence of encapsulation (P > 0.05; Table 1).
Figure 2.
High Sorcin expression is a potential prognosticator for HCC clinical outcome after liver resection. (A) A paired representative images for immunohistochemistry (IHC) staining of Sorcin protein in Ts and ANLTs from training and validation cohort. (B) Box-plot analyzed the immunohistochemical scores of Ts and ANLTs from training and validation cohort showed that Ts exhibited higher Sorcin protein expression than corresponding ANLTs. (C) According to IHC immunostaining score, HCC patients dichotomized into high Sorcin expression group (>3) and low Sorcin expression group (≤3). χ2 analysis was used to analyze the difference between Ts and ANLTs from training (C1) and validation cohort (C2). (D) Kaplan-Meier analysis of the correlation between Sorcin expression and the disease-free survival (DFS; D1), overall survival (OS; D2), early recurrence (ER; D3) of HCC patients in training cohort. (E) Kaplan-Meier analysis of the correlation between Sorcin expression and the disease-free survival (DFS; E1), overall survival (OS; E2), early recurrence (ER; E3) of HCC patients in validation cohort.
Table 1.
Correlations between Sorcin Expression in HCC Tissues and Clinicopathologic Variables of HCC patients in Training and Validation Cohort.
| Clinicopathologic variable | Training cohort | Validation Cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| No. | Sorcin expression levels | P value | No. | SORCIN expression level | P value | |||
| Low(n = 54) | High(n = 106) | Low(n = 44) | High(n = 76) | |||||
| Gender | ||||||||
| Female | 29 | 11 | 18 | 0.599 | 19 | 7 | 12 | 0.986 |
| Male | 131 | 43 | 88 | 101 | 37 | 64 | ||
| Age (years) | ||||||||
| ≤60 | 105 | 34 | 71 | 0.613 | 88 | 31 | 57 | 0.587 |
| >60 | 55 | 20 | 35 | 32 | 13 | 19 | ||
| Serum AFP level(ng/mL) | ||||||||
| ≤20 | 40 | 13 | 27 | 0.847 | 31 | 12 | 19 | 0.784 |
| >20 | 120 | 41 | 79 | 89 | 32 | 57 | ||
| HBsAg | ||||||||
| Negative | 32 | 15 | 17 | 0.079 | 27 | 10 | 17 | 0.964 |
| Positive | 128 | 39 | 89 | 93 | 34 | 59 | ||
| Liver cirrhosis | ||||||||
| Absence | 63 | 19 | 44 | 0.439 | 44 | 14 | 30 | 0.402 |
| Presence | 97 | 35 | 62 | 76 | 30 | 46 | ||
| Child-Pugh classification | ||||||||
| A | 139 | 46 | 93 | 0.651 | 104 | 36 | 68 | 0.235 |
| B | 21 | 8 | 13 | 16 | 8 | 8 | ||
| Tumor number | ||||||||
| Solitary | 69 | 33 | 36 | 0.001 | 54 | 29 | 25 | <0.001 |
| Multiple | 91 | 21 | 70 | 66 | 15 | 51 | ||
| Tumor size | ||||||||
| ≤5 cm | 58 | 30 | 28 | <0.001 | 45 | 23 | 22 | 0.011 |
| >5 cm | 102 | 24 | 78 | 75 | 21 | 54 | ||
| Capsular formation | ||||||||
| Presence | 65 | 25 | 40 | 0.297 | 48 | 17 | 31 | 0.817 |
| Absence | 95 | 29 | 66 | 72 | 27 | 45 | ||
| Microvascular invasion | ||||||||
| Absence | 60 | 40 | 20 | <0.001 | 55 | 33 | 22 | <0.001 |
| Presence | 100 | 14 | 86 | 65 | 11 | 54 | ||
| Edmondson-Steiner grade | ||||||||
| Low grade (I and II) | 62 | 30 | 32 | 0.002 | 49 | 25 | 24 | 0.007 |
| High grade (III and IV) | 98 | 24 | 74 | 71 | 19 | 52 | ||
| TNM Stage | ||||||||
| I | 57 | 36 | 21 | <0.001 | 50 | 29 | 21 | <0.001 |
| II - III | 103 | 18 | 85 | 70 | 15 | 55 | ||
| BCLC Stage | ||||||||
| 0-A | 50 | 28 | 22 | <0.001 | 44 | 26 | 18 | <0.001 |
| B-C | 110 | 26 | 84 | 76 | 18 | 58 | ||
Abbreviations: AFP, alpha-fetoprotein; TNM, tumor node metastasis; BCLC, Barcelona Clinic Liver Cancer.
Analyzed by the Kaplan-Meier method with log-rank test, results showed that HCC patients with high Sorcin expression had either shorter disease-free survival (DFS; P < 0.001; Fig. 2D1) or shorter overall survival (OS; P < 0.001; Fig. 2D2) than those with low Sorcin expression. In addition, HCC patients with the high Sorcin expression had higher early recurrence (within 2 years) than those with low Sorcin expression (P = 0.003; Fig. 2D3). Next, we further explored the prognostic value of Sorcin in specific subgroups of HCC patients. Patients were also classified into two subgroups based on microvascular invasion (MVI), which is a major prognostic factor for HCC and was recently identified as a better predictor of tumor recurrence15. MVI offered great significance in differential stratification of patients for DFS (P < 0.001; Supplementary Fig. 2A1) or OS (P < 0.001; Supplementary Fig. 2B1) in training cohort. The DFS (P = 0.004; Supplementary Fig. 2A2) or OS (P = 0.001; Supplementary Fig. 2B2) of patients with high Sorcin expression significantly decreased compared with those with low Sorcin expression in non-MVI group. However, for patients in the MVI group, no significant associations between high Sorcin expression and DFS (P = 0.760; Supplementary Fig. 2A3) or OS (P = 0.542; Supplementary Fig. 2B3) were observed. Finally, to determine whether high Sorcin expression was an independent prognostic factor for HCC, a univariate analysis was first performed followed by the multivariate Cox proportional hazards analysis. The data exhibited high Sorcin expression to be an independent risk factor for both DFS (HR 2.641, 95% CI 1.491 to 4.678, P = 0.009; Table 2) and OS (HR 2.860, 95% CI 1.836 to 4.455, P = 0.003; Table 2) in HCC.
Table 2.
The Cox Proportional Hazard Regression Analyses for Disease-free Survival and Overall Survival in Training Cohort.
| Variables | DFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Univariable Analysis | Multivariable Analysis | Univariable Analysis | Multivariable Analysis | |||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Gender | |||||||||
| Female | 29 | Reference | Reference | ||||||
| Male | 131 | 1.021(0.531–1.963) | 0.346 | NA | 1.081(0.702–1.665) | 0.335 | NA | ||
| Age (years) | |||||||||
| ≤60 | 105 | Reference | Reference | ||||||
| >60 | 55 | 1.124(0.872–1.449) | 0.239 | NA | 1.205(0.752–1.931) | 0.218 | NA | ||
| Serum AFP level(ng/mL) | |||||||||
| ≤20 | 40 | Reference | Reference | ||||||
| >20 | 120 | 1.168(0.634–2.152) | 0.202 | NA | 1.206(0.723–2.012) | 0.283 | NA | ||
| HBsAg | |||||||||
| Negative | 32 | Reference | Reference | ||||||
| Positive | 128 | 1.306(0.910–1.874) | 0.154 | NA | 1.375(0.901–2.098) | 0.171 | NA | ||
| Liver cirrhosis | |||||||||
| Absence | 63 | Reference | Reference | ||||||
| Presence | 97 | 1.288 (0.773–2.146) | 0.251 | NA | 1.136(0.682–1.892) | 0.248 | NA | ||
| Child-Pugh classification | |||||||||
| A | 139 | Reference | Reference | ||||||
| B | 21 | 1.242(0.704–2.360) | 0.195 | NA | 1.247(0.813–1.913) | 0.187 | NA | ||
| Tumor number | |||||||||
| Solitary | 69 | Reference | Reference | Reference | Reference | ||||
| Multiple | 91 | 2.097(1.392–3.159) | 0.006 | 1.841(1.209–2.803) | 0.019 | 2.196(1.809–2.665) | 0.009 | 1.832(1.311–2.560) | 0.034 |
| Tumor size | |||||||||
| ≤5 cm | 58 | Reference | Reference | Reference | |||||
| >5 cm | 102 | 1.316(0.772–1.894) | 0.084 | 1.114(0.628–1.976) | 0.104 | 1.617(0.883–2.308) | 0.132 | NA | |
| Capsular formation | |||||||||
| Presence | 65 | Reference | Reference | Reference | Reference | ||||
| Absence | 95 | 1.957(1.462–2.620) | 0.010 | 1.432(0.908–2.258) | 0.065 | 1.895(1.317–2.727) | 0.017 | 1.361(0.856–2.164) | 0.091 |
| Microvascular invasion | |||||||||
| Absence | 60 | Reference | Reference | Reference | Reference | ||||
| Presence | 100 | 4.135(2.758–6.199) | <0.001 | 2.917(1.809–4.704) | 0.006 | 3.781(2.528–5.655) | <0.001 | 3.015(1.936–4.695) | 0.001 |
| Edmondson-Steiner grade | |||||||||
| Low grade (I and II) | 62 | Reference | Reference | ||||||
| High grade (III and IV) | 98 | 1.077(0.604–1.920) | 0.207 | NA | 1.224(0.815–1.838) | 0.232 | NA | ||
| TNM Stage | |||||||||
| I | 57 | Reference | Reference | Reference | Reference | ||||
| II – III | 103 | 2.634(1.783–3.891) | 0.001 | 2.206(1.501–3.242) | 0.011 | 3.008(1.804–5.016) | 0.002 | 2.273(1.398–3.696) | 0.014 |
| BCLC Stage | |||||||||
| 0-A | 50 | Reference | Reference | Reference | Reference | ||||
| B-C | 110 | 2.163(1.743–2.684) | 0.004 | 1.752(1.419–2.163) | 0.030 | 2.306(1.732–3.074) | 0.005 | 1.904(1.425–2.544) | 0.023 |
| Sorcin expression | |||||||||
| Low | 54 | Reference | Reference | Reference | Reference | ||||
| High | 106 | 3.803(1.894–7.636) | <0.001 | 2.641(1.491–4.678) | 0.008 | 3.934(2.032–7.616) | <0.001 | 2.860(1.836–4.455) | 0.003 |
Abbreviations: NA, not adopt.
Validation of the Effect of Sorcin Expression on Predicting Prognosis in HCC
IHC assays of HCC samples from validation cohort (Supplementary Fig. 1 and Supplementary Table 1) further confirmed that the expression level of Sorcin was significantly higher in Ts than that in ANLT (Fig. 2B). High Sorcin expression was found in 63.33% (76/120) in Ts, as compared with only 7.5% (9/120) in ANLTs (P < 0.001; Fig. 2C2) from validation cohort. Sorcin expression was also significantly correlated with aggressive clinicopathological variables including multiple nodules (P < 0.001; Table 1), larger tumor size (P = 0.011; Table 1), microvascular invasion (P < 0.001; Table 1), higher Edmondson-Stainer grade (P = 0.007; Table 1), and advanced TNM (P < 0.001; Table 1) or BCLC stage (P < 0.001; Table 1). However, high Sorcin expression did not correlate with clinicopathological variables including gender, age, HBV infection, AFP, presence of cirrhosis and presence of encapsulation (P > 0.05; Table 1).
Analyzed by the Kaplan-Meier method with log-rank test, HCC patients with high Sorcin expression had either shorter DFS (P = 0.001; Fig. 2E1) or shorter OS (P < 0.001; Fig. 2E2) than those with low Sorcin expression. In addition, HCC patients with high Sorcin expression had higher early recurrence than those with Sorcin low expression (P = 0.002; Fig. 2E3). MVI offered great significance in differential stratification of patients for DFS (P < 0.001; Supplementary Fig. 3A1) or OS (P < 0.001; Supplementary Fig. 3B1) in validation cohort. Moreover, the prognostic significance of Sorcin occurred in HCC patients with non-MVI that in such clinical subgroups, patients with high expression of Sorcin showed significantly decreased DFS (P = 0.014; Supplementary Fig. 3A2) or OS (P = 0.001; Supplementary Fig. 3B2), but this significant associations between high Sorcin expression and DFS (P = 0.246; Supplementary Fig. 3A3) or OS (P = 0.284; Supplementary Fig. 3B3) were not observed in MVI subgroup. Furthermore, the multivariate Cox regression model indicated high Sorcin expression was an independent risk factor for DFS (HR 2.373; 95% CI 1.508 to 3.734, P = 0.008; Table 3) and OS (HR 2.412, 95% CI 1.685 to 3.453, P = 0.016; Table 3) in HCC.
Table 3.
The Cox Proportional Hazard Regression Analyses for Disease-free Survival (DFS) and Overall Survival (OS) in Validation Cohort.
| Variables | DFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Univariable Analysis | Multivariable Analysis | Univariable Analysis | Multivariable Analysis | |||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Gender | |||||||||
| Female | 19 | Reference | Reference | ||||||
| Male | 101 | 1.074 ERK1/2 (p-ERK1/2) increased when Sorcin was overexpressed in HepG2 cells(0.659–1.750) | 0.343 | NA | 1.091(0.608–1.958) | 0.409 | NA | ||
| Age (years) | |||||||||
| ≤60 | 88 | Reference | Reference | ||||||
| >60 | 32 | 1.288(0.801–2.071) | 0.271 | NA | 1.191(0.694–2.044) | 0.307 | NA | ||
| Serum AFP level(ng/mL) | |||||||||
| ≤20 | 31 | Reference | Reference | ||||||
| >20 | 89 | 1.207(0.780–1.868) | 0.360 | NA | 1.305(0.803–2.121) | 0.298 | NA | ||
| HBsAg | |||||||||
| Negative | 27 | Reference | Reference | ||||||
| Positive | 93 | 1.167(0.684–1.991) | 0.301 | NA | 1.254(0.734–2.142) | 0.314 | NA | ||
| Liver cirrhosis | |||||||||
| Absence | 44 | Reference | Reference | ||||||
| Presence | 76 | 1.326(0.807–2.179) | 0.288 | NA | 1.264(0.691–2.312) | 0.301 | NA | ||
| Child-Pugh classification | |||||||||
| A | 104 | Reference | Reference | ||||||
| B | 16 | 1.403(0.741–2.656) | 0.185 | NA | 1.394(0.769–2.527) | 0.190 | NA | ||
| Tumor number | |||||||||
| Solitary | 54 | Reference | Reference | Reference | Reference | ||||
| Multiple | 66 | 3.202(1.936–5.296) | <0.001 | 2.198(1.583–3.052) | 0.010 | 2.955(1.927–4.531) | <0.001 | 2.087(1.651–2.638) | 0.021 |
| Tumor size | |||||||||
| ≤5 cm | 45 | Reference | Reference | Reference | |||||
| >5 cm | 75 | 1.504(0.967–2.339) | 0.074 | 1.061(0.809–1.391) | 0.194 | 1.320(0.738–2.361) | 0.239 | NA | |
| Capsular formation | |||||||||
| Presence | 48 | Reference | Reference | Reference | Reference | ||||
| Absence | 72 | 1.834(1.972–7.624) | 0.010 | 1.366(0.828–2.254) | 0.078 | 1.506(1.033–2.196) | 0.040 | 1.123(0.809–1.562) | 0.109 |
| Microvascular invasion | |||||||||
| Absence | 55 | Reference | Reference | Reference | Reference | ||||
| Presence | 65 | 3.594(1.645–4.252) | <0.001 | 2.678(1.737–4.129) | <0.001 | 3.756(1.962–7.190) | <0.001 | 2.827(1.667–4.794) | 0.005 |
| Edmondson-Steiner grade | |||||||||
| Low grade (I and II) | 49 | Reference | Reference | ||||||
| High grade(III and IV) | 71 | 1.394(0.837–2.322) | 0.192 | NA | 1.412(0.854–2.335) | 0.153 | NA | ||
| TNM Stage | |||||||||
| I | 50 | Reference | Reference | Reference | Reference | ||||
| II – III | 70 | 2.429(1.709–3.452) | 0.007 | 1.802(1.326–2.449) | 0.030 | 2.658(1.917–3.685) | 0.005 | 1.914(1.381–2.653) | 0.042 |
| BCLC Stage | |||||||||
| 0-A | 44 | Reference | Reference | Reference | Reference | ||||
| B-C | 76 | 3.106(1.982–4.867) | 0.003 | 2.107(1.433–3.098) | 0.018 | 2.769(1.835–4.178) | 0.001 | 1.935(1.502–2.493) | 0.035 |
| Sorcin expression | |||||||||
| Low | 76 | Reference | Reference | Reference | Reference | ||||
| High | 44 | 3.500(2.101–5.831) | 0.001 | 2.373(1.508–3.734) | 0.008 | 3.693(2.807–4.859) | <0.001 | 2.412(1.685–3.453) | 0.016 |
Sorcin Enhances Invasion and Metastasis of HCC cells in Vitro and in Vivo
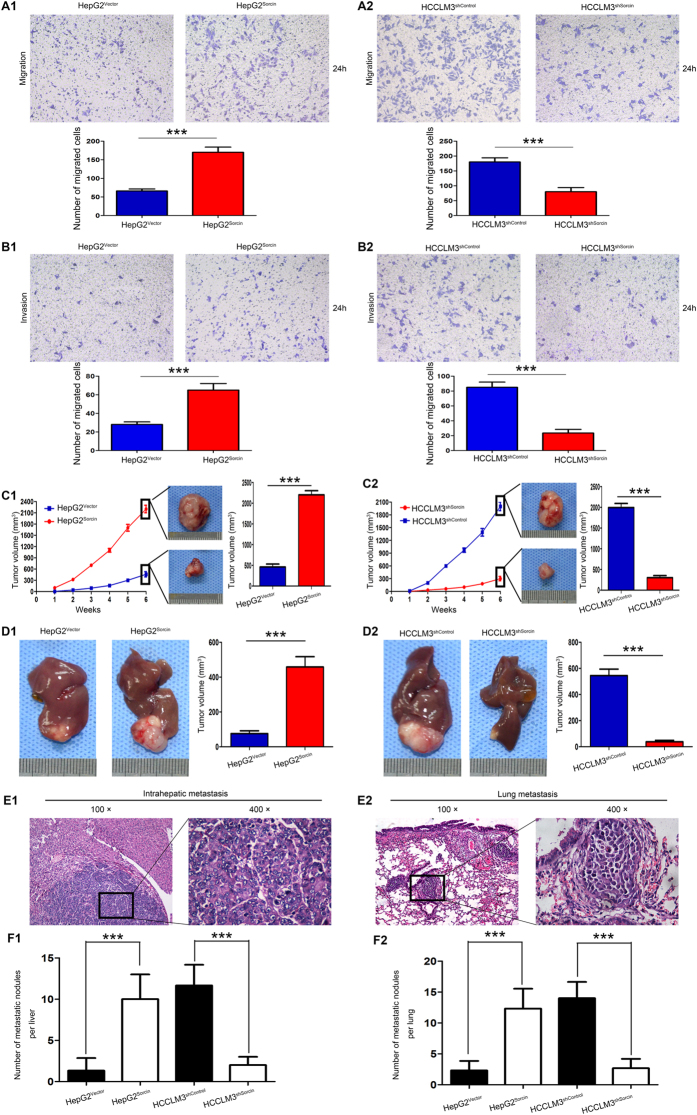
The above results indicated that high Sorcin expression was positively associated with metastasis in HCC, so we explored the role of Sorcin in the metastasis of HCC. Firstly, we determined the Sorcin expression in HCC cell lines (HepG2, PLC/PRF5, Bel7402, Hep3B, MHCC97-H and HCCLM3) and normal liver cells L02 cells. Compared with human normal liver cells L02 cells, Sorcin messenger RNA (mRNA) and protein was highly expressed in 6 human HCC cell lines (Supplementary Fig. 4A1,2). Notably, Sorcin expression in high metastatic HCC cell lines, such as Hep3B, MHCC97-H and HCCLM3 was higher than in low metastatic HCC cell lines HepG2, PLC/PRF5 and Bel740216, 17 (Supplementary Fig. 4A1,2). To investigate the role of Sorcin in the metastasis of HCC, we overexpressed Sorcin in HepG2 cells (Supplementary Fig. 4A1) and stably knocked down Sorcin in HCCLM3 (Supplementary Fig. 4B2) according to the expression level of Sorcin in HCC cell lines and biological characteristics of HCC cell lines16, 17. Transwell migration assays showed overexpression of Sorcin significantly increased the ability of migration in HepG2 cells (Fig. 3A1), whereas down-regulation of Sorcin decreased the ability of migration in HCCLM3 cells (Fig. 3A2). The similar results were observed by transwell invasion assays (Fig. 3B1,2). Next, we examined the effect of Sorcin on the proliferation of HCC cells. MTT assays showed that overexpression of Sorcin significantly accelerated HepG2 cell proliferation (Supplementary Fig. 5A). On the contrary, down-regulation of Sorcin dramatically decelerated HCCLM3 cell proliferation (Supplementary Fig. 5B). Colony formation assays also shown that the capacity of colony formation of HepG2Sorcin cell is significantly more potent than that of HepG2Vector cell (Supplementary Fig. 5C). However, the capacity of colony formation of HCCLM3shSocrin cell is significantly weaker than that of HCCLM3shcontrol cell (Supplementary Fig. 5D). Above of all, these data indicate that Sorcin displays an oncogenic function and is involved in invasion and metastasis in HCC.
Figure 3.
Sorcin promotes HCC cell invasion and metastasis in vitro and in vivo. (A&B) Sorcin promoted HCC cell migration and invasion in vitro. Transwell migration (A) and invasion (B) assays of HepG2 cells with Sorcin overexpression (HepG2Sorcin) or control (HepG2Vector), and HCCLM3 cells with Sorcin knockdown (HCCLM3shSorcin) or control (HCCLM3shcontrol). (C,D) Sorcin promoted HCC cell growth in vivo. (C) Left column: Linear graphs were used to analyze the rate of tumor growth in each group. Middle column: Representative subcutaneous tumors from HepG2Sorcin and HCCLM3shSorcin cells and their control cells were shown in the middle panel. Right column: Tumor volumes of each group were shown and compared in the bar charts. (D) Left and middle column: Representative pictures of orthotopic liver exnograft tumors from each indicated groups were shown. Right column: Tumor volumes of each group were shown and compared in the bar charts. (E) Representative pictures of intrahepatic (E1) and lung metastasis (E2). original magnification: left, 100×; right, 400×. (F) The number of metastatic nodules in each mice liver (F1) or lung (F2) was calculated and compared. ***P < 0.001. Error bars, SD.
To verify the in vitro results, the role of Sorcin was further evaluated by using subcutaneous xenograft tumor and subsequently by in situ xenograft metastasis mice models. After 6 weeks, HepG2Sorcin cell-derived tumors at the subcutaneous implantation site grew more rapidly and were larger than HepG2Vector cell-derived tumors (Fig. 3C1), while HCCLM3shSocrin cell-derived tumors grew more slowly and were smaller than HCCLM3shcontrol cell-derived tumors (Fig. 3C2). Consistently, by analyzing the tumor volume of orthotopic xenograft liver tumor, it showed that Sorcin overexpression promoted tumors growth (Fig. 3D1), while Sorcin knockdown inhibited tumors growth in vivo (Fig. 3D2). Further detected the metastatic nodules in livers (Fig. 3E1) and lungs (Fig. 3E2) by H&E, data showed the number of intrahepatic and pulmonary metastatic nodules in mice with tumors derived from HepG2Sorcin cells was significantly higher than in those with tumors derived from HepG2Vector cells (Fig. 3F1,2). In contrast, the number of metastatic nodules in mice with tumors generated from HCCLM3shSocrin cells was markedly smaller than in those with tumors generated from HCCLM3shcontrol cells (Fig. 3F1,2). Taken it together, our studies demonstrated that Sorcin could promote HCC growth and metastasis in vivo.
Sorcin Exerts the Function through Activating Extracellular Signal-regulated Kinase (ERK) Pathway in HCC
To elucidate the potential signaling by which Sorcin contributes to metastasis in HCC, a multi-pathway reporter array was performed. Sorcin overexpression significantly enhanced the activity of ERK signaling in HepG2 cells (Fig. 4A1), but Sorcin knockdown attenuated ERK signaling activity in HCCLM3 cells (Fig. 4A2). Growing evidence has showed that ERK signaling is crucial for progression of HCC18, 19, and its activation can be triggered by changing cytosolic Ca2+ level9, 20. As Sorcin also regulates Ca2+ homeostasis, so we focus on exploring whether Sorcin enhanced metastasis by activating ERK signaling in HCC. Western blot showed the level of phosphorylated ERK1/2 (p-ERK1/2) increased when Sorcin was overexpressed in HepG2 cells (Fig. 4B1). In contrast, knockdown of the level of p-ERK1/2 decreased when Sorcin was knocked down in HCCLM3 cells (Fig. 4B2). However, manipulation of Sorcin expression in HCC cells didn’t affect ERK1/2 expression (Fig. 4B1,2), implying Sorcin specifically activated ERK signaling. To confirm the activation of ERK signaling by Sorcin in HCC cells, cells were pretreated with U0126, an ERK pathway inhibitor, at the concentration of 10 µM for 30 minutes. Western blot showed U0126 treatment didn’t affect Sorcin expression in HepG2 or HCCLM3 cells (Fig. 4B1,2). However, U0126 treatment effectively inhibited Sorcin-induced ERK1/2 activation in HepG2 cells (Fig. 4B1). In HCCLM3 cells, as U0126 treatment decreased the level of p-ERK1/2, Sorcin knockdown also caused the reduced level of p-ERK1/2 (Fig. 4B2). These data proved that Sorcin activated ERK signaling, not its downstream molecules. IHC analysis also showed HCC tissues, with high Sorcin expression, also exhibited high level of p-ERK1/2 (Fig. 4C1). Spearman rank correlation analysis showed the positive correlation of Sorcin expression with the level of p-ERK1/2 (Figs. 4C2,3). These results concluded that Sorcin could activate ERK1/2 signaling in HCC.
Figure 4.
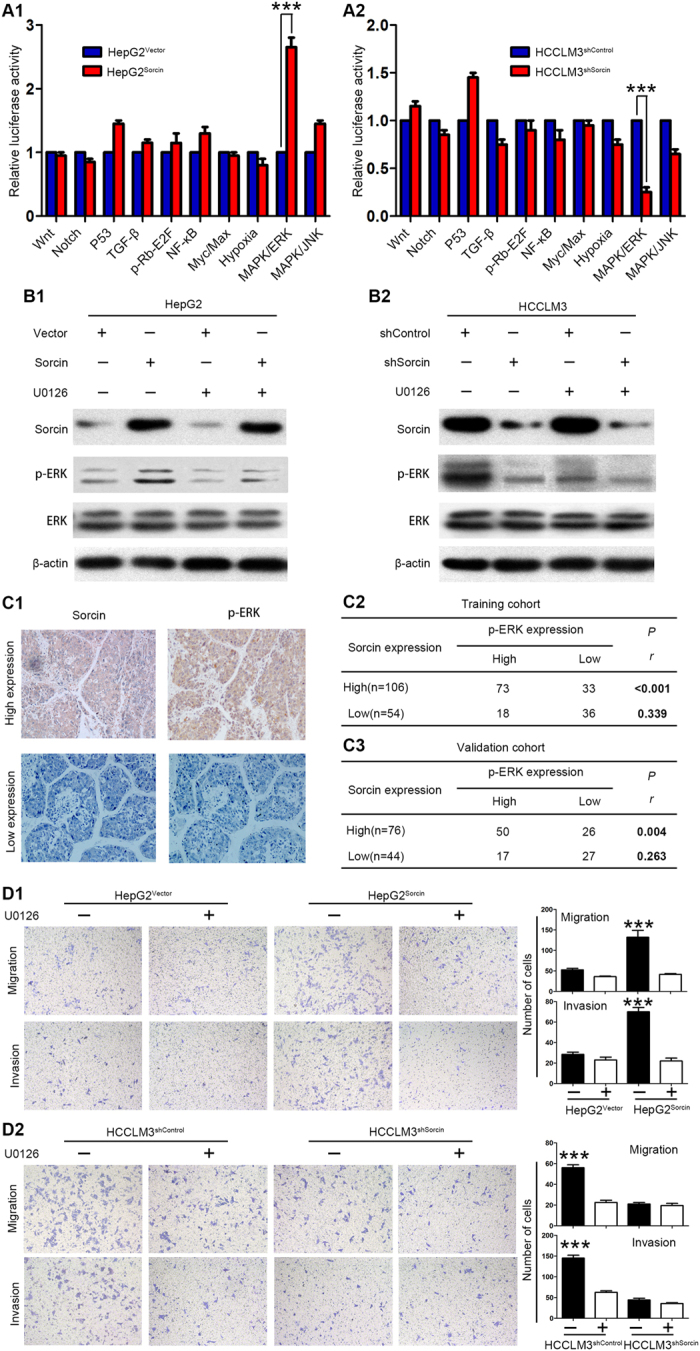
Sorcin activates ERK signaling in HCC. (A) 10-Pathway Reporter Array showed the signaling change in Sorcin-interfered cells. (B) The expression levels of key members of ERK signaling were detected by western blot. (C) Sorcin expression was positively correlated with p-ERK levels in HCC tissues. (C1) Representative IHC images of Sorcin and p-ERK expression in HCC tissues. magnification: 100×. (C2,3) Correlation of Sorcin and p-ERK expression levels was analyzed by Spearman rank correlation test in training (C2) and validation cohort (C3). (D) The migration and invasion assays for HepG2Sorcin, HCCLM3shSorcin and their control cells with/without U0126 treatment. ***P < 0.001. Error bars, SD.
Further, to study whether Sorcin promotes invasion and metastasis through activating ERK signaling in HCC cells, the effect of Sorcin on HCC cell migration and invasiveness interfered by U0126 was measured. Transwell migration and invasion assays showed that U0126 could significantly inhibited the effect of Sorcin on cell migration and invasion capacity in HepG2Sorcin cells (Fig. 4D1), while it had little effect on HepG2Vector cells in which Sorcin expression and ERK activation is low (Fig. 4D1). Similarly, U0126 treatment obviously inhibited the migration and invasion capacity of HCCLM3shcontrol cells with high Sorcin expression and high level of p-ERK, but it had little effect on HCCLM3shSorcin cells in which Sorcin expression and p-ERK level is low (Fig. 4D2). In addition, U0126 treatment obviously reduced the effect of Sorcin on the proliferation and colony formation capacity in Sorcin-overexpressed cells HepG2Sorcin (Supplementary Fig. 6A1 and B1) or high Sorcin expression cells HCCLM3shcontrol (Supplementary Fig. 6A2 and B2), but it had little effect on HepG2Vector cells (Supplementary Fig. 6A1 and B1) or HCCLM3shSorcin (Supplementary Fig. 6A2 and B2). Collectively, these results indicate that Sorcin promotes HCC invasion and metastasis by activating ERK signaling.
Sorcin Enhances Metastasis by Activating ERK Signaling via Facilitating EMT
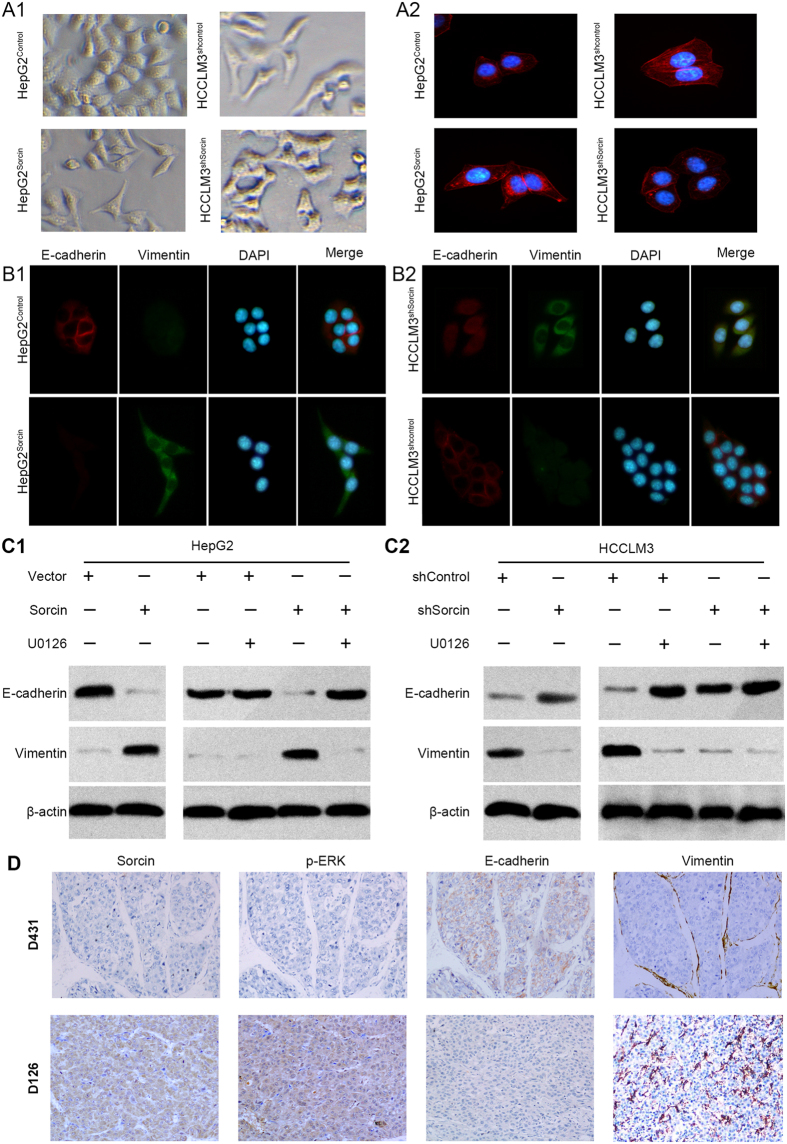
EMT, by which HCC cells gain cell motility and invasiveness, is defined by loss of epithelial cell polarity and E-cadherin expression, and by the acquisition of fibroblastic mesenchymal morphology and Vimentin expression21. It was observed that the HepG2Sorcin cells exhibited spindle-like mesenchymal morphology, while HepG2Vector cells mostly look like epithelial cobblestone appearance (Fig. 5A1). On the contrary, HCCLM3shSorcin cells changed to an epithelial phenotype as compared with mesenchymal like HCCLM3shControl cells (Fig. 5A1). F-actin immunofluorescence staining was used to analyze the cytoskeleton. Immunofluorescence (IF) analysis showed HepG2Sorcin cells presented the appearance of F-actin fibers comparing with HepG2Vector cells (Fig. 5A2). Inversely, HCCLM3shSorcin cells exhibited shrinkable F-actin fibers relative to HCCLM3shcontrol cells (Fig. 5A2). Thus, these indicated that Sorcin might be associated with EMT in HCC.
Figure 5.
Sorcin Enhances Metastasis by Activating ERK Signaling via Facilitating EMT. (A) Representative phase contrast images for cell morphology showed that Sorcin affected the HCC cell morphology (A1) and F-actin immunofluorescence staining was used to analyze the cytoskeleton in HepG2Sorcin, HCCLM3shSorcin and their control cells (A2). (B) Immunofluorescent (IF) showed the relative expression of E-cadherin (red), Vimentin (green) in HepG2Sorcin with Sorcin overexpression or HCCLM3shSorcin cells with Sorcin knockdown, their corresponding control cells. (C) Western blot analysis of E-cadherin and vimentin expressions in HepG2Sorcin, HCCLM3shSorcin and their control cells with/without U0126 treatment. (D) Representative IHC images of Sorcin, p-ERK, E-cadherin and Vimentin expression in HCC tissues. Magnification: 100×.
To further study the role of Sorcin in EMT in HCC, immnunofluorescence (IF) analysis revealed that overexpression of Sorcin in HepG2 cells significantly increased the expression level of mesenchymal marker Vimentin, but dramatically reduced the expression level of epithelial marker E-cadherin (Fig. 5B1). Conversely, knockdown of Sorcin in HCCLM3 significantly reduced the expression level of mesenchymal marker Vimentin, but significantly increased the expression level of epithelial marker E-cadherin (Fig. 5B2). Similar with the data of IF, western blot also showed that overexpression of Sorcin increased the expression of Vimentin and decreased the expression of E-cadherin (Fig. 5C1), whereas down-regulation of Sorcin decreased the expression of Vimentin and increased the expression of E-cadherin (Fig. 5C2). IHC analysis of orthotopic liver xenograft tumors of mice exhibited the similar changes of these EMT markers and Sorcin in protein expression (Supplementary Fig. 7A and B). Furthermore, high Sorcin expression co-located with low E-cadherin and high vimentin expression in HCC tissues was observed by IHC (Fig. 5D). A positive correlation of the expression of Sorcin with the expression of Vimentin and a negative correlation of the expression of Sorcin with the expression of E-cadherin was also observed in the HCC tissues (Supplementary Table 2). These results suggested that Sorcin could promote HCC invasion and metastasis via EMT.
Growing evidence has shown that EMT can be triggered by activation of ERK pathway18, 22. Further, we asked whether Sorcin promoted EMT through activation of ERK pathway. Interestingly, it was observed by western blot assays that U0126 treatment reversed the effect of Sorcin on decrease in E-cadherin expression, and increase in vimentin expression in HepG2Sorcin cells (Fig. 5C1) or HCCLM3shcontrol (Fig. 5C2), but they were not observed in low Sorcin expression cells HepG2Vector (Fig. 5C1) or HCCLM3shsorcin (Fig. 5C2) with relative low level of ERK activation, suggesting that activation of ERK is involved in Sorcin-induced EMT. IHC analysis of liver orthotopic transplantation tumors derived from HepG2Sorcin cells, with mesenchymal tissue phenotype, exhibited high level of p-ERK, whereas liver orthotopic transplantation tumors derived from HepG2Vector cells, with epithelial tissue phenotype, exhibited low level of p-ERK (Supplementary Fig. 7B). On the contrary, orthotopic liver exnograft tumors derived from HCCLM3shSorcin cells, with mesenchymal tissue phenotype, exhibited high level of p-ERK, whereas orthotopic liver exnograft tumors derived from HCCLM3shcontrol cells, with epithelial tissue phenotype, exhibited high level of p-ERK (Supplementary Fig. 7B). Moreover, IHC showed that Vimentin, p-ERK expressions level was high in HCC tissues with high level of Sorcin expression, but they were low in tumors with low expression of Sorcin (Fig. 5D). In contrast, the E-cadherin expression was opposite (Fig. 5D). Furthermore, the correlation analysis revealed Sorcin expression was positively correlative with p-ERK level in HCC samples (Fig. 4C2,3). p-ERK level reversely correlated with E-cadherin expression and positively with Vimentin in HCC tissues (Supplementary Table 3). Taken together, these data indicate that Sorcin can promote EMT by activating ERK pathway.
Discussion
In the present study, we demonstrated that both Sorcin mRNA and protein levels were significantly elevated in tumors compared with ANLTs in HCC. Elevated Sorcin expression was found to be positively correlated with the metastatic potential of HCC cells, suggesting Sorcin might play a role in HCC metastasis. Further analysis of the association of Sorcin expression with the clinicopathological characteristics in HCC patients revealed that Sorcin overexpression was significantly correlated with more aggressive clinicopathological characteristics including multiple tumor nodules, poor differentiation, MVI, advanced TNM stage and BCLC stage. Kaplan-Meier analysis showed that the HCC patients with high Sorcin expression in general had worse prognosis than those with low expression. And a multivariable Cox regression analysis indicated that high Sorcin expression is an independent risk factor for recurrence and survival. Notably, we proved that high Sorcin expression was associated with early recurrence. Stratifying the patients into subclinical group by MVI, the prognostic significance of Sorcin was still found to be existed in non-MVI groups. Consequently, our results suggested that the Sorcin expression could be used as a useful indicator for prognostic assessment, especial for early HCC that prognosis is very difficult to predict using conventional clinical indexes, which would substantially improve assessment of tumor prognosis and thus guide therapeutic strategy.
Our results also have shown that overexpression of Sorcin in HCC cells significantly enhanced cell proliferation, migration and invasion in vitro, and facilitated tumor growth and metastasis in vivo, whereas knockdown of Sorcin in HCC cells significantly decreased cell proliferation, migration and invasion in vitro, and inhibited tumor growth and metastasis in vivo. These results consisted with the involvement of Sorcin in progression in other malignancies11, 13, suggesting that Sorcin possesses oncogenic properties in HCC.
Having identified the role of Sorcin in HCC, we continued to explore the mechanism that Sorcin promote metastasis of HCC. With a multi-pathway reporter array, we screened out ERK signaling. ERK signaling has been widely associated with cell proliferation, differentiation, migration23. The core components of ERK signaling are ERK1 and ERK224, and can be activated by stimuli, including growth factors, cytokines, ligands for heterotrimeric guanine nucleotide–binding protein (G protein)–coupled receptors, transforming agents, carcinogens, et al.23, 24. Our results also provide a link between Sorcin and activation of ERK1/2. Data showed that the level of phosphorylated ERK1/2 was increased when Sorcin was up-regulated in HCC cells. In contrast, an obvious decrease of phosphorylated ERK1/2 was observed in Sorcin knockdown cells. U0126, an effective ERK pathway inhibitor, effectively decreased the levels of phosphorylated ERK induced by Sorcin. By functional experiment, we observed that U0126 treatment obviously reduced the effect of Sorcin on the invasiveness and proliferation in Sorcin-overexpressed cells HepG2Sorcin or high Sorcin expression cells HCCLM3shcontrol, but it had little effect on HepG2Vector or HCCLM3shSorcin cells. However, previous study found U0126 exerted the inhibition on growth of the parent HepG2 cells25. This inconsistent phenomenon may be associated with the following reasons: one reason, as previous studies reported, HCC cell proliferation is association with multiple signal pathways26, 27. Through selecting stable transfected cells by puromycin for 2 weeks, other signal pathways except for ERK signal pathway in HepG2Vector cells were activated and actively exert theirs function to make HepG2Vector resist cell death and enable cells proliferation. Second reason, HepG2 cells exist intra-cell heterogeneities. As microenvironmental discrepancies have been illuminated to affect cell plasticity and heterogeneity in cancer28, different culture condition (such as selecting stable transfected cells by puromycin for 2 weeks) may result in heterogeneity of HepG2Vector cell proliferation activity after cells passaged for several population doublings, comparing with its parent HepG2 cell that other studies used, though short tandem repeat (STR) DNA fingerprinting was used to authenticate all cell lines before the study. Third reason, the introduction of puromycin-resistant gene might make the HepG2Vector cells insensitive to U0126. Accumulated evidence suggests that activation of ERK1/2 is also linked to an imbalance of intracellular Ca2+ levels29, 30. Sorcin, a Ca2+-binding protein with multiple E-F hand domains, regulates intracellular Ca2+ homeostasis by relocates Ca2+ from the cytoplasm to the sarcoplasmic reticulum31. However, additional researches regarding a role of Sorcin-induced disruption of Ca2+ homeostasis linked to the activation of ERK in HCC are required.
EMT has been generally considered as a hallmark of cancer and found to play a crucial role in facilitating cancer cell invasion and migration21. In this study, we found ectopic Sorcin expression changed the epithelial morphology cell to mesenchymal phenotype, decreased the epithelial marker expression and increased the mesenchymal markers expression. However, Sorcin knockdown showed opposite impacts. These findings were confirmed by analysis of the expressions of Sorcin, E-cadherin and vimentin in xenograft tumors in vivo. Data showed that comparing with HepG2Vector cell-derived tumors tissues, the tissues of HepG2Sorcin cell-derived tumors showed decrease in E-cadherin staining and increase in vimentin staining. On the contrary, orthotopic liver xenograft tumors derived from HCCLM3shSorcin cells exhibited high level of E-cadherin expression and low level of vimentin expression, whereas orthotopic liver xenograft tumors derived from HCCLM3shcontrol exhibited low level of E-cadherin expression and high level of vimentin expression. Obviously, there were more fibroblast-like cells with positive vimentin staining in the tissues of HepG2Sorcin cell-derived tumors than in those of HepG2Vector cell-derived tumors, while the number of fibroblast-like cells with positive vimentin staining in tissues of HCCLM3shSocrin cell-derived tumors was dramatically smaller than in those of HCCLM3shcontrol cell-derived tumors. It has been proposed that fibroblast-like cells, named tumor-associated fibroblasts (TAF) in tumor microenvironment32, undergo an epithelial-mesenchymal transition (EMT) of carcinoma cells, during which cancer cells lose their epithelial properties and acquire a mesenchymal phenotype that consequently favors increased invasive and migratory capabilities. From above all, the data indicated that Sorcin could promote EMT in vivo. Alternatively, TAF may be recruited by cancer cells from resident fibroblasts or from circulating mesenchymal progenitor cells of bone marrow origin33. Indeed, vimentin, a major type III intermediate filament protein is also expressed in endothelial and other mesenchymal cells34, so part of vimentin staining could exist in cells of tumor blood vessels or TAF recruited by tumor cells. However, distinct recruited populations of stromal fibroblasts usually morphologically identified as fibroblasts surrounding the tumors displayed a thicker capsule with a diffuse border with the tumor parenchyma35. So the effect of Sorcin on EMT could be clearly observed as these vimentin-positive fibroblastic cells were not considered in semi-quantitative evaluation under a microscope. By this method, the effect of Sorcin on EMT was further confirmed by analysis of the expressions of Sorcin, E-cadherin and vimentin in HCC tissues. Growing evidence has demonstrated that EMT can be triggered by activation of ERK pathway36, 37. We also provide the evidence that Sorcin could activate ERK pathway, and thus induce EMT.
In conclusion, our study has identified for the first time the frequently aberrant expression of Sorcin in HCC tissues by quantitative real-time PCR, western blot and IHC. This expression pattern was associated with aggressive clinicopathological characteristics and poor prognosis in HCC. Furthermore, we have demonstrated that Sorcin could promote metastasis via activating ERK signaling in HCC. Collectively, our data indicates that Sorcin plays an important role in metastasis of HCC and provides a novel target for HCC drug discovery.
Methods
HCC Samples
The study was approved by the Ethics Committee of the institutional review boards of The First Affiliated Hospital of Nanchang University, The First Affiliated Hospital of Sun Yat-Sen University, Affiliated Cancer Hospital of Xiangya School of Medicine of Central South University, Xiangya Hospital of Central South University. Prior informed consent was obtained from all participants, and it was conducted in accordance with the Declaration of Helsinki and current ethical guidelines. Three cohorts of HCC samples were enrolled in this study (Supplementary Fig. 1). Firstly, 60 pairs of frozen fresh HCC tumor tissues and corresponding adjacent nontumorous liver tissues (ANLTs) were collected after radical surgical resection at the Department of General Surgery, The First Affiliated Hospital of Nanchang University between January 2012 and December 2014. In addition, normal liver tissues were obtained from 5 patients with hepatic hemangioma for hepatic resection at the same period. These tissues used to screen the expression of Sorcin mRNA and protein were set as screening cohort. Secondly, formalin-fixed, paraffin-embedded paired HCC samples (including tumors and ANLTs) obtained from 160 HCC patients undergoing radical surgical resection at the Department of General Surgery, the First Affiliated Hospital of Nanchang University from January 2006 to December 2010 were set as training cohort. Another HCC sample cohort containing 120 multicenter samples (including HCC tumors and ANLTs) from patients who underwent resection between June 2007 and June 2010 (including 40 patients at the Department of Surgery, The First Affiliated Hospital of Sun Yat-Sen University, 40 patients at Department of Pathology, Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, and 40 patients at Department of Surgery, Xiangya Hospital, Central South University) was set as validation cohort. These two independent cohorts of subjects were used for prognostic study according to ReMARK guidelines for reporting prognostic biomarkers in cancer14. The inclusion criteria used for all samples enrolled in the cohorts were histopathologically diagnosed, had completed clinicopathologic and follow-up data, and without anticancer therapies or distant metastasis before the operation. Clinicopathologic characteristics of the patients in three cohorts were shown in Supplementary Table 1.
Prognostic Study
All HCC patients were regularly followed-up by the experienced and well-trained researchers. The follow-up period was defined as the interval between the date of operation and that of the patient’s death or the last follow-up. The median follow-up was 38.5 months (range 3.0–108.0 months) for training cohort and 36.5 months (range 3.0–96.0 months) for validation cohort. Deaths from other causes were treated as censored cases. The recurrence and metastasis was surveillance by clinical examination, serial monitoring of alpha-fetoprotein (AFP) levels and ultrasonography (US) or computed tomography (CT) or magnetic resonance imaging (MRI) scan at a 3 months’ interval. Recurrence and metastasis were diagnosed by clinical examination, serial AFP level, and US or CT or MRI scan. Disease-free survival (DFS) was defined as the length of time after liver resection during which a patient survived without sign of HCC recurrence or metastasis. Overall survival (OS) was defined as the interval between operation and death or between operation and the last observation for surviving patients. Data of conventional clinical and pathological variables were also collected for analysis, including gender, age, serum AFP level, HBsAg, liver cirrhosis, Child-Pugh classification, tumor number, tumor size, capsular formation, microvascular invasion (MVI), Edmondson-Steiner grade, TNM stage and BCLC stage. MVI was defined as tumor cells forming a thrombus in peritumoral vessels, can only be assessed after careful histological assessment of the whole surgical specimen15, 38. The follow-up data were regularly updated in the database for each patient. Patients alive at the end of follow up or dead from causes without sign of recurrence or metastasis were censored.
Cell Lines
PLC/PRF/5, Hep3B and HepG2 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA). L02 and Bel7402 cells were gifted by the Liver Cancer Institute of Xiangya hospital, Central South University (Changsha, China). MHCC97-H and HCCLM3 cells were gifted from the Liver Cancer Institute of Fudan University (Shanghai, China). Short tandem repeat (STR) DNA fingerprinting was used to authenticate all cell lines before the study. All cell lines were routinely cultured with the high glucose DMEM supplemented with 10% fetal bovine serum, and maintained in 5% CO2 humidified incubator at 37 °C.
Vector Construction and Transfection
The lentiviral vectors (LV) encoding short hairpin RNAs (shRNAs) for Sorcin knockdown, and LV encoding Sorcin gene were purchased from OriGene Technologies Incorporation (Rockville, MD). The sequences of three shRNAs for Sorcin knockdown were as follow: Sorcin-shRNA-Seq. 1: Sense: 5′-CGACTACATCGCCTGCGTCAAACTGA-3′. Sorcin-shRNA-Seq. 2: Sense: 5′-TGACACAGTCTGGCATTGCTGGAGGATAC-3′. Sorcin-shRNA-Seq. 3: Sense: 5′-GTTACTTTGCTGTAGCTGGACAGAT-3′. HCCLM3 cells were transfected with lentiviral vectors encoding the shRNAs, and HepG2 cells were transfected with lentiviral vectors encoding the human Sorcin gene. An empty vector was used as the negative control and was designated as LV-control. The lentiviral vectors were transfected into the HCC cells with a proper multiplicity of infection (MOI). At 48 h after transfection, 2.5 μg/ml puromycin (OriGene) was added, and the cells were incubated for 2 weeks to select stable transfected cells. Overexpression or down-regulated expression of Sorcin was confirmed by qPCR and western blot (Supplementary Fig. 4B1,2). The inhibitory efficiency of three shRNAs was validated and the Sorcin-shRNA-Sequence2 (named shSorcin in the figures) was adopted for subsequent study because of highly effective inhibition of Sorcin expression in HCCLM3.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from HCC cell lines or fresh frozen tumor specimen by using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. qRT-PCR was performed using the SYBR® Green Realtime PCR Master Mix assay kit (Toyobo, Osaka, Japan) according to the manufacturer’s instructions. The primers for Sorcin were as follows: forward, 5′-GGTGATCTTTCCATTGGTG-3′; reverse, 5′-TCCGCTGTATGGTTACTTTG-3′. GAPDH was used as a control using the following primers: forward, 5′-GCACCGTCAAGGCTGAGAAC-3′; reverse, 5′-TGGTGAAGACGCCAGTGGA -3′. The results were analyzed using the 2−ΔΔCt method as the following formula: ΔΔCt = ΔCtT−ΔCt ANLT, ΔCt = CtSorcin−CtGAPDH.
Western blot
Total proteins were extracted. After protein concentration being determined, the same weight of protein of each sample separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto PVDF membranes (Millipore, Bedford, MA). The blotted membranes were incubated with the primary antibodies and then an appropriate HRP-conjugated secondary antibody (KPL, Gaithersburg, MD) in order. Band was detected with enhanced chemiluminescence regents (Thermo Scientific, Rockford, IL). Beta-actin protein was also determined by using the specific antibody (Sigma, St Louis, MO) as a loading control. Protein expression were quantified by BandScan software (BioRad, Hercules, CA) and defined as the ratio of target protein relative to Beta-actin. Antibodies for Sorcin, ERK, p-ERK, Vimentin, E-cadherin and corresponding secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz Biotechnology, Santa Cruz, CA).
Multi-pathway reporter array
A Cignal Finder 10-Pathway Reporter Array (SABiosciences, Valencia, CA) was employed for finding the potential pathway by which Sorcin contributed to HCC metastasis. Cells were infected with Sorcin expression, Sorcin knockdown or corresponding control lentivirus. Relative firefly luciferase activity was calculated and normalized to the constitutively expressed Renilla luciferase. The assay was performed as protocol provided by the kit.
Immunohistochemistry
The detailed Immunohistochemistry procedures performed as we described before17. Tumor tissues except for surrounding fibrosis or capsule and tumor tissues nearby without necrosis were used to made paraffin-embedded tissues. Paraffin-embedded tissues were sectioned into 4 μm slides. Then, the slides were dewaxed, tissues were rehydrated, and antigen retrieval was performed by microwave-pretreated EDTA buffer (1 mM, pH 8.0) for 10 minutes. After blocking, the slides were incubated for Sorcin antibody (Santa Cruz Biotechnology) at 4 °C overnight. The slides were incubated with biotin-labeled secondary and streptavidin-peroxidase (Zhong-shan Goldenbridge Biotechnology, Beijing, China) for 30 minutes. The samples were developed using 3,3′-diaminobenzidine substrate (Zhong-shan Goldenbridge Biotechnology) and counterstained with hematoxylin (Zhong-shan Goldenbridge Biotechnology). Negative controls were without primary antibody incubation during the procedure. The stainings were examined in a blinded fashion by two independent pathologists. The final immunostaining score (IS) defined by the consistency for the grading by two pathologists. The expression levels of Sorcin were scored based on staining intensity (SI) and percentage of positive cells (PP) using the immunostaining score as described before39. SI was classified four grades: 0, negative; 1, weak; 2, moderate; 3, strong. PP was defined into five categories: 0, 0% positive cells; 1, 0–25% positive cells; 2, 25–50% positive cells; 3, 50–75% positive cells, and 4, 75–100% positive cells. IS = SI × PP. For categorization of the continuous Sorcin values into low and high, we chose a commonly used cutoff point for the analysis (range 0–12, cut point ≤3 versus >3) as previous study described39. For ERK, p-ERK, vimentin, E-cadherin, the procedures as Sorcin and immunostaining score were adopt as described elsewhere40. For vimentin expression scoring, vimentin-positive fibroblast-like cells in surrounding fibrosis or capsule and vimentin-positive vascular cells were not considered in semi-quantitative evaluation. Antibodies for ERK, p-ERK, vimentin, E-cadherin were all purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell Proliferation and Colony Formation Assays
Methyl thiazolyl tetrazolium (MTT) assays were used to determine the level of cell proliferation. For MTT assays, cells were seeded into each well of 96-well plates at a density of 5 × 103 cells/well. Three wells of each group were detected every day. 100 μl fresh medium containing MTT (Sigma, St Louis, MO) 0.5 mg/ml was put into each well and incubated at 37 °C for 4 hrs, then the medium was replaced by 100 μl of DMSO and shaken at room temperature for 10 mins. The absorbance was measured at 570 nm. For colony formation assays, 500 cells were seeded into 35 mm dishes (Corning Costar Corp, Corning, NY) and cultured in 5% CO2 for 2 weeks at 37 °C. The number of colonies per dish was counted after staining with crystal violet. Only positive colonies (diameter >40 um) in the dishes were counted and compared41. These experiments were performed in triplicate.
Transwell Assays
Transwell migration and invasion assays were determined to HCC cell migration and invasion ability separately. For transwell invasion assays, the upper chamber of the insert was plated with matrigel (BD Biosciences, Franklin Lakes, NJ). But for transwell migration assays, the upper chamber of the insert was without matrigel (BD Biosciences). Briefly, after being preincubated with Mitomycin-C (10 μg/ml) for 1 h at 37 °C to suppress HCC cell proliferation, about 1 × 105 cells in serum-free medium were placed into the upper chamber of the insert. After 24 hours of incubation in 5% CO2 at 37 °C, the cells in upper chamber were removed with cotton swabs, following fixed by 20% methanol, and then stained with a solution containing 0.1% crystal violet (Beyotime Institute of Biotechnology, Beijing, China). The number of cells that adhered to the lower membrane of the inserts was counted. For each experimental group, the assays were performed in triplicates, and five random fields were chosen for analysis.
Cellular immunofluorescence
Cellular immunofluorescence was performed as we described previously17. Cells were seeded into the 6-well culture plate (Corning Costar Corp) to prepare for performing cell immunofluorescence (IF). After incubating with primary antibodies, cells then incubated with corresponding fluorescence labeled secondary antibody. After stained with DAPI (Beyotime Institue of Biotecchnology, Jiangsu, China), the slides were photographed using the inverted fluorescence microscope TE-2000S (Nikon, Tokyo, Japan). Primary antibodies for E-cadherin, vimentin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All fluorescence labeled secondary antibody were obtained from Beyotime Institute of Biotechnology (Shanghai, China). Rhodamine-conjugated phalloidin (Beyotime Institute of Biotechnology) was used to analyze to cytoskeleton. Cells grown on cover slides were fixed, then incubated with rhodamine-conjugated phalloidin (Beyotime Institue of Biotecchnology) and followed by DAPI (Beyotime Institue of Biotecchnology).
In Vivo Metastatic Assays
For the in vivo metastatic assays, HCC metastatic model in mice was constructed as previously described42. Briefly, 5 × 106 HCC cells were injected subcutaneously into the left upper flank regions of nude mouse (4 weeks of age, male, BALB/c). After 35 days, the subcutaneous tumors were removed and then divided into commensurate fragments of approximate 1 mm3, then implanted into the liver of nude mouse (6 mice in each group). After 7 weeks, mice were killed, and all livers and lungs were harvested. The tumor size was calculated as follows: tumor volume (mm3) = (L × W2)/243, where L = long axis and W = short axis. Then all livers and lungs were fixed with 10% phosphate-buffered neutral formalin, sectioned serially and stained with hematoxylin and eosin (H&E) for histological examination. Tumor tissues except for surrounding fibrosis or capsule and tumor tissues nearby were used to made paraffin-embedded tissue for immunohistochemistry. The expression of Sorcin, p-ERK, Vimentin, E-cadherin in orthotropic liver tumor tissues was also determined by immunohistochemistry. Mice were performed and housed in the Animal Institute of Nanchang University according to the protocols approved by the Medical Experimental Animal Care Commission.
Statistical Analysis
All data were analyzed using the statistical software SPSS 18.0 for Windows (SPSS Inc., Chicago, IL). The differences between groups were analyzed by Student’s t test between two groups or by one-way analysis of variance (ANOVA) in more than two groups when the variance is homogeneous. If the variance is not homogeneous, the differences between groups were analyzed by Mann-Whitney U test between two groups or by Kruskal-Wallis H test in more than two groups. χ2 analysis was used to analyze the correlation between Sorcin expression and clinicopathologic features. Spearman’s rank analysis was used to analyze the correlations between different protein expressions level. Survival curves were constructed by the Kaplan-Meier method and compared by the log-rank test. The Cox proportional hazards regression model was established to identify independent factors for overall survival (OS) and disease-free survival (DFS) of HCC patients. All the tests were two-tailed and P < 0.05 was considered statistically significant.
Electronic supplementary material
Acknowledgements
This work was supported by National Natural Science Foundation of China (81172018) and also partly supported by the Natural Science Foundation of Jiangxi, China (20141511040001).
Author Contributions
Taiyuan Li, Xiong Lei designed experiments. Xiong Lei, Yahang Liang, Jian Chen, Shuai Xiao, Jian Lei, Jinzhong Duanmu, Qunguang Jiang, Dongning Liu, Cheng Tang performed experiments and analyzed data. Taiyuan Li, Xiong Lei, Jian Chen, Shuai Xiao, Jian Lei, Jianfeng Li provided patient samples and collected data. Taiyuan Li, Xiong Lei wrote and revised the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
A correction to this article is available online at https://doi.org/10.1038/s41598-018-29892-8.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-10365-3
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/2/2018
This paper has been retracted at the request of the authors.
References
- 1.Torre LA, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Yang LY, et al. Mesohepatectomy for centrally located large hepatocellular carcinoma: Indications, techniques, and outcomes. Surgery. 2014;156:1177–87. doi: 10.1016/j.surg.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Zhong JH, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260:329–40. doi: 10.1097/SLA.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 4.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 5.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394–9. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844–855. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lokuta AJ, Meyers MB, Sander PR, Fishman GI, Valdivia HH. Modulation of cardiac ryanodine receptors by sorcin. J Biol Chem. 1997;272:25333–8. doi: 10.1074/jbc.272.40.25333. [DOI] [PubMed] [Google Scholar]
- 8.Farrell EF, Antaramian A, Rueda A, Gomez AM, Valdivia HH. Sorcin inhibits calcium release and modulates excitation-contraction coupling in the heart. J Biol Chem. 2003;278:34660–6. doi: 10.1074/jbc.M305931200. [DOI] [PubMed] [Google Scholar]
- 9.Tsai FC, Kuo GH, Chang SW, Tsai PJ. Ca2+ signaling in cytoskeletal reorganization, cell migration, and cancer metastasis. Biomed Res Int. 2015;2015:409245. doi: 10.1155/2015/409245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Q, et al. Overexpression of sorcin results in multidrug resistance in gastric cancer cells with up-regulation of P-gp. Oncol Rep. 2011;25:237–43. [PubMed] [Google Scholar]
- 11.Tong W, Sun D, Wang Q, Suo J. Sorcin Enhances Metastasis and Promotes Epithelial-to-Mesenchymal Transition of Colorectal Cancer. Cell Biochemistry and Biophysics. 2015;72:453–459. doi: 10.1007/s12013-014-0486-3. [DOI] [PubMed] [Google Scholar]
- 12.Qu Y, Yang Y, Liu B, Xiao W. Comparative proteomic profiling identified sorcin being associated with gemcitabine resistance in non-small cell lung cancer. Med Oncol. 2010;27:1303–8. doi: 10.1007/s12032-009-9379-5. [DOI] [PubMed] [Google Scholar]
- 13.Hu Y, et al. Sorcin silencing inhibits epithelial-to-mesenchymal transition and suppresses breast cancer metastasis in vivo. Breast Cancer Research and Treatment. 2014;143:287–299. doi: 10.1007/s10549-013-2809-2. [DOI] [PubMed] [Google Scholar]
- 14.McShane LM, et al. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–4. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 15.Pote N, et al. Imaging mass spectrometry reveals modified forms of histone H4 as new biomarkers of microvascular invasion in hepatocellular carcinomas. Hepatology. 2013;58:983–94. doi: 10.1002/hep.26433. [DOI] [PubMed] [Google Scholar]
- 16.Tang ZY, et al. A decade’s studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187–96. doi: 10.1007/s00432-003-0511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei X, et al. Ack1 overexpression promotes metastasis and indicates poor prognosis of hepatocellular carcinoma. Oncotarget. 2015;6:40622–41. doi: 10.18632/oncotarget.5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichikawa K, et al. MCRIP1, an ERK substrate, mediates ERK-induced gene silencing during epithelial-mesenchymal transition by regulating the co-repressor CtBP. Mol Cell. 2015;58:35–46. doi: 10.1016/j.molcel.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Lemieux E, et al. Constitutively active MEK1 is sufficient to induce epithelial-to-mesenchymal transition in intestinal epithelial cells and to promote tumor invasion and metastasis. Int J Cancer. 2009;125:1575–86. doi: 10.1002/ijc.24485. [DOI] [PubMed] [Google Scholar]
- 20.Moreno-Ortega AJ, et al. CALHM1 and its polymorphism P86L differentially control Ca2+ homeostasis, mitogen-activated protein kinase signaling, and cell vulnerability upon exposure to amyloid β. Aging Cell. 2015;14:1094–1102. doi: 10.1111/acel.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 22.OuYang H, et al. MEP1A contributes to tumor progression and predicts poor clinical outcome in human hepatocellular carcinoma. Hepatology. 2016;63:1227–1239. doi: 10.1002/hep.28397. [DOI] [PubMed] [Google Scholar]
- 23.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–44. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 25.Lin S, et al. MEK inhibition induced downregulation of MRP1 and MRP3 expression in experimental hepatocellular carcinoma. Cancer Cell Int. 2013;13:3. doi: 10.1186/1475-2867-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrieux LO, et al. GATA-1 is essential in EGF-mediated induction of nucleotide excision repair activity and ERCC1 expression through ERK2 in human hepatoma cells. Cancer Res. 2007;67:2114–23. doi: 10.1158/0008-5472.CAN-06-3821. [DOI] [PubMed] [Google Scholar]
- 27.Chung, T. W. Hepatitis B viral HBx induces matrix metalloproteinase-9 gene expression through activation of ERKs and PI-3K/AKT pathways: Involvement of invasive potential. The FASEB Journal (2004). [DOI] [PubMed]
- 28.Li L, Wang H. Heterogeneity of liver cancer and personalized therapy. Cancer Letters. 2016;379:191–197. doi: 10.1016/j.canlet.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 29.Wang C, et al. Surfactin-Induced Apoptosis Through ROS–ERS–Ca2+ -ERK Pathways in HepG2 Cells. Cell Biochemistry and Biophysics. 2013;67:1433–1439. doi: 10.1007/s12013-013-9676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Impey S, et al. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–83. doi: 10.1016/S0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 31.Xie X, Dwyer MD, Swenson L, Parker MH, Botfield MC. Crystal structure of calcium-free human sorcin: a member of the penta-EF-hand protein family. Protein Sci. 2001;10:2419–25. doi: 10.1110/ps.ps.36701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 33.Cadamuro M, et al. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology. 2013;58:1042–1053. doi: 10.1002/hep.26384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dave JM, Bayless KJ. Vimentin as an integral regulator of cell adhesion and endothelial sprouting. Microcirculation. 2014;21:333–44. doi: 10.1111/micc.12111. [DOI] [PubMed] [Google Scholar]
- 35.Anderberg C, et al. Paracrine signaling by platelet-derived growth factor-CC promotes tumor growth by recruitment of cancer-associated fibroblasts. Cancer Res. 2009;69:369–78. doi: 10.1158/0008-5472.CAN-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bao, R. et al. miR-101 targeting ZFX suppresses tumor proliferation and metastasis by regulating the MAPK/Erk and smad pathways in gallbladder carcinoma. Oncotarget (2016). [DOI] [PMC free article] [PubMed] [Retracted]
- 37.Lin, Z. H., Wang, L., Zhang, J. B., Liu, Y. & Li, X. Q. MST4 promotes hepatocellular carcinoma epithelial-mesenchymal transition and metastasis via activation of the p-ERK pathway. International Journal of Oncology (2014). [DOI] [PubMed]
- 38.Pote N, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62:848–54. doi: 10.1016/j.jhep.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Su S, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25:605–20. doi: 10.1016/j.ccr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 40.Xu J, et al. SIN1 promotes invasion and metastasis of hepatocellular carcinoma by facilitating epithelial-mesenchymal transition. Cancer. 2013;119:2247–57. doi: 10.1002/cncr.28023. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Echeverria C, et al. In vivo antitumor activity of NVP-AEW541-A novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5:231–9. doi: 10.1016/S1535-6108(04)00051-0. [DOI] [PubMed] [Google Scholar]
- 42.Lei, X. et al. JARID2 promotes invasion and metastasis of hepatocellular carcinoma by facilitating epithelial-mesenchymal transition through PTEN/AKT signaling. Oncotarget (2016). [DOI] [PMC free article] [PubMed]
- 43.Zhang JF, et al. Primate-specific microRNA-637 inhibits tumorigenesis in hepatocellular carcinoma by disrupting signal transducer and activator of transcription 3 signaling. Hepatology. 2011;54:2137–48. doi: 10.1002/hep.24595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.